Abstract
Internalizing pathology is related to alterations in amygdala resting state functional connectivity, potentially implicating altered emotional reactivity and/or emotion regulation in the etiological pathway. Importantly, there is accumulating evidence that stress exposure and genetic vulnerability impact amygdala structure/function and risk for internalizing pathology. The present study examined whether early life stress and genetic profile scores (10 single nucleotide polymorphisms within four hypothalamic-pituitary-adrenal axis genes: CRHR1, NR3C2, NR3C1, and FKBP5) predicted individual differences in amygdala functional connectivity in school-age children (9–14 year olds; N=120). Whole-brain regression analyses indicated that increasing genetic ‘risk’ predicted alterations in amygdala connectivity to the caudate and postcentral gyrus. Experience of more stressful and traumatic life events predicted weakened amygdala-anterior cingulate cortex connectivity. Genetic ‘risk’ and stress exposure interacted to predict weakened connectivity between the amygdala and the inferior and middle frontal gyri, caudate, and parahippocampal gyrus in those children with the greatest genetic and environmental risk load. Furthermore, amygdala connectivity longitudinally predicted anxiety symptoms and emotion regulation skills at a later follow-up. Amygdala connectivity mediated effects of life stress on anxiety and of genetic variants on emotion regulation. The current results suggest that considering the unique and interacting effects of biological vulnerability and environmental risk factors may be key to understanding the development of altered amygdala functional connectivity, a potential factor in the risk trajectory for internalizing pathology.
Keywords: amygdala, functional connectivity, stress, genetics, anxiety
Introduction
Major depressive disorder (MDD) and anxiety disorders are among the most prevalent and disabling psychiatric conditions (Kessler et al., 2005) and are characterized by deficits in emotional and overall adaptive functioning (American Psychiatric Association, 2013). Neuroimaging studies implicate the amygdala as a key region involved in emotional reactivity (e.g. Sergerie, Chochol, & Armony, 2008) and find that successful emotion regulation modulates amygdala reactivity (Lapate et al., 2012; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). Importantly, amygdala structure and function are altered in patients with depression and anxiety (for meta-analyses, see Etkin & Wager, 2007; Hamilton et al., 2012; Hamilton, Siemer, & Gotlib, 2008) and are linked to individual differences in emotional experience and regulation (Abler et al., 2010; Drabant, McRae, Manuck, Hariri, & Gross, 2009). Recent work has examined the functional connectivity of the amygdala with a variety of regions, particularly prefrontal cortex, cingulate cortex, the striatum, and the hippocampus (e.g. Gabard-Durnam et al., 2014; Roy et al., 2009), aiding our understandings of communication between regions that subserve effective emotion reactivity and regulation.
Increasingly in the literature, studies examine disrupted amygdala connectivity as a potential intermediate phenotype between risk factors and psychological outcomes. This type of work aims to identify mechanisms linking risk factors to pathology by understanding the relations between risk factors and more proximal outcomes, like brain function. Particularly, genetic predispositions and individual environmental factors, such as stressful life events, are among the most potent predictors of depression and anxiety onset (e.g. Kendler, Gardner, & Lichtenstein, 2008; Kendler, Hettema, Butera, Gardner, & Prescott, 2003; Kendler, Karkowski, & Prescott, 1999; Kendler, Neale, Kessler, Heath, & Eaves, 1992; Kessler, Davis, & Kendler, 1997). As such, the goal of the current study was to investigate whether stressful life events and/or genetic risk factors that influence stress responses also influence amygdala functional connectivity, which in turn may be a potentially mediating factor in the pathway to emotion regulation impairments and pathology, such as anxiety or depression. Here, we first briefly review the current literature on normative amygdala connectivity to set the stage for understanding alterations in connectivity. We then review the literature on alterations in connectivity associated with internalizing psychopathology, stress exposure, and genetic risk factors.
Normative Amygdala Connectivity
Resting state functional connectivity (rsFC) MRI measures correlations in intrinsic, low frequency fluctuations across brain areas (Biswal, Zerrin Yetkin, Haughton, & Hyde, 1995). Resting state connectivity has been suggested to represent the accumulated history of co-activation of brain areas (e.g. Dosenbach et al., 2007; Fair et al., 2007; Kelly et al., 2009). Many studies examine normative patterns of global rsFC across the brain (e.g. Greicius, Krasnow, Reiss, & Menon, 2003; Power et al., 2011) and provide much useful information about intrinsic functional brain networks and their changes across development (e.g. Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). Recently, several studies have begun to characterize normative rsFC specifically with the amygdala. For example, work in adults suggests that the amygdala shows normative patterns of positive connectivity (i.e. correlations) with a network of regions, including the hippocampus, insula, thalamus, striatum, and medial frontal gyrus (Roy et al., 2009). The amygdala also shows normative negative connectivity (anti-correlation) with a separate network of regions, including the superior frontal gyrus (SFG), middle frontal gyrus (MFG), posterior cingulate, parietal regions, and occipital regions (Roy et al., 2009).
These patterns are similar to regions often co-activated with the amygdala in fMRI studies of emotion processing, congruous with the idea that rsFC represents a history of co-activation (e.g. Dosenbach et al., 2007; Fair et al., 2007; Kelly et al., 2009). Specifically, many of the regions that typically show positive rsFC with the amygdala are seen in meta-analyses to be activated in response to emotional faces (Fusar-Poli et al., 2009) or during emotional memory tasks (Murty, Ritchey, Adcock, & Labar, 2010). Furthermore, many of the regions typically showing negative connectivity with the amygdala are involved in cognitive reappraisal of emotion and down-regulation amygdala activity (Buhle et al., 2013; Frank et al., 2014; Kohn et al., 2013), i.e. activity in regions down-regulating amygdala reactivity tend to be negatively correlated with amygdala activity.
Alterations in Amygdala Connectivity Associated with Internalizing Pathology
Internalizing psychopathology, i.e. affective and anxiety disorders (Krueger, 1999), is typically linked to weakened amygdala connectivity as summarized in Table 1. Particularly, weakened connectivity between the amygdala and regions often showing positive connectivity, e.g. ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), caudate, nucleus accumbens, and orbitofrontal cortex (OFC) is observed in adults or adolescents with generalized anxiety disorder (GAD) or adults with social and/or panic disorders (Etkin et al., 2009; Roy et al., 2013; Hahn et al., 2011). Weakened negative connectivity with ventrolateral PFC is also observed in GAD (Roy et al., 2013). Relatedly, individual differences in state anxiety relate to weakened positive connectivity with vmPFC and weakened negative connectivity with dmPFC (Kim et al., 2011). Similarly, increasing anxiety symptoms have been linked to decreased positive connectivity with dlPFC (Etkin et al., 2009). MDD is also linked to both weakened connectivity with the amygdala, e.g. in an ‘affective’ network of regions including the insula (Veer et al., 2010). Children with a personal (and/or maternal) history of depression show reduced connectivity across networks of positively (e.g. parahippocampal gyrus, putamen) and negatively (e.g. MFG, postcentral gyrus) connected regions (Luking et al., 2011). Several studies also examined functional coupling of the amygdala during emotional tasks (rather than at rest) and similarly noted reduced coupling between the amygdala and the cingulate, hippocampus, insula, putamen, and inferior and middle frontal cortex (Chen et al., 2007; Lui et al., 2001; Matthews et al., 2008). Furthermore, antidepressant treatment may recover amygdala coupling with a variety of these regions and others (Chen et al., 2007). Overall, this weakened connectivity between the amygdala and key regions involved in effective emotional function may contribute to some of the core dysfunctions in internalizing pathology.
Table 1.
Prior Work Examining Alterations in Amygdala Connectivity
| Study | Population Total (Affected) N | Positive Connectivity | Negative Connectivity |
|---|---|---|---|
| Etkin et al., 2009 | 64(16) GAD | ↓normative targets a | ↑ normative targets a |
| Roy et al., 2013 | 35(15) GAD in adolescents b | ↓vmPFC, sgACC, pgACC, caudate, NA; ↑ insula, STG | ↑ vlPFC |
| Hahn et al., 2011 | 37(10) Social and/or panic disorder | ↓mOFC | ↓PCC |
| Kim et al., 2011 | 29 STAI-S | ↓vmPFC | ↑dmPFC |
| Veer et al., 2010 | MDD | ↓insula c | |
| Dannlowski et al., 2009 | 65(34) MDD & MAOA-H c | ↓dACC, dlPFC | |
| Lui et al., 2011 | 108(32) non-refractory MDD (28) refractory MDD | ↓cingulate | |
| Matthews et al., 2008 | 31(15) MDD c | ↑sgACC, ↓ supragenual ACC | |
| Chen et al., 2007 | 38(19) MDD & antidepressant treatment c | ↓hippocampus, putamen, insula, inferior and middle frontal cortices | |
| Luking et al., 2011 | 51(37) MDD and/or maternal MDD in children | ↓STG, ITG, putamen, hippocampus, | ↑ MFG, SFG, precuneus, IPL, postcentral gyrus |
| van der Werff et al., 2012 | 88(44) CEM | ↓ OFC, insula, hippocampus, putamen | ↑ occipital cortex, precuneus |
| Herringa et al., 2013 | 64 CTQ in adolescents | ↓sgACC, postcentral gyrus, ↑dlPFC | |
| Burghy et al., 2012 | 57 cortisol in adolescents | ↓vmPFC | |
| Veer et al., 2012 | 20 cortisol in males | ↓perigenual ACC, medial frontal pole | |
| Gee et al., 2013 | 89(41) early institutional care in children/adolescents | ↓vmPFC | |
| Pezawas et al., 2005 | 94 5-HTTLPR short | ↓ sgACC | ↑ supragenual ACC |
This table summarizes main findings from prior studies citing alterations in amygdala connectivity related to internalizing psychopathology or risk factors. All studies examined adult populations except where noted otherwise. The population column describes the study sample including the total and affected Ns as well as the disorder or risk factor of interest. Increases (↑) or decreases (↓) in connectivity values are noted separated by regions that show positive or negative connectivity in each study.
