Abstract
Inflammatory Bowel Diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, relapsing inflammatory disorders of the gastrointestinal tract. Chronic inflammation of the intestine affects the normal fluid and electrolyte absorption leading to diarrhea, the hallmark symptom of IBD. The management of IBD associated diarrhea still remains to be a challenge, and extensive studies over the last two decades have focused on investigating the molecular mechanisms underlying IBD-associated diarrhea. These studies have shown that the predominant mechanism of diarrhea in IBD involves impairment of electroneutral NaCl absorption, with very little role if any played by anion secretion. The electroneutral NaCl absorption involves coupled operation of Na+/H+ exchanger 3 (NHE3 or SLC9A3) and Cl−/HCO3− exchanger DRA (Down Regulated in Adenoma or SLC26A3). Increasing evidence now supports the critical role of a marked decrease in NHE3 and DRA function and/or expression in IBD-associated diarrhea. This review provides a detailed analysis of the current knowledge related to alterations in NHE3 and DRA function and expression in IBD including the mechanisms underlying these observations, and highlights the potential of these transporters as important and novel therapeutic targets.
Keywords: Intestinal inflammation, NaCl Absorption, NHE3, DRA
[1] INTRODUCTION
The inflammatory bowel diseases (IBD) are multifactorial, complex diseases that include the entities of ulcerative colitis (UC) and Crohn’s Disease (CD) and are emerging as a major global health concern. Europe has the highest incidence closely followed by North America 1 ranging from 21 to 246 per 100,000 for UC and 8 to 214 per 100,000 for CD 2. The pathogenesis of IBD involves complex interactions between various environmental stimuli, luminal microflora and hereditary predisposition leading to gut immune dysregulation resulting in chronic mucosal inflammation and diarrhea 3, 4. The medical management of IBD is challenging, however, the primary therapeutic goals are still centered on maintaining remission and treating diarrhea. According to recent data, majority of the patients with UC and CD presents with diarrhea at the onset of disease 5. However, the severity of this diarrhea varies widely and is linked to the location, extent and degree of intestinal inflammation and can be used as an index of the disease activity and in determination of appropriate treatment strategies 5.
The mechanisms underlying IBD associated diarrhea include: i) altered expression and/or function of ion transporters and channels; ii) increased paracellular permeability; and iii) increased intestinal transit time (dysmotility) leading to inadequate water and solute absorption 5–10. The retention of electrolytes in the intestinal lumen leads to accumulation of water and initiation of diarrhea. Substantial evidence now supports the notion that dysregulation in electrolyte absorption rather than secretion is the major contributing factor to IBD associated diarrhea 6, 11, 12. The decrease in electrolyte absorption has been attributed to impaired function or reduced expression of Na+/H+ exchanger 3 (NHE3 or SLC9A3) and Cl−/HCO3− exchanger DRA (Down Regulated in Adenoma or SLC26A3), the key molecular species involved in electroneutral NaCl absorption in the ileum and colon 5, 13.
This review attempts to highlight the recent advances in our understanding about the role played by Na+ and Cl− transport dysfunction in the pathogenesis of diarrhea in IBD. Major emphasis will be placed on the molecular mechanisms underlying down regulation of ion transporters involved in electroneutral sodium chloride absorption (NHE3 and DRA). An understanding of the mechanisms of NHE3 and DRA dysregulation is important for identification of novel therapeutic targets for the treatment of IBD associated diarrhea.
[2] Mechanisms Underlying Diarrhea
2.1) Fluid Homeostasis in Intestine
Out of 8–10 L/day of fluid presented to the intestinal lumen (representing sum total of ingested food and biological secretions), the small intestine absorbs the majority of this fluid content and the remaining ~1.5–1.9 L of the fluid is absorbed by large intestine 6. Therefore, under normal conditions, <0.1–0.2 L/d of fluid volume is excreted in the stool. It is important to note that while it is the small intestine that absorbs most of this volume, the maximal absorptive capacity of the large intestine (4.5 to 5 liters per day) 13 is enough to compensate for an increased fluid delivery to the colon that may occur due to dysregulated absorption or motility in the small intestine. In IBD however, the absorptive capacity of the colon is significantly reduced leading to diarrhea.
2.2) Mechanism(s) of Electrolyte Transport in Intestine and Diarrhea
The gastrointestinal tract exhibits segmental heterogeneity in the expression pattern of various ion transporters and channels, which work in conjunction and determine the electrolyte content and fluid volume in the lumen. The movement of solutes, particularly sodium and chloride across the intestinal epithelium sets up an osmotic gradient along which water is absorbed. It follows that the nature and severity of diarrhea seen in IBD depends on which segment of the intestine is inflamed. In the intestine, Na+ and/or Cl− transport occurs via (1) nutrient coupled Na+ absorption; (2) electroneutral NaCl absorption; (3) electrogenic Cl− secretion by CFTR; and (4) electrogenic Na+ absorption by ENaC. Nutrient coupled sodium absorption involves the co-transport of sugars (glucose and galactose) and amino acids along with Na+, where the concentration gradient of sodium established by Na+/K+ ATPase facilitates the absorption of solute. Oral rehydration therapy, which is a life saving treatment in diarrhea, exploits the Na-glucose co-transport mechanism to promote water absorption that occurs osmotically along with solute and salt absorption 6, 15.
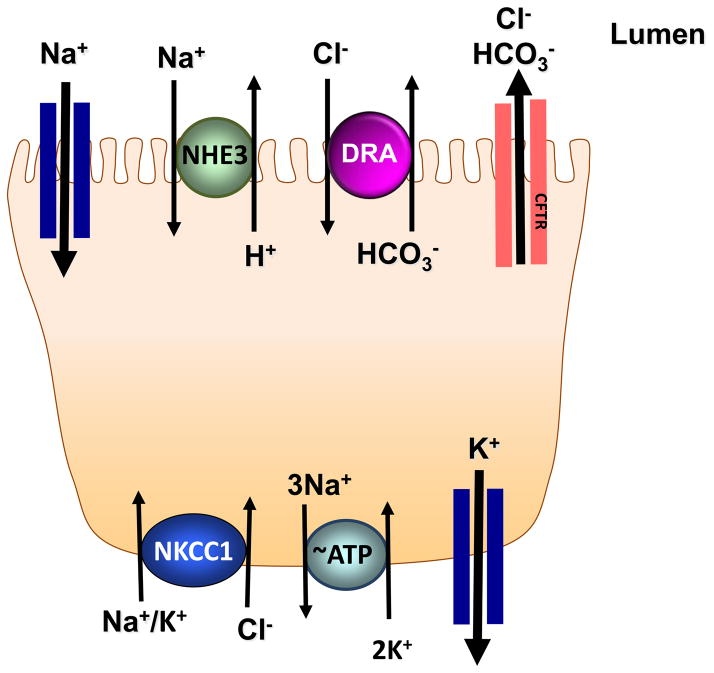
Electroneutral NaCl absorption is the predominant mechanism for Na+, Cl− and fluid absorption in the ileum and colon of the mammalian gastrointestinal tract (Fig 1). This process occurs via coupled functioning of Na+/H+ and Cl−/HCO3− exchangers and is not associated with the generation of transepithelial current 5, 13, 15–17. Recent studies in various animal models of IBD and biopsies from UC and CD patients report impairment of active sodium and chloride absorption as the prime feature of diarrhea in IBD 6, 11, 12, 18. Electrogenic sodium absorption is also reduced under inflammatory conditions, secondary to decreased expression of apical sodium channel ENaC (epithelial sodium channel) and basolaterally located cation transporter, Na+/K+ ATPase 6, 12.
