Abstract
The purpose of this study was to demonstrate that automated, continuous, curvilinear distraction osteogenesis (DO) in a minipig model is effective when performed bilaterally, at rates up to 3mm/day, to achieve clinically relevant lengthening.
A Yucatan minipig in the mixed dentition phase, underwent bilateral, continuous DO at a rate of 2 mm/day at the center of rotation; 1.0 and 3.0 mm/day at the superior and inferior regions, respectively. The distraction period was 13 days with no latency period. Vector and rate of distraction were remotely monitored without radiographs, using the device sensor. After fixation and euthanasia, the mandible and digastric muscles were harvested. The ex-vivo appearance, stability, and radiodensity of the regenerate were evaluated using a semi-quantitative scale. Percent surface area (PSA) occupied by bone, fibrous tissue, cartilage, and hematoma were calculated using histomorphometrics. The effects of DO on the digastric muscles and mandibular condyles were assessed via microscopy and degenerative changes were quantified.
The animal was distracted to 21 mm and 24 mm on the right and left sides, respectively. Clinical appearance, stability, and radiodensity were scored as ‘3’ bilaterally indicating osseous union. The total PSA occupied by bone (right = 75.53±2.19%; left PSA = 73.11±2.18%) approached that of an unoperated mandible (84.67±0.86%). Digastric muscles and condyles showed negligible degenerative or abnormal histologic changes.
This proof of principle study is the first report of osseous healing with no ill-effect on associated soft tissue and the mandibular condyle using bilateral, automated, continuous, curvilinear DO at rates up to 3 mm/day. The model approximates potential human application of continuous automated distraction with a semiburied device.
Keywords: automated continuous distraction osteogenesis, distraction wound, regenerate, mandibular distraction osteogenesis
Introduction
Distraction osteogenesis (DO) is a technique for skeletal lengthening that induces bone formation in an expanding skeletal gap.1-5 Discontinuous DO (i.e. distraction of 1 mm/day achieved through 1-4 activations/day) has become a preferred technique for large skeletal movements, but the treatment can be lengthy and remains dependent on patient and family compliance.6 Automated, continuous DO may minimize these limitations.7 Our group has recently demonstrated clinical, radiographic and histologic bone fill after distraction rates of up to 4.5 mm/day using an automated, continuous, curvilinear device in a minipig mandibular distraction model.8,9 DO in these studies was performed unilaterally with mandibular lengthening of 12 mm (i.e. critical size defect). To date, there have been no reports of an animal model in which continuous DO was performed bilaterally to achieve large movements, as would be required for micrognathia. This is a critical clinical application for continuous DO.8-11
The purpose of this study was to assess clinical, radiographic and histologic evidence of bone formation in a DO wound (lengthened greater than 20 mm) after bilateral automated, curvilinear, continuous DO at rates up to 3mm/day at the inferior border in a minipig model. Our hypothesis was that automated continuous DO at these rates would be well tolerated and that there would be bony union with negligible degenerative effects on the mandibular condyle and anterior digastric muscles. We also hypothesized that it would be possible to accurately monitor the vector and rate of DO, without radiographs, using a sensor incorporated in the device and Bluetooth® technology.
Materials and Methods
One female Yucatan minpig with an entry age of 75 days, weight of 9.7 kg and in the mixed dentition phase, was acclimated for 11 days to the environment and pureed diet. This animal study met the requirements of the Accreditation of Laboratory Animal Care standards and was approved by the Massachusetts General Hospital Subcommittee on Research Animal Care (SRAC 2009N0000073). The authors have read and are in full compliance with the Declaration of Helsinki.
Placement of Bilateral Distractors
The animal was sedated with telazol (4.4 mg/kg), xylazine (2.2 mg/kg), and atropine (0.04 mg/kg) intramuscularly. A submandibular approach was used to expose the lateral aspects of the mandible bilaterally. Osteotomies were created with a reciprocating saw extending from the retromolar region to the inferior border immediately anterior to the mandibular angle. An automated, curvilinear distraction device (5 cm radius of curvature, United States Patent #8177789) was rigidly fixed to the center of the mandible on each side using bicortical screws (Dupuy Synthes, West Chester, PA, USA) (Figure 1A). Marker screws were placed 12 mm apart, proximal and distal to the inferior aspect of the osteotomies, to monitor the magnitude of distraction. The wounds were closed in layers. Lateral oblique radiographs were obtained before and after distractor placement (Figure 1B).
Figure 1.
