Abstract
Background
Alzheimer’s disease (AD) is the most common type of dementia. It causes progressive brain disorder involving loss of normal memory and thinking skills. The transplantation of neural stem cells (NSCs) has been reported to improve learning and memory function of AD rats, and protects basal forebrain cholinergic neurons. Nerve growth factor – poly (ethylene glycol) – poly (lactic-co-glycolic acid)-nanoparticles (NGF-PEG-PLGA-NPs) can facilitate the differentiation of NSCs in vitro. This study thus investigated the treatment efficacy of NGF-PEG-PLGA-NPs combining NSC transplantation in AD model rats.
Material/Methods
AD rats were prepared by injection of 192IgG-saporin into their lateral ventricles. Embryonic rat NSCs were separated, induced by NGF-PEG-PLGA-NPs in vitro, and were transplanted. The Morris water-maze test was used to evaluate learning and memory function, followed by immunohistochemical staining for basal forebrain cholinergic neurons, hippocampal synaptophysin, and acetylcholine esterase (AchE) fibers.
Results
Rats in the combined treatment group had significantly improved spatial learning ability compared to AD model animals (p<0.05). The number of basal forebrain cholinergic neurons, hippocampal synaptophysin, and AchE-positive fibers were all significantly larger than in the NSC-transplantation group, with no difference from control animals.
Conclusions
NGF-PEG-PLGA-NPs plus NSC transplantation can significantly improve learning and memory functions of AD rats, replenish basal forebrain cholinergic neurons, and help form hippocampal synapses and AchE-positive fibers. These findings may offer practical support for and insight into treatment of Alzheimer’s disease.
MeSH Keywords: Alzheimer Disease, Neural Cell Adhesion Molecule L1, Neural Stem Cells
Background
Alzheimer’s disease (AD) involves the degeneration of basal forebrain cholinergic neurons. As a kind of pleiotropic potent stem cell, neural stem cells (NSCs) have self-renewal abilities, and can engage in proliferation and induced differentiation. The induced differentiation and transplantation of NSCs is currently a research focus in AD treatment. Available techniques of NSC transplantation include purified cell injection, NSC-derived tissue implantation, and genetic-modified NSCs transplantation [1–5]. Transplanted NSCs have been reported to successfully differentiate into cholinergic neurons, suggesting efficacy in alleviating cognitive deficits.
Nerve growth factor (NGF) is the first and most comprehensively studied neurotrophic factor. In vitro studies showed that NGF can induce various NSCs, embryonic stem (ES) cells, and mesenchymal stem cells (MSCs) from different species to differentiate into specific neurons [6]. In AD treatment using exogenous NSCs, a critical step is to induce the differentiation of NSCs into specific neurons. Wicklum et al. treated human ES-derived NSCs with 50 ng/mL NGF for 28~35 days and found significantly elevated number of ChAT-positive neurons [7]. The expressional profile of basal forebrain-specific transcriptional factor LIM-Homeobox (Lhx-8) was found to be consistent with those in ChAT, suggesting the importance of NGF in simulating the development of basal forebrain cholinergic neurons. The direct application of NGF in AD patients, however, has inherent complications due to its short half-life and tissue impermeability [8]. Therefore, it is critical to develop a novel method for delivering NGF to target cells. This issue may be solved by the encapsulation of NGF with a bio-degradable polymer coating [9–11]. Our previous research has optimized the protocol for preparing NGF-nanoparticles, and obtained NGF-poly (ethylene glycol)-poly (lactic-co-glycolic acid)-nanoparticles (NGF-PE-PLGA-NPs) with satisfactory physical and chemical properties, in addition to bioactivity in vitro. This complex can induce the differentiation of PC12 cells into neural precursors [12]. This study aimed to investigate the treatment efficacy of NGF-PE-PLGA-NPs combined with NSC transplantation in AD rats, by the observation of learning and memory function, basal forebrain cholinergic neurons, hippocampal synaptophysin, and acetylcholine esterase (AchE) fibers. This study may provide a theoretical basis for using NGF-PE-PLGA-NPs combined with NSC transplantation in AD patients.