Figure 1. Ion transporters and channels involved in Na+ and Cl− transport in intestinal epithelial cells.
Various ion transporters and channels work in conjunction to orchestrate electrolyte transport in the intestinal tract. Electroneutral NaCl absorption takes place by coupled operation of Na+/H+ exchanger 3 (NHE3) and Down Regulated in Adenoma (DRA) expressed apically at the luminal membrane of enterocytes. Epithelial sodium channel (ENaC) located at the apical membrane (predominantly in distal intestine) is involved in Na+ transport that is electrogenic in nature and is energized by Na+/K+ ATPase. Na+/K+ ATPase is a plasma membrane protein localized to the basolateral membrane of the enterocyte that sets up a Na+ gradient, which is necessary for several forms of secondary active transport in the intestine. Transepithelial chloride secretion is also electrogenic and is made up of several transmembrane transport pathways including cystic fibrosis transmembrane conductance regulator (CFTR) a cAMP-activated, apical Cl− channel, which plays the major role in chloride movement across the apical membrane and the basolateral Na+/K+/2Cl− cotransport system (NKCC1) that takes up Cl− from the serosal side in an electroneutral manner. K+ taken up by NKCC1 is recycled back to the basolateral side by K+ channels localized to the basolateral membrane. The expression and/or function of these transporters and channels (except for those involved in chloride secretion) is diminished in inflammatory disorders of the gut such as IBD resulting in associated diarrhea.
Ion transport mechanisms involved in Cl− secretion include (i) the apical cystic fibrosis transmembrane conductance regulator (CFTR)-type Cl− channel (ii) the basolateral Na+/K+/2Cl− cotransport system (NKCC1) (iii) the basolateral K+ channels and (iv) the Na+/K+ATPase, which energizes Cl− transport (Fig 1). In this regard, results obtained from electrophysiological studies conducted in the colonic mucosa from UC and CD patients show a lack of electrogenic Cl− secretion in response to secretagogues indicating that Cl− secretory pathway does not play any significant role in IBD associated diarrhea. The focus of the current review is, therefore, restricted to the alterations in the expression and/or activity of molecular species involved in electroneutral NaCl absorption under inflammatory conditions.
2.3) Molecular Basis for Electroneutral Sodium Chloride Absorption in Intestine
2.3.1.) Na+/H+ Exchangers
The Na+/H+ exchangers play a central role in Na+ and water absorption, maintenance of intracellular (cytosolic and organellar) pH and cell volume. There are 11 NHE isoforms identified in mammals (NHE1-11). In the intestine NHE1 (SLC9A1), NHE2 (SLC9A2), NHE3 (SLC9A3) and NHE8 (SLC9A8) have been shown to be present in the intestinal epithelium 19. NHE1 is located on the basolateral membrane of the intestinal epithelial cells and as such, does not contribute to luminal ion and water absorption 14. NHE8 is predominantly expressed during early life. NHE8 KO mice do not have diarrhea probably because of compensatory increase in NHE2 and NHE3 in the small intestine 20. NHE3 (SLC9A3) has received the most attention; and is believed to be the major transporter for Na+ absorption in the intestine 13 (Fig 1). This has been proven by studies in NHE3 knockout mice, demonstrating that the lack of functional NHE3 transporter manifests with altered intestinal Na+ and water absorption and a diarrheal phenotype 21, 22. NHE2 dysfunction does not cause such changes and double knockout mice lacking both NHE3 and NHE2 do not have a more severe diarrheal phenotype than single NHE3 KO 23. Interestingly, recent evidence alludes to the multiple mechanisms by which NHE3 dysfunction might play a role in IBD associated diarrhea as discussed in Section 3.
2.3.2.) Cl−/HCO3− Exchangers
Two members of the SLC26 gene family have been identified as candidate genes representing apical Cl−/HCO3− exchangers in mammalian intestinal epithelial cells namely DRA (SLC26A3) and PAT-1 (putative anion transporter, SLC26A6) 24, 25. DRA is predominantly expressed in the colon and duodenum and its expression is less abundant in jejunum and ileum whereas the expression pattern of PAT-1 is the inverse of DRA, except for the robust expression of both in the duodenum 26. Several lines of evidence suggest that NHE3 couples with DRA (the major luminal intestinal Cl−/HCO3− exchanger) to mediate electroneutral NaCl absorption in the ileum and colon (Fig 1). The role of DRA in mediating the vectorial Cl− absorption is evident from congenital chloride diarrhea (CLD), a rare genetic disorder caused by mutations in DRA, characterized by voluminous diarrhea, massive loss of Cl− in stool and metabolic alkalosis 27, 28. Similar to CLD phenotype, DRA knockout mice develop severe diarrhea, metabolic alkalosis as well as serum electrolyte imbalance 29. In contrast, PAT-1-deficient mice did not exhibit diarrheal phenotype suggesting that it is less important in mediating bulk intestinal Cl− absorption 24. Another study with Hepatocyte Nuclear Factor (HNF) KO mice exhibiting diarrheal phenotype also showed that expression of ileal and colonic DRA were substantially reduced in mice deficient in either intestinal HNF1α or HNF1β, and DRA was almost abolished in mice deficient in both factors in the intestine. This complete loss of DRA in double knockouts correlated with severe diarrhea 30. Borenshtein et al. 31 using Citrobacter rodentium-induced colitis model in mice also reported complete loss of DRA expression associated with severe diarrhea. Taken together, these studies indicate that loss of DRA function results in development of severe diarrheal phenotype.
