Abstract
Social experiences may moderate genetic influences on alcohol dependence (AD) symptoms. Consistent with this hypothesis, Park, Sher, Todorov, and Heath (2011) previously reported interactions between the dopamine D4 receptor gene (DRD4) and developmentally specific environments in the etiology of AD symptoms during emerging and young adulthood. Using a longitudinal cohort of n = 367 White participants followed from ages 18–27 we examine a series of similar interactions between DRD4 and developmentally sensitive contexts including childhood adversity and work and family roles. In contrast to previous results, we observed no significant interactions between DRD4 and childhood adversity. Overall, results further highlight the need for longitudinal studies of gene × environment interaction in the behavioral sciences and the difficulty of identifying candidate gene × environment interaction effects that are consistent across studies.
Keywords: DRD4, alcohol dependence, gene × environment interaction, longitudinal, childhood maltreatment, college
Our findings suggest that DRD4 genotype is unrelated to alcohol dependence in early adulthood. Additionally, we found no evidence for its interaction with childhood maltreatment or work and family roles, in the prediction of alcohol dependence during this period. These findings contrast with those from a similar study, highlighting the importance of replication studies, and ultimately meta analyses, for interpretation of candidate gene research.
Individual differences in the development of problematic patterns of alcohol use during young adulthood are partially due to genetic factors, but the specific variants that constitute genetic risk for alcohol problems are largely unknown (Kendler et al., 2012). The role of dopamine in the neurobiological basis of craving, dependence, and reward (DeYoung, 2013; Depue & Collins, 1999) has motivated research on polymorphisms in candidate dopamine-relevant genes, including the dopamine D4 receptor gene (DRD4). Yet it has been difficult to establish reliable associations between dopamine-related genes and alcohol use outcomes (Munafo, Matheson, & Flint, 2007; McGeary, 2009).
One possibility that might account for inconsistent associations between dopamine- related genes and alcohol use outcomes is potential gene by environment (G×E) interactions, in which the effect of genotype depends on environmental context (and the effect of environmental context depends on genotype). Behavioral genetic studies using twins and siblings have shown that heritability estimates of alcohol use are greater among individuals in college than among young adults not enrolled in college (Timberlake et al., 2007), as well as in single compared to married individuals (Heath, Jardine, & Martin, 1989). These results suggest that alcohol-conducive environments may amplify the expression of genetic differences between people. Consequently, dopamine-related genes may confer risk for problematic alcohol use only in concert with certain kinds of environments.
Consistent with this hypothesis, Park, Sher, Todorov, and Heath (2011), reported evidence for interactions between a polymorphism in DRD4 and developmentally-specific environments in a longitudinal sample of 234 White individuals followed from ages 18 to 34. Persistent alcohol dependence (AD) symptoms representing variance in AD symptoms across all seven waves of data collection was contrasted with symptoms specific to emerging adulthood and young adulthood, representing residual variance in AD symptoms from ages 18 to 21, and ages 25–34, respectively. Individuals who reported childhood adversity were more likely to demonstrate persistent AD symptoms than individuals who did not, and this association was stronger among those who had at least one “long” allele of the DRD4 exon III VNTR polymorphism.1 Overall, these results are consistent with quantitative genetic research on G×E interactions and with the general tenets of diathesis-stress models of psychopathology. Moreover, the Park et al. (2011) study was unique in its attention to development: alcohol dependence was measured longitudinally, and hypotheses about G×E interaction were formulated based on life-course theories regarding which environments would be most relevant at which point in the lifespan.
The literature on candidate gene associations and candidate gene × environment interactions has been criticized as containing an unacceptably high number of false positive findings (e.g., Duncan & Keller, 2011). In an editorial in Behavior Genetics, Hewitt (2012, p. 1) concluded that “it now seems likely that many of the published findings of the last decade are wrong or misleading and have not contributed to real advances in knowledge.” Given this situation, there is a pressing need for additional, developmentally-sensitive studies of G×E, in order to identify which candidate gene associations and interactions (if any) can be robustly identified across multiple studies. Consequently, the goal of the current paper is to provide results from a second longitudinal study of G×E interactions across emerging to young adulthood in a Caucasian cohort (n =367) assessed at 11 waves over ages 18 to 27.