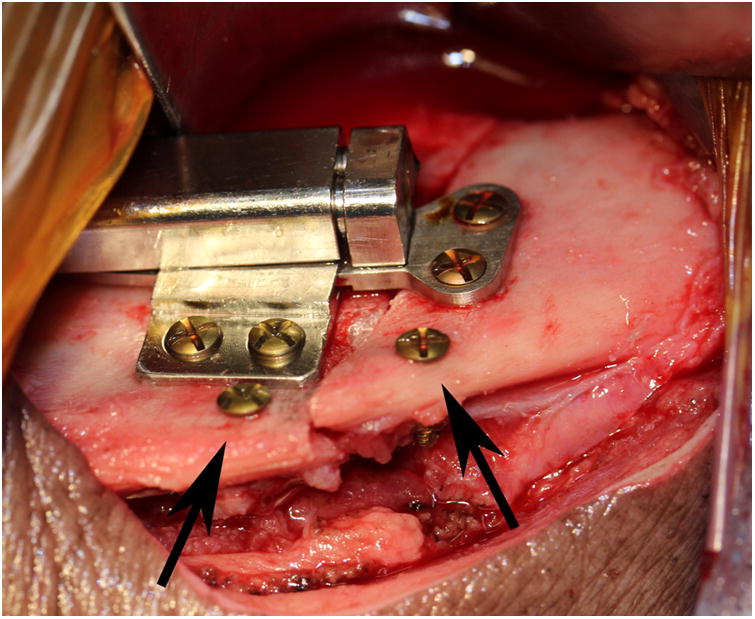
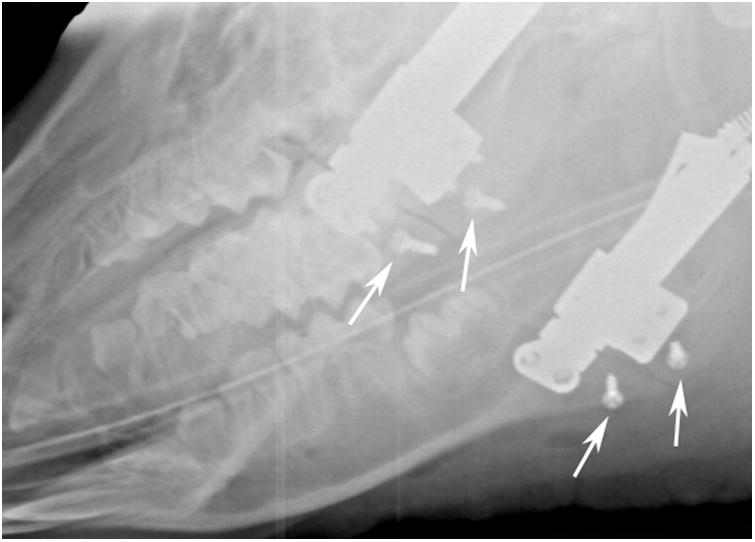
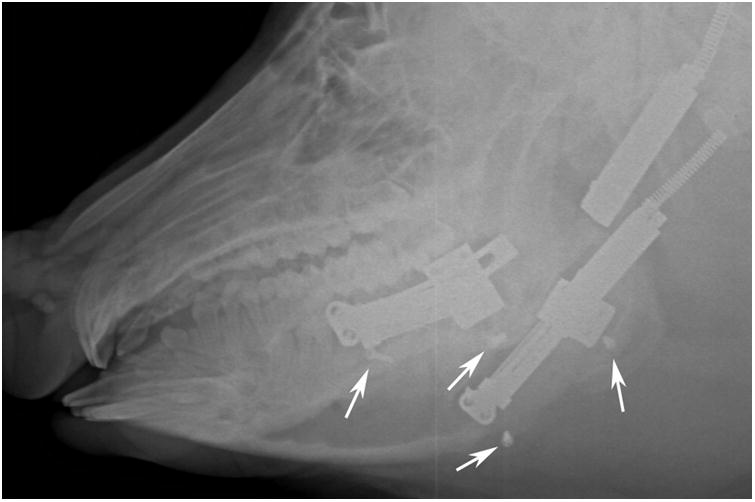
A, Intraoperative photograph of the device fixed across the osteotomy with markers screws (arrows) placed at the inferior border. B, Immediate postoperative radiograph showing bilateral osteotomies with marker screws (white arrows) C, End-Fixation radiograph showing the lengthening of the mandible with increased distance between marker screws (white arrows). The right side distractor became unattached during the fixation period with some loss of lengthening. The left side distractor is fully extended. The regenerate appears radiodense for both sides (scores of 3).
Distraction Protocol
No latency period was used and each device was set to a continuous distraction rate of 2 mm/day at the center of rotation. Curvilinear DO produces a gradient of distraction rates at the superior and inferior borders of the mandible (1 and 3 mm/day respectively).9, 12 The distraction period was 13 days followed by a 50 day fixation period. At mid-DO, end-DO, end-fixation, and ex-mortem, bilateral lateral oblique radiographs were obtained to confirm position recorded by the internal sensor (Figures 1B and 1C). The animal was euthanized at end-fixation and the bilateral hemimandibles including the condyles and digastric muscles were harvested.