[3] Mechanisms Underlying IBD Associated Diarrhea
A substantial body of literature derived from patients with IBD, knockout models, mouse models of IBD and in vitro models of inflammation has provided strong evidence for dysregulation of Na+ and Cl− absorption in intestine mediated via NHE3 and DRA in IBD-associated diarrhea. These findings are discussed below in detail.
3.1) NHE3 in IBD Associated Diarrhea
3.1.1) Alterations in NHE3 Function or Expression in UC Patients
Studies investigating alterations in NHE3 function and/or expression in patients with ulcerative colitis have indicated that decreased activity of NHE3 in inflamed mucosa may underlie diarrhea associated with ulcerative colitis. However, there are contradictory reports regarding the mechanisms causing the loss of NHE3 function in UC patients, with some studies showing decreased NHE3 activity despite correct cellular NHE3 localization and unaltered expression, while several others demonstrating reduced gene expression, as presented in Table 1 and outlined below. A number of studies have shown a decrease in NHE3 expression in human IBD 32, 33. In a study by Sullivan et al 33 quantitative analysis by Western blot in patients with CD and UC showed a decrease in NHE3 protein in 87% of sigmoid colonic biopsies (n=42) and 100% of ileal biopises (n=8). No decrease was found in intestinal alkaline phosphatase in inflamed samples, ruling out confounding effects due to epithelial cell loss secondary to inflammation. Siddique et al 32 also found that NHE3 protein and Na+ pump activity was decreased in UC and CD patient biopsies (n=13, treated IBD patients and n=13, untreated IBD patients), whereas, NHE3 mRNA was decreased only in CD and not in UC biopsies.
Table 1.
NHE3 in IBD
| mRNA | Protein | Function | ||
|---|---|---|---|---|
| IBD patients | UC and CD | ⇓ (32 only in CD) ⇔ (18, 34, 35) |
⇓ (32, 33) ⇔ (34) |
⇓ (32, 18, 34) |
| Animal models of inflammation | TnfΔARE+/− (mice) | ⇔ (36) | ⇔ (36) | ⇓ (36) |
| Rag2−/− CD4+ CD45RBhigh (mice) | ⇑ (36) | ⇔ (36) | ⇓ (36) | |
| DSS treated IL-10 KO (mice) | ⇔ (36) | ⇔ (36) | ⇓ (36) | |
| IFN-γ (rat) | ⇓ (42) | ⇓ (42) | ⇓ (42) | |
| IL-2 KO (mice) | ⇓ (37) | ⇓ (37) | ⇓ (37) | |
| DSS and TNBS (mice) | ⇓ (33) | |||
| In vitro models of inflammation | IFN-γ | ⇓ (42) | ⇓ (42) | ⇓ (42) |
| TNFα and IFN-γ | ⇓ (47) | |||
| Nitric Oxide | ⇓ (49) |
⇑ increase, ⇓ decrease, ⇔ no effect, Number in parenthesis indicate citation number
Furthermore, Farkas et al 18 using human sigmoid and rectal biopsies from patients with UC (n=69) showed a decrease in NHE3 activity in the apical and middle region of the colonic crypts. However, a corresponding decrease in NHE3 mRNA by quantitative PCR in the UC biopsies was not observed. Yeruva et al 34 also used human UC biopsies (n=40) with varying severity of inflammation. A significant decrease in Na+ absorption in moderately inflamed colon was seen, in parallel with a reduction in NHE3 activity. However, this decrease in NHE3 activity was not associated with a decrease in NHE3 gene expression or a change in membrane localization of NHE3 molecules in the crypts of the sigmoid colon biopsies in UC patients 34. Lohi et al 35 showed that there was no significant difference in NHE3 mRNA levels between inflamed and non-inflamed colonic biopsies from UC patients (n=10).
From above studies, it appears that the differences in NHE3 function and expression may be partly attributed to segmental variations along the length of the intestine, or to differences in disease severity between the various studies. The functional impairment in NHE3, despite normal expression and localization also suggested the potential involvement of other factors in UC associated diarrhea, such as regulatory proteins, discussed below (Section 3.1.5).
3.1.2) Alterations in NHE3 Function or Expression in Murine Inflammatory Models
Yeruva et al 36 studied NHE3 activity and mRNA levels in three different murine colitis models having differing segmental disease and severity of inflammation (TnfΔARE+/−, Rag2−/− CD4+ CD45RBhigh and DSS-induced IL-10−/− model). NHE3 mRNA expression was unchanged in the distal ileum of TnfΔARE+/− mouse model and the colon of IL-10−/− mouse model compared to their respective controls, and was increased in Rag2−/− CD4+ CD45RBhigh colon, but brush border localization of NHE3 was preserved in the mucosa in all 3 models. NHE3 activity was, however, decreased in all three murine models. This evidence again points to a functional defect in NHE3, possibly due to alterations in the PDZ domain-containing protein PDZK1 (NHERF3), discussed later in this review. Another study utilizing IL-2 knockout mice, which develop spontaneous colitis, showed a reduction in colonic Na+ absorption only partly due to the downregulation of NHE3 protein and mRNA levels 37. It was surprising to note that although net Na+ absorption was reduced by 80%, NHE3 mRNA and protein expression were reduced by only 41% and 24%, respectively 37. This pointed to other factors operating in the pathogenesis of diarrhea such as decreased Cl− anion exchange (coupled to Na+), decreased basal Na+/K+-ATPase, other defective Na+ transporters or defects in NHE3 endocytosis. In a study by Sullivan et al 33 in DSS and TNBS induced colitis in mice, a downregulation of NHE3 protein expression was found to occur in the mouse colon. Thus, there is strong evidence to suggest decreased NHE3 transporter activity in the presence of mucosal inflammation.
3.1.3) Lessons from NHE3 KO mice
Studies utilizing knockout mice suggest that deficiency of NHE3 may be a critical contributor to dysregulated immunomodulation, loss of barrier function and dysbiosis observed in IBD. For example, deletion of NHE3 in mice manifests with spontaneous colitis associated with mild diarrhea, occasional rectal prolapse and reduced body weight. Histological examination showed crypt hyperplasia, diffuse neutrophilic infiltrate with increase in matrix metalloproteinase 8 expression, a marked decrease in PAS positive goblet cells and induction of inducible nitric oxide synthase (iNOS) and TNF-α. These findings suggested a possible immunomodulatory role of NHE3 and its essential role in the maintenance of epithelial barrier integrity. Another significant observation supporting this theory was the increased bacterial adhesion and translocation found in the distal colon of NHE3−/− mice; and the fact that the colitis was ameliorated by oral administration of antibiotics 38.
