Abstract
Depression is a debilitating mental illness with clear developmental patterns from childhood through late adolescence. Here, we present data from the Gene Environment Mood (GEM) study, which used an accelerated longitudinal cohort design with youth (N = 665) starting in 3rd, 6th, and 9th grades, and a caretaker, who were recruited from the general community, and were then assessed repeatedly via semi-structured diagnostic interviews every 6-months over 3 years (7 waves of data) to establish and then predict trajectories of depression from age 8 to 18. First, we demonstrated that overall prevalence rates of depression over time, by age, gender, and pubertal status, in the GEM study closely match those trajectories previously obtained in past developmental epidemiological research. Second, we tested whether a genetic vulnerability-stress model involving 5-HTTLPR and chronic peer stress was moderated by developmental factors. Results showed that older aged adolescents with SS/SL genotype, who experienced higher peer chronic stress over 3 years, were the most likely to be diagnosed with a depressive episode over time. Girls experiencing greater peer chronic stress were the most likely to develop depression.
Keywords: depression trajectories, longitudinal, genetics, peer stress, development, gender
Depression is a chronic, debilitating disorder with clear developmental patterning. Rates of diagnosable depression surge in adolescence, and the gender difference in depression emerges around early adolescence (e.g., Avenevoli et al., 2015; Costello, Copeland, & Angold, 2011; Hankin et al., 1998). Behavioral genetic research indicates significant, albeit moderate, heritability for depression, and this genetic effect increases among older adolescents (Rice, Harold, Thapar, 2003; Silberg et al., 1999), especially in stressful environments (Uher & McGuffin, 2008, 2010). This suggests a complex interplay involving genetic risk, stress, and development. The primary purpose of this paper is to better understand the development of depression from childhood through late adolescence and examine the role that genetic risk, environmental stressors, and developmental factors (age, pubertal status) play in the onset of depression among youth.
Developmental epidemiology of depression across age, gender, and puberty
Between 13 and 18 years of age represents a critical period for depression onset and rising depression trajectories. A substantial portion of depression diagnoses have their initial origins in adolescence, and the vast majority (approximately 2/3 to 3/4) of lifetime cases among adults derive from adolescent-onset disorder (Kessler, Berglund, Demler, Jin, Merikangas, & Walters, 2005; Kim-Cohen et al., 2003). The longitudinal studies of relatively large, community-based samples with repeated measures of diagnosable depression reveal that depression rates rise dramatically during adolescence (e.g., Costello et al., 2003; Hankin et al., 1998; Lewinshon, Rohde, Seeley, Klein, & Gotlib, 2000). These studies also show that the gender difference in depression emerges during early adolescence (around age 12–13) or middle puberty (after Tanner Stage III; Angold, Costello, & Worthman, 1998). Finally, the most recent cross-sectional epidemiological studies with large-scale, nationally representative samples established a 2.7% past year prevalence rate among 8–15 year olds (Merikangas, He, Brody, Fisher, Bouron, & Koretz, 2010) and a 7.5% for past-year prevalence rate (11% lifetime prevalence) for 13–18 year olds (Avenevoli et al., 2015).
Most studies report depression rates over time as a function of age. But, age can be a general, nonspecific index of development (Rutter, 1989). Pubertal stage has also been investigated as a salient influence in depression, in large part because pubertal onset educes a confluence of physical, hormonal, social, emotional, and psychological changes (Hayward, 2003; Ladoucuer, 2012). Levels of depression, both symptoms and disorder, are higher among postpubertal, compared to prepubertal youth, (i.e., pubertal status; Angold, Worthman, & Costello, 2003; Ge & Natsuaki, 2009; Graber, 2013; Johnson et al., 2012; Negriff & Susma, 2011; Patton & Viner, 2007; Rudolph, 2014). These pubertal status effects are particularly pronounced among girls (Conley & Rudolph, 2009; Copeland et al., 2010; Galvao et al., 2014; Ge, Conger, & Elder, 2001; Graber et al., 1997, 2004; Hayward & Sanborn, 2002; Wichstrom, 1999) but they are also found for boys (Huddleston & Ge, 2003; Mendle & Ferrero, 2012). Moreover, pubertal status predicts enduring risk for depression throughout adolescence and into young adulthood (Copeland et al., 2010; Graber et al., 2004). Yet, few studies have systematically investigated which particular developmental influences (i.e., age and/ or pubertal status) accentuate individual differences that enhance vulnerability to onset of depression.
Why do more youth become depressed throughout adolescence?
The mechanisms underlying the surge in rates of depression from late childhood through adolescence are not well understood, although several theoretical models have been proposed. Common to many theories of the development of depression is the idea that adolescence is a significant period of social reorientation, when various vulnerabilities are hypothesized to potentiate the depressogenic impact of stressful life events (especially interpersonal events) (e.g., Cyranowksi et al., 2000; Hankin & Abramson, 2001; Nelson, Leibenluft, McClure, & Pine, 2005; Oldehinkel & Bouma, 2011). In this study, we draw from these models on the social reorientation of adolescence to specifically investigate why rates of depression skyrocket throughout this developmental period. In particular, we investigated 5-HTTLPR, as a prominent genetic susceptibility marker, and chronic peer stress, as one of the most developmentally salient stressful environmental contexts relevant for children and adolescents. We tested the hypothesis that this genetic vulnerability-stress interaction will be potentiated for older adolescents in the prediction of future depressive episodes.
Peer chronic stress
It is well-established that stressful life events potently predict later depression among youth (Grant, Compas, Thurm, McMahon, & Gipson, 2004; Hammen, 2009). Of the various domains in which stress occurs, exposure to peer stress has been shown to be one of the strongest, and most replicated, associations of depression among youth (e.g., Rudolph, Flynn, & Abaied, 2008). Depressed youth experience more peer victimization and more deleterious peer relationships (e.g., more rejection, lower popularity) (Nolan, Flynn, & Garber, 2003; Rudolph & Clark, 2001). They have less stable friendships, and their friendships are characterized by poorer quality (e.g., less intimacy; more conflict, hostility, criticism) (Borelli & Prinstein, 2006; Prinstein et al., 2005).
Still, despite these robust associations between depression among youth and peer stress, relatively few prospective studies have been conducted that examined the role of peer stress, especially when assessed via gold-standard contextual stress interview methods (Hammen, 2009) as a longitudinal predictor of future onset of depressive episodes. The longitudinal studies with interview-based assessment of peer stress and diagnostically ascertained depression show that peer stress is a significant predictor of future depression among high risk adolescents (Goodyer, Herbert, Tamplin & Altham, 2000), adolescents recruited from outpatient treatment (Williamson et al., 1998), and general community samples (e.g., Conley & Rudolph, 2009; Hammen, Shih, & Brennan, 2004; Monroe, Rodhe, Seeley, & Lewinsohn, 1999).
Finally, despite studies demonstrating a prospective, predictive main effect of peer stress on later diagnosable depression, there is a relative dearth of research investigating theoretically-derived moderators (Grant et al., 2006). As such, this study included genetic risk, specifically 5-HTTLPR, as an empirically supported moderator to further advance knowledge of processes predicting future depression among youth.
5-HTTLPR x stress interaction
Since Caspi and colleagues (2003) demonstrated that future depression in early adulthood (ages 21–26) was predicted by the interaction of major negative events and 5-HTTLPR, numerous GxE studies of 5-HTTLPR and various environmental risks have been conducted. A recent and comprehensive meta-analysis showed a robust, significant GxE in depression (Karg et al., 2011, but see Risch et al., 2009). Overall, more recent reviews show that significant GxE is more likely in higher quality studies (e.g., those with interview-based measures of stressful life events) as well as among older-aged adolescents and girls (Karg et al., 2011; Uher & McGuffin, 2008, 2010).
Still, key unaddressed questions remain, and there are particular limitations to past GxE studies. First, most GxE studies measured environmental stress with potentially subjective self-report stress checklists as opposed to gold-standard contextual stress interview methods (see Hammen et al., 2010; Jenness et al., 2011; Vrshek-Schallhorn et al., 2013, as notable exceptions). Indeed, studies utilizing interview-based measures of stress have been shown to be significantly more likely to obtain GxE effects in depression (Monroe & Reid, 2008; Uher & McGuffin, 2008, 2010). Second, and related, relatively few GxE studies have carefully selected and characterized developmentally appropriate, specifically relevant candidate contextual environments that would be most likely to predict onset of depression among youth at greatest genetic susceptibility. Prior work, including data from the GEM study (cf., Hankin et al., 2011; Hankin, Nederhoff, et al., 2011; Jenness et al., 2011; Oppenheimer et al., 2013), has examined the influence of family stress (Hammen, Brennan et al. 2010; Jenness et al., 2011; Nobile et al., 2009; Sjoberg et al., 2006; Vrshek-Schallhorn et al., 2013), exposure to elevated maternal depressive symptoms (Oppenheimer et al., 2013), maltreatment (e.g., Kaufman et al., 2006; Cicchetti, Rogosch, & Sturge-Apple, 2007), and general stressors (e.g., Eley et al., 2004; Hankin et al., 2011). Yet, surprisingly, we located no study that selected and specifically tested peer stress in interaction with 5-HTTLPR in the prediction of depression among adolescents. In this study we focused specifically on chronic peer stress given its central salience and contextual relevance for depression among youth. Last, most of the GxE studies of youth depression only investigated self-reported depressive symptoms as the outcome (see Vrshek-Schallhorn et al., 2013 for an exception with depression diagnoses), so the extent to which 5-HTTLPR interacts with stress, especially peer stress, to predict future diagnosable depression is an open question.