Assessment
Clinical and Radiographic Evaluation
The ex-vivo appearance of the DO wound was evaluated using a 4-point semi-quantitative scale: 3 - osteotomy not visible; 2 - osteotomy < 50% visible; 1 - osteotomy > 50% visible; 0 - osteotomy clearly visible. Bimanual examination of the ex-vivo mandible was graded as: 3 - no mobility; 2 - mobility in 1 plane; 1 - mobility in 2 planes; 0 - mobility in 3 dimensions (unstable). The radiodensity of the DO wounds as a reflection of bone formation was evaluated on a 5-point scale: 4 = 100% bone fill, 3 = >75% and < 100%, 2 = 50%-75%, 1 = <50%, or 0 - no bone fill (Figure 2).
Figure 2.
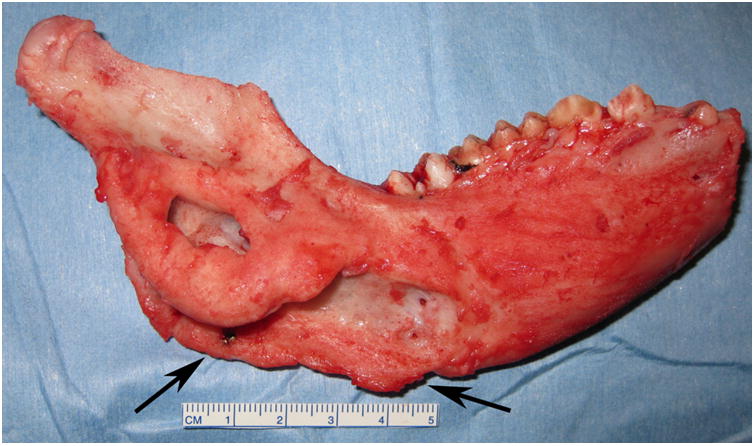
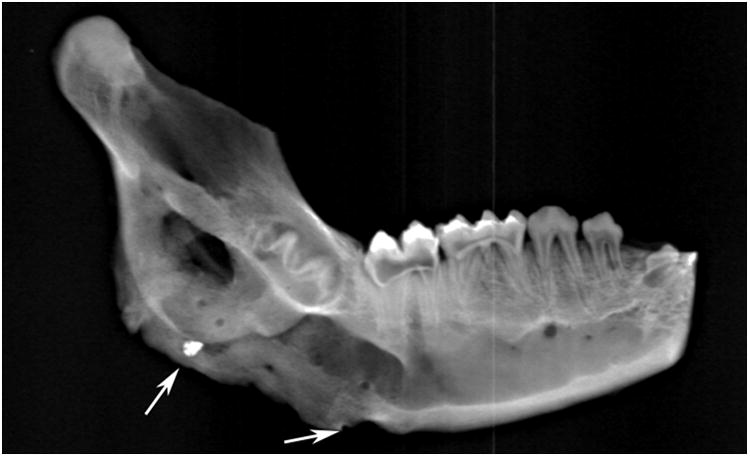
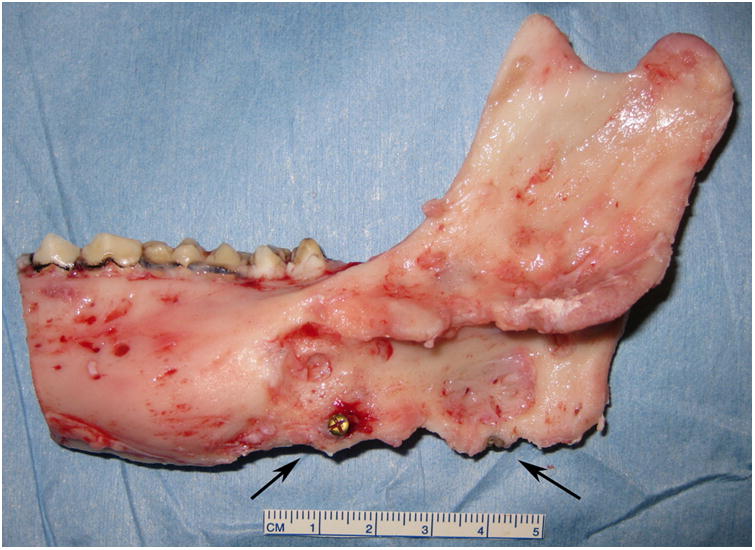
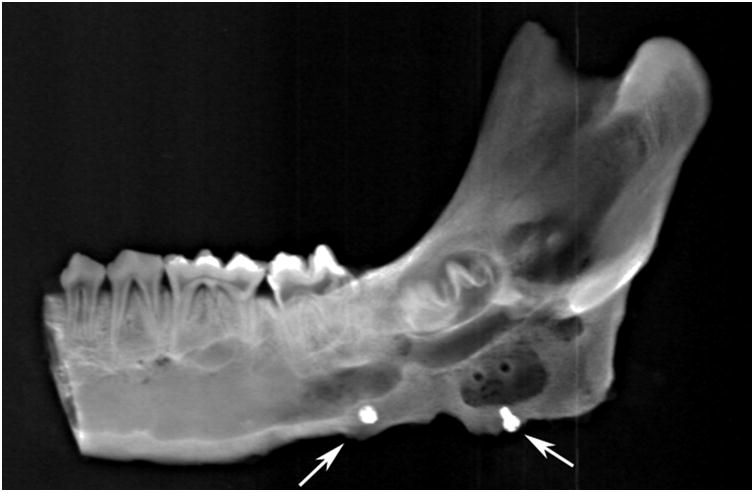
Right side hemimandible photograph (A) and radiograph (B) showing strong clinical bone fill (score of 3) and radiodensity (score of 3) within the distraction gap. The left side hemimandible photograph (C) and radiograph (D) showed similar results (score of 3 for each). Position of marker screws marked with black arrows.
