Synopsis
Borrelia miyamotoi disease (BMD) is a newly recognized borreliosis globally transmitted by ticks of the Ixodes persulcatus species complex. Once considered to be a tick symbiont with no public health implications, B. miyamotoi is increasingly being recognized as the agent of a nonspecific febrile illness often misdiagnosed as acute Lyme disease without rash, or as ehrlichiosis. The frequency of its diagnosis in the northeastern U.S. is similar to that of HGA. A diagnosis of BMD may be confirmed by PCR analysis of acute blood samples, or by seroconversion using a recombinant GlpQ enzyme immunoassay. As with Lyme disease or HGA, BMD is successfully treated with oral doxycycline or amoxicillin.
Keywords: Borrelia miyamotoi, Lyme disease, deer ticks, borreliosis, zoonotic infection
Introduction
Tick borne Borrelia spp. are now usually divided into two taxonomic groups, which correspond to the typical human disease manifestations, Lyme disease or relapsing fever, as well as to their tick vectors, prostriate ixodid ticks and argasid ticks, respectively (Table 1). Arnold Theiler (1904) demonstrated spirochetes in metastriate ixodid ticks infesting African cattle with a mild disease that was called “tick spirochetosis” or bovine borreliosis. Borrelia theileri subsequently has been globally reported, usually associated with cattle and their cosmopolitan tick Rhipicephalus (Boophilus) microplus and R. annulatus. Professor Kenji Miyamoto of Asahikawa Medical College, during 1990–1992, isolated spirochetes from I. persulcatus ticks collected in Hokkaido, Japan. These isolates were subsequently demonstrated by analysis of the 23S-5S rDNA and other genes, to be a new species related to the relapsing fever spirochetes (Fukunaga et al. 1995). The name Borrelia miyamotoi was applied to this new species, and subsequently, this spirochete has been detected wherever I. persulcatus species complex ticks (I. dammini, I. scapularis, I. pacificus, I. ricinus, herein referred to as Ixodes spp.) occur. In 1995, borreliae from American Lone Star ticks (Amblyomma americanum) were identified independently by two research groups (Armstrong et al. 1996; Barbour et al. 1996) and provided the names B. lonestari and B. barbouri; the former name has prevailed in the literature.1 Molecular phylogenetic analyses demonstrate that B. theileri, B. lonestari, and B. miyamotoi comprise a group together, deep within the relapsing fever spirochete clade (Barbour 2014) and not within the other ixodid (“hard”) tick maintained borreliae, namely those in Ixodes spp. that are recognized as B. burgdorferi sensu lato. Although phylogenetically considered to be relapsing fever spirochetes, the metastriate-transmitted Borrelia spp. should not be assumed to be biologically similar to the true relapsing fever spirochetes maintained by argasid (“soft”) ticks, nor to cause typical relapsing fever.
Table 1.
Kinds of ticks and their roles as vectors of Borrelia spp.
| Family | Subfamily | Colloquial name | Species example | Common name | Associated Borrelia sp. |
|---|---|---|---|---|---|
| Argasidae | Argasinae | Soft ticks | Ornithodoros moubata | Tampan tick | B. duttoni |
| Ixodidae | (division Prostriata) -- Ixodinae | Hard ticks | Ixodes persulcatus | Taiga tick | B. burgdorferi s.l., B. miyamotoi s.l. |
| Ixodidae | (division Metastriata) -- Rhipicephalinae, Amblyomminae, Haemaphysalinae, Hyalomminae | Hard ticks | Amblyomma americanum | Lone Star tick | B. lonestari |
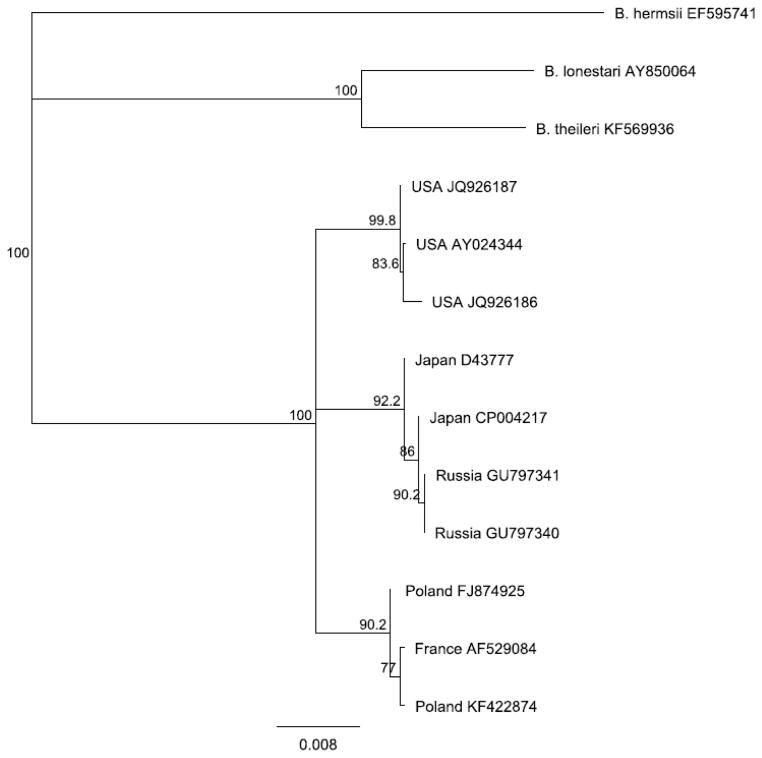
As with B. burgdorferi (see reviews in this issue), evidence is emerging that B. miyamotoi comprises a species complex or group of genospecies (Barbour 2014) and should be referred to as B. miyamotoi sensu lato. Asian, European, and American clades are apparent with phylogenetic analyses of typical gene targets such as flagellin (Figure 1). Unlike B. burgdorferi s.l., for which less than half of the recognized genospecies have been associated with human infection, all three B. miyamotoi clades recognized thus far have been associated with human clinical cases.