Method
Participants
Participants were drawn from a multi-wave longitudinal study of alcohol use and other behavioral risks (the “UT Experience!” UTE), which followed a cohort of first-time college students from the summer preceding matriculation to a large public university (2004) through five years post-entry (n = 2,245). In 2012, a target subset of 1,060 former UTE participants, now ages 26–27, was re-contacted to collect salivary DNA and survey data. From this target, n = 598 participants provided DNA and have valid genotype information (62% White, 14.8% Asian, 14% Hispanic, 6.3% Multi-racial, 2.9% Black). (Please see the Supplement for information on the recruitment of the original UTE sample, how participants were targeted for re-recruitment, participation rates, and comparisons between the genetic sample and the full sample.) Primary analyses were limited to Caucasian participants for whom valid genotypic data was available (n = 367, 65% female).
Measures
AD symptoms
AD symptoms were indexed by summing 12 dichotomously-scored items from the Abuse/Dependence factor of the Rutgers Alcohol Problem Index (RAPI; Martens, Neighbors, Dams-O’Connor, Lee, & Larimer, 2007; White & Labouvie, 1989). Summary statistics and alpha reliabilities are summarized in Table 1.
Table 1.
AD Symptoms by Wave: Summary Statistics and Reliabilities for the Caucasian Sample
| Wave | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Age | 18.4 | 18.8 | 19.2 | 19.8 | 20.2 | 20.8 | 21.2 | 21.8 | 22.8 | 23.8 | 26.9 |
| Mean AD | 0.66 | 0.97 | 0.89 | 0.84 | 0.80 | 0.78 | 0.80 | 0.72 | 0.66 | 0.77 | 1.26 |
| Standard Deviation | 1.41 | 1.73 | 1.71 | 1.68 | 1.87 | 1.64 | 1.74 | 1.65 | 1.54 | 1.68 | 2.07 |
| Range | 0–10 | 0–12 | 0–12 | 0–10 | 0–12 | 0–11 | 0–11 | 0–12 | 0–9 | 0–10 | 0–9 |
| n | 361 | 355 | 353 | 344 | 333 | 325 | 314 | 305 | 304 | 342 | 337 |
| Cronbach’s alpha | .76 | .80 | .82 | .83 | .88 | .83 | .86 | .84 | .81 | .83 | .80 |
Childhood adversity
Retrospective self-reports of physical and sexual abuse prior to age 18 were used to index childhood adversity. Six items were administered annually, over waves 8–10: How many times did… (a) a parent or guardian hit or beat you (with hands or with an object); (b) a parent or guardian cause cuts or bruises on you?; (c) you experience unwanted sexual contact by a parent, guardian, or relative?; (d) you experience unwanted sexual contact by an adult more than five years older than you?; (e) you feel coerced into having sex (through arguments, pressure, or physical force)?; (f) you experience unwanted sexual advances or sex play (e.g., fondling, kissing, petting, but not intercourse)? Items were dichotomized and a positive endorsement of any item over any of the three reporting periods was coded as an endorsement. A sum score was then calculated by taking the mean of these scored items multiplied by six (M = 0.92, SD = 1.27). Test-retest reliabilities for the six items across waves 8–10 ranged from .61–.85.
Work and Family Roles
This composite included items measuring employment, marital status, and parenthood status, and was coded in accordance with theoretical expectation for increasing AD risk. Employment was measured at wave 11; participants who endorsed a full-time job or homemaker status were coded 0, otherwise participants were coded 1. Participants who were married at wave 11 were coded 0 for marriage (n = 124 [34%]), non-married participants were coded 1. If participants endorsed “assumed primary responsibilities for a child” at any point in waves 4 through 10, or reported that they had become pregnant and had a live birth at wave 11, then parenting = 0 (n = 25 [6.8%]); otherwise, parenting = 1. Parenting, marriage, and employment were summed, resulting in a composite ranging from 0 to 3 (M = 1.79, SD = .79, median = 2, mode = 2).
Dopamine Receptor D4 (DRD4) polymorphism
DNA samples were collected using Oragene salivary self-collection kits and genotyped at the Institute for Behavior Genetics in Boulder, CO. Further description of genotyping procedures are detailed in Smolen et al. (2013). Our study focuses on the DRD4 gene which maps onto 11p15.5, and contains a 48-base-pair variable number tandem repeat (VNTR) in exon III (Van Tol et al., 1992). Repeats range from 2–11 and we coded the DRD4 genotype 0, 1, or 2 7-repeat alleles. The majority of the sample were non-carriers of the 7-repeat (White sample [WS]: n =227, 62.5%; Full: n = 396, 66.4%); approximately one-third were 7-repeat carriers (WS: n = 136, 37.5%; Full: n = 200, 33.6%). Among these, most were heterozygous (WS: n = 118, 32.5%; Full: n = 171, 29%) and a small proportion homozygous (WS: n = 18, 5%; Full: n = 29, 4.9%). Assumptions of Hardy-Weinberg Equilibrium were met (WS: p = .60, Full: p = .06).