Histologic Evaluation
The hemi-mandibles were fixed for 3 days in 10% phosphate-buffered formalin, decalcified in Cal-Ex® II (C5511-4D; Fisher Scientific, Pittsburg, PA, USA) solution (10% formic acid in 10% formalin solution) for 4 weeks, and then stabilized in 30% sucrose solution for 24 hours. Coronal sections through the middle of the regenerate (Figure 3A), sagittal sections of mandibular condyles, and sagittal and coronal sections of the digastric muscles were obtained, paraffinized, mounted onto slides (6 μm thickness), and stained with hematoxylin and eosin. Slides were imaged and the percent surface area (PSA) occupied by bone, fibrous tissue, cartilage, and hematoma within the regenerate was calculated using ImageJ® software (National Institutes of Health, Bethesda, MD) as previously described.8, 13 The condyles were assessed for early and late stage degenerative changes by a head and neck pathologist (WCW) as previously described.14, 15 The anterior digastric muscles were also evaluated for evidence of degeneration (i.e. presence of necrosis, fibrosis, central nuclei, inflammation, and atrophic or “moth-eaten fibers) and scored on the following semi-quantitative scale: 0 - no abnormal tissue, 1 -abnormal tissue evident in less than 5% of a specimen, 2 - abnormal tissue evident in 5% to 50% of a specimen, and 3 - abnormal histology evident in more than 50% of a specimen.16 All comparative statistics were calculated using SPSS (Statistical Package of Social Sciences, version 17.0; Chicago, IL) using independent 2-sample, 2-tailed t tests. P values ≤ 0.05 were considered statistically significant.
Figure 3.
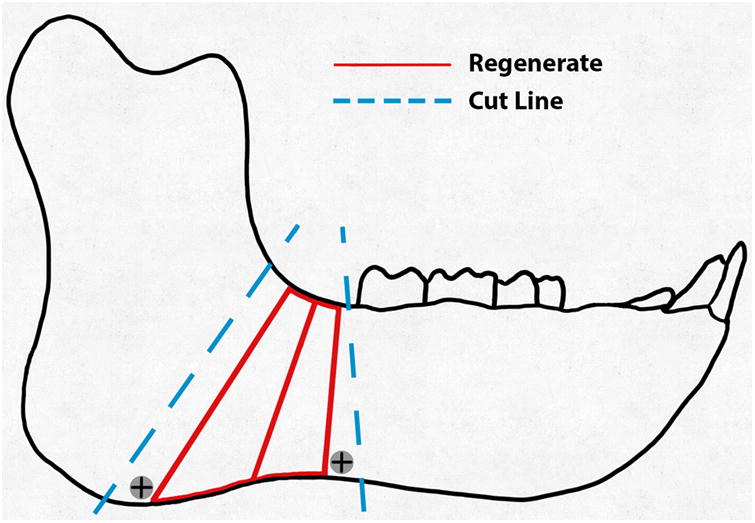
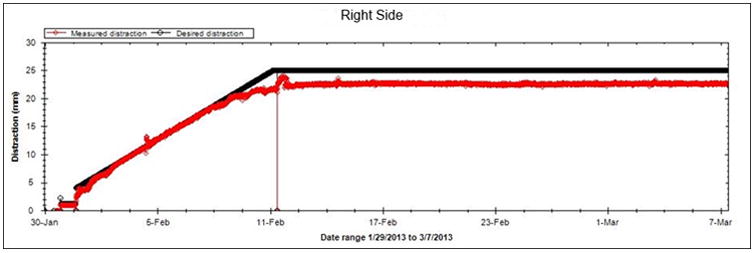
A, Schematic of the hemimandible (black), regenerate (red), and the intended cuts (blue).B, Graph of projected (black line) and measured distraction (red) for the right side. The measured position closely followed the projected 2 mm/day rate and remained at 21.5 mm during the consolidation period.