Figure 1.
Phylogenetic relationship of B. miyamotoi s.l. Neighbor-joining analysis of a 608 bp portion of the flagellin gene. Numbers at nodes represent proportion of 1000 bootstrap replicates consistent with the branching topology; branch lengths are proportional to nucleotide differences. Three B. miyamotoi clades are apparent: American, Japanese/Russian and European.
(Data from GenBank; Available at: http://www.ncbi.nlm.nih.gov/genbank/)
The biology of B. theileri has been well studied, particularly clinical aspects of bovine borreliosis (Callow 1967) and vector-pathogen interactions (Smith et al. 1985). “Borrelia lonestari” has been circumstantially associated with a disease manifesting mainly as erythema migrans (Southern Tick Associated Rash Illness [STARI] or Masters Disease), but its formal incrimination as the etiologic agent remains lacking (Feder et al. 2013). Further discussion of STARI/Masters Disease as a borreliosis is beyond the scope of this review. B. miyamotoi, despite its global distribution in Lyme disease vectors, remained as an incidental finding in field surveys and was thought to be an endosymbiont of Ixodes spp. ticks. In 2011, a case series comprising febrile Russian patients, some with a recurrent fever, was presented with polymerase chain reaction (PCR) evidence that implicated B. miyamotoi as the etiologic agent (Platonov et al. 2011). This was the first suggestion that B. miyamotoi was capable of causing human disease, and was followed in 2013 by the index case for North America (Gugliotta et al. 2013) and soon thereafter that for the European Union (Hovius et al. 2013). We now know that at least in the northeastern U.S., B. miyamotoi disease (BMD) may be relatively common (Molloy et al. in press).
Epidemiology
BMD appears to be a common infection in sites that are intensely zoonotic for Lyme disease. For the Russian case-series, BMD was diagnosed as frequently as tick borne encephalitis (TBE) and about half as frequently as erythema migrans due to B. burgdorferi s.l. (Platonov et al. 2011). Active case detection using PCR of acute phase blood samples from 11,515 febrile patients in southern New England demonstrated that 3.1% had evidence of infection by Babesia microti, 1.4% by Anaplasma phagocytophilum, and 0.8% by B. miyamotoi (Molloy et al. in press). It should be noted that blood was sampled only from patients whose signs and symptoms were sufficient to cause them to seek medical attention; it is likely that subclinical B. miyamotoi infections occur. Indeed, an enzyme immunoassay using the recombinant B. miyamotoi antigen GlpQ (rGlpQ), suggested that the serum of about 4% of participants from cross sectional serosurveys in New England Lyme disease endemic sites was reactive; about 9% of these sera reacted to antigens of B. burgdorferi (Krause et al. 2014).
The Russian case series presented from May–August (Platonov et al. 2011), had an estimated 12–16 day incubation period. Adult I. persulcatus, the Lyme disease and TBE vector in the Yekaterinburg area, seek hosts during May and June (Fillipova 1985), with nymphs and larvae appearing during June and activity extending into August. It is thus not clear which I. persulcatus developmental stage transmits B. miyamotoi to people in Russia. In southern New England, about 70% of all the BMD cases were diagnosed during July and August (Molloy et al. in press), which incriminates larval I. dammini as the main BMD vector because nymphal I. dammini have their main period of activity during May to mid-July. Two-thirds of all Lyme disease cases in the northeastern U.S. are reported during June and July (Bacon et al. 2008), which demonstrates that nymphal I. dammini are the main vector stage. Accordingly, BMD in the northeastern U.S. has a different epidemiologic pattern than does Lyme disease. Education about preventing Lyme disease typically focuses on temporal risk during early summer (June) and with the emergence of adult deer ticks during the fall (October–November). Larval deer ticks would be found in the same habitats as nymphs or adults, but would require more intimate contact with the ground and leaf litter for a person to become infested. As with Lyme disease, nymphal and adult Ixodes spp. may transmit infection, with prevalence rates of B. miyamotoi infection typically ranging from 1%–10% (Scoles et al. 2001; Richter et al. 2003; Mun et al. 2006; Ogden et al. 2011). However, for most tick borne infections, an infected tick is a necessary but insufficient condition for risk: risk is instead determined by whether a tick is allowed to feed for an extended duration. Adult Ixodes spp. tend to seek hosts during the colder months when people are dressed in a manner that inhibits tick attachment. Adults are also much bigger and more easily detected and removed. Nymphal Ixodes spp. are empirically very efficient vectors for Lyme disease, and the finding that more BMD cases coincide with the larval tick season of activity in the northeastern U.S. suggests that nymphs may not transmit B. miyamotoi as efficiently as do larvae.