Most of the GxE research with youth has studied adolescents with relatively less attention paid to potential developmental changes across different age-linked periods. Given the clear developmental trends and surge in depression from childhood into adolescence, the lack of GxE research in samples of youth across different developmentally salient epochs is a notable gap. Presently, it is unknown whether developmental factors, such as older age and/or pubertal status, moderate GxE effects in depression. The few studies suggest that GxE in depression may be accentuated by development, although it is unclear whether age or pubertal status is more influential. For instance, in one study, 5-HTTLPR interacted with maternal reports of child stress to predict trajectories of broad-band internalizing trajectories from age 12 to 17, and this GxE effect became more significant with increasing age (Petersen et al., 2012). On the other hand, in behavioral genetic research, pubertal status affected the heritability of and latent GxE influences for depression in youth (Rice, Harold, Thapar, 2003; Silberg et al., 1999). A cross-sectional study with a small sample of adolescents showed that depressive symptoms were associated with the interaction of 5-HTTLPR and pubertal status after controlling for gender, such that low-expressing 5-HTTLPR alleles were related to depressive symptoms only among postpubertal youth (Salum et al., 2012).
Gender differences in genetics, peer stress, and implications for depression
Girls are more relationally oriented and exhibit greater affiliative needs, especially in adolescence (Cyranowski et al., 2000; Rose & Rudolph, 2006). Adolescent girls experience significantly more peer stress compared to boys (e.g., Hankin et al., 2007; Rudolph, 2002; Shih, Eberhart, Hammen, & Brennan, 2006). Moreover, girls are more reactive to peer stress and are more likely to become depressed as a consequence of peer stress exposure than boys (e.g., Conley & Rudolph, 2009; Hankin et al., 2007; Rudolph & Flynn, 2007), but not all studies find that gender moderates peer stress prediction on later depression among postpubertal youth.
Some behavioral genetic studies find a mean gender difference in depression heritability, although the findings are inconsistent (Lau & Eley, 2008). Longitudinal twin research (Silberg et al., 1999) shows that latent genetic risk increased the likelihood of depression and experiencing more stress for girls after, but not before, puberty. A few molecular genetic studies have found that the interaction of 5-HTTLPR with negative events was associated with depressive symptoms in girls, but not boys (e.g., Eley et al., 2004; Hammen et al., 2010; Priess-Groben & Hyde, 2013; Sjoberg et al., 2006), whereas the other GxE studies with 5-HTTLPR have not found gender to be a significant moderator. Given these mixed findings, and the importance of peer stress in the prediction of depression, especially for girls, gender was explored as a possible moderator of the 5-HTTLPR x chronic peer stress interaction.
The present investigation
Here, we present data from the Gene-Environment Mood (GEM) study. The overarching goal of the GEM study was to predict longitudinal trajectories of depression over time to better understand the ontogeny of depression among youth. The GEM study was a 3-year multi-wave prospective study that used an accelerated longitudinal cohort design (Duncan, Duncan, & Hops, 1996) with a sample of youth, initially recruited in the 3rd, 6th, and 9th grades, who were then followed prospectively for 3 years with repeated diagnostic interviews every 6 months to ascertain diagnosable depression trajectories over time. In this report, we first investigated descriptive trajectories of depression by gender and developmental status (age, grade cohort, and pubertal status) to confirm that the expected surge in adolescent depression and the emergence of the gender difference in depression occurred in the GEM study. Then, we specifically examined the hypothesis that chronic peer stress over the three years would interact with 5-HTTLPR to predict prospective onsets of depressive episodes among children and adolescents. Further, we expected that this GxE would be accentuated by developmental factors, and we examined both age and pubertal status as potential developmental moderators. We hypothesized that older adolescents at enhanced genetic susceptibility (i.e., either S/S or S/L genotype in an additive genetic framework) and who experience more chronic peer stress would be the most likely to prospectively experience an episode of depression. Given the emergence of the gender difference in depression during early adolescence, we explored whether gender affected the anticipated G x E x Development interaction predicting prospective depressive diagnosis onset over the three-year follow-up. Finally, we examined the role of chronic peer stress and developmental factors (age and pubertal status), independent of 5-HTTLPR, as influences affecting the gender difference in adolescent depression.
Method
Participants and Procedures
Children and adolescents were recruited at two sites: University of Denver (DU) and Rutgers University (RU). Brief information letters were sent home directly to the participating school districts of families, around either DU (broader Denver metropolitan area) or RU (central New Jersey area), with a child in third, sixth, or ninth grades. Of the families to whom letters were sent, 1108 parents responded to the letter and called the laboratory for more information. Parent report established that both the parent and child were fluent in English, the child did not have an autism spectrum or psychotic disorder, and the child had an IQ > 70. Of the families who initially contacted the laboratory, 665 (60% participation rate) qualified as study participants, as they met criteria and arrived at the laboratory for the assessment. The remaining 460 (40%) were considered nonparticipants for the following reasons: 4 (1%) were excluded because the parents reported that their child had an autism spectrum disorder or low IQ; 13 (3%) were non–English-speaking families; 330 (71%) declined after learning about the study’s requirements; and 113 (25%) were scheduled but did not arrive for assessment.
The final sample consisted of 665 youth who ranged in age from 7 to 16 years (mean = 11.6, SD = 2.4) as well as one caretaker. Table 1 shows descriptive demographic characteristics. The sample was comparable to the community and school districts from which it was recruited. The sample was also generally comparable to the ethnic and racial characteristics of the overall population of the United States, although there were relatively fewer Hispanic participants in the GEM study than found in the overall population of the United States.
Table 1.
Demographic Characteristics of GEM study population
| Characteristic | Percent (n) or Median |
|---|---|
| Gender | |
| Female | 55.0% (366) |
| Male | 45.0% (299) |
| Grade | |
| Third | 29.5 %(196) |
| Sixth | 37.3% (248) |
| Ninth | 33.2% (221) |
| Race/ Ethnicity | |
| White | 62.2% (414) |
| African American | 11.3% (75) |
| Hispanic | 7.5% (50) |
| Asian/Pacific Islander | 9.6% (64) |
| Other/ Mixed ethnicity and race | 9.3% (62) |
| Caregiver’s marital status | |
| Married | 77.0% (512) |
| Single | 7.0% (46) |
| Divorced or separated | 15.0% (100) |
| Widowed | 1.0% (7) |
| Annual Income | $86, 500 |
| Free/reduced lunch | 18.3% (122) |
The caretaker and youth visited the laboratory for an in-person, in-depth assessment at baseline and then at 18- and 36-month follow-ups. Caretakers provided informed written consent for their child’s participation; youth provided written assent. After the initial baseline assessment, regular phone follow-up assessments took place every six months for the next three years, for a total of seven repeated measures assessments. Both the caretaker and youth were interviewed with a semi-structured diagnostic interview (K-SADS mood disorder section; see below). Youth and their caretaker returned to the laboratory for an 18- and 36-month in-depth laboratory-based visit. At these three time points, the chronic stress portion of the contextual stress interview was administered. Retention rate from baseline to 36-month follow-up was 93%; 618 youth, or a caregiver, provided follow-up data over the course of the study. The institutional review boards at both the University of Denver and Rutgers University approved all procedures. Both youth and the caretaker were compensated monetarily for their participation.
Measures
Genotyping
Saliva samples were obtained from all study participants with Oragene™ (DNA Genotek, Ontario, Canada) collection kits, and DNA was extracted using standard salting-out and solvent precipitation methods. The method for 5-HTTLPR and SNP rs25531 is detailed in Whisman, Richardson, and Smolen (2011). The successful call-rate was 97.5% for 5-HTTLPR. 5-HTTLPR was in Hardy-Weinberg Equilibrium (p = .28). Three groups of participants were formed based on their genotyping: children homozygous for the lower expressing S or LG alleles (i.e., S/S), those heterozygous (i.e., S/L), and those homozygous for the higher expressing LA allele (i.e., L/L). Genotype did not vary significantly by race (χ2 (2) = 2.33, p = .32).
Depression Diagnoses
Trained interviewers administered the Mood Disorders and Psychosis subsections of the well-validated Schedule for Affective Disorders and Schizophrenia for School Age Children (K-SADS-PL; Kaufman et al., 1997) to youth and a caretaker about their child to assess for current and past episodes of depression and mania at baseline and each follow-up. No youth was diagnosed with a bipolar spectrum disorder or psychosis.
Interviewers and graduate students were trained by Ph.D. level, licensed clinical psychologists to conduct the diagnostic interviews. Diagnostic interviewers completed an intensive training program for administering the K-SADS and for assigning DSM–IV and research diagnostic criteria diagnoses. The training program consisted of attending approximately 40 hours of didactic instruction, listening to audiotaped interviews, and conducting practice interviews. The principal investigators (PIs) at each site held weekly supervision sessions for the interviewers. The PIs also reviewed interviewers’ notes and tapes to confirm the presence or absence of a diagnosis. Discrepancies were resolved through consensus meetings and best estimate procedures. Diagnostic interview inter-rater reliability was good (κ = .91) based on approximately 20% of interviews being reviewed for reliability. Interviewers utilized both youth report and parent report on the K-SADS to determine youths’ diagnostic status using best estimate diagnostic procedures (Klein et al., 2004).