GAD = generalized anxiety disorder, MDD = major depressive disorder, STAI-S = State-Trait Anxiety Inventory – State, MAOA-H = monoamine oxidase A – high-activity, CEM = childhood emotional maltreatment, CTQ = Childhood Trauma Questionnaire, 5-HTTLPR = serotonin transporter-linked polymorphic region, PFC = prefrontal cortex, ACC = anterior cingulate cortex, STG = superior temporal gyrus, ITG = inferior temporal gyrus, MFG = middle frontal gyrus, SFG = superior frontal gyrus, IPL = inferior parietal lobule, OFC = orbitofrontal cortex
Connectivity was reduced in GAD patients between basolateral and centromedial amygdala seeds and targets identified in a healthy sample but was increased with the other seed’s normative targets
Connectivity with the centromedial amygdala
Connectivity assessed during an emotion task rather than during rest
decreased connectivity in ‘affective’ network defined by independent components analysis
Alterations in Amygdala Connectivity Associated with Stress Exposure
Risk factors for internalizing disorders are also associated with altered amygdala functional connectivity. For example, as noted above, work by Luking et al., (2011) found that children with a personal and/or maternal history of depression (who are at increased risk for future depression) show reductions in amygdala connectivity with regions typically showing both positive or negative connectivity. Other risk key factors for internalizing pathology, e.g. early life stress, are also linked to weakened connectivity. As summarized in Table 1, childhood maltreatment and trauma are associated with decreased negative amygdala connectivity with the precuneus and decreased positive connectivity with the OFC, insula, hippocampus, putamen, sgACC, and postcentral gryus (van der Werff et al., 2012; Herringa, Birn, & Ruttle, 2013). Importantly, this altered connectivity between the amygdala (and hippocampus) and the sgACC partially mediated associations between maltreatment and internalizing symptoms (Herringa et al., 2013). In contrast, other work suggests that higher cortisol levels predict stronger negative amygdala-ventromedial PFC connectivity among females, mediating effects of early life stress on connectivity (Burghy et al., 2012). Another study related elevated baseline cortisol among adults to stronger negative amygdala-mPFC connectivity (Veer et al., 2012). Finally, other work suggests that deprivation during early institutional care predicts more negative amygdala-mPFC connectivity, mediated by cortisol (Gee, Gabard-Durnam, et al., 2013a).
Alterations in Amygdala Connectivity Associated with Genetic Variants
Commonly occurring genetic variants, like the short allele of serotonin transporter promoter polymorphism (Pezawas et al., 2005) and the higher active alleles (3.5 or 4 repeats) of the monoamine oxidase A variable number tandem repeat polymorphism (Dannlowski et al., 2009), are suggested to exert effects on amygdala connectivity. Specifically, individuals carrying these ‘risk’ variants (these alleles have shown main effects or interactions with environmental factors in predicting increased risk for psychopathology) tend to have weaker amygdala connectivity with regions of the ACC and PFC.
The Hypothalamic-Pituitary-Adrenal (HPA) Axis, Genetic Variation, and Internalizing Pathology
As described above, the small literature relating genetic variants to rsFC centers mainly on the monoamine/serotonin system. However, genetic variants influencing the HPA axis, which is activated by stress and leads to cortisol release, is another potentially important system of interest given the links between stress, pathology, and amygdala connectivity described above. Internalizing pathology is linked to alterations in HPA axis functioning (e.g. Heuser et al., 1994) and genetic variants in this system. Particularly, four genes of interest here – CRHR1, NR3C2, NR3C1, and FKBP5 – are linked to depression (Kuningas et al., 2007; Lavebratt et al., 2010; Lekman et al., 2008; Lui et al., 2006; van West et al., 2005), depression with comorbid anxiety (Minelli et al., 2013), and anxiety disorders, like PTSD (Binder, 2009; Xie et al., 2010). These genes are integral to the reactivity and regulation of the HPA axis and therefore cortisol function. Thus, these genes may themselves be risk factors for psychopathology or may moderate effects of life stress. Prior work also relates variants in these genes to decreases in volume and increases in reactivity to emotional stimuli in the amygdala and hippocampus (e.g. Pagliaccio et al., 2013; 2015), which could suggest an impact on amygdala connectivity as well. While these putative relationships have not yet been tested, they build on prior work relating cortisol to amygdala connectivity (e.g. Burghy et al., 2012; Gee, Gabard-Durnam, et al., 2013a; Veer et al., 2012) and showing that corticosteroid can induce weakening of amygdala connectivity (Henckens, van Wingen, Joëls, & Fernández, 2012).
Summary and Goals
Overall, the networks of regions showing either positive or negative connectivity with the amygdala largely include regions involved in emotion reactivity and regulation, respectively, as per recent meta-analyses. Further, weakened amygdala connectivity with both networks is found with internalizing disorders and environmental/genetic risk factors. While a variety of studies have examined how environmental or genetic factors predict alterations in amygdala connectivity, there are no prior studies to our knowledge that examine the interaction of such factors predicting alterations in amygdala connectivity or testing whether such alterations predict future internalizing disorder symptoms or emotional functioning. Importantly, the role of gene x environment or stress-diathesis interactions is being increasingly considered in studies of risk for psychopathology (e.g. Belsky & Pluess, 2009; Caspi & Moffitt, 2006; Caspi et al., 2003; Moffitt, Caspi, & Rutter, 2006; Rutter, Moffitt, & Caspi, 2006) as these interactions can account for significant variance over and above main effects of genotype and environment. In parallel, intermediate phenotypes, like brain structure and function, are an increasing focus in the literature as they may provide a powerful means of elucidating the mechanistic pathway from biological and/or environmental risk factors to psychiatric outcomes (for examples of studies examining interactions of stress and HPA axis genes on brain structure/function, see Bogdan, Williamson, & Hariri, 2012; Pagliaccio et al., 2013; 2015; White et al., 2012).
Given these considerations, the goal of the current study was to test whether stress-related environmental and/or genetic risk factors predicted amygdala connectivity in school-age children (N=120 9–14-year-olds) and whether connectivity patterns related to psychiatric outcomes. First, we characterized the normative rsFC patterns observed in this age range. Second, we tested three effects on amygdala connectivity: a main effect of early life stress exposure, a main effect of HPA axis genetic variants, and their interaction. Based on prior literature, we hypothesized that both early life stress exposure and genetic variants that modulate HPA axis function would predict weaker connectivity between the amygdala and regions typically showing negative connectivity, such as the dorsomedial and lateral PFC and the cingulate, and typically showing positive connectivity, such as the hippocampus and striatum. Finally, in an exploratory analysis, we examined whether altered amygdala connectivity patterns predicted psychopathology or emotion regulation concurrent to scan or at a 1-year follow-up.
Methods
Participants
A subsample of participants enrolled in the prospective longitudinal Preschool Depression Study (PDS; total N=306) were included in the current analyses. The PDS is being conducted by the Washington University in St. Louis School of Medicine Early Emotional Development Program (WUSM EEDP); its broad goals are to explore clinical and neural outcomes related to preschool-onset depression. The details of the study methods were published previously (see, Luby, Si, Belden, Tandon, & Spitznagel, 2009). Briefly, 3- to 5-year old children and their primary caregivers were recruited from the St. Louis metropolitan area to complete in-depth clinical interviews annually and three neuroimaging sessions with the children. The first imaging wave occurred when children were 7–12 years of age; the current study examines data from the second wave of imaging when children were 9–14 years old (current subsample: mean=11.21 ± 1.23 years). Pubertal status at the time of scan was assessed using the Tanner Pubertal Staging Questionnaire (Tanner, 1955). Parental written consent and child assent were obtained prior to study participation and the Institutional Review Board at Washington University approved all experimental procedures.
Of the 182 children who completed the second scan wave, 6 were excluded for poor quality structural images or missing functional connectivity scan runs. 32 children were excluded during due to excessive head motion (see fMRI pre-processing section below). Nine children were excluded for missing key measures of interest. Finally, an additional 15 participants who identified as ethnicities other than White or African American were removed from the current analysis to reduce population stratification leaving a final sample size of 120 participants (65 White, 55 African American), i.e. allowing us to control for and compare two ethnic groups. Further, while self-reported ancestry works well as a control with White and African American participants, we do not have ancestry informative markers to distinguish the ancestry of the remaining participants who identified as other ethnicities. Thus, removing these additional participants helps to minimize spurious genetic association signals (population admixture) that can arise from as natural variations in allele frequencies across ethnic groups.
Diagnostic Assessments
Trained WUSM EEDP staff conducted up to seven in-person assessments (median=6 assessments) with participants and their parents/guardians from study enrollment through the time of scan and most children had completed a follow-up assessment ~1 year after the scan session (13.73±4.65 months). A reliable and age-appropriate semi-structured parent-report diagnostic interview was used to assess psychiatric symptoms in children younger than 8 years of age, the Preschool-Age Psychiatric Assessment (PAPA; Egger, Ascher, & Angold, 2003). The Childhood and Adolescent Psychiatric Assessment (CAPA; Angold & Costello, 2000) was used when children were 8 years or older, which also includes child-report. Interviews were audiotaped, reviewed for reliability, and calibrated for accuracy (Luby et al., 2009). These measures show good test-retest reliability and strong construct validity in prior work (Angold & Costello, 1995, 2000). This data was used to assess whether children met criteria for relevant psychiatric disorders through the time of scan (Table S1). Data from the CAPA was also used to create continuous measures of depressive disorder (41 items), anxiety disorder (31 items: generalized anxiety disorder, separation anxiety, post-traumatic stress disorder), and externalizing disorder (41 items: attention-deficit hyperactivity disorder, conduct disorder, oppositional defiant disorder) symptom severity. These scores indicate the sum number of symptoms endorsed for each domain.
Data from the PAPA/CAPA were also used to assess the child’s experience of stressful and traumatic life events from birth through the scan session (a full list of events and their frequencies is reported in Table 2). We examined the sum count of instances of these life events in the current analyses. As we had no a priori method for weighting individual events and as counts of stressful versus traumatic events were highly correlated (r(118) = 0.443, p<0.001), all events were summed equally. Finally, parents reported on their child’s emotion regulation abilities using the Emotion Regulation Checklist (ERC; Shields & Cicchetti, 1997) at the assessment wave closest to scan and at the follow-up assessment. We focused on the emotion regulation subscale of the ERC (8 items – 4-point Likert scale) where higher sum scores indicated better emotion regulation skills in the children (Cronbach’s α = 0.810). A summary of demographic and clinical characteristics of the sample is presented in Table 3.