As with B. burgdorferi s.l., diverse kinds of warm-blooded animals may serve as amplifying hosts for B. miyamotoi. B. miyamotoi is easily propagated in the laboratory in both sigmondontine and murine rodents (Scoles et al. 2001). A common reservoir host for B. burgdorferi in the eastern U.S., the white footed mouse (Peromyscus leucopus), is naturally infected by B. miyamotoi (Barbour et al. 2009). European and Japanese rodents known to maintain B. burgdorferi s.l. are also competent hosts for B. miyamotoi (Taylor et al. 2013; Burri et al. 2014). B. miyamotoi has been detected in blood or in ticks removed from passerine or galliform birds (Hamer et al. 2012) as well as from ticks attached to deer (although it is not clear whether the tick was infected prior to infesting the deer or acquired infection from the deer). Whether specific vertebrate reservoirs are required for B. miyamotoi perpetuation is not axiomatic.
Transmission
B. miyamotoi was first isolated from host seeking, unfed adult I. persulcatus ticks (Fukunaga et al. 1995), which implied that spirochetes had been maintained through the molt from fed nymph to adult stage ticks (i.e.,transstadial transmission). The comprehensive report that established the endemicity of B. miyamotoi in North America (Scoles et al 2001) confirmed transstadial transmission as well as by inheritance (i.e., transovarial transmission). B. burgdorferi s.l., in contrast, is inefficiently inherited, if at all (Rollend et al. 2013), while the classic relapsing fever spirochetes (e.g., B. hermsi, B. turicata, B. crocidurae, B. duttoni) can be readily maintained by this mode of transmission, at least for as many as 5 generations (Burgdorfer and Varma 1967). Vertebrate hosts can serve to increase the prevalence of infection in the general tick population, and may even be required to maintain full pathogenicity. However, they may not be necessary for spirochetal persistence from generation to generation. The agent of bovine borreliosis, B. theileri, is also maintained in this manner, although the hard ticks that transmit B. theileri, Rhipicephalus microplus, are “one host” ticks which (unlike most ticks that detach after each bloodmeal) attach as larvae, feed and quickly develop into nymphs and then adults in situ without leaving the individual host. B. lonestari is found in host-seeking larval Amblyomma americanum (Telford, unpublished) and is likely to have inheritance as an important mode of perpetuation. Thus, the metastriate hard tick borreliae (B. theileri, B. lonestari, and B. miyamotoi) share aspects of the biology of the soft tick-transmitted relapsing fever spirochetes.
Although B. burgdorferi s.l. requires reactivation within the feeding Ixodes spp. prior to attaining infectivity, the true relapsing fever spirochetes apparently do not; isolates are readily made by homogenizing and injecting infected soft ticks into mice. Reactivation was first described for Rickettsia rickettsii, the agent of Rocky Mountain spotted fever (Spencer and Parker 1923). Host seeking wood ticks, Dermacentor andersoni, collected from zoonotic sites failed to induce productive infection in guinea pigs when homogenized and intraperitoneally injected, but those that were allowed to pre-feed for 24 hours on uninfected animals, then homogenized and injected into uninfected guinea pigs induced a typical fatal infection. Heating ticks to 37C for several hours also induced the activation of the dormant rickettsiae, as demonstrated by their capacity to cause lethal infection. Subsequently, virtually all hard tick-transmitted pathogens were recognized to require a similar reactivation to attain infectivity (Katavolos et al. 1998). The molecular basis of reactivation has been elegantly studied in B. burgdorferi s.l. (Rosa et al. 2005) and is the reason for the minimum 24 hour delay between tick attachment and delivery of an infectious dose to initiate Lyme disease. Reactivation does not seem to occur with the soft tick-transmitted relapsing fever spirochetes, perhaps because soft ticks require only a few hours for feeding, in contrast to hard ticks, which require days. It is not known whether B. miyamotoi requires reactivation during transmission by Ixodes spp. Accordingly, it is not clear whether prompt removal of feeding ticks may reduce the risk of acquiring BMD, as it does Lyme disease, although prompt removal might influence the quantum of infection (dose) delivered.
Because B. miyamotoi causes spirochetemia and can be detected by PCR in acute blood samples of febrile individuals, it is possible that transfusion-transmitted cases will be described. Further supporting this possibility is the finding that, infected mouse blood retains infectivity by syringe passage under storage conditions that are similar to those used in blood banking (Krause et al. 2015). On the other hand, the capacity of B. miyamotoi to persist in people who have recovered from acute illness remains unknown.
Clinical presentation
The BMD index cases in North America (Gugliotta et al. 2013) and in the European Union. (Hovius et al 2013) presented with meningoencephalitis. Both patients were elderly and had recently undergone chemotherapy for malignancy. BMD in both of these individuals was progressive, comprising memory and cognitive deficits, and spirochetes were detected in cerebrospinal fluid samples, demonstrating active intrathecal infection. For both patients, acute disease was entirely resolved following antibiotic treatment. How frequently B. miyamotoi breaches the blood-brain barrier of otherwise healthy individuals remains to be determined, though in our large case series no sign of meningeal irritation or encephalitis was observed (Molloy et al. in press)..