Family history of alcohol problems
Using the Family Tree Questionnaire (Mann, Sobell, Sobell, & Paven, 1985), participants rated the drinking behavior of each first degree biological relative and grandparents as: never drinker, social drinker, possible problem drinker, definite problem drinker or don’t know. Participants were coded High Risk = 1 if any of the following were met: (a) any first-degree biological relative as a definite problem drinker; (b) maternal/paternal possible problem drinker in conjunction with at least one corresponding-side maternal/paternal grandparent designated as a definite problem drinker; (c) two or more grandparents as definite problem drinkers (High Risk: WS = 28%, FS = 24%). Participants meeting none of these conditions were coded 0.
Controls
All analyses controlled for sex (males = 0). Age was not controlled due to the homogeneity of ages within wave. In sensitivity analyses, self-reported race/ethnicity was included as a dummy coded covariate.
Analyses
Structural equation modeling was conducted using Mplus version 7.1. Model fit was evaluated using chi-square (χ2), Root Mean Square Error of Approximation (RMSEA), and the Bentler Comparative Fit Index (CFI). Analyses and missing data were handled using full information maximum likelihood, and confidence intervals were estimated using bootstrapping with 1,000 draws.
Results
Latent Growth Curve Models
We fit a series of four latent growth curve models (LGM)2 to determine which model best captured our AD symptoms data over time: (1) linear growth between waves 1 and 11; (2) linear and quadratic growth between waves 1 and 11, (3) a latent basis model, in which the basis coefficients between waves 2 and 11 were freely estimated from the data, and (4) a spline model that specified quadratic change between waves 1–10 and a latent difference score between wave 10 and wave 11. Model fit statistics from all LGMs are summarized in Table 2.
Table 2.
Model Fit Statistics for Latent Growth Curve Models of AD Symptoms
| Model | χ2 | df | p | c | CFI/TLI | RMSEA | SRMR |
|---|---|---|---|---|---|---|---|
| Linear | 115.53 | 61 | 0.0001 | 2.53 | .85/.87 | 0.05 | 0.09 |
| Quadratic | 90.23 | 57 | 0.0001 | 2.50 | .91/.91 | 0.04 | 0.07 |
| Latent basis quadratic | 96.21 | 52 | 0.0001 | 2.39 | .88/.87 | 0.05 | 0.08 |
| Quadratic + wave 11 factor | 69.17 | 53 | 0.07 | 2.59 | .96/.96 | 0.03 | 0.06 |
Note. df = degrees of freedom, p = p-value, c = scaling correction factor, CFI/TLI = comparative fit index/Tucker-Lewis index; RMSEA = root mean square error approximation; SRMR = standardized root mean square residual.
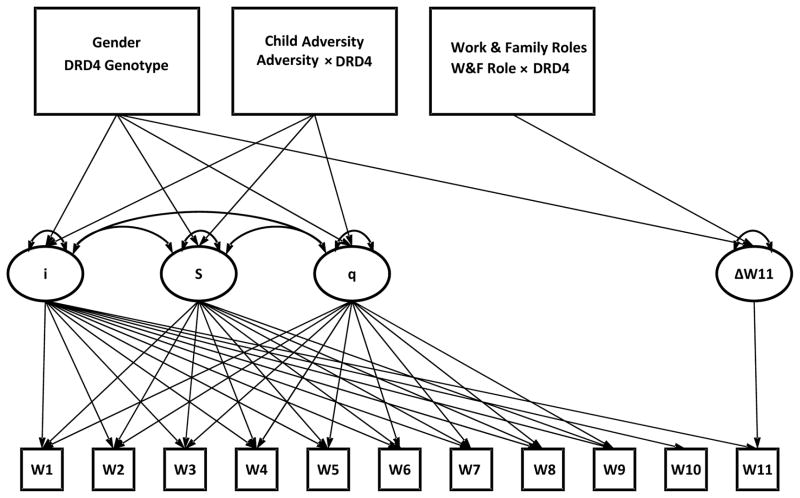
The best fitting model was Model 4, the spline model [χ2 (53) = 69.17, p = .07, RMSEA = .03, CFI/TLI = .96]. Spline models are useful for modeling non-parametric functions of latent variables and can be particularly useful for modeling data in which there is a gap in measurement intervals, such as waves 10 and 11 in the current study which correspond to mean ages 24 and 27, respectively. In this model basis coefficients from the linear slope (s) factor to observed AD symptoms were fixed in descending order from 9 to 1 at waves 1 through 9, respectively (and [9:1]2 for basis coefficients from the quadratic slope [q] factor) and were fixed to 0 at waves 10 and 11 (See Figure 1). Because our index of AD limited to young adulthood had only a single indicator (W11), factor identification required the residual variance in the observed W11 AD indicator to be fixed to zero and the factor loading fixed to 1. Parameterized as such, this factor represents variance specific to W11 (labeled Δw11 in Figure 1), that is, AD unique to young adulthood. This model implied that, on average, AD symptoms increased from waves 1 to 5, plateaued and gradually declined from waves 5 to 10, and then increased from wave 10 to wave 11. Parameter estimates presented in Table 3.