Results
The minipig survived the acclimation, distraction, and fixation periods without complication. The right and left sides were distracted 21 and 24 mm, respectively (Figure 3B). At end-fixation, the right and left distraction gaps were 21.5 mm and 17 mm, respectively. The distractor body became detached from the left side footplate during the fixation period resulting in the loss of some distraction length (Figure 1C). Gross appearance, stability, and radiodensity were all scored ‘3’ (i.e. osteotomy not visible, no mobility, and >75% radiographic bone fill) bilaterally indicating clinical and radiographic union (Figures 1 and 2).
Histologic analysis by computer morphometrics demonstrated a mean PSA occupied by bone of 75.53±2.19 on the right side and 73.11± 2.18 on the left. The best bone formation occurred in the superior (right PSA=82.09±3.49; left PSA=73.23± 4.25) region of the wound, above the inferior alveolar canal, in the area of the developing tooth bud, followed by the middle region (right PSA=73.64± 3.82; left PSA=76.24± 3.61), and the least bone occurred near the inferior border (right PSA=70.85± 3.80; left PSA=69.84± 3.46). The control PSA occupied by bone for the unoperated minipig mandible (n=10) was 84.67 ± 0.86.6 Bone formation was similar on the endosteal (right PSA=75.02± 2.96; left PSA=73.44± 2.74) and periosteal (right PSA=76.29± 3.29; left PSA=72.61± 3.64) regions. PSA occupied by bone was higher on the lingual (right PSA=79.83± 5.06; left PSA=83.86±4.09) compared to the buccal regions (right PSA=72.74± 4.15; left PSA=61.37± 3.95) of the mandible. (Table 1) The regions of the wounds devoid of bone exhibited fibrous tissue (right side PSA=24.47±2.19) and left side (PSA=26.89± 2.18) with no cartilage or hematoma present.
Table 1. Percent surface area (PSA) of bone found in the different regions of the regenerate.
| Bilateral DO: 2 mm/day | Right Side PSA(%) | Left Side PSA (%) |
|---|---|---|
| Total | 75.53 ± 2.19 | 73.11 ± 2.18 |
| Superior Third | 82.09 ± 3.49 | 73.23 ± 4.25 |
| Middle Third | 73.64 ± 3.82 | 76.24 ± 3.61 |
| Inferior Third | 70.85 ± 3.80 | 69.84 ± 3.46 |
| Endosteum | 75.02 ± 2.96 | 73.44 ± 2.74 |
| Periosteum | 76.29 ± 3.29 | 72.61 ± 3.64 |
| Lingual Periosteum | 79.83 ± 5.06 | 83.86 ± 4.09 |
| Buccal Periosteum | 72.74 ± 4.15 | 61.37 ± 3.95 |
Histologic analysis of the digastric muscles demonstrated normal muscle mass and morphology with less than 5% of the surface area of muscle occupied by abnormal muscle cells and matrix (necrosis, fibrosis, central nuclei, inflammation, and atrophic or “moth-eaten fibers,” mean score 0.06).
Both condyles were uniformly symmetric with evenly spaced bony trabeculae, and a regular articulating surface with normal thickness fibrocartilage surface and no erosions. No early or late stage degenerative changes were observed (Figure 4).
Figure 4.