In the Russian case series described above (Platonov et al. 2011), it was noted that BMD generally presented with more systemic signs and symptoms, particularly headache and fever, compared to Lyme disease. Virtually all patients presented with fever (axillary temperature >37.2C), fatigue, and headache, a statistically significant difference from the presentation of Lyme disease patients. The next most common signs and symptoms were myalgia, chills, nausea and arthralgia, characterizing 30%–60% of the patients. Similarly, a series of PCR positive patients from New England (Molloy et al. in press) almost always demonstrated fever, chills, headache, myalgia and fatigue. Referring physicians often noted that patients with BMD exhibited more significant symptoms than patients with Lyme disease. In fact, BMD can be misdiagnosed as ehrlichiosis due to A. phagocytophilum or Ehrlichia spp. (Chowdri et al. 2014). In both case series, rashes were observed for fewer than 10% of the patients.
Although recurrent fever has been reported from as many as 10% of the Russian and American BMD cases, classical relapsing fever is a very different disease clinically and biologically. Relapsing fever has a sudden onset of two or more episodes of high fever (i.e., >39C), with an inter-febrile period of a few days. The fevers end with half an hour of rigors, hyperpyrexia, and hypertension followed by hours of diaphoresis and hypotension. Often the patient is also delirious during this period. The spleen is enlarged and jaundice may be apparent in these patients (Southern and Sanford 1969). In most respects, relapsing fever is a very severe, acute disease compared to either BMD or Lyme disease. With respect to pathobiology, relapsing fever spirochetes are characterized by their evasion of the host immune response by antigenic variation (Barbour 1987), with progressive replacement of dozens of variable membrane proteins (VMPs). No evidence has been presented to suggest the phenomenon of antigenic variation by B. miyamotoi. Accordingly, even though B. miyamotoi is phylogenetically a relapsing fever spirochete, BMD should not be considered relapsing fever.
We present 3 case vignettes, representing the spectrum of BMD that we have observed.
Case 1. Uncomplicated BMD
In late November, an active 70 year old woman from southeastern Massachusetts presented to an urgent care center with 2 days of fever to 38.9C, shaking chills, rigors and headache. She denied nausea, vomiting, diarrhea or upper respiratory symptoms. A “big” tick had been imbedded in her flank two weeks prior. Her past medical history was significant for hypertension, hyperlipidemia, reflux disease and osteopenia. Blood was drawn and she was advised to take nonsteroidal anti-inflammatory drugs (NSAIDs), rest, and hydrate. Here complete blood cell count (CBC) was normal and a rapid influenza test was negative. PCR demonstrated B. miyamotoi DNA in the blood sample and 5 days after the initial presentation, the patient reported symptomatic improvement with the use of NSAIDs without antimicrobials. Her body aches and chills resolved, and the patient was otherwise well,but she continued to complain of residual fatigue. Skin examination revealed residual erythema at the site of the tick bite but no other rashes. She was started on doxycycline 100mg twice daily and a repeat blood sample taken at this time was negative for B. miyamotoi or other deer tick-borne infections. She was re-evaluated in late December after completion of the doxycycline course and complained of residual fatigue. She was seen again in late January feeling much better with her fatigue resolving.
Case 2. Human anaplasmosis or BMD?
A healthy 57 year old woman from southeastern Massachusetts became acutely ill in early August with fevers and chills, headache, joint and muscle pain. Two days after onset, she went to the community hospital emergency room due to increasing lethargy, where she was found to be febrile to 38.9C and tachycardic (HR = 116). She reported a tick bite three weeks prior. Peripheral blood smears, blood and urine cultures were negative and her CSF was normal. A CBC revealed a white blood cell (WBC) count of 7,600 with 71% polymorphonuclear leukocytes and 16% neutrophils, a platelet count of 120,000 cells/μl and elevated liver transaminases. The patient’s chest x-ray was normal, however she was admitted due to her septic appearance, and human anaplasmosis was presumptively diagnosed based on the clinical presentation and lab data. She was hydrated with IV fluids and started on IV doxycycline. A CBC the next day revealed WBC 6,200 and platelets of 80,000 cells/μl. She improved within 24 hours and although her headaches persisted, she was discharged on oral doxycycline.
Blood taken prior to treatment was positive by PCR for B. miyamotoi and negative for both A. phagocytophilum and B. microti. At her outpatient follow-up, the patient stated that although her fever resolved within 24 hours of the IV doxycycline, she continued to have fatigue and headaches, unrelieved by oxycodone with acetaminophen. She was alert and oriented but complained of severe headache. Vital signs and physical exam were normal. She was advised to continue doxycycline for a period of three weeks and was given a prescription for butalbital/acetaminophen and caffeine for her headaches. She was re-evaluated 9 days later and all of her symptoms had resolved, including the headaches. A CBC drawn at that time showed a WBC 11,200/uL, HCT 42.8% and PLT 397,000/uL. She has since remained well and is back to her normal activities.