For the GEM study, youth participants were deemed to have a depressive episode if they met DSM-IV criteria for Major Depressive Disorder (MDD) Definite, MDD-Probable (four threshold depressive symptoms with at least 2 weeks duration), or minor Depressive Disorder (mDD) Definite (two or three threshold depressive symptoms with at least 2 weeks duration) in the past 6 months as ascertained between each K-SADS follow-up interview. We combined these diagnoses together into a single variable as each of these depressive diagnoses lasted a minimum of two weeks and youth were required, per the K-SADS, to demonstrate significant distress and/or impairment. Moreover, we based the decision on prior evidence showing that depression is dimensionally distributed at the latent level (Hankin et al., 2005) and that these various depression diagnoses (i.e., MDD Definite, MDD Probable, and mDD Definite) all are associated with significant distress, impairment, and psychosocial problems (Avenevoli et al., 2015; Gotlib et al., 1995).
We interviewed both the youth and their caretaker every 6 months over the 3 years to enhance reliability and validity of diagnostic data and to create more accurate depression trajectories over the 3 year study follow-up given well-known memory and recall biases with retrospective recall over longer periods of time (Compton & Lopez, 2014; Costello, Erkanli, & Angold, 2006). Given our prospective ascertainment of depressive episodes repeatedly with a 6-month interviewing time frame, we anticipated that our depression rates would be higher than those reported in cross-sectional studies with lifetime reporting given prior evidence demonstrating that prevalence rates can be doubled when prospectively assessed in contrast to retrospectively measured (Moffitt et al., 2010).
Chronic peer stress
The Youth Life Stress Interview (YLSI; Rudolph & Flynn, 2007), a revised version of the UCLA Child Episodic Life Stress and Chronic Stress Interview (Rudolph & Hammen, 1999) was used. The YLSI is a reliable, valid, semi-structured contextual stress interview used to assess youths’ ongoing stress level. The YLSI has demonstrated excellent reliability and validity (e.g., Conley & Rudolph, 2009; Rudolph & Flynn, 2007). For this study, we concentrated on chronic stress in peer relationships. Interviewers ascertained from youth information relevant to chronic peer stress over the past 18 months, including standardized questions assessing aspects of friendships (e.g., poor quality friendships; lack of friendships overall; lack of trust, support, closeness in relationships; severity of conflict in relationships). Severity information based on responses to these questions were presented to a team of three or more blind raters, who came to an agreed upon severity score, from 1 (little/no stress), 2 (average/normal stress), 3 (moderate stress), 4 (serious stress), to 5 (severe stress).
As our primary purpose was to predict onset of depressive episodes over the three-year follow-up based on chronic peer stress exposure, we combined together the peer chronic stress ratings to cover the three years of the prospective study. We then dichotomized the total peer chronic stress score, that spanned the 3 years of the study, into none/little-average/normal (ratings on the severity score of 1–2) peer stress (coded as 0) and moderate-severe (ratings on the severity score that were greater than 2) peer stress (coded as 1) groupings for analysis. We created these dichotomous stress groupings for the GxE analyses because our a priori power analyses were based on stress groupings that contained sufficient proportions in each stress group (i.e., approximately 30% in a stress group) across the different genotype groups to produce reliable, robust estimates of GxE effects. Note that the results were highly similar regardless of whether dimensional or dichotomized chronic stress scores were used for all analyses for which there was sufficient dimensional stress data to adequately model1. Finally, we used the peer chronic stress data from the full three years of the study in the main analyses reported here to predict onset of depressive episode over the three year period; we also repeated all analyses using peer chronic stress experienced through 18 months of the study to ensure that our results applied to a prospective test of peer chronic stress predicting onset of depressive onset with stronger confidence in the temporal precedence of effects. In all cases, the pattern of findings was the same when peer chronic stress over 18 months was used, as when over 36 months, to predict onset of depressive episode over the 3 years of the study1.
Pubertal Development
At baseline of the GEM study, youth were administered the Pubertal Development Scale (PDS; Petersen et al., 1988), which includes five questions about physical development, scored from 1 (no) to 4 (development complete). Reliability and validity of the PDS is high (Petersen et al., 1988; Shirtcliff et al., 2009), as PDS scores relate significantly with physical examination for pubertal development (Shirtcliff et al., 2009). We followed standard PDS scoring to create prepubertal and postpubertal groups separately for girls and boys.
Data Analytic Plan
We first examined the pattern of depression by gender, grade and age, and pubertal status. We report these profiles for both prior depression history as well as prospectively ascertained trajectories of depressive episodes, as assessed repeatedly every 6-months via K-SADS over the 3-year longitudinal follow-up.
Then, we used logistic regression to investigate which factors predicted onset of new episodes of depression over time. Our a priori power analyses for testing GxE2 showed that we had sufficient power to detect a 3-way interaction, so we focused on our hypothesized 5-HTTLPR x peer chronic stress x development interaction. In one model, we used age as the indicator for development while controlling for the effect of pubertal status; and in the second model we used pubertal status as the developmental indicator and controlled for age. We also conducted an exploratory analysis to see if gender moderated the 3-way interaction, but this was viewed as exploratory given our lack of power to detect a reliable 4-way interaction. We used logistic regression to examine demographic and psychosocial predictors, including gender, development (age and pubertal status), and peer chronic stress, to predict depression rates over the 3 years without inclusion of genetic risk.
In all of these analyses, we entered prior history of depression (assessed at baseline to ascertain depression history before entry into the GEM study) as a control because substantial prior evidence demonstrates that depression is a recurrent disorder, and we were interested in investigating the prediction of prospectively assessed, new onsets of depression over the 3 years follow-up. Additionally, by controlling for prior depression, we removed statistically the relation between past depression and our primary predictors, especially chronic peer stress, which was significantly associated with past depression (see Table 3). We note here that we investigated whether prior depression history moderated any of the GxE (and GxExDevelopment) effects, but no significant interactions were obtained with prior depression history. Thus, we included only the main effect to control for past depression history in the results reported. By doing this and using onsets of depressive episodes, as prospectively ascertained by K-SADS interview every 6 months over the 3 years follow-up, as our primary dependent outcome, we were able to rigorously investigate the longitudinal prediction of the development of depression over time. Finally, self-reported ethnicity was included as covariate for the GxE analyses to manage concerns about ethnic population stratification. Self-reported ethnicity correlates nearly perfectly with genetic ancestry and addresses concerns about population stratification (Tang, Quertermous, Rodriguez, et al., 2005).
Table 3.
Pearson correlations among primary variables
| Gender | Age | Puberty | 5-HTTLPR | Peer Stress | Past Depression | Future Depression | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Gender | 1.0 | ||||||
| Age | .02 | 1.0 | |||||
| Puberty | .27** | .60*** | 1.0 | ||||
| 5-HTTLPR | .02 | .005 | .01 | 1.0 | |||
| Peer Stress | .09* | .02 | .06 | −.05 | 1.0 | ||
| Past Depression | .08* | .21*** | .19*** | .02 | .12** | 1.0 | |
| Future Depression | .14** | .23*** | .22*** | .03 | .31*** | .24*** | 1.0 |
Note: N = 618.
Puberty = Prepubertal status (0) or pospubertal status (1). 5-HTTLPR = functional variation in the Transporter-Linked Polymorphic Region of the gene SLC6A4, which codes for the serotonin transporter; 0=LL, 1=SL, 2=SS. Peer Stress = Chronic Peer Stress codes summed across the 3 time points, as assessed via Youth Life Stress Interview. Past Depression = Clinically significant depressive episode, as diagnosed via K-SADS prior to baseline. Future Depression = Clinically significant depressive episode, as diagnosed via K-SADS administered repeatedly every 6-months, occurred at some point after baseline through 36-months follow-up.
RESULTS
Preliminary Descriptive Results
Means and standard deviations for all primary variables, overall as well as separated by gender and pubertal status, are presented in Table 2. Pearson correlations among all primary variables are provided in Table 3. It is notable that the zero-order correlations of relevant variables for depression were of similar magnitude whether development was indexed by age or pubertal status. Polychoric correlations showed no significant rGE with peer stress (r = −.04).
Table 2.
Descriptive statistics overall and by gender and pubertal status.
| Variable | Full sample M (SD) |
Girls M (SD) |
Boys M (SD) |
Prepubertal M (SD) |
Postpubertal M (SD) |
|---|---|---|---|---|---|
| Age | 11.6 (2.4) | 11.65 (2.4) | 11.53 (2.4) | 10.67 (2.07) | 13.75 (1.45) b |
| Puberty | 31.7% | 43.4% (.49) | 17.5% (.37)a | -- | -- |
| 5-HTTLPR | |||||
| SS | 21.1% | 19.3% | 22% | 20.8% | 20.9% |
| SL | 46.2% | 50.3% | 41.1% | 45% | 46.9% |
| LL | 30.5% | 27.9% | 34% | 31.2% | 30.1% |
| High Peer Stress | 25.9% | 28.6% | 22.6% a | 25% | 26.3% |
| Past Depression | 20% | 23% | 17% a | 15% | 32% b |
| Future Depression | 25.6% | 31% | 19% a | 18.75% | 40% b |
Note. Full sample N = 665; Girls n = 365; boys n = 300; Prepubertal n = 450; Postpubertal n = 215.
Denotes significant gender difference.
Denotes significant pubertal group difference.
Puberty = Prepubertal status (0) or pospubertal status (1). 5-HTTLPR = functional variation in the Transporter-Linked Polymorphic Region of the gene SLC6A4, which codes for the serotonin transporter. High Peer Stress Group = Little to Normal Stress (0); Moderate to High Stress (1), as assessed by the Youth Life Stress Interview. Past Depression = Clinically significant depressive episode, as diagnosed via K-SADS prior to baseline. Future Depression = Clinically significant depressive episode, as diagnosed via K-SADS administered repeatedly every 6-months, that occurred at some point after baseline through 36-months follow-up.