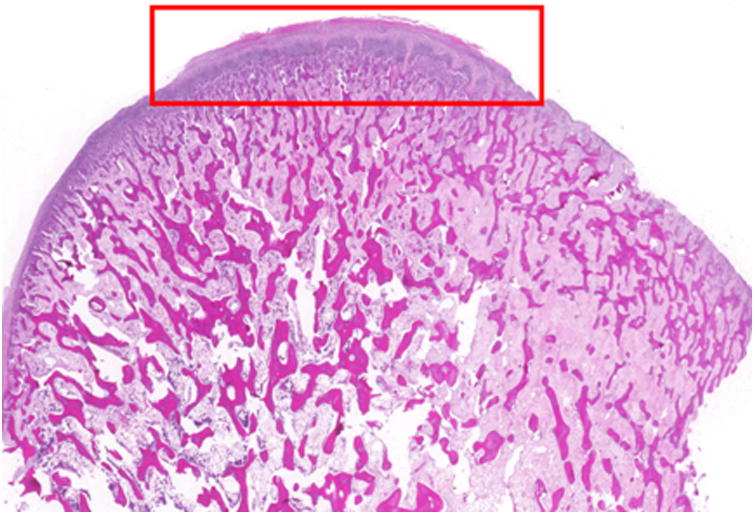
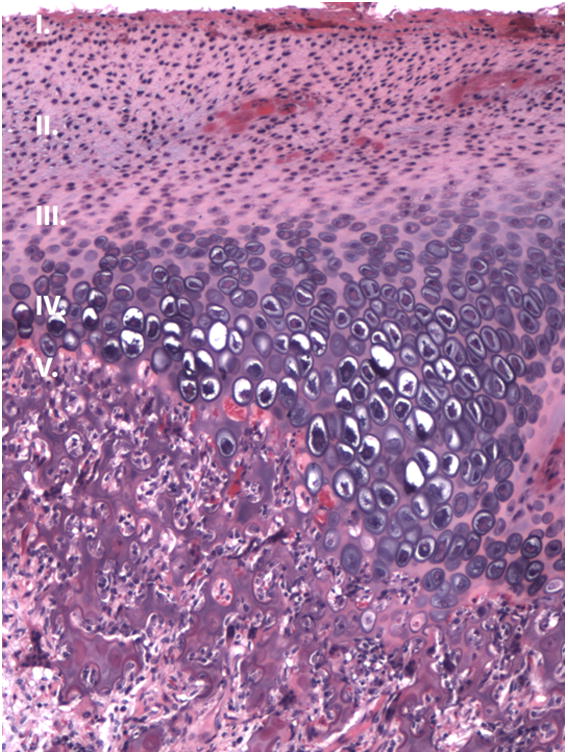
Condyle histology. (H&E, sagittal view). A-B, Photomicrograph of the (A) right and (B) left condylar head showing evenly spaced trabeculae and a regular articulating surface (10× magnification). C-D, Representative images from the right (D) and left (E) condyle (200 × magnification of the red box from figure 5A) that appear similar to a normal condyle.
Discussion
The purpose of the current study was to demonstrate for the first time, bilateral, automated, continuous curvilinear DO in a minipig model to approximate potential clinical applications. We hypothesized that continuous bilateral DO at rates up to 3 mm/day with large movements would produce adequate bone formation and satisfactory osseous union, confirmed by clinical, radiographic and histologic examination. We also hypothesized that continuous DO, at the rates in this study, would not produce degenerative changes in the digastric muscles as they lengthen parallel to the vector of distraction or degenerative changes in the mandibular condyles as a result of compressive forces.
To our knowledge, this is the first reported proof of principle study using automated, continuous distraction for bilateral mandibular lengthening, in which the movements were comparable to those that would be used to correct severe micrognathia in humans. Previous investigators have only reported unilateral continuous distraction8, 9, 17-27 and limited mandibular lengthening to 12 mm.8-11, 13-16, 28-31 In addition, this is the first time rate of distraction was monitored successfully without radiographs, using a sensor incorporated in the device and Bluetooth® technology.
The increased magnitude of distraction resulted in osseous union with a higher PSA occupied by bone in each zone of the regenerate than that found in previous studies. The PSA occupied by bone was also greater than that reported for discontinuous DO at 1 mm/day.8, 13 Bone formation was greater on the lingual surface compared to the buccal surface likely due to the presence of the device rigidly fixed between the buccal cortex and periosteum. This was consistent with previous experiments using this model.8, 13
Although the duration of distraction was shorter because of the faster rates, the fixation period utilized in this study, twice the number of mm of DO in days, was the same as that used for discontinuous DO at 1 mm/day. Additional studies are underway to assess the sequence of healing with continuous DO assessing bone formation at shorter fixation periods with the hypothesis that bone formation may occur faster with continuous DO.
Investigators studying continuous and discontinuous DO have previously focused and reported on device safety, accuracy of the vectors distraction and bone formation in unilateral DO models.21, 25, 31-37 In the current study, we sought to replicate a common clinical scenario. Children with micrognathia (e.g. bilateral craniofacial microsomia, Treacher Collins syndrome or Robin sequence) often require curvilinear DO with lengthening up to 30 mm. With this single application of bilateral continuous DO at rates ranging from 1 (superior) to 3.0 mm/day (inferior) at the mandibular angle, we have demonstrated by clinical, radiographic and histologic evaluations, adequate bone healing in the DO wound.8, 9 With this experiment, we also proved the principle that it is possible to monitor the vector and rate of DO using a sensor embedded in the device and Bluetooth® technology. This is important because it may potentially eliminate the need for serial radiographs, some of which require sedation in children, to monitor the progress of DO.