Case 3. Recurrent fever presentation
A 57 year old female nurse from southeastern Massachusetts felt ill at the end of July with generalized malaise and body aches. She is an avid gardener and has dogs, but did not recall any tick bites. None of her family members had been ill. Her past medical history is significant for hypertension and anxiety. She developed fevers, shaking chills, night sweats and headaches and was evaluated in a local community hospital emergency room where she was given 2 liters of IV fluids and sent home. She continued to be febrile with nausea, fatigue and body aches and went to her primary care physician a few days later. A CBC demonstrated leukopenia and elevated transaminases. She gave a history of allergy to amoxicillin and tetracycline and was sent home without treatment. A whole blood specimen was negative by PCR for B. microti and A. phagocytophilum, but serum was reactive for IgM-class antibodies to B. burgdorferi using a whole cell sonicate EIA, although confirmatory testing by immunoblot was negative. She continued to feel poorly with recurrent fever, chills and headaches associated with anorexia and weight loss. She had multiple visits to her primary care provider, who continued to monitor her clinically with serial CBCs. No specific diagnosis was made.Due to persistent symptoms, including fever, generalized arthralgias, malaise, headaches, nausea and loss of appetite, the patient was examined by an infectious disease specialist approximately 5–6 weeks after her initial presentation. She stated her fevers resolved for several days and then relapsed, causing a recurrence of her symptom complex. Physical examination revealed an alert but pale and ill looking woman. All other systems were unaffected and the patient was started on doxycycline while awaiting the results of the lab tests.
Blood samples drawn on 8/2 and 8/30 yielded evidence of B. miyamotoi DNA. She was re-evaluated on 9/27; the fever, headache and joint pain had all resolved. She complained of extreme fatigue and the inability to function at her normal pace. Her exam at that visit was normal and her CBC had returned to normal values. She was advised to complete the 4 weeks of doxycycline given that she was able to tolerate it with no side effects. The patient called the office on Oct 16 stating all of her symptoms had resolved and she was feeling markedly better.
Diagnosis
If Lyme disease is known to be zoonotic where the patient may have acquired infection, BMD is also likely to be endemic and thus should be considered in the differential diagnosis for a febrile, tick-exposed patient. As with the other deer tick-transmitted infections (i.e., Lyme disease, babesiosis, HGA, or Powassan/deer tick virus fever), patients with BMD present with an undifferentiated febrile illness, which may include acute headaches, fever, chills, myalgia, arthralgia, fatigue, or malaise. BMD patients often appear septic and laboratory studies reveal leukopenia, thrombocytopenia, and elevated transaminases. Such findings, along with clinical presentation, usually requires providers to exclude HGA.
Specialized laboratory testing, particularly PCR testing of blood samples collected during acute infection and ideally prior to initiation of treatment, is useful for confirming a BMD diagnosis. Acute anticoagulated blood samples (EDTA or citrate, not heparin) taken prior to treatment may be analyzed for evidence of B. miyamotoi DNA by PCR. Commercial testing laboratories are increasingly offering specific PCR testing for B. miyamotoi, but physicians need to consider a clinical laboratory experience’s with PCR in general when interpreting results. Like all other PCR assays, the failure to detect specific DNA or RNA in the blood does not exclude the possibility of BMD. In addition to B. burgdorferi, concurrent infections with B. microti, A. phagocytophilum, or even deer tick virus are possible and should be ruled out.
Serology is a useful complementary method to PCR for confirming a BMD diagnosis (Molloy et al. in press). Importantly, the recommended two-tiered serologic protocol (Wormser et al. 2006) for confirming a B. burgdorferi infection (EIA followed by immunoblot of reactive samples) is not useful for diagnosis of BMD. However, 23% of acute and 90% of convalescent sera from PCR confirmed BMD cases were reactive for IgM antibodies to B. burgdorferi using a sonicate antigen EIA. Few of these reactives were considered positive when immunoblots are interpreted as recommended (MMWR 1995). No more than 20% of convalescent BMD sera were reactive in the IgG EIA and of these, only 7% were confirmed by immunoblot. Thus, an IgM reactive sample by Lyme ELISA, but not confirmed by immunoblot, may represent a response to B. miyamotoi instead of B. burgdorferi. Convalescent sera from BMD patients rarely demonstrate IgG reactivity to B. burgdorferi antigens.
The B. burgdorferi EIA, when reactive for IgM but not confirmed for specific reactivity, may be effectively complemented by an EIA using a recombinant glycerophosphodiester phosphodiesterase (GlpQ) protein. GlpQ is a 39–42kDa protein that was identified by screening a B. hermsi genomic library with high titered human sera collected from a patient with relapsing fever. GlpQ was demonstrated in all Borrelia spp. examined except for B. burgdorferi s.l., thus allowing for serologic discrimination of relapsing fever from Lyme disease (Schwan et al. 1996). Over 90% of convalescent sera from relapsing fever cases were reactive in EIAs using the recombinant GlpQ (rGlpQ) protein, but none were reactive from either Lyme disease or syphilis cases. Given that B. miyamotoi is more closely related to relapsing fever spirochetes than to B. burgdorferi s.l., GlpQ was a natural target to serve as the basis for the development of an immunoassay to confirm a diagnosis of BMD.