Figure 1.
Path Diagram for Latent Growth Curve Model of AD Symptoms with Covariates.
Note. Basis coefficients from intercept (i) factor to observed AD symptoms fixed to 1 at all waves. Basis coefficients from linear slope (s) factor to observed AD symptoms fixed at 9:1 at waves 1 through 9, respectively, and were fixed to 0 at waves 10 and 11. Basis coefficients from quadratic slope (q) factor to observed AD symptoms fixed to (9:1)2 at waves 1 through 9, respectively. Basis coefficient from wave 11 change factor (Δw11) fixed to 1.0. Residual variances estimated and allowed to vary across waves 1–10. Residual variance at wave 11 fixed to zero.
Table 3.
Parameter Estimates from Latent Growth Curve Models of AD Symptoms
| Means and Variances for Growth Factors with No Covariates | ||
|---|---|---|
| Growth Factor | Mean | Variance |
| Intercept | 0.72 (.55, .89) | 1.52 (.70, 2.34) |
| Linear Slope | 0.06 (−.01, .13) | 0.24 (.13, .35) |
| Quadratic Slope | −0.006 (−.01, .001) | 0.002 (.001, .003) |
| Wave 11 Change | 0.55 (.35, .76) | 2.92 (1.87, 3.98) |
| Effects of Covariates on Growth Factors (bs and 95% Confidence Intervals)
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Main Effects Only | Main Effects + Interactions | ||||||
| Intercept | Linear Slope | Quadratic Slope | Wave 11 Change | Intercept | Linear Slope | Quadratic Slope | Wave 11 Change | |
| Gender | −.26 (−.62, .11) | −.16 (−.32, .001) | .024 (.01, .04) | −.15 (−.66, −.35) | −.26 (−.62, .11) | −.16 (−.32, .00) | .02 (.01, .04) | −.15 (−.66, .36) |
| DRD4 | −.08 (−.38, .22) | .001 (−.12, .13) | .000 (−.01, .01) | .31 (−.07, .70) | −.10 (−.39, .20) | −.01 (−.16, .14) | .003 (−.01, .02) | .48 (−.16, 1.12) |
| Childhood Adversity (CA) | .20 (.06, .34) | −.04 (−.10, .03) | .003 (−.003, .009) | -- | .19 (.01, .38) | −.04 (−.13, .04) | .004 (−.004, .01) | -- |
| Work & Family Roles | -- | -- | -- | .18 (−.03, .38) | -- | -- | -- | .22 (−.05, .49) |
| CA × DRD4 | -- | -- | -- | -- | .02 (−.28, .31) | −.12 (−.06, .13) | −.003 (−.01, .01) | -- |
| TA × DRD4 | -- | -- | -- | -- | -- | -- | -- | −.10 (−.46, .27) |
Note. Bootstrapped confidence intervals are in parenthesis. Parameter estimates with 95% CIs that do not include zero are shown in bold.
Genetic Association Results
We first examined the main effects of childhood adversity, work & family roles, and DRD4 genotype on the developmentally appropriate growth factors. Next, we tested for interactions by regressing the growth factors on DRD4 genotype, environmental contexts, and interactions between DRD4 and each environmental context (Figure 1). See parameter estimates in Table 3.