Depression Diagnoses: Past History and Prospective Trajectories
In total, 20% of the entire sample reported a past depression diagnosis. Logistic regression analysis was used to examine the effects of gender and grade (in one analysis) and then gender and pubertal status (in the second analysis) on past depression diagnosis. For the first analysis, both gender (b = 0.42, p < .05, Wald = 3.91, odds ratio = 1.51) and grade (b = 0.26, p < .001, Wald = 31.40, odds ratio = 1.30) were significantly associated with past depression, although the gender x grade interaction was not significant. For the second analysis, only pubertal status (b = 0.91, p < .001, Wald = 18.05, odds ratio = 2.48), but not gender (b = 0.19, p = .39, Wald = .73, odds ratio = 1.21), was significantly associated with past depression; the gender x pubertal status interaction was not significant. Descriptively, 23% of girls and 16.6% of boys were diagnosed with a prior depressive episode; 10% of 3rd graders, 16.6% of 6th graders, and 33% of 9th graders had a past depression; and 15% of prepubertal youth and 32% of postpubertal adolescents had a past diagnosis.
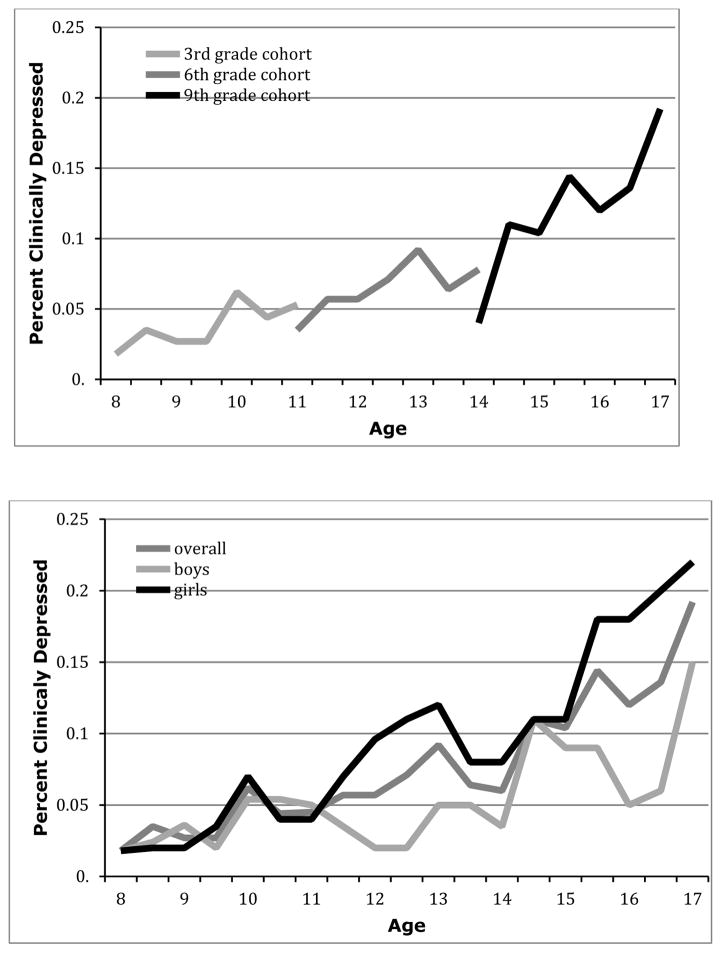
Overall, 25.6% of youth (n = 158) experienced an onset of depression over the 36 months; 17.5% were truly new episodes (n=108), and 8.1% (n=50) were recurrences. Figure 1 shows the descriptive trajectories of prospectively assessed depressive episodes over the course of the study. The top panel displays these trajectories by grade cohort. As the GEM study used an accelerated longitudinal cohort design, an important assumption to verify was that the incidence of depression at the same time point when grades overlap did not statistically differ between grade cohorts (Duncan et al., 1996). Importantly, this assumption was met, as there was no significant difference between adjacent grade cohorts for clinical depression rates at the overlapping time point (highest F = 2.41); thus, we were justified in combining data across cohorts.
Figure 1.
Descriptive depression trajectories across time by grade cohort (top panel) and by gender (bottom panel).
The bottom panel of Figure 1 shows these trajectories for the sample overall as well as separately for boys and girls as a function of age. As suggested by the Figure and shown in prior developmental epidemiological research, multilevel modeling verified that there was a significant gender x age interaction affecting rates of depression over time, F (1, 614) = 3.83, p < .05. Logistic regression showed that the gender difference in depression significantly emerged starting at age 12.5, χ2 = 5.11, p < .05, and differed at all ages thereafter except 14.5. Indeed, descriptive rates showed that at least twice as many girls were diagnosed with depression, compared to boys, at each age time point from age 12.5 through age 17.5, except at age 14.5. Also, repeated measures ANOVA showed that there was a significant gender x grade interaction accounting for rates of depression over time, F (2, 617) = 3.25, p < .05. Consistent with the finer-grained analyses by age, decomposition of this interaction showed that the gender difference was significant in 6th (F = 7.67, p < .01) and 9th (F = 5.82, p < .01) grade cohorts.
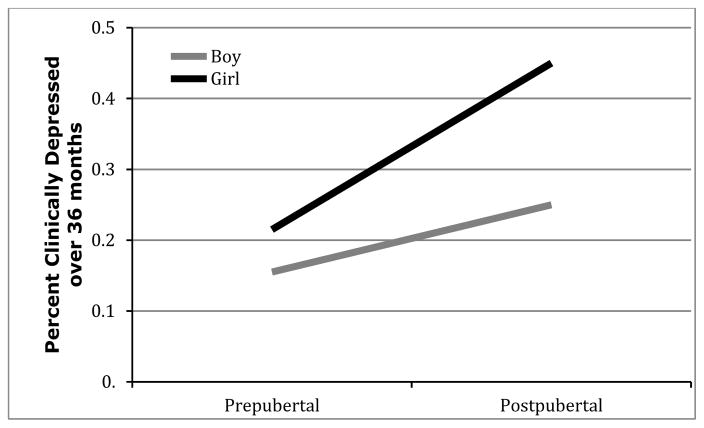
Next, both main effects of gender (b = 0.52, p = .01, Wald = 6.06, odds ratio = 1.67), and pubertal status (b = 0.92, p < .001, Wald = 21.02, odds ratio = 2.50), significantly predicted future onsets of depression, but these were qualified by a significant gender x pubertal status interaction (b = 0.54, p < .05, Wald = 2.41, odds ratio = 1.71). Decomposition of this interaction revealed a significant pubertal effect only among girls (b = 1.07, p < .001, Wald = 19.83, odds ratio = 2.93) but not boys (b = 0.54, p = .15, Wald = 2.00, odds ratio = 1.71). Figure 2 illustrates this significant gender X puberty interaction: postpubertal girls experienced the greatest prospective surge in depression diagnoses over the three years.
Figure 2.
Gender x Pubertal status interaction in the prospective prediction of depression onsets over the 3-year follow-up.
Prediction of Future Depressive Episodes: Effects of Gender, Peer Chronic Stress, Development, and 5-HTTLPR
Before examining the 3-way G x E x Development interaction, we first used logistic regression to test whether the expected G x E predicted later onsets of depression diagnoses after controlling for youths’ prior history of depression. 5-HTTLPR significantly interacted with Peer Stress to predict depression onset (b = .23, p = .006, Wald = 7.59, odds ratio = 1.26)3. Given this, we examined the G x E x Development interaction in prediction of prospective onset of depressive episodes over the three years.
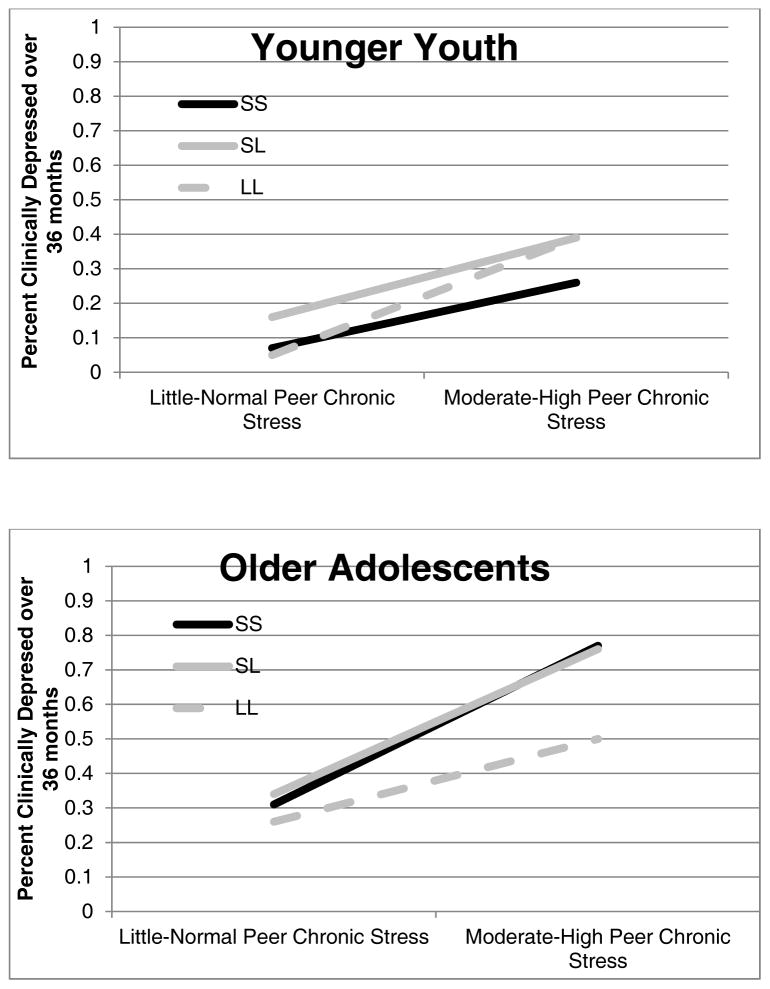
The 3-way interaction of Pubertal Status x 5-HTTLPR x Peer Stress did not significantly predict future onset of depression (Wald = 1.92, p = .16), whereas the 3-way interaction of Age x 5-HTTLPR x Peer Stress did significantly predict future onset of depression (b = .07, p < .05, Wald = 4.02, odds ratio = 1.15). (See Supplementary Table 1 for complete reporting of the final Age x 5-HTTLPR x Peer Stress analysis with all covariates and predictors entered at each step). Figure 3 illustrates these results for each age group separately (under age 13, top; over age 13, bottom). Consistent with our hypothesis, older adolescents who experienced more chronic peer stress over the three years and who were most genetically susceptible (i.e., SS and SL genotypes) were the most likely to be diagnosed with a new onset of a depressive episode: (b = .29, p = .01, Wald = 6.39, odds ratio = 1.33), whereas this GxE was not found in the younger group (b = .05, p = .58, Wald = .30, odds ratio = 1.05).