The rGlpQ EIA appears to be sensitive and specific for confirming BMD (Molloy et al. in press). Lyme disease patient sera did not react with rGlpQ, nor did those from HGA or monocytic ehrlichiosis patients. A low frequency (14%) of IgM reactivity was observed with B. microti patient sera; the biologic basis for this finding remains unexplored. Some (3.6%) serum samples from 250 healthy individuals residing west of the Mississippi River were considered IgG reactive; this puzzling finding may reflect exposure to relapsing fever spirochetes other than B. miyamotoi. Although GlpQ homologs may be found in Haemophilus influenzae and even Escherichia coli, their sequences are divergent enough so that crossreactivity with B. miyamotoi GlpQ is not likely (Krause et al. 2014). Of a well-studied BMD case series, 86% were considered to have seroconverted to rGlpQ (Molloy et al. in press); convalescent sera comprised those taken at least 5 days after initial presentation.
The need for a recombinant protein antigen was determined by the lack of availability of whole cell B. miyamotoi antigens. Although the Japanese B. miyamotoi type strain HT31 was originally isolated in BSK II medium and apparently can be productively maintained in vitro, until very recently no one has succeeded in culturing the American or Eurasian strains. The only means of propagating these other strains has been by serial blood passage in SCID mice. These immunodeficient mice sustain spirochetemias approaching 1000–5000 bacteria/mL for long durations, and they are very useful in making primary isolates by inoculating blood from BMD patients. However, the cost of purchasing SCID mice and maintaining them for the purpose of harvesting spirochetes for use as serologic reagents would be prohibitive. HT31 itself is not available through ATCC or other biological repositories and must be requested from a handful of research laboratories within the U.S. or Europe.
Successful cultivation of B. miyamotoi has recently been reported (Wagemakers et al. 2014; Margos et al. 2015). Both research groups report passaging HT31 and a North American B. miyamotoi strain in variants of Kelly-Pettenkofer medium (Kelly’s medium is the base for BSK II medium, which is used to cultivate B. burgdorferi). In one system, 10% fetal calf serum and slight modifications of the concentrations of bovine serum albumin, CMRL-1066 base, HEPES, glucose, and rabbit serum allowed for propagation within standard conditions used for B. burgdorferi. In the other system, the critical factors included 50% fetal calf serum in a 6% carbon dioxide atmosphere. In both systems, cell densities did not exceed 107 bacteria/ mL, wherease B. burgdorferi in BSK II medium will attain densities of 109 cells/mL (Barbour 1985). Both research groups report failing to maintain B. miyamotoi viability in unmodified BSKII medium but Fukunaga et al. (1996) originally propagated HT31 in BSKII medium to densities sufficient to harvest cells for preparing immunoblots or DNA for southern blots or pulse field gel electrophoresis. It may be that the 3 members of B. miyamotoi s.l. may differ in their in vitro growth requirements, or that batches of BSK vary with respect to their capacity to sustain HT31 growth. In any event, the possibility of propagating B. miyamotoi in vitro allows for generating more traditional serologic reagents such as whole cell sonicate EIAs and immunoblots.
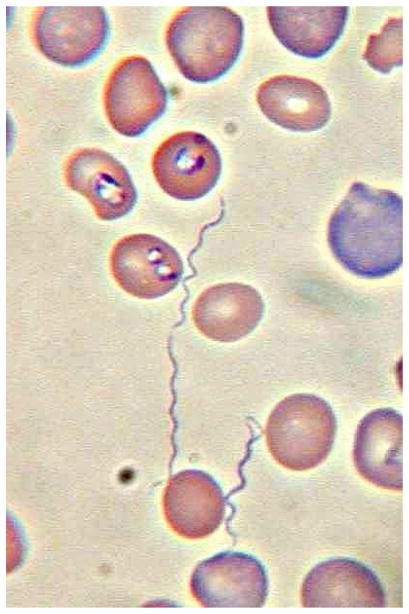
SCID mice could be inoculated with acute blood samples and borreliae may be visualized by peripheral blood smears of the mice 7–14 days after inoculation (Figure 2). The sensitivity of this procedure has not yet been determined and this method would be restricted to a limited number of research laboratories. Spirochetes were demonstrable in the CSF of the American and European index cases, but these were unusual findings in elderly immunocompromised individuals. However, because B. miyamotoi causes peripheral blood spirochetemia, Giemsa stained blood smears made from acute blood samples may allow for a microscopic confirmation of the diagnosis. Thick smears, as performed for malaria diagnosis, are the most likely method for successful detection; by quantitative PCR, a range of 5–53,000 (mean 8313) spirochetes were estimated per milliliter of whole blood from our recent case series (Molloy et al., in press). For comparison, the sensitivity of thick blood smears for malarial parasites is approximately 15,000 infected red blood cells per milliliter (Bruce Chwatt 1985).
Figure 2.
Giemsa stained (pH 7.0) thin blood smear of blood from a CB17-SCID mouse concurrently infected by Babesia microti and Borrelia miyamotoi. Three spirochetes are seen in this field. X630, bright field illumination.