Along the same lines, Kiela et al 39 showed that NHE3 KO mice, when exposed to even low concentrations of DSS, developed severe colitis resulting in early death secondary to intestinal bleeding, hypovolemic shock and sepsis. They also showed evidence of small intestinal injury in these mice by microarray analysis with an induction of numerous proinflammatory genes in small intestinal mucosa in response to DSS. This was surprising since DSS is not known to cause inflammation in the small intestine in wild type mice. Further, treatment of the NHE3 KO mice with broad-spectrum antibiotics resulted in decreased cytokine expression in the small intestinal mucosa, suggesting a possible role played by intestinal microbiome in this process 39. Another study showed that NHE3 KO mice when housed in a conventional facility spontaneously developed distal colitis with mild diarrhea; whereas, the KO mice in an ultraclean facility showed improvement in colitis symptoms 40. This indicates the important role played by altered gut microbiome in colitis.
Further evidence for the immunomodulatory effect of NHE3 downregulation in IBD in addition to its transporter function defect was shown in the following study. Larmonier et al 41 created double knockouts of NHE3 and IL-10 in mice and compared the immunohistochemistry and chemokine profile of the colitis that occurred in these mice with either NHE3 or IL-10 single knockout mice. Double KO mice displayed a more severe degree of inflammation with a significant increase in neutrophils and mononuclear cell infiltrate possibly due to increased expression of chemokines 41.
In summary, NHE3 deficiency in IBD appears to contribute to the pathogenesis of diarrhea in multiple ways including decreased Na+ absorption, disruption of barrier integrity, immunomodulation and dysbiosis.
3.1.4) Mechanisms Underlying NHE3 Dysregulation in Experimental Models of Colitis
3.1.4.1) Role of pro-inflammatory cytokines
With respect to mechanisms, in vitro cell culture models and experimental models of colitis have provided valuable insights into the possible mediators that decrease NHE3 function and/or expression in IBD. Rocha et al 42 demonstrated a decrease in NHE2 and NHE3 protein and mRNA expression in both human C2/bbe cells as well as in rat intestine (ileum and colon) following treatment with IFN-γ, a cytokine known to be increased in IBD affected intestine 43–46. Another study by our group showed that TNF-α and IFN-γ decreased NHE3 transcription in C2/bbe cells by repressing NHE3 promoter activity via a protein Kinase A (PKA) mediated phosphorylation of Sp1 and Sp3 47. In addition, there is evidence showing overproduction of NO, another inflammatory mediator, in IBD patients and experimental models of colitis 48. In this regard, we have earlier demonstrated that NO decreased NHE3 function by the activation of a soluble guanylate cyclase and protein kinase G (PKG) in intestinal epithelial cells 49. These studies indicated that the proinflammatory mediators (cytokines and NO) decrease NHE3 function and expression mainly via PKA and PKG pathways, respectively.
3.1.4.2) Role of NHE Regulatory Factors (NHERFs)
In the context of IBD, recent studies have focused on the role played by NHERF family members in NHE3 dysfunction. To date, four NHERF proteins have been identified: NHERF1 (EBP50), NHERF2 (E3KARP), PDZK1 (NHERF3) and IKEPP. Each of these proteins harbors 2 – 4 PDZ domains, and while they are homologous; they each contribute in a unique fashion to regulation of NHE3 or other substrate proteins. This uniqueness, partly depends upon their differing localization in the intestinal epithelium and different binding partners of these 4 proteins 50. The NHERF proteins co-localize with NHE3 and serve as scaffolding proteins to help form NHE3 containing signaling complexes, thereby affecting NHE3 function in a signal dependent and cell specific manner. Sullivan et al 33 showed that along with NHE3 protein, NHERF1 mRNA and protein were also downregulated in sigmoid mucosal biopsies in most of the patients of UC (n=13) and/or Crohn’s disease (n=8). Similar decrease in NHE3, NHERF1 and NHERF2 protein expression was also demonstrated in DSS and TNBS treated mice colon 33. On the other hand, Lenzen et al 51 using IL-10 KO mice showed diminished mRNA and protein of NHERF2 and PDZK1; whereas, NHERF1 expression was unchanged.
Yeruva et al 36 found a significant downregulation of PDZK1 mRNA and protein expression in colon biopsies from UC patients, as well as in inflamed murine ileum and colon. In addition, as described above in Sections 3.1.1 and 3.1.2, NHE3 activity was also decreased in both murine and human biopsies despite correct brush border localization and unaltered protein expression of NHE3. This finding indicates that PDZK1 downregulation might be a contributing factor to the functional defect of NHE3 observed in intestinal inflammation.
Overall, it appears that either decreased NHE3 function and/or expression results in decreased Na+ absorption in IBD. The decreased function could be secondary to altered signaling pathways, and/or NHERF expression which may involve altered localization of NHE3 on enterocyte surface membrane. In addition, altered expression of NHE3 is attributed to the effects of proinflammatory cytokines via PKA, Sp1 and Sp2 transcription factors. Also, the direct effect of NHE3 loss on the disruption of gut barrier integrity and dysbiosis cannot be ruled out.
3.2) Altered Cl− Absorption in IBD
As the inflammatory mediators are predominantly pro-secretory in nature, increased net chloride secretion (electrogenic) was initially thought to play a major role in the development of diarrhea in IBD. However, recent studies pertaining to dysregulated electrolyte transport in IBD 6, 8, 11, 12, 17, 52, 53 have indicated that decreased sodium and chloride absorption is the major contributor to diarrhea associated with gut inflammation. This was attributed to several important studies obtained from both animal models and humans. For example, in vitro flux studies conducted in colonic mucosa and rectum of UC patients exhibited a significant reduction in net chloride absorption rather than enhanced chloride secretion 54, 55. Further, transmucosal electrical potential difference was reported to be either significantly low or absent 55–57 in the mucosa of UC patients. A decrease in electrical potential difference is consistent with defective chloride absorption. Roediger et al 58 reported that in UC patients, Cl− uptake by the colon is significantly diminished, which goes well with the elevated levels of colonic and fecal Cl− content 58–60. Along the same lines, Farkas et al 18 reported significantly lower Cl−/HCO−3 exchange activity in the colonic crypts isolated from UC patients. Collectively, all these studies reflect the notion that impaired Cl− absorption in IBD represents one of the critical events in IBD-associated diarrhea.