Figure 3.
5-HTTLPR x Chronic Peer Stress x Age interaction in the prospective prediction of depression onsets over the 3-year follow-up.
As the 5-HTTLPR x Peer Stress was significantly moderated by child age, we then conducted a few exploratory analyses to better understand the robustness and specificity of this effect. First, gender did not significantly moderate the GxExAge interaction: the 4-way interaction of Gender x Development x 5-HTTLPR x Peer Stress was not significant (for age: Wald = .16, p = .68; for pubertal status: Wald = .03, p = .88). Second, we conducted additional analyses to examine whether the GxExAge effect was specific for chronic peer-stress by including non-peer chronic stress, specifically academic, parent-child, and romantic forms of chronic stress, as obtained by the YLSI ratings for these domains, as covariates in the logistic regressions to predict onset of depressive episode. It should be noted that inclusion of these non-peer forms of chronic stress provides a very stringent and risky test of the GxExAge effect with specificity for peer chronic stress because considerable prior research shows that academic, parent-child, and romantic chronic stress are very strong, potent predictors of adolescent depression (e.g., Hammen; 2009; Monroe et al., 1999), and several prior studies show that 5-HTTLPR moderates the effect of parent-child chronic stress on later depressive symptoms during adolescence (Hammen et al., 2010; Jenness et al., 2011; Vrshek-Schallhorn et al., 2013). After controlling for non-peer chronic stress (significant predictor of depression among older adolescents: b = .27, p = .02, Wald = 5.37, odds ratio = 1.31, and younger children: b = .28, p = .01, Wald = 6.27, odds ratio = 1.32), 5-HTTLPR x Peer Stress significantly predicted later depressive episodes among the older adolescents (b = .28, p = .05, Wald = 3.76, odds ratio = 1.32) but not younger children (b = .03, p = .76, Wald = .09, odds ratio = 1.03).
While gender did not moderate the 3-way interaction, we examined gender and developmental effects on peer stress. Univariate analysis of variance (ANOVA) showed that there was a significant gender difference in peer chronic stress exposure over the three years: F (1, 644) = 6.35, p = .01, partial η2 = .01. As expected and consistent with prior research, girls experienced significantly higher levels of peer chronic stress. The main effects of age [F (9, 644) = 1.55, p = .12, partial η2 = .02] and pubertal status [F (1, 644) = .63, p = .43, partial η2 = .001] were not significant, nor did they moderate the effect of gender on peer chronic stress.
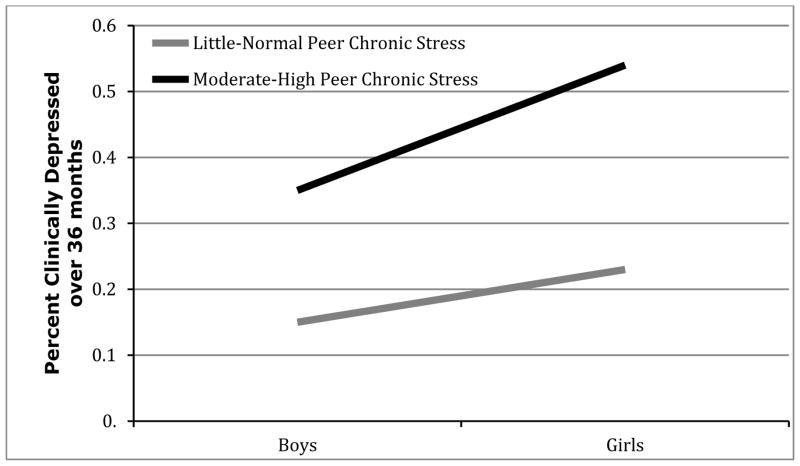
Then, we used logistic regression analyses to examine whether gender moderated effects of peer chronic stress and development in the prediction of prospective onset of depression diagnosis over the three years. Only the 2-way interaction between Gender X Peer Stress significantly predicted later depression onset (b = .18, p < .001, Wald = 13.38, odds ratio = 1.20). After decomposing this interaction, results showed that peer chronic stress significantly predicted prospective onset of depressive diagnosis more strongly among girls (b = 1.43, p < .001, Wald = 24.54, odds ratio = 4.17), while the effect was still significant, albeit less so, among boys (b = 1.27, p = .001, Wald = 10.84, odds ratio = 3.58). Figure 4 shows this effect.
Figure 4.
Gender x Chronic Peer Stress interaction in the prospective prediction of depression onsets over the 3-year follow-up.
Discussion
We planned the GEM study to delineate and predict depression trajectories from approximately ages 8 through 18. Using an accelerated longitudinal cohort design, we used repeated semi-structured diagnostic interviews to assess for onsets of depressive episodes every 6 months over 3 years with a moderately large sample of youth recruited from different grade cohorts (initially 3rd, 6th, and 9th grades). Three main sets of findings were obtained that have relevance for understanding developmental trajectories of depression from childhood through late adolescence. First, the overall rates of depression diagnoses and the emergence of the gender difference in depression closely matched established developmental epidemiological data on age-related trends in depression among youth. Second, we investigated explanations to predict prospectively assessed new onsets of depression over the 3 years of the GEM study. Consistent with past meta-analyses, we found a significant GxE in the prediction of later depression. Moreover, age moderated this GxE such that older aged, genetically susceptible (i.e., S carriers of 5-HTTLPR) adolescents who experienced more chronic peer stress over time were the most likely to become depressed. Finally, girls with greater chronic peer stress were the most likely to be diagnosed with a depressive episode.
Depression rates: Age, pubertal status, and gender effects
Prevalence rates of depressive episode onset found over time in the GEM study were remarkably similar to those found in other studies utilizing representative samples of youth recruited from the general community. In the two largest cross-sectional studies of youth depression, to date, that used rigorous, epidemiologically sound methods, one-year prevalence rate for clinical depression was 2.7% for 8 to 15 year olds (Merikangas et al., 2010) and 7.5% for 13–18 year olds (Avenevoli et al., 2015). Our prevalence data from the GEM study closely matches these estimates as our rate for past year depression was 3.3% for 8 to 15 year olds and 7.9% for 13 to 17 year olds. Moreover, our overall longitudinal depression trajectories similarly converge with the past longitudinal studies designed to ascertain patterns of depression over time from childhood into adolescence. In particular, overall rates of depression in the current study were relatively low and stable at around 3–5% from ages 8 to 14, and then surged dramatically from about 5% to 20% from ages 14 to 17.
Similar to the concordance of overall depression rates found in the literature, the gender divergence in depression in the GEM study also closely corresponded to the patterning repeatedly obtained in past developmental epidemiological studies. We found that significantly more girls than boys experienced a depressive episode starting at age 12½, and this pattern of twice as many girls being depressed relative to boys was maintained at nearly every 6-month follow-up through late adolescence and the end of our assessments.
Our results also showed that pubertal status was significantly associated with greater overall rates of depression and the gender difference in depression. Postpubertal youth were 2½ times more likely to experience an episode of depression over three years, and this was especially so for postpubertal girls. These findings are consistent with past research showing that depression increases significantly after puberty and that the gender difference in depression emerges after the pubertal transition (e.g., Copeland et al., 2010; Graber, 2013, Rudolph, 2014). Thus, both age and pubertal status significantly affected the developmental unfolding of depression diagnoses and the gender difference in depression.
Taken as a whole, these descriptive prevalence data regarding overall depression trajectories, the timing and patterning of the gender difference in depression, and the impact of development on depression, provide strong confidence in the depression trajectory data obtained from the GEM study using an accelerated longitudinal cohort design with repeated diagnostic assessment of depression occurring every 6 months over 3 years. Moreover, establishing that the depression trajectory findings hew closely to and converge with these other, established developmental epidemiological studies of similar aged youth enhances confidence in the explanatory factors, including genetic risk and peer chronic stress, assessed and examined in the GEM study to account for the development in depression over time.