In summary, BMD should be considered in febrile patients from Lyme-endemic regions of the northeast United States, who present between April to November with evidence of a systemic illness or who appear septic. Other infections to be considered in these patients include Lyme disease without rash, babesiosis, HGA, deer tick virus fever and West Nile fever. Elevated transaminases, leukopenia, and thrombocytopenia narrow the list of deer tick transmitted infections to BMD and HGA; in sites with dog ticks or Lone Star ticks, rickettsiosis, monocytic ehrlichiosis, and tularemia may also be considered. PCR testing of an acute, pre-treatment blood sample for Borrelia spp. (“pan-borrelia”), preferably followed by a species specific PCR assay as a confirmatory identification, is the most efficient way to demonstrate active infection. As with Lyme disease or virtually any other tick-transmitted infection, acute serology for BMD is not sensitive, but it is useful as an archived comparison to a convalescent sample collected 3 to4 weeks later. BMD convalescent serum is usually IgM reactive in B. burgdorferi EIA (whole cell sonicate), but the confirmatory Lyme immunoblot using the two-tiered protocol will not be interpreted as positive. Only a fifth of convalescent BMD patient sera will react in the B. burgdorferi IgG EIA (and usually are negatively by the confirmatory immunoblot), but more than 80% will react in the rGlpQ EIA for IgG. Hence, the confirmation of BMD rests on elevated transaminases, acute blood PCR, and seroconversion to rGlpQ.
Treatment
It is very likely that BMD has been occurring in Lyme-endemic sites from the very beginning of our recognition of Lyme disease as an American zoonosis, but presumptively has been diagnosed as atypical Lyme disease and treated successfully on that basis. Oral doxycycline (100 mg twice a day for 10–21 days) or amoxicillin (500 mg three times a day for 14–21 days) as currently recommended for early disseminated Lyme disease (Wormser et al. 2006) has been sufficient to treat BMD (Molloy et al. in press). Treatment failure has not been documented. The clinical implications of milder infections and failure to treat remains undetermined; mild or subclinical infections clearly occur given the evidence for 5%–10% prior exposure in New England communities suggested by cross-sectional serosurveys (Krause et al. 2014). The manner in which BMD presents in children remains to be described.
Prevention would be as for any other tick borne infection, namely personal protection using repellents such as DEET, clothing treatment with permethrin, daily showers, tick checks, prompt tick removal, and vigilance for unexplained fevers when living in or visiting Lyme-endemic sites.
Key Points.
Borrelia miyamotoi was first described from Japanese Ixodes persulcatus ticks. Subsequently, it was detected as an inherited infection of I. dammini ticks in the northeastern United States.
The index case of B. miyamotoi disease (BMD) in the United States comprised meningoencephalitis in an elderly immunocompromised patient. BMD is likely a common, underdiagnosed zoonosis wherever Lyme disease is reported.
Cases typically present with headache, fever, chills, fatigue, and myalgia.
BMD should not be considered a relapsing fever; there is no crisis with rigors or hyperpyrexia followed by diaphoresis and hypotension.
BMD may be confirmed by PCR of acute blood samples or by seroconversion to a recombinant GlpQ antigen.
Treatment is identical to that for Lyme disease.
Acknowledgments
SRT and HKG are supported, in part, by grants from the National Institutes of Health (U01AI109656, R41AI078631); the Tufts Innovation Institute; the Evelyn Lilly Lutz Foundation; the Dorothy Harrison Egan Foundation; and the Bill and Melinda Gates Foundation.
Footnotes
The name B. lonestari technically should be presented in quotation marks or referred to as Candidatus Borrelia lonestari, inasmuch as the requirements for naming a new bacterial taxon have not been fulfilled, in particular, formal publication in the IJSEM and its propagation in vitro that would enable deposition of cultures into an accepted biological repository.
POTENTIAL CONFLICTS OF INTEREST. VPB is an associate director of laboratory science and CEO of Imugen, Inc. PJM and HKG are employees of Imugen, Inc. HRC is a clinical consultant and SRT is a consultant and scientific advisor to Imugen, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sam R. Telford, III, Email: sam.telford@tufts.edu.
Heidi K. Goethert, Email: heidi.goethert@tufts.edu.
Philip Molloy, Email: pmolloy@imugen.com.
Victor Berardi, Email: vberardi@imugen.com.
Hanumara Ram Chowdri, Email: hchowdri@hawthornmed.com.
Joseph L. Gugliotta, Email: Gugliotta.Joseph@hunterdonhealthcare.org.
Timothy J. Lepore, Email: tjalepore@hotmail.com.