Table 2.
Reported Instances of Stressful and Traumatic Life Events
| Stressful Life Events | Count of Participants by # of Instances | |||||||
|---|---|---|---|---|---|---|---|---|
| Missing | 0 | 1 | 2 | 3 | 4 | 5 | 6+ | |
| Broke Up with Best Friend | 26 | 86 | 8 | |||||
| Broke Up with Boy/Girlfriend | 1 | 114 | 4 | 1 | ||||
| Change Daycare/School | 0 | 18 | 40 | 34 | 14 | 7 | 4 | 3 |
| Conflict Between Parents/Family | 28 | 82 | 2 | 4 | 1 | 1 | 2 | |
| Death of Pet | 0 | 26 | 17 | 1 | ||||
| Forced Separation from Home | 0 | 112 | 6 | 1 | 1 | |||
| Lived/Attended School in Unsafe Environment | 0 | 108 | 10 | 1 | 1 | |||
| Loss of Home Without Family Separation | 28 | 86 | 6 | |||||
| Lost Significant Person Through Moving | 0 | 78 | 34 | 6 | 1 | 1 | ||
| Moving House | 0 | 28 | 32 | 21 | 18 | 12 | 4 | 5 |
| New Child in Home | 0 | 38 | 38 | 31 | 4 | 6 | 2 | |
| New Parental Figure | 0 | 92 | 21 | 4 | 3 | |||
| Parental Arrest | 0 | 92 | 19 | 7 | 1 | 1 | ||
| Parental Divorce | 0 | 102 | 16 | 2 | ||||
| Parental Hospitalization | 28 | 37 | 40 | 9 | 2 | 3 | 1 | |
| Parental Separation | 0 | 77 | 33 | 8 | 2 | |||
| Reduction in Standard of Living | 0 | 81 | 21 | 12 | 6 | |||
| Separation From Parent (1 week or more) | 28 | 55 | 26 | 6 | 2 | 3 | ||
| Traumatic Life Events | ||||||||
| Accident or Crash with Automobile, Plane, or Boat | 0 | 92 | 26 | 2 | ||||
| Accidental Burning, Poisoning, or Drowning | 9 | 80 | 9 | 2 | ||||
| Attacked by an Animal | 29 | 84 | 7 | |||||
| Death of Adult Loved One | 1 | 29 | 21 | 23 | 21 | 11 | 7 | 7 |
| Death of Sibling or Peer | 0 | 97 | 17 | 4 | 2 | |||
| Diagnosed with Physical Illness | 0 | 92 | 23 | 4 | 1 | |||
| Domestic Violence | 114 | 3 | 1 | 1 | 1 | |||
| Hospitalization, Emergency Room Visit, or Invasive Medical Procedure | 0 | 62 | 32 | 11 | 4 | 1 | 2 | 4 |
| Learned about Traumatic Event | 0 | 86 | 22 | 10 | 2 | |||
| Man-made Disasters (fire, war, terrorism) | 0 | 115 | 2 | 2 | 1 | |||
| Natural Disasters (flood, hurricane, tornado, earthquake) | 0 | 86 | 25 | 7 | 1 | 1 | ||
| Physical Abuse | 0 | 116 | 4 | |||||
| Sexual Abuse, Sexual Assault, or Rape | 0 | 116 | 2 | 1 | 1 | |||
| Victim of Physical Violence | 0 | 117 | 3 | |||||
| Witnessed Another Person Being Threatened with Harm, Seriously Injured, or Killed | 0 | 90 | 22 | 8 | ||||
This table presents a list of stressful and traumatic life events assessed during the diagnostic interviews. The count of participants reporting different cumulative numbers of instances of each event through the time of scan are presented along with the number of missing/not reported values.
Table 3.
Demographic and Clinical Variables
| Mean | Std | Minimum | Maximum | |
|---|---|---|---|---|
|
|
||||
| Genetic Profile Scores | 5.508 | 1.193 | 3 | 8 |
| Stressful and Traumatic Life Events | 15.917 | 10.398 | 1 | 54 |
| Age at Scan (years) | 11.240 | 1.225 | 9 | 14 |
| Age at Scan (months) | 140.200 | 14.941 | 109 | 179 |
| Depression Symptoms | 2.284 | 2.841 | 0 | 15 |
| Anxiety Symptoms | 1.530 | 1.749 | 0 | 13 |
| Externalizing Symptoms | 2.930 | 4.136 | 0 | 24 |
| ERC Emotion Regulation Scores | 28.040 | 3.785 | 17 | 32 |
|
Counts for Each Subgroup
|
||||
| Sex | Female = 58, Male = 62 | |||
| Ethnicity | White = 65, African American = 55 | |||
| Tanner Pubertal Stage | Pre =27, Early=30, Mid=40, Late=18, Missing =5 | |||
Mean, standard deviation (std), minimum, and maximum values are presented for predictors of interest, age at scan, symptoms, and emotion regulation scores. Counts of participants by sex, ethnicity, and pubertal status are also presented.
Genetic Profile Scores (GPS)
Extensive details on the rationale, methods, and limitations of our HPA axis genetic profile score (GPS) creation were published previously (Pagliaccio et al., 2013). In short, prior work documented the utility of additively combining genetic variants to study their polygenic effects on brain structure and function, whereas a single polymorphism alone may not be a significant predictor (Nikolova, Ferrell, Manuck, & Hariri, 2011). The current additive genetic profile scores sum across 10 SNPs within 4 integral HPA axis genes where higher scores indicate more alleles previously associated with increased cortisol, depression prevalence/severity, and/or related phenotypes (e.g. antidepressant treatment response, suicidality, etc.). These SNPs were narrowed down from a larger selection to reduce linkage disequilibrium (all pairwise r2<0.49). In prior work, higher GPS predicted elevated cortisol reactivity to a stressor, indicative of their construct validity (Pagliaccio et al., 2013). The variants of interest included SNPs from CRHR1 (rs4792887, rs110402, rs242941, rs242939, rs1876828), NR3C2 (rs5522), NR3C1 (rs41423247, rs10482605, rs10052957), and FKBP5 (rs1360780). For more background on each SNP and linkage disequilibrium plots, see (Pagliaccio et al., 2013).
MRI Scanning
Participants completed a neuroimaging battery including high-resolution structural, diffusion imaging, functional task, and resting state scans collected using a 3.0 Tesla TIM TRIO Siemens whole body scanner at Washington University in St. Louis. The resting state data were the focus of the current analysis. T1-weighted structural images were acquired in the sagittal plane using an MPRAGE 3D sequence (TR=2400ms, TE=3.16ms, flip angle=8°, slab=176mm, 176 slices, matrix size=256x256, field of view (FOV)=256 mm, voxel size=1x1x1 mm; interslice skip=0). T2-weighted images were collected for registration purposes using a 3D SPACE acquisition (TR=3200ms, TE=497ms, 160 slices, FOV=256, voxel size=1x1x1mm).
Two resting state fMRI scan runs were acquired (where able – five children ran out of time or were unable to stay still during the second run), each including 164 frames (each lasting ~6.8 minutes). Participants were instructed to rest with their eyes closed and to remain awake during the resting state scan runs. Data were acquired using an asymmetric spin-echo, echo-planar sequence, which was maximally sensitive to blood oxygenation level–dependent (BOLD) contrast (T2*) (TR=2500ms, TE=27ms, FOV=256mm, flip=90°, voxel size=4x4x4mm, slices=36).
fMRI pre-processing
Imaging data were preprocessed using the following steps: (1) correction for slice-dependent time shifts; (2) removal of first 4 images of each run to allow BOLD signal to reach steady state; (3) elimination of odd/even slice intensity differences due to interpolated acquisition; (4) realignment of data acquired from each participant within and across scan runs to compensate for rigid body motion (Ojemann et al., 1997); (5) image intensity normalization to a whole-brain mode value of 1000; (6) registration of the 3D structural volume (T1) to an atlas template (WU “711-2B”) in the Talairach coordinate system (Talairach & Tournoux, 1988) using a 12-parameter affine transform and re-sampling to 1mm cubic representation (Buckner et al., 2004; Ojemann et al., 1997); (7) co-registration of the 3D fMRI volume to the T2, and the T2 to the participant’s structural image; and (8) transformation of the fMRI data to 3x3x3mm voxel atlas space using a single affine 12-parameter transform.
Functional Connectivity Data Processing
Resting state functional connectivity scan runs were concatenated and then processing occurred in three stages using in-house software. First, nuisance variables were regressed from the BOLD data (average signal from the ventricles, white matter, and whole brain as defined by FreeSurfer segmentation as well as 6 head realignment parameters and their derivates [24 parameters from Volterra series expansion]), a temporal band-pass filter was applied (0.009 Hz < f < 0.08 Hz), and spatial smoothing was applied (6 mm full width at half maximum). Further, average global signal and its derivate were regressed out of the BOLD data, which has been shown to reduce motion and signal artifacts (Power et al., 2012; Power, Barnes, Snyder, Schlaggar, & Petersen, 2013; Power et al., 2014; Satterthwaite et al., 2013).