3.3) DRA in IBD Associated Diarrhea
3.3.1) Alterations in DRA Function or Expression in IBD Patients
An early study of Yang et. al. 61 provided the first evidence of altered DRA expression in UC patients. Although limited in size to only 3 specimen pairs of healthy and moderately inflamed UC tissue, a significant reduction in DRA mRNA expression and complete absence of DRA protein expression in the inflamed surface epithelium was reported. In another study by Lohi et al. 35 pre-operative colonic samples were taken from 10 UC patients and 4 control subjects and the mRNA and protein expression of DRA was examined. Cases of UC with severe inflammation (with the exception of mild and moderate UC) resulted in a 2.6 fold decrease in DRA mRNA expression. However, a corresponding decrease in DRA protein levels was not observed. Another study reported no significant change in the expression of DRA mRNA or cytoplasmic immunoreactivity for DRA protein in 6 UC, 5 CD and 4 ischemic colitis patients. But, the characteristic apical membrane staining of DRA was absent in UC as compared to normal individuals or Crohn’s, and ischemic colitis patients 62. This suggests impairment in membrane targeting event(s) that could reduce DRA surface expression leading to a decrease in Cl− absorption. In this regard, a recent study correlated the alterations in DRA expression with Cl−/HCO3− exchange activity in crypts of 128 healthy individuals and 69 IBD patients (with active UC and diarrheal symptoms) 18. In this study, a robust reduction in Cl− absorption with a parallel decrease of ~50% in DRA mRNA expression was demonstrated in the surface of the colonic crypts in UC patients. These studies collectively indicate that diminished Cl− absorption in UC patients can be attributed to decreased DRA expression.
Overall, the patient data clearly pinpoints to the down regulation of DRA function and/or expression as one of the critical features that underlies the pathogenesis of diarrhea associated with UC (Table 2). However, there is still very limited information on abnormalities in DRA function and expression in CD patients. In addition to the role of DRA in diarrhea, the upcoming evidence now suggests that downregulation of DRA expression may also be involved in the pathogenesis of IBD. For example, a two-stage GWAS (Genome Wide Association Studies) conducted in Japan; using 1384 UC patients and 3057 control individuals identified single-nucleotide polymorphism in SLC26A3 gene, which was associated with lower DRA expression, constituting a risk factor for the development of ulcerative colitis 63. However, direct correlation of genetic deficiency in inducing inflammatory diarrhea needs further investigation.
Table 2.
DRA in IBD
| mRNA | Protein | Function | ||
|---|---|---|---|---|
| IBD patients | UC and CD | ⇓ (18, 35, 61) ⇔ (62) |
⇓ (61) ⇓ (62) ⇔ (35) |
⇓ (18) |
| Animal models of inflammation | HLA-B27/β2m (rats) | ⇓ (61) | ||
| IL-10 KO (mice) | ⇓ (61) | |||
| TNF+/ΔARE (mice) | ⇓ (66) | ⇓ (66) | ⇓ (66) | |
| DSS (mice) | ⇓ (64, 65) | ⇓ (64, 65) | ||
| Citrobacter rodentium infection (mice) | ⇓ (31) | ⇓ (31) | ||
| In vitro models of inflammation | IL-1β | ⇓ (61) | ||
| IFN γ | ⇓ (64, 70) | ⇓ (64, 70) | ⇓ (64, 70) | |
| TNFα | ⇓ (71) | ⇓ (71) | ||
| Hydrogen peroxide | ⇓ (73) |
⇓ decrease, ⇔ no effect, Number in parenthesis indicate citation number
3.3.2) Alterations in DRA Function or Expression in Mouse Models of IBD
Studies in different murine inflammatory models have further advanced our current knowledge about the role of DRA in IBD pathophysiology. In this regard, Yang et al. 61 showed a significant reduction (5–7 fold) in DRA mRNA expression in cecum, proximal and distal colon of HLA-B27/β2m transgenic rats as compared to the wild type rats. In addition, a similar decrease in DRA mRNA expression was also shown in IL-10 knockout mice housed in conventional conditions after developing colonic inflammation 61. It should be noted that DRA expression was preserved in IL-10 KO mice housed in germ-free environment as these mice failed to develop colonic inflammation. This phenomenon clearly indicated that downregulation of DRA expression is the direct consequence of intestinal inflammation and is not secondary to the absence of IL-10 expression.
In another model of colitis induced by dextran sulphate sodium (DSS), we have 64 reported ~50–60% decrease in DRA mRNA and protein expression in the distal colon (but not in ileum or proximal colon). Also, immunofluorescence staining revealed substantial reduction in DRA expression on the apical membrane in the colonic sections of DSS mice. This study utilized 3% DSS which resulted in mild colitis with no significant loss of surface epithelium, suggesting that decreased DRA expression is the direct consequence of intestinal inflammation (as observed in UC patients, Section 3.3.1). Along the same lines, Xu et al. 65 also demonstrated a decrease in intestinal DRA protein expression in DSS mice associated with diarrheal phenotype.
In another study, a significant decrease in DRA mRNA and protein expression in both ileum and mid distal colon of TNF+/ΔARE mice (TNF-α overexpressing mouse model that closely resembles CD and displays strong expression of TH1 cytokines) was demonstrated by Xiao et al. 66. In another model of Citrobacter rodentium induced colitis in mice, a dramatic decrease in DRA mRNA and protein expression was observed. This was associated with significant increase in fecal chloride levels, hypochloremia and fatal diarrhea 31.
Therefore, the above studies from the animal models of IBD further attest to the conclusion derived from IBD patients showing that a downregulation of DRA clearly underlies IBD associated diarrhea.
3.3.3) Lessons from DRA KO Mice
Studies with DRA knockout mice demonstrated a significantly reduced apical Cl−/HCO3− exchange activity, higher chloride levels and water content in the stool (diarrhea), distended large intestine, retarded growth and a short life span of ~4–5 months 29. Further, Xiao et al 67 reported that DRA KO mice were extremely susceptible to DSS induced colitis, exhibited severe disease symptoms and markedly reduced survival. These studies in DRA KO mice highlighted other important roles played by DRA in the pathogenesis of IBD, outside of its transporter function. In this regard, Schweinfest et al. 29 reported a striking feature of “conjoined crypts” in the colon of DRA KO mice (wherein the crypt orifices were merged) with an enhanced rate of proliferation. In contrast to the wild type mice where the proliferative cells were confined only at the bottom of the crypts, the proliferative zone in these knockout mice occupied 30–50% of the crypt axis. Thus, loss of DRA expression in the knockout mice altered the overall proliferative homeostasis of the colonic crypts, similar to the pattern observed in colitis patients 68.
Also, the absence of an adherent inner mucus layer in the colon of DRA knockout mice with no significant difference in the number of goblet cell count as compared to the wild type mice was reported 67. This study suggested that although the machinery involved in mucus production was intact in DRA knockout mice, the low luminal pH in the knockout mice (due to reduced bicarbonate secretion) inhibited the expansion and sheet formation of the mucin granules secreted from the goblet cells. The absence of protective colonic mucus layer such as observed in DRA KO mice, is considered as a potential mechanism in the development of colonic inflammation in both murine colitis models and IBD patients 69. Further, improper formation of the adherent mucus layer may also result in dysbiosis due to “ease of penetration” by pathogenic bacteria in absence of a barrier 52. Based on the above observations, it can be stated that DRA deficiency not only results in decreased absorptive efficiency of colon but also in impaired mucus layer formation resulting in aggravated inflammation and diarrhea associated with IBD.