Main effect only models showed a significant effect of childhood adversity on the latent intercept of AD symptoms, such that greater adversity predicted higher mean AD symptoms across all waves (β = .20, p < .01). There was also a significant main effect of gender on the quadratic slope (β = .20, p < .01) such that females decreased in AD symptoms more quickly than males. Interaction models also showed a main effect of childhood adversity across all waves (β = .19, p < .05) and a main effect of gender on the quadratic slope (β = .24, p < .05). None of the DRD4 × environment interaction terms attained statistical significance.
Sensitivity Analyses
To assess the robustness of parameter estimates, we conducted four sets of sensitivity analyses: (1) recoding DRD4 to reflect a dominance versus additive model of genetic risk (one or two 7-repeat alleles versus 0); (2) recoding childhood adversity as a dichotomized variable; (3) broadening the sample to include all genotyped participants; and (4) testing a multiple group model stratifying by familial risk for AD. Complete results for the sensitivity analyses are described in the Supplement.
Sensitivity analyses 1 and 2 were largely consistent with results from the original analysis (though two minor exceptions are noted in the Supplement). Results from sensitivity analysis 3 were consistent with the Caucasian-only sample, with two exceptions. First, female participants endorsed lower mean AD symptoms across all waves (as did Asian and Black participants; Blacks participants also showed lower increases in AD symptoms at wave 11 relative to Whites). Second, in the main effect only model, participants who endorsed fewer work and family roles by wave 11 (age 27) exhibited greater AD symptoms unique to wave 11 (β = .10, p = .02), but this effect was not significant in the interaction model (β = .10, p = .06). Note, however, that race/ethnicity was controlled based on self-report as ancestry informative markers were not available.
For sensitivity analysis 4, we assessed the effect of DRD4 by family background stratifying the sample according to familial risk and conducting multiple-group models. Chi-square difference tests indicated that the regression coefficients of the predictor variables could be constrained to equality across high and low risk groups without loss in model fit (main effect: Δχ2 = 13.83, Δdf = 12, p > .05; interaction model: Δχ2 = 16.47, Δdf = 16, p > .05).
Discussion
We tested a series of developmentally sensitive gene × environment interactions on AD symptoms in a large longitudinal sample of college students. Specifically, we tested for main effects and interactions between DRD4 genotype and childhood adversity and work and family roles on overall levels of and change in AD symptoms. We found that greater childhood adversity predicted greater AD symptoms that persisted across emerging and young adulthood. We also found that women tended to decrease in AD symptoms at a greater rate than men during emerging adulthood. None of the gene × environment interactions attained statistical significance.
One unique aspect of our findings was a departure from the widely cited “maturing out” pattern reported in some prior cohorts (e.g., Johnston, O’Malley, & Bachman, 2001b; Park et al., 2011). Specifically, average AD symptoms increased from wave 10 to wave 11. This pattern may be due to the fact that participants who showed high or increasing levels of binge drinking in previous waves were more likely to be targeted for re-recruitment in our genotyping study, compared to participants with stably low levels of binge drinking. But recent large studies from the UK and US suggest the continuation of heavy drinking into young adulthood may be increasingly common. Integrating longitudinal data from nine cohorts (N = 59,397), a landmark study from the UK found that alcohol consumption peaked around age 25, and frequency of drinking increased through young adulthood before declining in midlife (Britton, Ben-Shlomo, Benzeval, Kuh, & Bell, 2015). Furthermore, recent data from the Centers for Disease Control shows that binge drinking has become increasingly prevalent among young adults, particularly those with high income. According to their data, 20% of young adults in the $75,000 or greater income bracket endorsed binge drinking in the past month, and the average number of monthly binges was 3.7 (Center for Disease Control, 2010), a notable finding given that 40% of our Caucasian sample reported annual income greater than $70,000.
We did not detect any main effects of DRD4 genotype, or any significant interactions between environments (childhood adversity or work and family roles) and DRD4, in predicting AD symptoms. These results contrast with results from a previous longitudinal study by Park et al. (2011) that examined similar developmentally sensitive G × E interactions in a large cohort. Although it is tempting to speculate about how differences between the studies produced differences in the results, the most parsimonious explanation is randomness. The role of sampling error is particularly pertinent here, as neither study was well-powered to detect a very small effect. We had >99% power to detect an interaction effect as large as the DRD4 × childhood adversity interaction effect reported in Park et al., and 80% power to detect an interaction effect of .11 magnitude. However, some have suggested that a biologically plausible effect size for a single polymorphism or single interaction is much, much smaller (e.g., Chabris, Lee, Cesarini, Benjamin, & Laibson, 2015; Duncan & Keller, 2011). Given the pragmatic difficulty of collecting in-depth longitudinal data on massive numbers of participants, future developmental research on G×E may benefit from using polygenic risk scores (e.g., Salvatore et al., 2015), which are expected to have bigger effects than single genetic variants.