Next, frames with excess head motion artifact were censored based on frame-wise displacement (FD) as previously described Power et al., (2012). FD is a sum of the absolute values of the 6 linear and rotational head displacement values from the realignment parameters estimated in Step 4 of the above preprocessing (the 3 rotational values are converted to millimeters as displacement on the surface of a sphere of radius 50mm). Volumes with FD greater than 0.2 were censored from all subsequent analyses. Furthermore, any scan runs with less than 40 frames remaining after censoring and participants with less than 110 total frames remaining across all available runs were excluded from further analyses. Finally, the initial rs-fcMRI processing (nuisance regressors, band-pass filtering, smoothing) was reapplied to the raw data (output of the initial preprocessing) interpolating over the frames censored in the previous stage Power et al., (2013).
fMRI Analysis
We used FreeSurfer v5.1 (Fischl et al., 2004; 2002) to create anatomical region of interest (ROI) masks. The amygdala was segmented bilaterally from each participant’s T1 anatomical image, down-sampled to match the functional resolution of the atlas space (3x3x3mm), and registered to the common atlas space. These images were summed and a group-level anatomical mask was created by thresholding the region where at least half of participants had overlap in their amygdala segmentations, allowing a more anatomically precise ROI than relying on atlas ROIs. The center of mass of the ROI is indicated on a surface rendering in Figure 1; slices through the ROI are presented in Figure S3.
Figure 1. Normative Left Amygdala Connectivity and Regions Showing Significant Regression Effects.
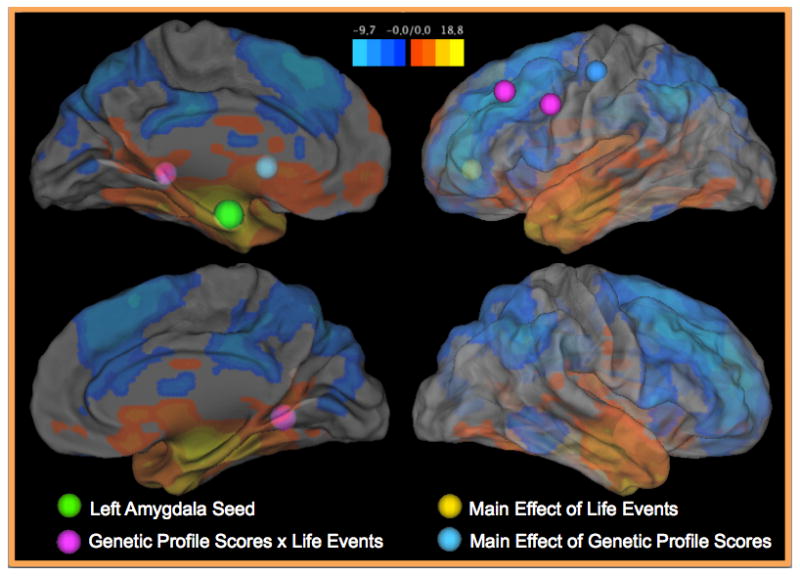
This figure presents a surface rendering of the normative resting state connectivity patterns found with the left amygdala. Specifically, colors on the surface indicate z-statistics for the whole-brain one-sample t-test indicating areas that show significant connectivity with the left amygdala. The normative connectivity results are also presented in axial slices in Figure S1. The center of the left amygdala seed is indicated by a green sphere. Other spheres indicate the peaks of regression effects: blue = main effects of genetic profile scores; yellow = main effects of life events; purple = genetic profile score x life events interactions. Axial slices through these regions showing altered connectivity are presented in Figure S3.
The time-series from these two ROIs were correlated with the time-series at every other voxel in the brain to create two whole brain voxel-wise correlation maps for each participant. Values in these maps were converted to z-statistics using Fisher’s r-to-z transform.
Statistical Analysis
Normative Connectivity Patterns
First, to establish the overall normative patterns of amygdala connectivity in our sample, two whole-brain one-sample t-tests (null hypothesis = zero) were run using in-house software (FIDL analysis package, http://www.nil.wustl.edu/labs/fidl/index.html; Ollinger, Corbetta, & Shulman, 2001) to characterize significant voxel-wise resting state functional connectivity (r-to-z transformed) with the left or right amygdala. Whole-brain t-test results were thresholded based on Monte Carlo simulations (3dClustSim, afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) at z≥3 and ≥17 contiguous voxels to achieve a whole-brain false positive rate of p<0.05. A summary of peak locations was created using a peak finding program to isolate local maxima/minima in these whole brain thresholded maps and to consolidate nearby peaks less than 20mm from each other.
Effects of Stress-Related Risk Factors
Next, to test our main hypotheses, we examined two whole-brain regression analyses predicting voxel-wise rsFC with the left or right amygdala. GPS, life events, and their interaction (GPS x LE) were the predictors of interest, controlling for ethnicity (White vs. African American), sex (females vs. males), and interactions between these covariates and GPS and life events (for discussion of controlling for interactions with covariates, see (Keller, 2013). The GPS and life events variables were z-scored to center and normalize both variables. Whole-brain z-maps for the effects of GPS, life events, and the GPS x life events interaction were thresholded as above based on Monte Carlo simulations at z≥3 and ≥21 contiguous voxels to achieve a whole-brain false positive rate of p<0.05 (these contrasts were the only whole brain regression results examined). Average connectivity values within each significant cluster were extracted for each participant. Effects of the predictors of interest in the regions identified at the whole brain level were further corrected for multiple comparisons using Bonferonni correction (p<0.008; 0.05/6 tests – 3 predictors x 2 seed regions). Extracted values for regions passing Bonferonni correction were used to perform control analyses. Further, we used the moderation model from the PROCESS tool for SPSS (Hayes, 2013) to parse significant GPS x life events interaction effects by isolating simple slopes.
Control Analyses
We used linear regressions in IBM SPSS Statistics v20 (Armonk, NY: IBM Corp.) to extract statistics for the above regressions predicting average cluster activity. In post-hoc regressions, we then tested whether the effects observed at the whole-brain level remained significant when controlling for a variety of covariates. Particularly, we controlled for effects of dimensional scores of depression, anxiety, or externalizing disorder symptom severity at scan and ERC emotion regulation skills. In further supplementary control analyses, we tested whether effects remained significant when controlling for age at scan and interactions between age and GPS or life events or pubertal status at scan and interactions between puberty and GPS or life events.
Exploratory Analyses Predicting Symptoms at Follow-Up
In cases where there was an association between symptom severity or emotion regulation and connectivity, we tested whether that region’s connectivity with the amygdala predicted symptom severity (MDD N=98, externalizing N=90, anxiety N=91) or regulation (N=98) by the time of the follow-up wave in a final exploratory analysis. This follow-up wave was ~1 year (13.73±4.65 months) after the scan when connectivity was examined. To do this, we ran a linear regression with connectivity predicting the follow-up outcome. In a subsequent step, we tested whether connectivity predicted change in scores by controlling for concurrent severity or regulation skills and the number of months between the scan and the follow-up. In a final step, we controlled for all other factors in the main regressions, i.e. sex, ethnicity, GPS, life events, and their interactions. In cases where connectivity predicted future outcomes, we tested whether connectivity mediated the association between GPS or life events (whichever predictor identified the ROI) and outcome scores using the PROCESS tool.
Results
Characterizing Normative Amygdala Connectivity Patterns
Figures 1 and S1 present the results of whole-brain one-sample t-tests exploring left amygdala connectivity in this sample. Peak coordinates are presented in Table S2. Right amygdala connectivity is presented in Figure S2 and Table S3. Consistent with the prior literature, both the left and right amygdala show strong positive connectivity with much of the subcortex, including the bilateral hippocampus, striatum, and contralateral amygdala as well as the brain stem, posterior insula, and vmPFC. Additionally, the amygdala shows strong negative connectivity with much of the dmPFC, lateral PFC, anterior insula, cingulate cortex, and parietal lobe. The patterns of connectivity for the left and right amygdala were very similar/overlapping.
Effects of Stressful Life Events and HPA Genetic Variation
The whole-brain regression analyses identified regions showing effects of life events, genetic variants, or their interaction predicting connectivity with the left and right amygdala at a whole-brain false positive rate of p<0.05; only effects of these predictors that passed a further stringent Bonferonni correction were examined (p<0.008; 0.05/6 tests – 3 predictors x 2 seed regions). The whole-brain regression predicting left amygdala connectivity revealed two significant clusters showing a main effect of GPS (putamen and postcentral gyrus), one cluster in the ACC/mPFC showing a main effect of life events, and four clusters showing a significant GPS x life events interaction (parahippocampal gyrus, caudate tail, MFG, IFG). Figure 1 and S3 display these regions and Table 4 presents coordinates, voxel extents, and t-statistics from one-sample t-tests examining whether rsFC significantly differed from zero on average. No significant clusters were found when predicting right amygdala connectivity. Table 5 presents regression results predicting connectivity between the left amygdala and each of these regions (averaged across the region) controlling for symptom severity and emotion regulation skills at scan (Table S4 presents unstandardized regression coefficient and confidence intervals for these result). The residuals from these main regressions were all normally distributed (Shapiro-Wilk W≥0.985, p ≥0.197). Table S5 controls for age effects and Table S6 controls for effects of puberty. We discuss the effects of interest here in the main text and provide more discussion of effects/interactions of covariates in the Supplementary Materials. Additionally, we present regressions separately by ethnicity in Table S7.
Table 4.
Summary of Clusters Showing Effects in Whole-Brain Regressions
| Cluster | X | Y | Z | Voxels | BA | Mean r-to-z | t | Effect | Concurrent | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Main Effects of GPS | ↑ GPS | |||||||||
| Left Putamen | −16 | 9 | 3 | 41 | - | 0.043 | 3.338** | ↓/weaker connectivity | - | - |
| Left Post-Central Gyrus | −50 | −15 | 48 | 47 | 3 | −0.011 | −0.891 | ↑/weaker connectivity | Ext, Reg | Reg |
| Main Effect of Life Events | ↑ LE | |||||||||
| Left Anterior Cingulate | −20 | 42 | 3 | 23 | 32 | −0.101 | −7.346*** | ↑/weaker connectivity | Anx, Ext | Anx |
| GPS x LE Interactions | ↑ LE with high GPS, ↓LE with low GPS | |||||||||
| Right Parahippocampal Gyrus | 28 | −48 | 0 | 129 | 19 | 0.053 | 4.756*** | ↓/weaker connectivity | Reg | - |
| Left Caudate Tail | −34 | −33 | 0 | 22 | - | 0.093 | 7.683*** | ↓/weaker connectivity | - | - |
| Left Middle Frontal Gyrus | −40 | 27 | 39 | 66 | 8 | −0.139 | −11.563*** | ↑/weaker connectivity | - | - |
| Left Inferior Frontal Gyrus | −50 | 6 | 33 | 22 | 9 | −0.045 | −3.048** | ↑/weaker connectivity | Dep, Anx | - |
Clusters showing significant effects of genetic profile scores (GPS), life events (LE), or their interaction in the whole brain regressions are listed here. Their peak Talairach co-ordinates (X,Y,Z) for each cluster, voxel extent, and Brodmann area (BA) are presented. The mean r-to-z connectivity values for each cluster with the left amygdala and associated one sample t-statistic testing a null hypothesis of mean zero connectivity are also presented. The direction of effects are summarized along with relationships with concurrent and follow-up scores (Dep=depressive, Anx=anxiety, Ext=externalizing disorder symptoms, Reg=ERC emotion regulation scores) ↑/↓ = higher or lower values of a variable.
p<0.05,
p<0.01,
p<0.001
Table 5.