3.3.4) Mechanisms Underlying DRA Dysregulation in IBD
3.3.4.1) Role of inflammatory mediators
In order to better understand the mechanisms directly affecting DRA function and/or expression in IBD, few in vitro studies have investigated the direct modulation of DRA by pro-inflammatory mediators. The study by Yang et al. 61 demonstrated a four-fold decrease in DRA mRNA expression in response to IL-1β treatment in Caco-2 cells. The results obtained from nuclear run on assays indicated that IL-1β mediated inhibition of DRA expression occurred at the transcriptional level. Furthermore, our group showed that IFN-γ directly decreased DRA function and expression by downregulating DRA promoter activity via a JAK/STAT1 pathway in Caco2 cells 70. In addition, the study identified that IFN-γ responsive region was located in the -933 to -925 bp region harboring a GAS (γ-activated site) cis element. These studies provided a mechanistic link between increased expression and activation of STAT1 in colonic mucosa of UC patients and diminished DRA function and expression. Our group also demonstrated a significant decrease in DRA mRNA and protein expression in response to direct TNF-α treatment in vitro 71. These studies further showed the involvement of TNF-α induced activation of NF-κB pathway in inhibiting DRA expression at the transcriptional level, which may partly contribute to IBD associated diarrhea 71. Besides proinflammatory cytokines, chronic intestinal inflammation is also associated with augmented production of reactive oxygen and nitrogen species (ROS/RNS). An increased production of ROS/RNS has been shown to play a critical role in pathophysiology of IBD in both humans and animal models of IBD 72. In this regard, we earlier reported an inhibition of apical Cl−/OH− exchange activity by H2O2, a highly reactive oxygen metabolite 73. However, this inhibition of Cl−/OH− exchange activity was found to be independent of changes in the surface expression of DRA and may have involved other post-translational modifications such as phosphorylation, lipid-raft dependent pathways or involvement of accessory proteins such as NHERFs, which remain to be studied. Further, pathologically elevated nitric oxide (NO) levels have been demonstrated in IBD 48, 74. In this context, we have previously shown that nitric oxide inhibited apical Cl−/OH− exchange activity via a cGMP-PKG and PKC mediated pathway in Caco-2 cells 75. This study indicated that dysregulated chloride absorption associated with IBD could be partly secondary to observed increase in NO in the inflamed intestine. Though the direct involvement of DRA in this study was not examined, however, it is well established that DRA is the key anion transporter involved in chloride absorption in intestinal epithelial cells.
In summary, loss of DRA function and/or expression in IBD leads to decreased chloride absorption and associated diarrhea in IBD. This decrease in DRA function/expression during gut inflammation can be attributed to direct modulation of surface DRA levels involving post-translational mechanisms or activation of proinflammatory pathways that can alter DRA expression at the transcriptional level. In addition, DRA also appears to play an important role in maintaining a protective mucus barrier, however, identification of DRA as a critical intermediate in development of dysbiosis warrants further detailed investigations.
[4.] CONCLUSIONS
Understanding the pathophysiological basis of IBD associated diarrhea have evolved significantly during the recent past predominantly due to many advances in our knowledge about the molecular mechanisms underlying NaCl absorption in the gut in health and disease. This has been possible due to the identification of molecular isoforms of SLC9 and SLC26 gene families involved in gut NaCl absorption. It is now well established that a coupled operation of NHE3 (SLC9A3) and DRA (SLC26A3) underlies the electroneutral NaCl absorption in the intestine. In addition, molecular studies with UC and CD patients as well as with various animal models of IBD in mice including those with NHE3 and DRA KO mice have further increased our understanding of the role of NHE3 and DRA in IBD associated diarrhea. The studies reviewed here clearly show that a decrease in NHE3 and DRA function and/or expression are the fundamental defects which explain the basis of defective electrolyte and fluid absorption in IBD associated diarrhea, whereas the anion secretion plays very limited role if any in this process. Therefore, these transporters represent important and novel therapeutic targets for IBD associated diarrhea. Overwhelming evidence now suggests that gut inflammation mediated downregulation of DRA expression secondary to the potential effect of cytokines appears to be the predominant mechanism involved in reduction in chloride absorption in IBD. In contrast, the effects of gut inflammation on NHE3 appear to be mainly affecting its function that could be secondary to the alterations in regulatory NHERF proteins. However, the role of repressing NHE3 expression could not be ruled out. In addition, it appears that the basis for some of the contradictory reports in the literature with respect to a decrease in DRA and NHE3 function and/or expression may also be due to the involvement of different segments of the intestine and severity of inflammation. Studies from NHE3 and DRA KO mice further indicate that in addition to their respective roles in intestinal Na+ and Cl− absorption, these transporters may have additional roles in the pathogenesis of IBD which include effects on gut barrier, immunomodulation and dysbiosis. Further studies are needed to understand these other than the “transporter roles” of NHE3 and DRA. Also, strategies to upregulate function and/or expression of these key transporters would represent novel therapeutic approaches for IBD associated diarrhea..