5-HTTLPR, peer stress, and developmental influences
The primary etiological hypothesis tested in this study was that 5-HTTLPR would interact with higher chronic peer stress to predict prospective onsets of depressive episodes, and that this GxE effect would be potentiated across development. Consistent with this hypothesis, we found that older aged SS/ SL genotype adolescents who experienced greater chronic peer stress were the most likely to experience a depressive episode over the three years. Among the younger aged youth, we obtained the expected and well-replicated main effect of peer stress predicting depression, but 5-HTTLPR did not moderate this association. Moreover, only age, but not pubertal status, moderated this GxE effect in predicting later depression. This suggests that more general developmental processes, as opposed to pubertal status as a specific factor, affect the synergestic GxE effect in depression. Future research is needed that explicitly assesses developmentally sensitive age-related processes to explain why the 5-HTTLPR x chronic peer stress effect becomes accentuated over age throughout adolescence.
Our findings are consistent with other GxE studies showing that 5-HTTLPR interacts with environmental risk to predict depressive symptoms, especially so among older youth. Petersen and colleagues (2012) reported that the GxE effect became increasingly more significant with older age in their longitudinal study following youth from ages 12 to 17 and predicting broad-band internalizing symptoms. Prior reviews show that the GxE effect predicting depression is most robust among older adolescents and young adults relative to younger-aged youth (Uher & McGuffin, 2008, 2010). Our findings replicate and extend past GxE studies using methodologically rigorous contextual stress interviews to assess youths’ stressful environment and with a longitudinal design to predict prospective increases in depression. Previous studies showed that 5-HTTLPR interacted with family stress (Hammen et al., 2010; Jenness et al., 2011) to predict prospective elevations in depressive symptoms, and general interpersonal stress (Vrshek-Schallhorn et al., 2013) interacted with 5-HTTLPR to predict depression diagnoses among older adolescents. Thus, 5-HTTLPR interacts with developmentally salient and relevant environmental risk—chronic peer stress in this study—to predict prospective onsets of depression among youth.
Why might the interaction between 5-HTTLPR and peer stress become significant among older adolescents? Adolescence brings about a cascade of neural, physical, hormonal, social, emotional, and psychological changes, but most youth pass through this transition without substantial emotional or behavioral problems. The accentuation hypothesis offers a possible explanation about why adolescence confers risk for some, but not all, individuals (Caspi & Moffitt, 1991). The accentuation model proposes that youth without pre-existing vulnerabilities will traverse the turmoil of the adolescent transition without significant emotional or behavioral problems, whereas individuals possessing risk characteristics will react less adaptively to developmentally salient and contextually prominent environmental influences. Our findings are consistent with the accentuation hypothesis in that 5-HTTLPR clearly constitutes a pre-existing, stable susceptibility that can potentiate the impact of peer stress to precipitate depression onset. There is increasing recognition that a coordinated set of stress regulation systems and processes (e.g., cortisol reactivity, immune function, autonomic response, neural activity) is involved with responding to challenges that occur during adolescence. Normally this coordinated system can adaptively cope with these stressors. However, for those with pre-existing risk, such as 5-HTTLPR as genetic susceptibility that confers dysregulated stress response under negative environmental contexts (Hankin, Nederhoff, et al., 2011; Miller, Wankerl, Stalder, Kirschbaum, & Alexander, 2013; Munafo, Brown, & Hariri, 2008), and for those exposed to elevated peer stress (a developmentally salient context given the social reorientation of adolescence; Nelson et al., 2005), the adolescent transition can accentuate these vulnerabilities and stress-reactive processes to enhance the likelihood that susceptible youth will experience depression.
Gender differences in youth depression
Gender did not moderate the interaction among development, 5-HTTLPR, and peer stress in the prediction of depression. This suggests that the 3-way GxExAge interaction applies equally to boys and girls, and implies that this 3-way interaction is not likely to account for the emergence of the gender difference in depression, although a larger sample size that has sufficient power to detect a potential 4-way interaction would be needed. Our results revealing no moderation by gender are consistent with some other research showing that peer stress amplifies the link between development and psychopathology equally for boys and girls (Sontag, Graber, Klemans, 2011; Teunissen et al., 2011).
We found that gender interacts with chronic peer stress to predict prospective episodes of depression. That girls react more strongly to peer stress exposure, than boys, is consistent with other studies using contextual threat methods to assess peer stress, although these studies either measured depressive symptoms longitudinally or depression concurrently (e.g., Hankin et al., 2007; Rudolph et al., 2000). Importantly, then, our findings extend this past work by using prospective, repeated measures of interview-based assessment of peer chronic stress and diagnostic interviewing to ascertain onset of depression episodes. Taken together, there is growing evidence that girls are more likely to respond to heightened peer stress with elevated depressive symptoms as well as depressive episodes over time.
Strengths, weaknesses, and future directions
Our findings need to be considered in light of strengths and weaknesses. The GEM study used multiple methods, informants, and repeated measures to provide a rigorous mapping of depression trajectories over time and to test etiological predictions of a developmentally contextualized GxE model. We used gold-standard contextual stress interview methods to assess peer chronic stress, and this approach provides more exacting measurement of environmental risk, which is necessary for more optimal tests of GxE influences (Monroe & Reid, 2008; Uher & McGuffin, 2008, 2010). Our results on GxE are based on prospective prediction of future onsets of depression because we controlled for past depression history in analyses, and our depression outcome was constructed via episodes that were longitudinally and repeatedly ascertained every 6 months by K-SADS over the 3 years of the study. Finally, the GEM study recruited a general community sample of youth and caretaker who were selected based only on child’s grade to provide a relatively equal proportion of cohorts that cover the critical developmental periods from childhood through adolescence. This allowed us to test developmental trajectories of depression before and throughout the known high-risk adolescent period for onset of depression. Use of a general population sample provides more accurate, less biased estimates and reduces known limitations and biases inherent from samples recruited from psychiatric clinics, or youth diagnosed with current depression, all of whom differ from unselected, general community samples in terms of treatment seeking, greater severity, and comorbidity patterns (e.g., Goodman et al., 1997).
At the same time, particular study limitations provide avenues for future inquiry. First, we only examined one candidate gene—5-HTTLPR. Psychiatric disorders, especially clinical depression, involve many genetic influences, each of small effect (e.g., Kendler, 2013). Future research is needed to investigate the role of other genetic susceptibilities, as informed and analyzed by modern genetic approaches. Second, we did not formally investigate mechanisms underlying the GxExAge effect. We speculated on various possible processes above, but future research is needed to examine potential hormonal, neural, psychosocial, affective, epigenetic, and stress physiological mechanisms. Third, we used a self-report measure of pubertal status, so we do not know the precise processes underlying effects found with pubertal status. Past research has identified pubertal timing, development, and tempo as significant, albeit non-redundant, pubertal influences affecting risk to depression (Keenan et al., 2014; Mendle, 2014; Rudolph, 2014). Ideally future research would repeatedly collect multiple pubertal assessments over time to enable disaggregation of these different hypothesized pubertal mechanisms. Fourth, we cannot make claims about causal direction between chronic peer stress and depression onset over the three years of the study. Stress and depression have bi-directional and transactional associations over time (Hankin & Abramson, 2001; Rudolph et al., 2000). Controlling for prior depression and prospectively assessing depressive episodes every 6 months over 3 years cannot rule out more complex, dynamic associations between stress and depression. Finally, we did not examine the specificity of prediction to depression versus known co-occurring disorder and symptom trajectories, especially anxiety and externalizing problems.
In summary, depression is a burdensome, debilitating disorder with clear developmental patterns across age, gender, and pubertal status. Findings from the GEM study showed that depression rises dramatically in middle to late adolescence, or after puberty. The gender difference in depression emerged in early adolescence, and post-pubertal status predicted the gender divergence in depression. Older aged, genetically susceptible youth who experienced greater peer stress, were the most likely to be diagnosed with a depressive episode. Girls experiencing higher peer stress were also the most likely to receive a depression diagnosis.
Supplementary Material
General Scientific Summary.
This study used repeated assessments of diagnostic interviewing in a moderately large sample of youth over 3 years to show that depression rates increase in middle to late adolescence, or post-pubertally, and that the gender difference in depression emerges earlier in adolescence (age 12 ½), or post-pubertally. Additionally, genetically susceptible older adolescents who experience chronic peer stress were the most likely to become depressed over time.
Acknowledgments
This work was supported by NIMH grants R01-MH 077195 and R01-MH 077178 awarded to Benjamin L. Hankin and Jami F. Young. We would like to thank the many staff members and students who assisted with data collection, interviewing, and project management. In particular, we thank Heather Harbison and Dana Sheshko who served as project managers for the majority of the GEM study. Also, we would like to thank the families for their participation in this longitudinal study. Finally, John R.Z. Abela sadly died early in the course of this study, but his efforts, energy, and dedication were invaluable for the research.
Footnotes
Contact the first author for these analyses.
Power for the genetic vulnerability X stress interactions was based on past research finding significant GxE (Caspi’s n = 847; Kendler’s n = 549; Kaufman et al 2006’s n = 196), so our total planned N, before the study was initiated, was 650. This sample size was calculated to provide sufficient power, especially given that we were using multiple assessments of stressors and depression over time that would enhance power (Snijders & Bosker, 1999). More specifically, in our power calculations prior to study initiation, we assumed that environmental stress would be present in approximately 30% of the participants and that each additional copy of the risk allele would increase the depression score by an additional 0.5 SD in participants with the vulnerability compared to those without the vulnerability (corresponding to a R2 of 0.032 for this gene x stress interaction); therein, the power to detect this interaction was 87%. With inclusion of one additional variable (e.g., age), we estimated power to be 83% to test a 3-way interaction of genetic vulnerability x stress x age.