Results of Main Linear Regression Models
| Predictor | Left Putamen | Left Post-Central Gyrus | Left Anterior Cingulate | Right Parahippocampal Gyrus | Left Caudate Tail | Left Middle Frontal Gyrus | Left Inferior Frontal Gyrus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t | β | t | β | t | β | t | β | t | β | t | β | t | |
| Sex | 0.258 | 1.436 | 0.038 | 0.206 | −0.203 | −1.184 | 0.043 | 0.255 | 0.209 | 1.156 | −0.140 | −0.742 | −0.245 | −1.308 |
| Ethnicity | −0.808 | −3.986*** | 0.755 | 3.63*** | −0.229 | −1.181 | −0.039 | −0.203 | −0.043 | −0.210 | 0.142 | 0.663 | 0.034 | 0.161 |
| GPS | −0.421 | −4.345*** | 0.438 | 4.411*** | −0.074 | −0.798 | −0.110 | −1.194 | −0.124 | −1.269 | 0.051 | 0.504 | 0.075 | 0.738 |
| Life Events | 0.141 | 1.435 | 0.168 | 1.676# | 0.501 | 5.347*** | 0.095 | 1.029 | 0.199 | 2.024* | 0.040 | 0.384 | −0.078 | −0.760 |
| GPS x Sex | 0.197 | 1.177 | −0.129 | −0.749 | 0.202 | 1.263 | −0.237 | −1.494 | −0.221 | −1.310 | 0.136 | 0.772 | 0.047 | 0.267 |
| LE x Sex | 0.298 | 1.635 | −0.099 | −0.529 | 0.203 | 1.167 | 0.075 | 0.433 | 0.183 | 1.000 | −0.104 | −0.540 | −0.321 | −1.689# |
| GPS x Ethnicity | 0.107 | 0.562 | 0.272 | 1.391 | 0.235 | 1.285 | −0.170 | −0.940 | −0.062 | −0.325 | 0.455 | 2.259* | 0.426 | 2.137* |
| LE x Ethnicity | −0.179 | −0.876 | 0.447 | 2.134* | 0.417 | 2.134* | −1.048 | −5.417*** | −0.730 | −3.550** | 0.891 | 4.136*** | 0.326 | 1.526 |
| GPS x LE | −0.348 | −2.693*** | 0.245 | 1.844# | 0.167 | 1.348 | −0.755 | −6.161*** | −0.631 | −4.853*** | 0.665 | 4.876*** | 0.500 | 3.703*** |
| Depressive Symptoms | 0.041 | 0.444 | 0.020 | 0.207 | −0.031 | −0.352 | −0.101 | −1.155 | −0.150 | −1.621 | 0.029 | 0.298 | 0.166 | 1.721# |
| Anxiety Symptoms | 0.095 | 0.934 | −0.016 | −0.154 | 0.366 | 3.759*** | 0.040 | 0.413 | 0.093 | 0.903 | 0.031 | 0.292 | −0.192 | −1.799# |
| Externalizing Symptoms | −0.141 | −1.290 | −0.294 | −2.612* | −0.298 | −2.843** | 0.069 | 0.669 | −0.094 | −0.852 | −0.030 | −0.260 | −0.042 | −0.365 |
| Emotion Regulation | 0.105 | 1.117 | −0.175 | −1.809# | −0.106 | −1.171 | 0.167 | 1.865# | 0.036 | 0.384 | −0.051 | −0.511 | −0.056 | −0.570 |
| R2 | 0.313 | 0.276 | 0.372 | 0.383 | 0.304 | 0.236 | 0.251 | |||||||
| Adjusted R2 | 0.229 | 0.189 | 0.296 | 0.308 | 0.220 | 0.143 | 0.160 | |||||||
| Model F | 3.746*** | 3.145** | 4.882*** | 5.114*** | 3.600*** | 2.544** | 2.752** | |||||||
Standardized regression coefficients (β) and their associated t-values are presented for all predictors in the main regression results predicting all seven regions of interest. Model R2, adjusted R2, and model F values are presented for each model. Effects that identified each region are shaded gray. Effects with p<0.10 are in bold. GPS= genetic profile scores, LE= life events.
GxE effect not significant at whole-brain threshold level.
p<0.10,
p<0.05,
p<0.01,
p<0.001
Main Effect of Genetic Profile Scores
First, GPS negatively predicted connectivity between the left amygdala and the cluster in the putamen. As this region tended to show positive connectivity with the left amygdala on average (Table 4), higher GPS predicted weakened connectivity. As shown in Tables 5, S5, and S6, this effect remained significant when controlling for all other factors. The post-hoc regression also notes a GPS x life events interaction predicting amygdala-putamen connectivity (simple slopes presented in Table S8), though this effect was not significant at the level of the whole-brain multiple comparisons cluster correction. In addition, GPS positively predicted connectivity between the left amygdala and the postcentral gyrus. Though this postcentral gyrus cluster showed near zero connectivity at the group level with the left amygdala (Table 4), children with high GPS tended to have weak positive connectivity whereas those with low GPS tended to have weak negative connectivity. This effect of GPS held when controlling for all other factors (Tables 5, S5, and S6). Additionally, there was a negative association between amygdala-postcentral gyrus connectivity and emotion regulation skills (remained significant when controlling for effects of age [Table S5] and puberty [Table S6]), i.e. more negative connectivity predicted better emotion regulation skills. Finally, we noted a negative association between amygdala-postcentral gyrus connectivity and concurrent externalizing disorders symptoms, i.e. more negative connectivity correlated with increased symptoms.
Main Effect of Life Events
Negative life events showed a strong positive association with left amygdala-ACC connectivity, which remained significant when controlling for all other factors (Tables 5 and S5). This region showed strong negative connectivity with the amygdala at the group-level (Table 4), and thus the experience of more negative life events predicted weaker/less negative connectivity. Importantly, we also found a significant negative association between connectivity and concurrent externalizing symptoms and a significant positive association with anxiety, i.e. weaker negative connectivity related to greater anxiety symptoms but fewer externalizing symptoms. This effect also interacted with ethnicity where the relationship between connectivity and life events experience was slightly stronger among the White children (Table S6).
Interaction of Genetic Profile Scores and Life Events
Four clusters showed significant GPS x life events interaction, all of which remained significant when controlling for diagnostic, emotion regulation, age, and puberty effects (Tables 5, S5, and S6). These interactions all took a similar form where greater life events experience predicted weaker connectivity among children with higher GPS and predicted stronger connectivity among those with low GPS (Figure 2, Table S8). This interaction predicted left amygdala connectivity with the parahippocampal gyrus, which was positive at the group level (Table 4). Additionally, there was a trend-level positive association between amygdala-parahippocampal gyrus connectivity and emotion regulation skills (Table 5) that reached significance when controlling for age effects (Table S5), i.e. stronger connectivity predicted better emotion regulation. Connectivity with the caudate tail was also strongly positive at the group level (Table 4) and showed a similar GPS x life events interaction. Further, life events experience positively predicted amygdala-caudate connectivity at mean levels of GPS. Finally, this GPS x life events interaction predicted connectivity between the left amygdala and two left PFC regions, MFG and IFG, which both show strong negative connectivity at the group level (Table 4). These interaction effects mirror effects on the parahippocampal gyrus and caudate but with signs reversed (Figure 2), i.e. children with the highest GPS and life events experience showed the weakest connectivity (least negative/closest to zero).
Figure 2. Simple Slope Plots for Interaction Effects.
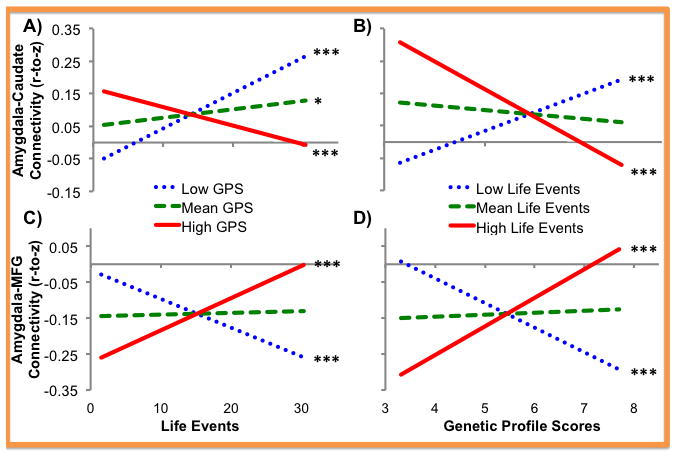
Simple slope effects of life events (top row) and genetic profile scores (bottom row) predicting two regions showing a genetic profile score x life events interaction on left amygdala connectivity are shown here, specifically a cluster in the caudate (left column) and the middle frontal gyrus (right column). These regions were chosen to exemplify the interaction patterns predicting regions showing typically positive connectivity, e.g. the caudate, or negative connectivity, e.g. the middle frontal gyrus. Simple slopes for each variable were presented at high (mean + 1 SD), mean, and low (mean − 1 SD) levels of the interacting variable and effects controlled for all other variables in the regressions (Table 5). * p<0.05, ** p<0.01, *** p<0.001
Predicting Symptoms and Emotion Regulation at Follow-Up
Next, in an exploratory analysis, we examined whether any regions that showed an association with concurrent symptoms or emotion regulation skills also predicted future outcomes (Table S9). Particularly, given the association between left amygdala-postcentral gyrus connectivity and externalizing symptoms and emotion regulation skills, we tested associations with these variables at a follow-up assessment. We found that amygdala-postcentral gyrus connectivity did not predict externalizing symptoms at follow-up in any step of the regression. On the other hand, amygdala-postcentral gyrus connectivity negatively predicted emotion regulation skills at follow-up and, importantly, continued to predict when controlling for concurrent emotion regulation, i.e. stronger negative connectivity predicted better emotion regulation skills at follow-up. Furthermore, postcentral gyrus connectivity significantly mediated the association between GPS and improvements in emotion regulation skills (Figure 3A).