Nevertheless, we can compare and contrast key features of the two studies. Similarities between the two studies include longitudinal, college-based cohorts and multiple assessments throughout the college years and into young adulthood. Both studies tested for interactions between DRD4 and developmentally specific environments with relevance to the etiology of AD (childhood adversity, work and family roles). Both studies used Caucasian samples, and both modeled longitudinal data in a factor analytic framework. However, the moderators and outcomes were not measured in exactly the same way in the two studies. Park et al.’s (2011) childhood adversity composite included more abandonment and neglect items, and adversity data were obtained using a single face-to-face structured interview, whereas we used surveys over three waves. The composite indexing work and family roles contained similar content across studies, but the age span and number of measurement occasions differed. A further consideration is that the current study assessed AD more frequently at earlier ages (10 versus 4 assessments before age 25) and less frequently at later ages (1 versus 3 assessments after age 25) than Park et al. (2011). Finally, sample participants from Park et al. (2011) were selected so that half would be high risk for AD (biological father who met criteria for alcoholism) and half very low risk (no first or second degree biological relative that has met/meets criteria for alcoholism or substance abuse, and no first degree relative meeting for antisocial personality disorder). Although group comparisons stratified by family risk in our sample suggested interaction effects could be constrained to be equal in both high and low risk participants (and were significant in neither group), our operationalization of risk was less stringent than Park et al.’s (2011). Therefore, if the DRD4 × environment interaction effects observed in Park et al. (2011) are true effects, these interaction effects appear to be sensitive to changes in measures, in the temporal density of assessments, or in the prevalence of severe familial risk for AD.
Park et al. (2011) used a sophisticated study design that uniquely addressed developmental mechanisms relevant to drinking behavior – an important and valuable approach for gene × environment interaction studies. To date, this approach has been relatively rare in the candidate gene interaction literature – particularly as it relates to AD – which positions their study as an important target for replication efforts. We did not observe interactions between DRD4 genotype and environments in predicting AD symptoms in our data. Given the conceptual similarities between these two studies, our study constitutes an important contribution to the literature regarding how genes and environments combine in the etiology of alcohol-related problems.
Supplementary Material
Acknowledgments
This research was supported by Grant 1-R01-AA020637 to Dr. Kim Fromme, Grant 1-R21-AA020588 to Dr. Paige Harden, Grant 5-R24-HD042849 to the Population Research Center at the University of Texas at Austin, and Grant T32-AA07471 to the Waggoner Center for Alcohol and Addiction Research.
Footnotes
The 7-repeat (7R) allele of DRD4 is typically considered the risk-conferring allele. In Park et al. (2011), the 7-, 8-, and 9-repeat alleles were grouped together, although the majority of “long” allele carriers (95%) were 7R allele carriers.
Rather than estimating at latent growth curve model, Park et al. (2011) fit a bifactor model, in which all waves loaded on a single common factor (“Alcohol dependence throughout emerging and young adulthood”), covariation among residual variances at ages 18–21 were modeled with a factor labeled “Alcohol dependence limited to emerging adulthood”, and covariation among residual variances at ages 25, 28, and 34 were modeled with factor labeled “Alcohol dependence limited to young adulthood.” However, this bifactor model is not suitable for our data, because our participants completed only one assessment in the young adulthood period. Additionally, the bifactor model approach is limited because it does not capture individual differences in intra-individual change. Although our modeling approach was designed to be sensitive to the unique design features of our dataset, the growth factors in this model capture similar information, conceptually, as the factors in Park et al. (2011). To summarize, the Δw11 factor represents AD symptoms specific to W11 and is most analogous to AD limited to young adulthood. The intercept factor (labeled i in Figure 1, with a reference point at wave 10) has loadings from AD symptoms at all waves and is most analogous to persistent AD symptoms. The remaining factors (labeled s and q in Figure 1) reflect how individuals change in AD symptoms between 18–24 and are most analogous to AD limited to emerging adulthood. As a sensitivity analysis, we fit a model that represented the covariation among the waves with a single general factor; such a model assumes that there is no mean change in AD symptoms across the study period. Not surprisingly, this model fit poorly (no covariate model: χ2 (54) = 386.22, p = .0001, RMSEA = .13, CFI/TLI = .73/.62, SRMR = .096; main effect [ME] model: χ2 (82) = 400.8, p = .0001, RMSEA = .10, CFI/TLI = .74/.69, SRMR = .08; ME + Interaction: χ2 (103) = 437.04, p = .0001, RMSEA = .09, CFI/TLI = .73/.68, SRMR = .08). There were no significant interaction effects in any model using the general factor.