This study is limited by use of a single animal. Because of the great expense of such a project, we elected to demonstrate the proof of principle before proceeding with a larger series. We are now in the midst of a study with 19 animals to demonstrate the biology and biomechanical properties of the continuous DO wound. We were unable to maintain 20 mm (lengthening was 21.5 and 17 mm on the right and left side, respectively) of lengthening on the left side as a result of unusual device failure (the first time in 25 animals with this device). Nonetheless, the strong clinical, radiographic, and histologic results further support the possible benefit of bilateral continuous DO. Based on this proof of principle study we are undertaking a series of experiments in larger numbers of animals to further document the properties of the continuous distraction wound with varying rates of distraction and lengths of consolidation.
In conclusion, bilateral, automated, continuous, curvilinear DO results in osseous union with lengthening up to 21.5 mm matching a common clinical application. No detrimental effects were found in the surrounding soft tissue or condyles.
Acknowledgments
The authors would like to acknowledge, Adam G. Patenaude, for his assistance with the histomorphometrics and figure preparation.
Funding: This work was funded by NIH/NIDCR SBIR grant (5R44DE019047-03), the Massachusetts General Hospital Department of Oral and Maxillofacial Surgery Education and Research Fund, and the Hanson Foundation (Boston, MA, USA).
References
- 1.Codivilla A. The classic: On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity 1905. Clin Orthop Relat Res. 2008;466:2903–2909. doi: 10.1007/s11999-008-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48:1–11. [PubMed] [Google Scholar]
- 3.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 4.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I.The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 5.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8–26. [PubMed] [Google Scholar]
- 6.Troulis MJ, Kaban LB. Complications of mandibular distraction osteogenesis. Oral Maxillofac Surg Clin North Am. 2003;15:251–264. doi: 10.1016/S1042-3699(02)00101-2. [DOI] [PubMed] [Google Scholar]
- 7.Goldwaser BR, Papadaki ME, Kaban LB, et al. Automated continuous mandibular distraction osteogenesis: review of the literature. J Oral Maxillofac Surg. 2012;70(407) doi: 10.1016/j.joms.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Peacock ZS, Tricomi BJ, Lawler ME, et al. Skeletal and Soft Tissue Response to Automated, Continuous, Curvilinear Distraction Osteogenesis. J Oral Maxillofac Surg. 2014;72:1773–1787. doi: 10.1016/j.joms.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock ZS, Tricomi BJ, Murphy BA, et al. Automated Continuous Distraction Osteogenesis May Allow Faster Distraction Rates: A Preliminary Study. J Oral Maxillofac Surg. 2013;71:1073–1084. doi: 10.1016/j.joms.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldwaser BR, Magill J, Papadaki ME, et al. Continuous mandibular distraction osteogenesis: novel device and preliminary results in minipigs. J Oral Maxillofac Surg. 2013;71:e168–e177. doi: 10.1016/j.joms.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Magill JC, Byl MF, Goldwaser B, et al. Automating skeletal expansion: An implant for distraction osteogenesis of the mandible. J Med Device. 2009;3:14502. doi: 10.1115/1.3071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaban LB, Seldin EB, Kikinis R, et al. Clinical application of curvilinear distraction osteogenesis for correction of mandibular deformities. J Oral Maxillofac Surg. 2009;67:996–1008. doi: 10.1016/j.joms.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawler ME, Tayebaty FT, Williams WB, et al. Histomorphometric analysis of the porcine mandibular distraction wound. J Oral Maxillofac Surg. 2010;68:1543–1554. doi: 10.1016/j.joms.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Thurmuller P, Troulis MJ, Rosenberg A, et al. Microscopic changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2006;64:249–258. doi: 10.1016/j.joms.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Thurmuller P, Troulis MJ, Rosenberg A, et al. Changes in the condyle and disc in response to distraction osteogenesis of the minipig mandible. J Oral Maxillofac Surg. 2002;60:1327–1333. doi: 10.1053/joms.2002.35733. [DOI] [PubMed] [Google Scholar]
- 16.Lawler ME, Hansen GM, Williams WB, et al. Serial histologic and immunohistochemical changes in anterior digastric myocytes in response to distraction osteogenesis. J Oral Maxillofac Surg. 2012;70:168–178. doi: 10.1016/j.joms.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Ayoub AF, Richardson W, Barbenel JC. Mandibular elongation by automatic distraction osteogenesis: the first application in humans. Br J Oral Maxillofac Surg. 2005;43:324–328. doi: 10.1016/j.bjoms.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Ayoub AF, Richardson W, Koppel D, et al. Segmental mandibular reconstruction by microincremental automatic distraction osteogenesis: an animal study. Br J Oral Maxillofac Surg. 2001;39:356–364. doi: 10.1054/bjom.2001.0658. [DOI] [PubMed] [Google Scholar]