Figure 3. Mediation Models Predicting Outcomes at Follow-Up.
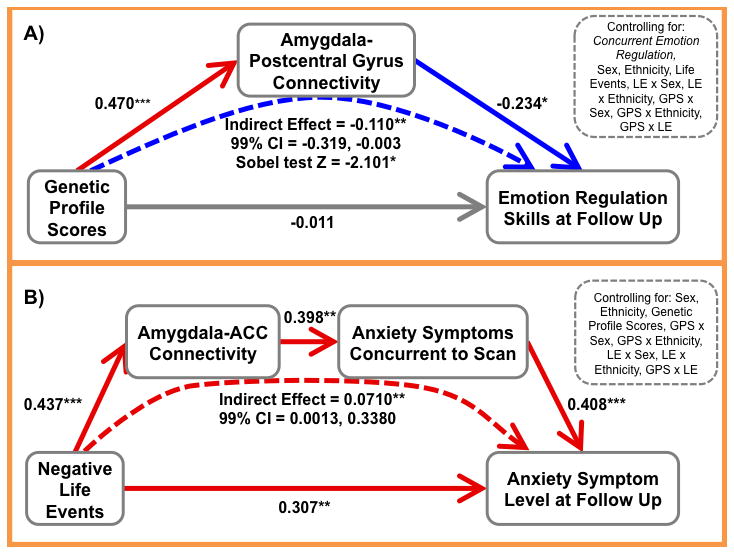
This figure presents a schematic of the mediation results testing two model: (A) left amygdala-postcentral gyrus connectivity mediates the relations between genetic profile scores (GPS) and emotion regulation skills at follow-up and (B) left amygdala-anterior cingulate cortex (ACC) connectivity and concurrent anxiety symptoms act as serial mediators of the effects of life events (LE) on follow-up anxiety symptoms. Standardized regression coefficients (β) are presented for all effects. The path from the independent to dependent variable represents the total effect. * p<0.05, ** p<0.01, *** p<0.001
Similarly, we examined associations between left amygdala-ACC connectivity and externalizing and anxiety symptoms at follow-up (Table S9). Left amygdala-ACC connectivity did not significant predict externalizing symptoms at follow-up in any step of the regression. On the other hand, amygdala-ACC connectivity positively predicted anxiety symptoms at follow-up, i.e. weaker/less negative connectivity predicted increased anxiety symptoms. This did not remain significant when controlling for concurrent symptoms, i.e. amygdala-ACC connectivity predicted future anxiety symptoms but not change in symptom level (current symptoms were highly predictive of symptom level at follow-up). Given this relationship and the finding that amygdala-ACC connectivity was predicted by both life event exposure and anxiety symptoms, we tested whether connectivity and concurrent symptoms acted as serial mediators between life events and later symptoms. Here, we found evidence for a significant indirect effect where greater life events exposure predicted weaker amygdala-ACC connectivity which predicted higher concurrent anxiety which in turn predicted higher future anxiety (Figure 3B). Thus, amygdala-ACC connectivity and concurrent anxiety symptoms shared variance in predicting future symptoms.
Discussion
Summary
The goal of the current study was to test whether normal variation in HPA axis genes and childhood stress exposure predicted or interacted to predict resting state functional connectivity with the amygdala in school-age children. Further, we examined how this connectivity related to concurrent depressive, externalizing, and anxious symptoms and emotion regulation skills and whether connectivity predicted these outcomes ~1 year later. We found that (1) greater HPA axis genetic profile scores predicted weaker/less positive connectivity with the putamen and predicted more positive connectivity with the postcentral gyrus, that (2) greater negative life events experience predicted weaker/less negative connectivity with ACC, and that (3) genetic profile scores and life events experience interacted to predict connectivity with the parahippocampal gyrus, caudate tail, MFG, and IFG where children with the highest GPS and life events showed the weakest connectivity. Finally, (4) connectivity with the postcentral gyrus related to concurrent externalizing symptoms and concurrent and future emotion regulation skills while connectivity with the ACC related to concurrent externalizing symptoms and concurrent and future anxiety symptoms. Importantly, alterations in amygdala connectivity could result from several different factors. For example, alterations in the anatomical connectivity of the brain (e.g., white matter pathways) could contribute, though functional connectivity is not isomorphic with structural connectivity (Damoiseaux & Greicius, 2009). Additionally, altered functional co-activation of different brain regions (e.g., hyper-reactivity of the amygdala and/or impaired activation of prefrontal and or cingulate regions) could lead to altered functional connectivity over time and across development.
Stressful Life Events and HPA Genetic Variation Predict Weakened Connectivity
The current results indicate that HPA axis genetic variation and early life stress exert main and interacting effects on amygdala resting state connectivity in children. Particularly, increasing risk from these stress-related factors related to weakened connectivity across several frontal and subcortical regions, some of which have shown depression- and anxiety-related alterations in function and connectivity in prior work (e.g. Dannlowski et al., 2009; Kim, Gee, Loucks, Davis, & Whalen, 2011a; Lui et al., 2011; Matthews et al., 2008). Importantly, while research has related commonly occurring genetic variants modulating the serotonin system to weakened amygdala rsFC (Dannlowski et al., 2009; Pezawas et al., 2005), the current results suggest a key role for HPA axis genetic variation as well. We found main effects of GPS similar to these prior studies, such that increasing genetic ‘risk’ (more variants previously associated with increased depression and/or cortisol) predicted a weakening of typically positive amygdala-putamen connectivity and predicted more positive amygdala-postcentral gyrus connectivity, which is typically negative in adults (Roy et al., 2009). Furthermore, we found that genetic profile scores interacted with childhood negative life events experience to predict weakened amygdala connectivity, i.e. less positive connectivity with regions typically showing positive connectivity (parahippocampal gyrus and caudate) and less negative connectivity with regions typically showing negative connectivity (MFG and IFG). Specifically, this interaction indicated that increasing life events exposure predicted weaker connectivity particularly among children with high genetic profile scores and vice versa.
Further, we noted a crossover interaction such that in the presence of elevated genetic risk, high life events exposure predicted weak connectivity with the amygdala while in the presence of low genetic risk, high life events exposure predicted stronger connectivity. This type of cross-over interaction has been observed previously in the literature, particularly between environmental stress and several of the genes in our profile scores in prior work (e.g. Bogdan et al., 2012; Klengel et al., 2012). These type of results have pushed the field to re-conceptualize many genetic ‘risk’ factors as ‘for-better-or-for-worse’ plasticity factors, which may be detrimental in poor environmental conditions, but adaptive in healthy/beneficial environments (Belsky et al., 2009). Additionally, it is important to point out that only one of these four regions showed a significant main effect of life events or GPS in the regressions (life events predicted amygdala-caudate connectivity at means levels of GPS). Thus, examining gene x environment interactions can be critical, as these stress-related alterations may not have been identified in a study examining only environmental or genetic risk factors independently.
Weakened Negative Connectivity with the Amygdala
The current results are in line with many prior studies linking depression/anxiety to weakening of both typical positive and negative amygdala connectivity. For example, prior work found that children with a personal and/or maternal history of depression showed reductions in amygdala connectivity with similar regions, including the parahippocampal gyrus, MFG, putamen and postcentral gyrus (Luking et al., 2011). Based on the idea that rsFC represents a cumulative history of co-activation, weakened negative amygdala-PFC connectivity potentially can be understood in the context of poor emotion regulation skills, e.g. less PFC down-regulation of amygdala reactivity relates to less successful emotion regulation (Wager et al., 2008) potentially leading to weaker negative rsFC over time. Particularly, the regions identified in the current study that showed negative connectivity with the amygdala, i.e. the ACC, MFG, and IFG, have been implicated in the regulation of emotion (and of amygdala activity) (e.g. Ochsner, Bunge, Gross, & Gabrieli, 2002; Ochsner et al., 2004, for meta-analysis see Frank et al., 2014). Further, regulation-related activity in postcentral gyrus, MFG, and other regions tends to show normative change across development (McRae et al., 2012). Our results regarding the postcentral gyrus also support this explanation as stronger negative amygdala-postcentral gyrus connectivity predicted better emotion regulation skills at scan and improvements in emotion regulation at the follow-up assessment. While we did not observe strong negative connectivity at the group level between the amygdala and postcentral gyrus, negative connectivity is typical of healthy adults (Roy et al., 2009), and development of this negative connectivity is thus potentially adaptive, relating to improve emotion regulation skills.
Weakened Positive Connectivity with the Amygdala
As noted above, prior work has implicated weakened positive connectivity in internalizing disorders, though the functional meaning of this need to be explored further in the future. Particularly, typical limbic hyper-reactivity to emotional stimuli in depression/anxiety (for meta-analyses, see Etkin & Wager, 2007; Groenewold, Opmeer, de Jonge, Aleman, & Costafreda, 2012) might suggest the hypothesis that greater co-activation of the amygdala and other subcortical regions over time in patients with internalizing disorders would predict stronger positive connectivity between the amygdala and these regions. However, given the evidence for weakened positive connectivity observed here with regions often implicated in processing of emotional face stimuli (Fusar-Poli et al., 2009) and similar findings in previous literature focused on internalizing psychopathology (e.g. Chen et al., 2007; Hahn et al., 2011), alternative explanations are needed. For example, one possibility is that specific disruptions in PFC regulation of amygdala activity could lead to uncoupling of amygdala activity from other subcortical responses to emotional stimuli. Further, it will be important to explore whether this is an alteration in intrinsic amygdala connectivity or whether it develops with age and experience.