Acknowledgments
Supported by Department of Veterans Affairs Merit Award # BX002011 (P.K. Dudeja), Merit Award # BX000152 (W.A. Alrefai), and a Senior Research Career Scientist Award (P.K. Dudeja), and the NIDDK grants DK54016, DK81858 and DK92441 (P.K. Dudeja), DK96254 (S. Saksena), DK 98170 (R. K. Gill) and DK 71596 (W. A. Alrefai)
References
- 1.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 2.Ellinghaus D, Bethune J, Petersen BS, et al. The genetics of Crohn’s disease and ulcerative colitis--status quo and beyond. Scand J Gastroenterol. 2015;50:13–23. doi: 10.3109/00365521.2014.990507. [DOI] [PubMed] [Google Scholar]
- 3.Engelhardt KR, Grimbacher B. IL-10 in humans: lessons from the gut, IL-10/IL-10 receptor deficiencies, and IL-10 polymorphisms. Curr Top Microbiol Immunol. 2014;380:1–18. doi: 10.1007/978-3-662-43492-5_1. [DOI] [PubMed] [Google Scholar]
- 4.O’Toole A, Korzenik J. Environmental triggers for IBD. Curr Gastroenterol Rep. 2014;16:396. doi: 10.1007/s11894-014-0396-y. [DOI] [PubMed] [Google Scholar]
- 5.Wenzl HH. Diarrhea in chronic inflammatory bowel diseases. Gastroenterol Clin North Am. 2012;41:651–675. doi: 10.1016/j.gtc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann N Y Acad Sci. 2009;1165:285–293. doi: 10.1111/j.1749-6632.2009.04039.x. [DOI] [PubMed] [Google Scholar]
- 7.Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1099–1109. doi: 10.1097/MIB.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidler U, Lenzen H, Cinar A, et al. Molecular mechanisms of disturbed electrolyte transport in intestinal inflammation. Ann N Y Acad Sci. 2006;1072:262–275. doi: 10.1196/annals.1326.024. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Forsyth CB, Keshavarzian A. New molecular insights into inflammatory bowel disease-induced diarrhea. Expert Rev Gastroenterol Hepatol. 2011;5:615–625. doi: 10.1586/egh.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urayama S, Chang EB. Mechanisms and treatment of diarrhea in inflammatory bowel diseases. Inflamm Bowel Dis. 1997;3:114–131. [PubMed] [Google Scholar]
- 11.Barkas F, Liberopoulos E, Kei A, et al. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann Gastroenterol. 2013;26:23–28. [PMC free article] [PubMed] [Google Scholar]
- 12.Sandle GI. Pathogenesis of diarrhea in ulcerative colitis: new views on an old problem. J Clin Gastroenterol. 2005;39:S49–52. doi: 10.1097/01.mcg.0000155520.04253.37. [DOI] [PubMed] [Google Scholar]
- 13.Gill RK, Dudeja PK. Recent research developments in physiology. Trivandrum (India): Research Signpost; 2003. Mechanisms and regulation of NaCl absorption in the human intestine; pp. 643–677. [Google Scholar]
- 14.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na(+)/H(+) exchangers. Mol Aspects Med. 2013;34:236–251. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghishan FK, Kiela PR. Small intestinal ion transport. Curr Opin Gastroenterol. 2012;28:130–134. doi: 10.1097/MOG.0b013e32834e7bc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Augustin O, Romero-Calvo I, Suarez MD, et al. Molecular bases of impaired water and ion movements in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:114–127. doi: 10.1002/ibd.20579. [DOI] [PubMed] [Google Scholar]
- 18.Farkas K, Yeruva S, Rakonczay Z, Jr, et al. New therapeutic targets in ulcerative colitis: the importance of ion transporters in the human colon. Inflamm Bowel Dis. 2011;17:884–898. doi: 10.1002/ibd.21432. [DOI] [PubMed] [Google Scholar]
- 19.Kiela PR, Xu H, Ghishan FK. Apical NA+/H+ exchangers in the mammalian gastrointestinal tract. J Physiol Pharmacol. 2006;57 (Suppl 7):51–79. [PubMed] [Google Scholar]
- 20.Xu H, Zhang B, Li J, et al. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G335–343. doi: 10.1152/ajpgi.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 22.Gawenis LR, Stien X, Shull GE, et al. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G776–784. doi: 10.1152/ajpgi.00297.2001. [DOI] [PubMed] [Google Scholar]
- 23.Ledoussal C, Woo AL, Miller ML, et al. Loss of the NHE2 Na(+)/H(+) exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1385–1396. doi: 10.1152/ajpgi.2001.281.6.G1385. [DOI] [PubMed] [Google Scholar]
- 24.Kato A, Romero MF. Regulation of electroneutral NaCl absorption by the small intestine. Annu Rev Physiol. 2011;73:261–281. doi: 10.1146/annurev-physiol-012110-142244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- 26.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Aspects Med. 2013;34:494–515. doi: 10.1016/j.mam.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makela S, Kere J, Holmberg C, et al. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2002;20:425–438. doi: 10.1002/humu.10139. [DOI] [PubMed] [Google Scholar]
- 28.Wedenoja S, Pekansaari E, Hoglund P, et al. Update on SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2011;32:715–722. doi: 10.1002/humu.21498. [DOI] [PubMed] [Google Scholar]
- 29.Schweinfest CW, Spyropoulos DD, Henderson KW, et al. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281:37962–37971. doi: 10.1074/jbc.M607527200. [DOI] [PubMed] [Google Scholar]
- 30.D’Angelo A, Bluteau O, Garcia-Gonzalez MA, et al. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development. 2010;137:1573–1582. doi: 10.1242/dev.044420. [DOI] [PubMed] [Google Scholar]
- 31.Borenshtein D, Schlieper KA, Rickman BH, et al. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infect Immun. 2009;77:3639–3650. doi: 10.1128/IAI.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddique I, Hasan F, Khan I. Suppression of Na+/H+ exchanger isoform-3 in human inflammatory bowel disease: lack of reversal by 5′-aminosalicylate treatment. Scand J Gastroenterol. 2009;44:56–64. doi: 10.1080/00365520802321253. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan S, Alex P, Dassopoulos T, et al. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis. 2009;15:261–274. doi: 10.1002/ibd.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeruva S, Farkas K, Hubricht J, et al. Preserved Na(+)/H(+) exchanger isoform 3 expression and localization, but decreased NHE3 function indicate regulatory sodium transport defect in ulcerative colitis. Inflamm Bowel Dis. 2010;16:1149–1161. doi: 10.1002/ibd.21183. [DOI] [PubMed] [Google Scholar]
- 35.Lohi H, Makela S, Pulkkinen K, et al. Upregulation of CFTR expression but not SLC26A3 and SLC9A3 in ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2002;283:G567–575. doi: 10.1152/ajpgi.00356.2001. [DOI] [PubMed] [Google Scholar]
- 36.Yeruva S, Chodisetti G, Luo M, et al. Evidence for a causal link between adaptor protein PDZK1 downregulation and Na/H exchanger NHE3 dysfunction in human and murine colitis. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barmeyer C, Harren M, Schmitz H, et al. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G244–252. doi: 10.1152/ajpgi.00141.2003. [DOI] [PubMed] [Google Scholar]