All results were the same when rs25531 was used as for 5-HTTLPR as reported here in the main text.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
The authors report no conflicts of interest.
Contributor Information
Benjamin L. Hankin, Department of Psychology, University of Denver
Jami F. Young, Department of Clinical Psychology, Graduate School of Applied and Professional Psychology, Rutgers University
John R. Z. Abela, Department of Clinical Psychology, Graduate School of Applied and Professional Psychology, Rutgers University
Andrew Smolen, University of Colorado-Boulder, Institute of Behavior Genetics.
Jessica L. Jenness, Department of Psychology, University of Denver
Lauren D. Gulley, Department of Psychology, University of Denver
Jessica R. Technow, Department of Psychology, University of Denver
Andrea Barrocas Gottlieb, Department of Psychology, University of Denver.
Joseph R. Cohen, Department of Psychology, University of Illinois
Caroline W. Oppenheimer, Western Psychiatric Institute and Clinics, University of Pittsburgh
References
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Angold A, Worthman C, Costello EJ. Puberty and depression. In: Hayward C, editor. Gender differences at puberty. New York, NY: Cambridge University Press; 2003. pp. 137–164. [Google Scholar]
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major Depression in the National Comorbidity Survey-Adolescent Supplement: Prevalence, Correlates, and Treatment. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54(1):37–44. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli JL, Prinstein MJ. Reciprocal, longitudinal associations among adolescents’ negative feedback-seeking, depressive symptoms, and peer relations. Journal of Abnormal Child Psychology. 2006;34(2):159–169. doi: 10.1007/s10802-005-9010-y. [DOI] [PubMed] [Google Scholar]
- Carter JS, Garber J, Ciesla JA, Cole DA. Modeling relations between hassles and internalizing and externalizing symptoms in adolescents: A four-year prospective study. Journal of Abnormal Psychology. 2006;115(3):428–442. doi: 10.1037/0021-843X.115.3.428. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Individual differences are accentuated during periods of social change: The sample case of girls at puberty. Journal of Personality and Social Psychology. 1991;61:157–168. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: Depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and Psychopathology. 2007;19(4):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, Rojas M, Brook J, Streuning EL. An epidemiologic study of disorders in late childhood and adolescence .1. age-specific and gender-specific prevalence. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1993;34(6):851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Cole DA, Nolen-Hoeksema S, Girgus J, Paul G. Stress exposure and stress generation in child and adolescent depression: A latent trait-state-error approach to longitudinal analyses. Journal of Abnormal Psychology. 2006;115(1):40–51. doi: 10.1037/0021-843X.115.1.40. [DOI] [PubMed] [Google Scholar]
- Compton WM, Lopez MF. Accuracy in reporting past psychiatric symptoms: The role of cross-sectional studies in psychiatric research. The Journal of the American Medical Association. 2014;71:233–234. doi: 10.1001/jamapsychiatry.2013.4111. [DOI] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD. The emerging sex difference in adolescent depression: Interacting contributions of puberty and peer stress. Development and psychopathology. 2009;21(02):593–620. doi: 10.1017/S0954579409000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CS, Rudolph KD, Bryant FB. Explaining the longitudinal association between puberty and depression: sex differences in the mediating effects of peer stress. Development and Psychopathology. 2012;24(02):691–701. doi: 10.1017/S0954579412000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B. Outcomes of early pubertal timing in young women: A prospective population-based study. American Journal of Psychiatry. 2010;167:1218–1225. doi: 10.1176/appi.ajp.2010.09081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Copeland W, Angold A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? Journal of Child Psychology and Psychiatry. 2011;52(10):1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? Journal of Child Psychology and Psychiatry. 2006;47(12):1263–1271. doi: 10.1111/j.1469-7610.2006.01682.x. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frnak E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression - A theoretical model. Archives of General Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Duncan SC, Duncan TE, Hops H. Analysis of longitudinal data within accelerated longitudinal designs. Psychological Methods. 1996;1(3):236. [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9(10):908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Prevelance and Comorbidity of DSM III-R Diagnoses In A Birth Cohort of 15 Year Olds. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(6):1127–1134. doi: 10.1097/00004583-199311000-00004. [DOI] [PubMed] [Google Scholar]
- Galvao TF, Silva MT, Zimmermann IR, Souza KM, Martins SS, Pereira MG. Pubertal timing in girls and depression: A systematic review. Journal of Affective Disorders. 2014;155:13–19. doi: 10.1016/j.jad.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki M. In search of explanations for early pubertal timing effects on developmental psychopathology. Current Directions in Psychological Science. 2009;18:327–331. http://dx.doi.org/10.1111/j.1467-8721.2009.01661.x. [Google Scholar]
- Gibb BE, Uhrlass DJ, Grassia M, Benas JS, McGeary J. Children’s inferential styles, 5-HTTLPR genotype, and maternal expressed emotion-criticism: An integrated model for the intergenerational transmission of depression. Journal of Abnormal Psychology. 2009;118(4):734. doi: 10.1037/a0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Lahey BB, Fielding B, Dulcan M, Narrow W, Regier D. Representativeness of clinical samples of youths with mental disorders: A preliminary population-based study. Journal of Abnormal Psychology. 1997;106:3–14. doi: 10.1037//0021-843x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PME. First-episode major depression in adolescents - Affective, cognitive and endocrine characteristics of risk status and predictors of onset. British Journal of Psychiatry. 2000;176:142–149. doi: 10.1192/bjp.176.2.142. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Lewinsohn PM, Seeley JR. Symptoms versus a diagnosis of depression- differences in psychosocial functioning. Journal of Consulting and Clinical Psychology. 1995;63(1):90–100. doi: 10.1037//0022-006x.63.1.90. [DOI] [PubMed] [Google Scholar]
- Graber JA. Pubertal and neuroendocrine development and risk for depressive disorders. In: Allen NB, Sheeber L, editors. Adolescent Emotional Development and the Emergence of Depressive Disorders. Cambridge University Press; Cambridge, UK: 2008. pp. 74–91. [Google Scholar]
- Graber JA. Pubertal timing and the development of psychopathology in adolescence and beyond. Hormones and Behavior. 2013;64(2):262–269. doi: 10.1016/j.yhbeh.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: Measurement issues and prospective effects. Journal of Clinical Child and Adolescent Psychology. 2004;33(2):412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY, Campbell AJ, Krochock K, Westerholm RI. Stressors and child and adolescent psychopathology: Evidence of moderating and mediating effects. Clinical Psychology Review. 2006;26(3):257–283. doi: 10.1016/j.cpr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Hammen C. Adolescent Depression: Stressful Interpersonal Contexts and Risk for Recurrence. Current Directions in Psychological Science. 2009;18(4):200–204. doi: 10.1111/j.1467-8721.2009.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene-environment interactions predicting depression symptoms in youth. Journal of Child Psychology and Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Shih JH, Brennan PA. Intergenerational transmission of depression: Test of an interpersonal stress model in a community sample. Journal of Consulting and Clinical Psychology. 2004;72(3):511–522. doi: 10.1037/0022-006X.72.3.511. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KA. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10 year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–141. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Fraley RC, Lahey BB, Waldman ID. Is depression best viewed as a continuum or discrete category? A taxometric analysis of childhood and adolescent depression in a population-based sample. Journal of abnormal psychology. 2005;114(1):96. doi: 10.1037/0021-843X.114.1.96. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Jenness J, Abela JRZ, Smolen A. Interaction of 5HTTLPR and idiographic stressors predicts prospective depressive symptoms specifically among youth in a multi-wave study. Journal of Child and Adolescent Clinical Psychology. 2011;40:572–585. doi: 10.1080/15374416.2011.581613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness JL, Young JF, Abela JRZ, Smolen A, Ormel J, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect “for better and worse. Translational Psychiatry (Nature publication) 2011;1:1–7. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Wetter E, Cheely CA. Sex differences in child and adolescent depression: A developmental psychopathological approach. In: Abela JRZ, Hankin BL, editors. Handbook of Child and Adolescent Depression. New York, NY: Guilford Press; 2008. pp. 377–414. [Google Scholar]
- Hayward C. Gender differences at puberty. NY: Cambridge University Press; 2003. [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. Journal of Adolescent Health. 2002;30:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Nolen-Hoeksema S. Gender differences in depression. In: Hammen C, Gotlib I, editors. Handbook of Depression. 3. New York: The Guilford Press; 2014. [Google Scholar]
- Huddleston J, Ge X. Boys at puberty: Psychosocial implications. In: Hayward C, editor. Gender differences at puberty. New York, NY: Cambridge University Press; 2003. pp. 113–134. [Google Scholar]
- Jenness J, Hankin BL, Abela JRZ, Young JF, Smolen A. Chronic family stress interacts with 5-HTTLPR to predict prospective depressive symptoms among youth. Depression and Anxiety. 2011;28:1074–1080. doi: 10.1002/da.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Heron J, Araya R, Paus T, Croudace T, Rubin M, Marcus M, Lewis G. Association between pubertal development and depressive symptoms in girls from a UK cohort. Psychological Medicine. 2012;12:1–11. doi: 10.1017/S003329171200061X. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The Serotonin Transporter Promoter Variant (5-HTTLPR), Stress, and Depression Meta-analysis Revisited Evidence of Genetic Moderation. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Keenan K, Culbert KM, Grimm KJ, Hipwell AE, Stepp SD. Timing and Tempo: Exploring the Complex Association Between Pubertal Development and Depression in African American and European American Girls. Journal of Abnormal Psychology. 2014;123(4):725–736. doi: 10.1037/a0038003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. What psychiatric genetics has taught us about the nature of psychiatric illness and what is left to learn. Molecular Psychiatry. 2013;18:1058–1066. doi: 10.1038/mp.2013.50. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Archives of general psychiatry. 2003;60(7):709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Cogner RD, Elder GH, Lorenz FO. Reciprocal influences between stressful life events and adolescent internalizing and externalizing problems. Child Development. 2003;74(1):127–143. doi: 10.1111/1467-8624.00525. [DOI] [PubMed] [Google Scholar]