Associations with Symptoms and Emotion Regulation
The current results also suggest associations between amygdala resting state connectivity and concurrent or future psychiatric outcomes. Particularly, weaker/less positive connectivity with the parahippocampal gyrus related to worse emotion regulation skills at the time of the scan. As the parahippocampal gyrus typically shows positive connectivity with the amygdala in adults (Roy et al., 2009) and in this sample, the current results suggest that weakened connectivity or decoupling of these regions is associated with poor emotional outcomes. As noted above, the functional implications of this weakened connectivity need to be explored further, particularly to understand its role in the development of internalizing symptoms. Relatedly, less negative connectivity with the postcentral gyrus related to having fewer externalizing symptoms at scan but worse emotion regulation skills at scan and worsening of emotion regulation over time. Furthermore, amygdala-postcentral gyrus connectivity served as a mediator of the effect of GPS on worsening of emotion regulation skills. This result presents a potential mechanism by which HPA axis genetic variation may influence one’s emotional functioning via alterations in amygdala connectivity, likely by moderating one’s intrinsic HPA axis reactivity/regulation in the face of environmental stressors.
Finally, weaker/less negative connectivity with the ACC related to having increased anxiety symptoms but fewer externalizing symptoms. While amygdala-ACC connectivity did not significantly predict future externalizing symptoms, it did predict future anxiety symptoms. Further, connectivity and concurrent anxiety acted as serial mediators of the effect of life events on later anxiety symptoms, i.e. greater negative life events exposure predicted weaker amygdala-ACC connectivity, which in turn predicted increased anxiety symptoms at scan and at the subsequent follow-up. Thus, amygdala-ACC connectivity shared variance with concurrent symptoms in predicting later anxiety. Nonetheless, amygdala connectivity likely plays a role in the effects of childhood stress experience on the development of anxiety. Prior work has, for example, suggested that changes in amygdala-mPFC may mediate normative age-related changes in anxiety (Gee, Humphreys, et al., 2013b). Thus, early stress may act on this circuit to perturb normative developmental trajectories. Overall, we find that weaker amygdala connectivity, be that less positive connectivity with the parahippocampal gyrus or less negative connectivity with the ACC or postcentral gyrus, related to poor emotional outcomes, i.e. worse emotion regulation scores or greater anxiety. Interestingly, we find the opposite effect with externalizing symptoms, though connectivity did not predict externalizing symptoms at follow-up. This should be examined further to determine the specificity and generalizability of these associations. Additionally, it is important to note symptoms and emotion regulation likely relate to amygdala connectivity with other regions not identified here, as our focus was on connectivity patterns relating to stress-related risk factors. Thus, other normative relations to symptoms or emotional regulation in children should be explored further in future studies.
Patterns of Normative Connectivity in School-Age Children
While the goal of the current study was not specifically to characterize normative resting state connectivity patterns of the amygdala in school-age children, we presented this data for reference to aid future work. Consistent with prior work (Gabard-Durnam et al., 2014; Roy et al., 2009), we found that the left and right amygdala exhibited significant positive connectivity with much of the subcortex (e.g. hippocampus and striatum), the brainstem, the posterior insula, the anterior temporal lobe, and part of the vmPFC whereas the left and right amygdala exhibited negative connectivity with much of the dmPFC, lateral PFC, anterior insula, cingulate cortex, and parietal lobe. The left and right amygdala show very similar patterns of connectivity only differing slightly in the strength of association with contra/ipsilateral regions, i.e. the left amygdala tended to show slightly stronger connectivity with left hemisphere regions than the right amygdala and vice versa. Despite this very similar connectivity, we only noted associations between stress-related risk factors and left amygdala connectivity. This type of left-lateralization was also observed in prior work, e.g. examining effects of antidepressant treatment on amygdala functional coupling (Chen et al., 2007).
These normative connectivity patterns may be useful for future research given that the literature characterizing normative resting state amygdala connectivity has focused primarily on adults (Roy et al., 2009) or consistencies/differences across development (Gabard-Durnam et al., 2014). The current patterns suggest that amygdala connectivity in childhood is quite similar to that shown in adulthood (Roy et al., 2009). While normative connectivity in this specific age range has not been established previously, the current patterns are also consistent with patterns of connectivity previously observed across development controlling for age (Gabard-Durnam et al., 2014). Gabard-Durnam et al., (2014) also noted age-related differences in amygdala connectivity, specifically more positive connectivity with regions of MFG and ACC with increasing age and more negative connectivity with posterior cingulate, insula, superior temporal gyrus, inferior parietal lobe, and parahippocampal gyrus. We did not observe any significant main effects of age (or pubertal status) on connectivity with any of the regions identified in the current study. This is consistent with prior work as the regions identified here generally fell within the connectivity patterns observed by Gabard-Durnam et al., controlling for age (rather than changing with age). Nonetheless, there are likely age-related differences in amygdala connectivity in the current sample/age range but with regions other than those identified based on relations with life events and/or GPS.
Limitations and Future Directions
First, there are several limitations to using single summary variables for genetic variation or stressful/traumatic life events, as has been discussed previously in greater detail (Pagliaccio et al., 2013). While combining across multiple sources of variance and reducing the number of tests performed can increase power, it assumes that the effects of stressors/SNPs sum additively with equal weights. Optimizing the relative weighting of events or SNPs can be very useful for future studies; to this end, we have previously presented SNP-wise relations with cortisol reactivity, amygdala and hippocampus volumes (Pagliaccio et al., 2013), and amygdala reactivity to fearful-neutral faces (Pagliaccio et al., 2015). Additionally, as we did not have an a priori method for weighting different life events or differential hypotheses about stressor severity/trauma, we combined across all events assessed. This could be explored further in the future to assess the specificity or magnitude of effects of certain types of stressors/traumas or to assess the effect of stressor timing during development on connectivity alterations.
We were also limited in our ability to examine change in diagnostic status across development. While examining change in more continuous variables can be more powerful, studying the onset of or presence/absence of a diagnosis has been a focus in the field to date. While we were limited in our ability to examine this in the current study (e.g. only four children with no prior history of MDD through the time of scan that had developed MDD by the time of the follow-up assessment), this could be examined in the future when further diagnostic longitudinal data is available. Finally, our sample size was reduced in the exploratory analyses examining the ~1 year follow-up; this type of longitudinal analyses across development should be tested rigorously in the future.
Conclusions
The current study finds that increasing negative life events exposure and HPA axis genetic ‘risk’ factors predict and interact to predict weakened amygdala resting state functional connectivity in school-age children. Particularly, these factors predicted weaker negative connectivity between the amygdala and regions of prefrontal cortex and postcentral gyrus and weakened positive connectivity with the parahippocampal gyrus and striatum. Further, these connectivity patterns were associated with anxiety disorder symptoms and emotion regulation skills. Overall, these results suggest that amygdala connectivity may place a key role in the mechanism between stress-related risk factors and the development of internalizing psychopathology.
Supplementary Material
Table 6.
Hierarchical Regressions of Connectivity Predicting Outcomes at Follow-up
| Step | Predictor | Parahippocampal Gyrus - Emotion Regulation |
Post-Central Gyrus-Emotion Regulation |
Postcentral Gyrus- Externalizing Symptoms |
Anterior Cingulate- Externalizing Symptoms |
Anterior Cingulate-Anxiety Symptoms |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | t | β | t | β | t | β | t | β | t | ||
| 1 | Connectivity | 0.010 | 0.099 | −0.194 | −1.992* | −0.051 | −0.515 | 0.029 | 0.282 | 0.226 | 2.303* |
|
| |||||||||||
| 2 | Connectivity | −0.015 | −0.190 | −0.178 | −2.298* | 0.092 | 1.239 | −0.033 | −0.440 | 0.075 | 0.756 |
| Concurrent Scores | 0.593 | 7.174*** | 0.587 | 7.319*** | 0.684 | 8.831*** | 0.666 | 8.707*** | 0.378 | 3.937*** | |
| Months between Scan and Follow-up | −0.038 | −0.361 | −0.035 | −0.340 | −0.069 | −0.710 | −0.066 | −0.677 | −0.008 | −0.065 | |
|
| |||||||||||
| 3 | Connectivity | −0.006 | −0.059 | −0.239 | −2.612* | 0.052 | 0.606 | −0.013 | −0.155 | −0.006 | −0.056 |
| Concurrent Scores | 0.577 | 6.141*** | 0.539 | 5.981*** | 0.610 | 6.518*** | 0.598 | 6.498*** | 0.409 | 3.932*** | |
| Months between Scan and Follow-up | −0.014 | −0.116 | −0.051 | −0.451 | −0.062 | −0.594 | −0.068 | −0.652 | −0.028 | −0.219 | |
| Sex | 0.139 | 0.789 | 0.213 | 1.244 | −0.278 | −1.68# | −0.268 | −1.609 | 0.004 | 0.021 | |
| Ethnicity | 0.178 | 0.829 | 0.375 | 1.71# | −0.054 | −0.276 | −0.018 | −0.097 | 0.067 | 0.286 | |
| GPS | −0.010 | −0.092 | 0.110 | 0.997 | 0.154 | 1.537 | 0.177 | 1.92# | −0.040 | −0.353 | |
| Life Events | 0.001 | 0.012 | 0.021 | 0.238 | 0.076 | 0.821 | 0.093 | 0.932 | 0.234 | 2.105* | |
| GPS x Sex | 0.192 | 0.997 | 0.114 | 0.616 | −0.007 | −0.039 | −0.018 | −0.105 | −0.133 | −0.637 | |
| LE x Sex | −0.221 | −1.242 | −0.216 | −1.270 | −0.274 | −1.71# | −0.274 | −1.671# | −0.292 | −1.452 | |
| GPS x Ethnicity | −0.112 | −0.486 | 0.012 | 0.058 | −0.025 | −0.136 | 0.003 | 0.017 | −0.055 | −0.241 | |
| LE x Ethnicity | −0.047 | −0.224 | 0.049 | 0.241 | 0.077 | 0.418 | 0.096 | 0.524 | 0.170 | 0.747 | |
| GPS x LE | 0.074 | 0.472 | 0.149 | 1.170 | 0.206 | 1.777# | 0.218 | 1.908# | 0.163 | 1.090 | |
Standardized regression coefficients (β) and their associated t-values are presented for hierarchical regression models. Connectivity showing relations with concurrent symptoms or emotion regulation skills were tested as predictors of that outcome at follow-up in Step 1. Concurrent scores and months between scan and follow-up were added in Step 2. All other predictors were controlled for in Scan 3. Model R2, adjusted R2, and model F values are presented for each model. Effects of connectivity are shaded gray. Effects with p<0.10 are in bold. GPS= genetic profile scores, LE= life events.
p<0.10,
p<0.05,
p<0.01,
p<0.001
Lay Summary.