We have no conflicting interests to disclose.
References
- Britton A, Ben-Shlomo Y, Benzeval M, Kuh D, Bell S. Life course trajectories of alcohol consumption in the United Kingdom using longitudinal data from nine cohort studies. BMC medicine. 2015;13(1):47. doi: 10.1186/s12916-015-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casswell S, Pledger M, Pratap S. Trajectories of drinking from 18 to 26 years: identification and prediction. Addiction. 2002;97(11):1427–1437. doi: 10.1046/j.1360-0443.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- Chabris CF, *, Lee JJ, *, Cesarini D, Benjamin DJ, Laibson DI. The Fourth Law of Behavior Genetics. Current Directions in Psychological Science. doi: 10.1177/0963721415580430. in press. *These authors contributed equally to the work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Vaughan EL, Fromme K. Ethnic differences and the closing of the sex gap in alcohol use among college-bound students. Psychology of Addictive Behaviors. 2008;22(2):240. doi: 10.1037/0893-164X.22.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22(03):491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- DeYoung CG. The neuromodulator of exploration: A unifying theory of the role of dopamine in personality. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168(10):1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Jardine R, Martin NG. Interactive effects of genotype and social environment on alcohol consumption in female twins. Journal of Studies on Alcohol and Drugs. 1989;50(01):38. doi: 10.15288/jsa.1989.50.38. [DOI] [PubMed] [Google Scholar]
- Hewitt JK. Editorial policy on candidate gene association and candidate gene-by-environment interaction studies of complex traits. Behavior Genetics. 2012;42(1):1–2. doi: 10.1007/s10519-011-9504-z. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, Riley B. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nature Neuroscience. 2012;15(2):181–189. doi: 10.1038/nn.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens MP, Neighbors C, Dams-O’Connor K, Lee CM, Larimer ME. The factor structure of a dichotomously scored Rutgers Alcohol Problem Index. Journal of Studies on Alcohol and Drugs. 2007;68(4):597. doi: 10.15288/jsad.2007.68.597. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary psychological research. Handbook of Psychology 2003 [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: a review. Pharmacology Biochemistry and Behavior. 2009;93(3):222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo M, Matheson I, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case–control studies and evidence of publication bias. Molecular Psychiatry. 2007;12(5):454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Muthén L, Muthén B. BO 1998–2007. Mplus User’s Guide. n.d;5 [Google Scholar]
- Park A, Sher KJ, Todorov AA, Heath AC. Interaction between the DRD4 VNTR polymorphism and proximal and distal environments in AD during emerging and young adulthood. Journal of Abnormal Psychology. 2011;120(3):585. doi: 10.1037/a0022648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Wightman P, Schoeni RF, Schulenberg JE. Socioeconomic status and substance use among young adults: a comparison across constructs and drugs. Journal of Studies on Alcohol and Drugs. 2012;73(5):772. doi: 10.15288/jsad.2012.73.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen A, Whitsel EA, Tabor J, Killeya-Jones LA, Cuthbertson CC, Hussey JM, Harris KM. Add Health wave IV documentation: Candidate Genes 2013 [Google Scholar]
- Soper DS. A-priori Sample Size Calculator for Structural Equation Models [Software] 2015 Available from http://www.danielsoper.com/statcalc.
- Timberlake DS, Hopfer CJ, Rhee SH, Friedman NP, Haberstick BC, Lessem JM, Hewitt JK. College attendance and its effect on drinking behaviors in a longitudinal study of adolescents. Alcoholism: Clinical and Experimental Research. 2007;31(6):1020–1030. doi: 10.1111/j.1530-0277.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan H-C, Ohara K, Bunzow JR, Civelli O, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. 1992 doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol and Drugs. 1989;50(01):30. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.