References
- 1.Theiler A. Spirillosis of cattle. J Comp Pathol. 1904;17:47–55. [Google Scholar]
- 2.Fukunaga M, Takahashi Y, Tsuruta Y, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov. isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. International Journal Of Systematic Bacteriology. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong PM, Rich SM, Smith RD, Hartl DL, Spielman A, Telford SR., III A new Borrelia infecting Lone Star ticks. Lancet. 1996;347:67–68. doi: 10.1016/s0140-6736(96)91604-9. [DOI] [PubMed] [Google Scholar]
- 4.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick, Amblyomma americanum. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 5.Barbour AG. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect Genet Evol. 2014;27:551–8. doi: 10.1016/j.meegid.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callow LL. Observations on tick-transmitted spirochaetes of cattle in Australia and South Africa. British Veterinary Journal. 1967;123:492–7. doi: 10.1016/s0007-1935(17)39704-x. [DOI] [PubMed] [Google Scholar]
- 7.Smith RD, Miranpuri GS, Adams JH, Ahrens EH. Borrelia theileri: Isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. American Journal Of Veterinary Research. 1985;46:1396–1398. [PubMed] [Google Scholar]
- 8.Feder HM, Jr, Hoss DM, Zemel L, Telford SR, 3rd, Dias F, Wormser GP. Southern Tick-Associated Rash Illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142–146. doi: 10.1093/cid/cir553. [DOI] [PubMed] [Google Scholar]
- 9.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerging Infectious Diseases. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gugliotta JL, Goethert HK, Berardi VP, Telford SR. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. NEJM. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovius JWR, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, et al. A case of meningoencephalitis by the relapsing fever spirochete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy PJ, Telford SR, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi disease in the northeastern United States: a case series. Ann Intern Med. doi: 10.7326/M15-0333. in press. [DOI] [PubMed] [Google Scholar]
- 13.Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillipova NA. Taiga Tick: Ixodes persulcatus Schulze (Acarina: Ixodidae) Nauka Publishers; Leningrad: 1985. p. 416. [Google Scholar]
- 15.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease – United States, 1992–2006. MMWR. 2008;57(SS10):1–9. [PubMed] [Google Scholar]
- 16.Scoles GA, Papero MA, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Diseases. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 17.Richter D, Schlee DB, Matuschka FR. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerging Infectious Diseases. 2003;9:697–701. doi: 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group Spirochete from Ixodes pacificus in California. Journal Of Medical Entomology. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- 19.Ogden NH, Margos G, Aanensen DM, et al. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Applied And Environmental Microbiology. 2011;77:3244–3254. doi: 10.1128/AEM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. American Journal Of Tropical Medicine And Hygiene. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burri C, Schumann O, Schumann C, Gern L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R monacensis and Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2014;5:245–51. doi: 10.1016/j.ttbdis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Taylor KR, Takano A, Konnai S, Shimozuru M, Kawabata H, Tsubota T. Borrelia miyamotoi infections among wild rodents show age and month independence and correlation with Ixodes persulcatus larval attachment in Hokkaido, Japan. Vector Borne Zoonotic Dis. 2013;13:92–7. doi: 10.1089/vbz.2012.1027. [DOI] [PubMed] [Google Scholar]
- 23.Hamer SA, Hickling GJ, Keith R, Sidge JL, Walker ED, Tsao JI. Associations of passerine birds, rabbits, and ticks with Borrelia miyamotoi and Borrelia andersonii in Michigan, U.S.A. Parasit Vectors. 2012;5:231. doi: 10.1186/1756-3305-5-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Burgdorfer W, Varma MGR. Transstadial and transovarial development of disease agents in arthropods. Annu Rev Entomol. 1967;12:347–376. doi: 10.1146/annurev.en.12.010167.002023. [DOI] [PubMed] [Google Scholar]
- 26.Spencer RR, Parker RR. Rocky Mountain spotted fever: infectivity of fasting and recently fed ticks. Publ Health Rep. 1923;38:333. [Google Scholar]
- 27.Katavolos P, Armstrong PM, Dawson JE, Telford SR., III Duration of tick attachment required for transmission of human granulocytic ehrlichiosis. J Infect Dis. 1998;177:1422–1425. doi: 10.1086/517829. [DOI] [PubMed] [Google Scholar]
- 28.Rosa PA, Tilly K, Stewart PE. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol. 2005;3:129–43. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- 29.Krause PJ, Hendrickson JE, Steeves TK, Fish D. Blood transfusion transmission of the tick-borne relapsing fever spirochete Borrelia miyamotoi in mice. Transfusion. 2015;55:593–7. doi: 10.1111/trf.12879. [DOI] [PubMed] [Google Scholar]
- 30.Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, Sterling SL, Telford SR. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med. 2013;159:21–7. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 31.Southern P, Sanford J. Relapsing fever. A clinical and microbiological review. Medicine. 1969;48:129–149. [Google Scholar]
- 32.Barbour AG. Immunobiology of relapsing fever. Contrib Microbiol Immunol. 1987;8:125–127. [PubMed] [Google Scholar]
- 33.Paddock CD, Telford SR. Critical Needs and Gaps in Understanding Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases: The Short-Term and Long-Term Outcomes - Workshop Report. Institute of Medicine, National Academy of Sciences; 2011. Through a glass, darkly: global incidence of tick borne diseases; pp. 1–41. [PubMed] [Google Scholar]
- 34.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Recommendations for test performance from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. 1995;44:590–591. [PubMed] [Google Scholar]
- 36.Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Konkel ME. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors. 2014;7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margos G, Stockmeier S, Hizo-Teufel C, Hepner S, Fish D, Dautel H, Sing A, Dzaferovic E, Rieger M, Jungnick S, Binder K, Straubinger RK, Fingerle V. Long-term in vitro cultivation of Borrelia miyamotoi. Ticks Tick Borne Dis. 2015;6:181–4. doi: 10.1016/j.ttbdis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Bruce Chwatt LJ. Essential Malariology. 2. John Wiley and Sons; NY: 1985. p. 452. [Google Scholar]
- 40.Pollack RJ, Telford SR, III, Spielman A. Standardisation of a complex medium for the growth of Borrelia burgdorferi. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telford SR, Goethert HK. Emerging and emergent tick borne infections. In: Chappell LH, Bowman AS, Nuttall PA, editors. Ticks: Biology, Disease and Control. Cambridge University Press; 2008. pp. 344–376. [Google Scholar]