Connections between the amygdala, a key region for emotion processing, and other regions of the brain are important to healthy functioning and development. Stress-related risk factors for depression and anxiety predict weakened connections between the amygdala and other regions of the brain in children. These weakened connections predict a worsening of children’s emotional skills and anxiety over time.
Acknowledgments
This work was supported by the National Institute of Mental Health (Grant Nos. MH64769 to J.L.L. and MH090786 to J.L.L., D.M.B., and K.N.B.) and a NARSAD award to J.L.L. A.C.B.’s work was supported by a grant from the National Institute of Mental Health (No. 1K01MH090515-01). M.S.G.’s work was supported by a grant from the National Institute of Mental Health (No. K23MH098176). D.P.’s work was supported by a grant from the National Institute of General Medical Sciences (No. 5T32GM081739). The NIMH had no further role in the design and conduct of the study (collection, management, analysis, or interpretation of the data) or in the preparation, review, or approval of the manuscript. Dr. Luby has also received research support from Communities Healing Adolescent Depression and Suicide (CHADS) Coalition, and the Sydney R. Baer Foundation. Dr. Luby reports receiving royalties from Guilford Press for a book on Preschool Mental Health (2006). Dr. Barsch reports consulting for Pfizer, Roche, Takeda, and Amgen. D.P. had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. This work comprised a portion of D.P.’s dissertation: Pagliaccio, D. (2015). The Effects of HPA Axis Genetic Variation and Early Life Stress on Cortisol Levels in Preschool Age Children and on Amygdala and Hippocampus Volumes, Reactivity, and Connectivity at School Age (Doctoral dissertation). We thank all participants and their families who provided time and effort to making this study possible.
References
- Abler B, Hofer C, Walter H, Erk S, Hoffmann H, Traue HC, Kessler H. Habitual emotion regulation strategies and depressive symptoms in healthy subjects predict fMRI brain activation patterns related to major depression. Psychiatry Research: Neuroimaging. 2010;183(2):105–113. doi: 10.1016/j.pscychresns.2010.05.010. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders: DSM 5 2013 [Google Scholar]
- Angold A, Costello EJ. A test–retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPAC) Psychological medicine. 1995;25(04):755–762. doi: 10.1017/s0033291700034991. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) Jaac. 2000;39(1):39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34:S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. The American Journal of Psychiatry. 2012;169(5):515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex (New York, NY: 1991) 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nature Reviews Neuroscience. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Fu CHY, Williams SCR, Walsh ND, et al. Functional Coupling of the Amygdala in Depressed Patients Treated with Antidepressant Medication. Neuropsychopharmacology. 2007;33(8):1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Structure and Function. 2009;213(6):525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, et al. Reduced amygdala–prefrontal coupling in major depression: association with MAOA genotype and illness severity. The International Journal of Neuropsychopharmacology. 2009;12(01):11. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Dosenbach N, Fair D, Miezin F, Cohen A, Wenger K, Dosenbach R, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual Differences in Typical Reappraisal Use Predict Amygdala and Prefrontal Responses. Bps. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H, Ascher B, Angold A. The Preschool Age Psychiatric Assessment: Version 1.4. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. Duke University Medical Center; Durham, NC: 2003. [Google Scholar]
- Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. The American Journal of Psychiatry. 2007;164(10):1–13. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater K, Schatzberg A, Menon V, Greicius M. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66(12):1361. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl BB, Salat DHD, van der Kouwe AJWA, Makris NN, Ségonne FF, Quinn BTB, Dale AMA. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwartz N, et al. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews. 2014:1–34. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience: JPN. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, et al. The Development of Human Amygdala Functional Connectivity at Rest from 4 to 23 Years: a cross-sectional study. NeuroImage. 2014;95:1–15. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the …. 2013a doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. Journal of Neuroscience. 2013b;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: Evidence from a meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews. 2012;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. The American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry. 2008;13(11):993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. Guilford Press; 2013. [Google Scholar]
- Henckens MJAGM, van Wingen GAG, Joëls MM, Fernández GG. Corticosteroid induced decoupling of the amygdala in men. Cerebral Cortex. 2012;22(10):2336–2345. doi: 10.1093/cercor/bhr313. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Presented at the Proceedings of the National Academy of Sciences; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28(4):341–356. doi: 10.1016/0022-3956(94)90017-5. http://doi.org/10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Keller MC. Gene Environment Interaction Studies Have Not Properly Controlled for Potential Confounders: The Problem and the (Simple) Solution. Bps. 2013:1–7. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of Anterior Cingulate Functional Connectivity from Late Childhood to Early Adulthood. Cerebral Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38(11):1567. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life Event Dimensions of Loss, Humiliation, Entrapment, and Danger in the Prediction of Onsets of Major Depression and Generalized Anxiety. Archives of General Psychiatry. 2003;60(8):1–8. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal Relationship Between Stressful Life Events and the Onset of Major Depression. The American Journal of Psychiatry. 1999;156(6):837. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Archives of General Psychiatry. 1992;49(9):716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27(5):1–19. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56(10):921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cerebral Cortex (New York, NY: 1991) 2011a;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. Behavioural Brain Research. Behavioural Brain Research. 2011b;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nature Neuroscience. 2012;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation - An ALE meta-analysis and MACM analysis. NeuroImage. 2013;87C:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuningas M, de Rijk RH, Westendorp RGJ, Jolles J, Slagboom PE, van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2007;32:1295–1301. doi: 10.1038/sj.npp.1301260. [DOI] [PubMed] [Google Scholar]
- Lapate RC, Lee H, Salomons TV, van Reekum CM, Greischar LL, Davidson RJ. Amygdalar Function Reflects Common Individual Differences in Emotion and Pain Regulation Success. Journal of Cognitive Neuroscience. 2012;24(1):148–158. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C, Aberg E, Sjöholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. Journal of Affective Disorders. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]
- Lekman M, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJM, Lipsky R, Wisniewski SR, Manji H, McMahon FJ, et al. The FKBP5-gene in depression and treatment response--an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biological Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang HTJ. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neuroscience. 2006 doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Luby J, Si X, Belden A, Tandon M, Spitznagel E. Preschool depression: Homotypic continuity and course over 24 months. Archives of General Psychiatry. 2009;66(8):897. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RCK, et al. Resting-state functional connectivity in treatment-resistant depression. The American Journal of Psychiatry. 2011;168(6):642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional Connectivity of the Amygdala in Early-Childhood-Onset Depression. Jaac. 2011;50(10):1027–1041.e3. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S, Strigo I, Simmons A. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. Journal of affective disorders. 2008;111(1):13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Maffioletti E, Cloninger CR, Magri C, Sartori R, Bortolomasi M, Gennarelli M. Role of allelic variants of FK506-binding protein 51 (FKBP5) gene in the development of anxiety disorders. Depression and anxiety. 2013;30(12):1170–1176. doi: 10.1002/da.22158. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Measured Gene-Environment Interactions in Psychopathology Concepts, Research Strategies, and Implications for Research, Intervention, and Public Understanding of Genetics. Perspectives on Psychological Science. 2006;1(1):5–27. doi: 10.1111/j.1745-6916.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, Labar KS. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48(12):3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36(9):1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Ray R, Cooper J, Robertson E, Chopra S, Gabrieli J, Gross J. For better or for worse: neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ojemann JGJ, Akbudak EE, Snyder AZA, McKinstry RCR, Raichle MEM, Conturo TET. Anatomic Localization and Quantitative Analysis of Gradient Refocused Echo-Planar fMRI Susceptibility Artifacts. NeuroImage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating Processes within a Trial in Event-Related Functional MRI. NeuroImage. 2001;13(1):218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, et al. Stress-System Genes and Life Stress Predict Cortisol Levels and Amygdala and Hippocampal Volumes in Children. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, et al. HPA axis genetic variation, pubertal status, and sex interact to predict amygdala and hippocampus responses to negative emotional faces in school-age children. NeuroImage. 2015;109:1–11. doi: 10.1016/j.neuroimage.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JSA, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(3):290–299.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(3–4):226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CDC, Ingram RER, Segal ZVZ. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25(4):487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Emotion regulation among school-age children: the development and validation of a new criterion Q-sort scale. Developmental Psychology. 1997;33(6):906–916. doi: 10.1037//0012-1649.33.6.906. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging 1988 [Google Scholar]
- Tanner J. Growth at Adolescence. Blackwell Scientific; Oxford, England: 1955. [Google Scholar]
- van der Werff SJA, Pannekoek JN, Veer IM, van Tol M-J, Aleman A, Veltman DJ, et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychological Medicine. 2012:1–12. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- van West D, Van Den Eede F, Del-Favero J, Souery D, Norrback KF, Van Duijn C, Sluijs S, Adolfsson R, Mendlewicz J, Deboutte D, et al. Glucocorticoid Receptor Gene-Based SNP Analysis in Patients with Recurrent Major Depression. Neuropsychopharmacology. 2005;31:620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- Veer IM. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IMI, Oei NYLN, Spinhoven PP, van Buchem MAM, Elzinga BMB, Rombouts SARBS. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37(7):1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-Subcortical Pathways Mediating Successful Emotion Regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Muñoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, Brain and Behavior. 2012;11(7):869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