- 19.Djasim UM, Mathot BJ, Wolvius EB, et al. Histomorphometric comparison between continuous and discontinuous distraction osteogenesis. J Craniomaxillofac Surg. 2009;37:398–404. doi: 10.1016/j.jcms.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Djasim UM, Wolvius EB, Bos JA, et al. Continuous versus discontinuous distraction: evaluation of bone regenerate following various rhythms of distraction. J Oral Maxillofac Surg. 2009;67:818–826. doi: 10.1016/j.joms.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Kessler P, Neukam FW, Wiltfang J. Effects of distraction forces and frequency of distraction on bony regeneration. Br J Oral Maxillofac Surg. 2005;43:392–398. doi: 10.1016/j.bjoms.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Kessler P, Wiltfang J, Neukam FW. A new distraction device to compare continuous and discontinuous bone distraction in mini-pigs: a preliminary report. J Craniomaxillofac Surg. 2000;28:5–11. doi: 10.1054/jcms.2000.0101. [DOI] [PubMed] [Google Scholar]
- 23.Kessler PA, Merten HA, Neukam FW, et al. The effects of magnitude and frequency of distraction forces on tissue regeneration in distraction osteogenesis of the mandible. Plast Reconstr Surg. 2002;109:171–180. doi: 10.1097/00006534-200201000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Ploder O, Kanz F, Randl U, et al. Three-dimensional histomorphometric analysis of distraction osteogenesis using an implanted device for mandibular lengthening in sheep. Plast Reconstr Surg. 2002;110:130–137. doi: 10.1097/00006534-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Schmelzeisen R, Neumann G, von der Fecht R. Distraction osteogenesis in the mandible with a motor-driven plate: a preliminary animal study. Br J Oral Maxillofac Surg. 1996;34:375–378. doi: 10.1016/s0266-4356(96)90090-x. [DOI] [PubMed] [Google Scholar]
- 26.Shetsov VI, Popkov AV. Limb lengthening in automatic mode. Ortop Traumatol Rehabil. 2002;4:403–412. [PubMed] [Google Scholar]
- 27.Isefuku S, Joyner CJ, Simpson AH. A murine model of distraction osteogenesis. Bone. 2000;27:661–665. doi: 10.1016/s8756-3282(00)00385-9. [DOI] [PubMed] [Google Scholar]
- 28.Castano FJ, Troulis MJ, Glowacki J, et al. Proliferation of masseter myocytes after distraction osteogenesis of the porcine mandible. J Oral Maxillofac Surg. 2001;59:302–307. doi: 10.1053/joms.2001.21000. [DOI] [PubMed] [Google Scholar]
- 29.Glowacki J, Shusterman EM, Troulis M, et al. Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone. Plast Reconstr Surg. 2004;113:566–573. doi: 10.1097/01.PRS.0000101061.99577.09. [DOI] [PubMed] [Google Scholar]
- 30.Hansen GM, Lawler ME, Williams WB, et al. BMP4 localization and PCNA expression during distraction osteogenesis of the porcine mandible. Int J Oral Maxillofac Surg. 2012;41:867–373. doi: 10.1016/j.ijom.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 31.Troulis MJ, Glowacki J, Perrott DH, et al. Effects of latency and rate on bone formation in a porcine mandibular distraction model. J Oral Maxillofac Surg. 2000;58:507–513. doi: 10.1016/s0278-2391(00)90012-0. [DOI] [PubMed] [Google Scholar]
- 32.Ploder O, Mayr W, Schnetz G, et al. Mandibular lengthening with an implanted motor-driven device: preliminary study in sheep. Br J Oral Maxillofac Surg. 1999;37:273–276. doi: 10.1054/bjom.1999.0115. [DOI] [PubMed] [Google Scholar]
- 33.Wiltfang J, Kessler P, Merten HA, et al. Continuous and intermittent bone distraction using a microhydraulic cylinder: an experimental study in minipigs. Br J Oral Maxillofac Surg. 2001;39:2–7. doi: 10.1054/bjom.2000.0564. [DOI] [PubMed] [Google Scholar]
- 34.Glowacki J, Shusterman EM, Troulis M, et al. Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone. Plast Reconstr Surg. 2004;113:566–573. doi: 10.1097/01.PRS.0000101061.99577.09. [DOI] [PubMed] [Google Scholar]
- 35.Yeshwant K, Seldin EB, Gateno J, et al. Analysis of skeletal movements in mandibular distraction osteogenesis. J Oral Maxillofac Surg. 2005;63:335–340. doi: 10.1016/j.joms.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 36.Ritter L, Yeshwant K, Seldin EB, et al. Range of curvilinear distraction devices required for treatment of mandibular deformities. J Oral Maxillofac Surg. 2006;64:259–264. doi: 10.1016/j.joms.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Park JT, Lee JG, Kim SY, et al. A piezoelectric motor-based microactuator-generated distractor for continuous jaw bone distraction. J Craniofac Surg. 2011;22:1486–1488. doi: 10.1097/SCS.0b013e31821d196b. [DOI] [PubMed] [Google Scholar]