- Klein DN, Dougherty LR, Olino TM. Toward guidelines for evidence-based assessment of depression in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:412–432. doi: 10.1207/s15374424jccp3403_3. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman SA, Lewinsohn PM, Seeley JR. Subthreshold depressive disorder in adolescents: predictors of escalation to full-syndrome depressive disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(7):703–710. doi: 10.1097/CHI.0b013e3181a56606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Eley TC. New behavioral genetic approaches to depression in childhood and adolescence. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. NY: Guilford Press; 2008. pp. 124–148. [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology. 1993;102:133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- Lewinshon PM, Rohde P, Seeley JR, Klein DN, Gotlib LH. Natural course of adolescent major depressive disorder in a community sample: Predictors of recurrence in young adults. American Journal of Psychiatry. 2000;157(10):1584–1591. doi: 10.1176/appi.ajp.157.10.1584. [DOI] [PubMed] [Google Scholar]
- Mendle J. Why Puberty Matters for Psychopathology. Child Development Perspectives. 2014;8(4):218–222. [Google Scholar]
- Mendle J, Ferrero J. Detrimental psychological outcomes associated with pubertal timing in adolescent boys. Developmental Review. 2012;32:49–66. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and Treatment of Mental Disorders Among US Children in the 2001 2004 NHANES. Pediatrics. 2010;125(1):75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, Poulton R. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine. 2010;40:899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-Environment Interactions in Depression Research: Genetic Polymorphisms and Life-Stress Polyprocedures. Psychological Science. 2008;19(10):947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Rohde P, Seeley JR, Lewinsohn PM. Life events and depression in adolescence: Relationship loss as a prospective risk factor for first onset of major depressive disorder. Journal of Abnormal Psychology. 1999;108(4):606–614. doi: 10.1037//0021-843x.108.4.606. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression, and externalizing problems: a framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011;213:717–746. [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(02):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, Vanzon L, Molteni M, Battaglia M. The influence of family structure, the TPH2 G-703T and the 5-HTTLPR serotonergic genes upon affective problems in children aged 10–14 years. Journal of Child Psychology and Psychiatry. 2009;50(3):317–325. doi: 10.1111/j.1469-7610.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- Nolan SA, Flynn C, Garber J. Prospective relations between rejection and depression in young adolescents. Journal of Personality and Social Psychology. 2003;85(4):745–755. doi: 10.1037/0022-3514.85.4.745. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Bouma EMC. Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: A review of gender differences. Neuroscience and Biobehavioral Reviews. 2011;35(8):1757–1770. doi: 10.1016/j.neubiorev.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Oppenheimer CW, Hankin BL, Young JF, Abela JRZ, Smolen A. Youth Genetic Vulnerability to Maternal Depressive Symptoms: 5-HTTLPR as Moderator of Intergenerational Transmission Effects in a Multiwave Prospective Study. Depression and Anxiety. 2013;30:190–196. doi: 10.1002/da.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status- reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petersen IT, Bates JE, Goodnight JA, Dodge KA, Lansford JE, Pettit GS, Dick DM. Interaction between serotonin transporter polymorphism (5-HTTLPR) and stressful life events in adolescents’ trajectories of anxious/depressed symptoms. Developmental Psychology. 2012;48(5):1463. doi: 10.1037/a0027471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess-Groben HA, Hyde JS. 5-HTTLPR X stress in adolescent depression: moderation by MAOA and gender. Journal of Abnormal Child Psychology. 2013;41(2):281–294. doi: 10.1007/s10802-012-9672-1. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Cheah CSL, Guyer AE. Peer victimization, cue interpretation, and internalizing symptoms: Preliminary concurrent and longitudinal findings for children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34(1):11–24. doi: 10.1207/s15374424jccp3401_2. [DOI] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Pakiz B, Silverman AB, Frost AK, Lefkowitz ES. Psychosocial Risks for Major Depression in Late Adolescence- A Longitudinal Community Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(6):1155–1163. doi: 10.1097/00004583-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. Negative life events as an account of age-related differences in the genetic aetiology of depression in childhood and adolescence. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44(7):977–987. doi: 10.1111/1469-7610.00182. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. The Journal of the American Medical Association. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132(1):98–131. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. Relation of implicit theories to the construction of personal histories. Psychological Review. 1989:341–357. [Google Scholar]
- Rudolph KD. Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health. 2002;30(4):3–13. doi: 10.1016/s1054-139x(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Rudolph KD. Developmental influences on interpersonal stress generation in depressed youth. Journal of Abnormal Psychology. 2008;117(3):673. doi: 10.1037/0021-843X.117.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD. Handbook of developmental psychopathology. Springer; US: 2014. Puberty as a developmental context of risk for psychopathology; pp. 331–354. [Google Scholar]
- Rudolph KD, Clark AG. Conceptions of relationships in children with depressive and aggressive symptoms: Social-cognitive distortion or reality? Journal of Abnormal Child Psychology. 2001;29(1):41–56. doi: 10.1023/a:1005299429060. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology. 2007;19(02):497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M, Abaied JL. A developmental perspective on interpersonal theories of youth depression. In: Abela JRZ, Hankin BL, editors. Handbook of Depression in Children and Adolescents. NY: Guilford Press; 2008. pp. 79–102. [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Development and Psychopathology. 2000;12(02):215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Rutter M. Age as an ambiguous variable in developmental research: Some epidemiological considerations from developmental psychopathology. International Journal of Behavioral Development. 1989;12(1):1–34. [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Salum GA, Bortoluzzi A, Silveira PP, Bosa VL, Schuch I, Goldani M, Manfro GG. Is puberty a trigger for 5HTTLPR polymorphism association with depressive symptoms? Journal of Psychiatric Research. 2012;46(6):831–833. doi: 10.1016/j.jpsychires.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Shanahan L, Copeland WE, Costello EJ, Angold A. Child-, adolescent- and young adult-onset depressions: differential risk factors in development. Psychological Medicine. 2011;41:2265–2274. doi: 10.1017/S0033291711000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2006;35:103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberg J, Pickles A, Rutter M, Hewitt J, Simonoff E, Maes H, Carbonneau R, Murrelle L, Foley D, Eaves L. The influence of genetic factors and life stress on depression among adolescent girls. Archives of General Psychiatry. 1999;56(3):225–232. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. International Journal of Neuropsychopharmacology. 2006;9(4):443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Sontag LM, Graber JA, Clemans KH. The role of peer stress and pubertal timing on symptoms of psychopathology during early adolescence. Journal of youth and adolescence. 2011;40(10):1371–1382. doi: 10.1007/s10964-010-9620-8. [DOI] [PubMed] [Google Scholar]
- Squire LR. On the Course of Forgetting in Very Long-Term-Memory. Journal of Experimental Psychology-Learning Memory and Cognition. 1989;15(2):241–245. doi: 10.1037//0278-7393.15.2.241. [DOI] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguesz B, Kardia SLR, Zhu XF, Brown A, Pankow JS, Province MA, Hunt SC, Boerwinkle E, Schork NJ, Risch NJ. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. American Journal of Human Genetics. 2005;76(2):268–275. doi: 10.1086/427888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen HA, Adelman CB, Prinstein MJ, Spijkerman R, Poelen EA, Engels RC, Scholte RH. The interaction between pubertal timing and peer popularity for boys and girls: An integration of biological and interpersonal perspectives on adolescent depression. Journal of Abnormal Child Psychology. 2011;39(3):413–423. doi: 10.1007/s10802-010-9467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CP, Skowrosnki JJ, Lee DJ. Reconstructing the Date of a Personal Event. Practical Aspects of Memory; Current Research and Issues. 1988;1:241–246. [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular Psychiatry. 2008;13(2):131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular psychiatry. 2010;15(1):18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Mineka S, Zinbarg RE, Craske MG, Griffith JW, Sutton J, Adam EK. Refining the Candidate Environment Interpersonal Stress, the Serotonin Transporter Polymorphism, and Gene-Environment Interactions in Major Depression. Clinical Psychological Science. 2013:2167702613499329. doi: 10.1177/2167702613499329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Richardson ED, Smolen A. Behavioral inhibition and triallelic genotyping of the serotonin transporter promoter (5-HTTLPR) polymorphism. Journal of Research in Personality. 2011;45(6):706–709. [Google Scholar]
- Wichstrom L. The emergence of gender difference in depressed mood during adolescence: The role of intensified gender socialization. Developmental Psychology. 1999;35:232–245. [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Frank E, Anderson BP, Matty MK, Kupfer DJ. Nature of life events and difficulties in depressed adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(10):1049–1057. doi: 10.1097/00004583-199810000-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.