- 38.Laubitz D, Larmonier CB, Bai A, et al. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G63–G77. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiela PR, Laubitz D, Larmonier CB, et al. Changes in mucosal homeostasis predispose NHE3 knockout mice to increased susceptibility to DSS-induced epithelial injury. Gastroenterology. 2009;137:965–975. 975 e961–910. doi: 10.1053/j.gastro.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larmonier CB, Laubitz D, Hill FM, et al. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G667–677. doi: 10.1152/ajpgi.00189.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larmonier CB, Laubitz D, Thurston RD, et al. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G998–G1009. doi: 10.1152/ajpgi.00073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha F, Musch MW, Lishanskiy L, et al. IFN-gamma downregulates expression of Na(+)/H(+) exchangers NHE2 and NHE3 in rat intestine and human Caco-2/bbe cells. Am J Physiol Cell Physiol. 2001;280:C1224–1232. doi: 10.1152/ajpcell.2001.280.5.C1224. [DOI] [PubMed] [Google Scholar]
- 43.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki T, Hiwatashi N, Yamazaki H, Noguchi M, Toyota T, et al. The role of interferon-gamma in the pathogenesis of crohn’s disease. Gastroenterol Jpn. 1992;27:29–36. [PubMed] [Google Scholar]
- 45.Colgan SP, Parkos CA, Matthews JB, et al. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994;267:C402–410. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- 46.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 47.Amin MR, Malakooti J, Sandoval R, et al. IFN-gamma and TNF-alpha regulate human NHE3 gene expression by modulating the Sp family transcription factors in human intestinal epithelial cell line C2BBe1. Am J Physiol Cell Physiol. 2006;291:C887–896. doi: 10.1152/ajpcell.00630.2005. [DOI] [PubMed] [Google Scholar]
- 48.Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:179–189. doi: 10.1097/00054725-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Gill RK, Saksena S, Syed IA, et al. Regulation of NHE3 by nitric oxide in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G747–756. doi: 10.1152/ajpgi.00294.2001. [DOI] [PubMed] [Google Scholar]
- 50.Donowitz M, Cha B, Zachos NC, et al. NHERF family and NHE3 regulation. J Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenzen H, Lunnemann M, Bleich A, et al. Downregulation of the NHE3-binding PDZ-adaptor protein PDZK1 expression during cytokine-induced inflammation in interleukin-10-deficient mice. PLoS One. 2012;7:e40657. doi: 10.1371/journal.pone.0040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bachmann O, Seidler U. News from the end of the gut--how the highly segmental pattern of colonic HCO(3)(-) transport relates to absorptive function and mucosal integrity. Biol Pharm Bull. 2011;34:794–802. doi: 10.1248/bpb.34.794. [DOI] [PubMed] [Google Scholar]
- 53.Greig E, Sandle GI. Diarrhea in ulcerative colitis. The role of altered colonic sodium transport. Ann N Y Acad Sci. 2000;915:327–332. doi: 10.1111/j.1749-6632.2000.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 54.Hawker PC, McKay JS, Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980;79:508–511. [PubMed] [Google Scholar]
- 55.Sandle GI, Hayslett JP, Binder HJ. Effect of glucocorticoids on rectal transport in normal subjects and patients with ulcerative colitis. Gut. 1986;27:309–316. doi: 10.1136/gut.27.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edmonds CJ, Pilcher D. Electrical potential difference and sodium and potassium fluxes across rectal mucosa in ulcerative colitis. Gut. 1973;14:784–789. doi: 10.1136/gut.14.10.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandle GI, Higgs N, Crowe P, et al. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- 58.Roediger WE, Lawson MJ, Kwok V, et al. Colonic bicarbonate output as a test of disease activity in ulcerative colitis. J Clin Pathol. 1984;37:704–707. doi: 10.1136/jcp.37.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caprilli R, Frieri G, Latella G, et al. Faecal excretion of bicarbonate in ulcerative colitis. Digestion. 1986;35:136–142. doi: 10.1159/000199359. [DOI] [PubMed] [Google Scholar]
- 60.Schilli R, Breuer RI, Klein F, et al. Comparison of the composition of faecal fluid in Crohn’s disease and ulcerative colitis. Gut. 1982;23:326–332. doi: 10.1136/gut.23.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H, Jiang W, Furth EE, et al. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. Am J Physiol. 1998;275:G1445–1453. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 62.Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, et al. The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol. 2000;113:279–286. doi: 10.1007/s004180000131. [DOI] [PubMed] [Google Scholar]
- 63.Asano K, Matsushita T, Umeno J, et al. A genome-wide association study identifies three new susceptibility loci for ulcerative colitis in the Japanese population. Nat Genet. 2009;41:1325–1329. doi: 10.1038/ng.482. [DOI] [PubMed] [Google Scholar]
- 64.Singh V, Kumar A, Raheja G, et al. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol. 2014;307:G623–631. doi: 10.1152/ajpgi.00104.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu L, Xiao F, He J, et al. Lysophosphatidic acid increases SLC26A3 expression in inflamed intestine and reduces diarrheal severity in C57BL/6 mice with dextran-sodium-sulfate-induced colitis. Chin Med J (Engl) 2014;127:1737–1743. [PubMed] [Google Scholar]
- 66.Xiao F, Juric M, Li J, et al. Loss of downregulated in adenoma (DRA) impairs mucosal HCO3(−) secretion in murine ileocolonic inflammation. Inflamm Bowel Dis. 2012;18:101–111. doi: 10.1002/ibd.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao F, Yu Q, Li J, et al. Slc26a3 deficiency is associated with loss of colonic HCO3 (−) secretion, absence of a firm mucus layer and barrier impairment in mice. Acta Physiol (Oxf) 2014;211:161–175. doi: 10.1111/apha.12220. [DOI] [PubMed] [Google Scholar]
- 68.Serafini EP, Kirk AP, Chambers TJ. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981;22:648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johansson ME, Gustafsson JK, Holmen-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saksena S, Singla A, Goyal S, et al. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-{gamma} Am J Physiol Gastrointest Liver Physiol. 2010;298:G159–166. doi: 10.1152/ajpgi.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anoop Kumar TG, Gill Ravinder K, Coffing Hayley, Anbazhagan Arivarasu Natarajan, Borthakur Alip, Alrefai Waddah A, Dudeja Pradeep K. TNF Inhibits SLC26A3 Expression via Activation of NF-κB Pathway. Gastroenterology. 2014 May;146(Supplement 1):S–650. [Google Scholar]
- 72.Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood) 2012;237:474–480. doi: 10.1258/ebm.2011.011358. [DOI] [PubMed] [Google Scholar]
- 73.Saksena S, Gill RK, Tyagi S, et al. Role of Fyn and PI3K in H2O2-induced inhibition of apical Cl-/OH- exchange activity in human intestinal epithelial cells. Biochem J. 2008;416:99–108. doi: 10.1042/BJ20070960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427–437. doi: 10.1111/j.1365-2567.2004.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saksena S, Gill RK, Syed IA, et al. Modulation of Cl-/OH- exchange activity in Caco-2 cells by nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2002;283:G626–633. doi: 10.1152/ajpgi.00395.2001. [DOI] [PubMed] [Google Scholar]