Material and Methods
Animals
A total of 40 healthy SD rats (body weight 200~250 g) were provided by the Experimental Animal Center, Guangdong Province and were used for AD models and transplantation therapy. Another cohort of 10 pregnant SD rats (E13.5~15.5) was purchased from the same source and was used for primary culture of NSCs.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Separation and culture of NSCs
Pregnant SD rats (E13.5~15.5) were euthanized and embryos were extracted. Whole brains were washed in D-Hanks buffer and separated for septal areas. After gentle rinsing in D-Hanks buffer, brain tissues in the septal area was incised and digested. Isolated neural progenitors were re-suspended in neurobasal culture medium (Gibco, USA) containing 20 ng/mL b-fibroblast growth factor (b-FGF, PeproTech, USA), 20 ng/mL epidermal growth factor (EGF, PeproTech, USA) and 2% B27 additives (Gibco, USA). Cells (1×105/ml) were inoculated into a 25-mL flask and incubated at 37°C in a chamber with 5% CO2. Cells were passed every 4~5 days. P2 generation NSCs were rinsed and induced for differentiation. Cells were examined for their differentiation potency using cyto-immunofluorescent assays [13].
Animal groups and AD model
A total of 40 male SD rats were randomly divided into 4 groups: 1) control group (n=16); 2) AD model group (n=16); 3) NSC transplantation group (n=8); and 4) NGF-PEG-PLGA-NP2 combined with NSC transplantation group (n=8). Groups 2, 3, and 4 were prepared for AD using IgG-saporin by slow intraventricular injection to injure basal forebrain cholinergic neurons [14]. In brief, rats were anaesthetized by intraperitoneal injection of 3% pentobarbital sodium and fixed in a stereotaxic apparatus. The left lateral ventricle was located with reference to the bregma (coordinates: left 0.8 mm, posterior 1.5 mm, depth 5.5 mm). A micro-syringe was used to slowly inject 5 μL 192 IgG-saporin into lateral ventricles. After the injection, head skins were sutured and sterilized, followed by single housing of animals. After 3 weeks for AD modeling, rats were taken from control (n=8) and AD model (n=8) groups to detect learning and memory ability by the Morris water maze test, and the basal forebrain cholinergic neurons and changes of hippocampal synaptophysin of rats were tested by cellular immunohistochemical staining, while the changes of hippocampal AchE fibers were studied by histochemistry.
Intracranial transplantation
After 3 weeks, with successful generation of AD model, rats in the NSC transplantation group received intra-hippocampal and basal forebrain injection of NSCs suspensions (5×105/ml in DMEM/F12, Gibco, USA), while rats in the NGF-PEG-PLGA-NP2 combined with NSC transplantation group received 5×105/ml NSCs suspensions plus NGF-PEG-PLGA-NP2 mixture (50 ng/μL). Control and AD model rats received equal volume (5 μL) of saline in the same brain regions. Intracranial injection adopted the same procedure as those for saporin. Coordinates for basal forebrain were: left 0.6 mm, anterior 0.5 mm, depth 5.5 mm relative to bregma. Coordinates for hippocampus were: left 1.8 mm, posterior 1.8 mm, depth 2.9 mm relative to bregma. After 4-week single housing, rats were tested for treatment efficacy using behavioral assays as described below.
Morris water-maze
The water tank has a 120 cm diameter and 50 cm height, with both inner walls and underwater platform in black color. On the inner wall of the tank, various symbols were marked to divide the water surface into 4 equal quadrants. A hidden platform (12 cm diameter, 30 cm height) was placed 35 cm away from the inner wall and 2 cm beneath the water face. The water temperature was maintained at 22±2°C during the whole experiment. A video camera was placed above the water tank and was connected to the computer for analyzing movement traces. The water-maze test paradigm includes a navigation task and spatial exploration session. During the navigation task, the rat was first placed on the platform for 30-s acclimation. Then the rat was released randomly from 1 quadrant with the head facing the wall. The time for animals searching and climbing onto the platform was recorded. A maximum cut-off time of 120 s was used for those rats who cannot locate the platform. Four repeated sessions were performed for each rat on 4 consecutive days, with the location of the platform being fixed. The average time latency was recorded to evaluate the spatial learning ability. One day after the end of the navigation task, the hidden platform was removed and the animal was released from each quadrant. The movement traces during 120 s were recorded. The time spent in the target quadrant, which is the original place of the hidden platform, was analyzed in addition to the total distance in the target quadrant, and crossing times were also recorded to evaluate the spatial memory of rats.
Immunohistochemical (IHC) staining
After the end of last water-maze spatial exploration session, rats were anesthetized and perfused with saline (200 mL) followed by 4% paraformaldehyde (250 mL) via the aorta cannulation. The whole brain was removed, fixed for 8 hours, embedded at −80°C, and sectioned into 30-μm thickness coronal slices.
P75 and synaptophysin staining
Basal forebrain and hippocampal slices were rinsed in PBS and incubated in 3% hydrogen peroxide. After blocking in 10% bovine serum albumin (BSA, Sigma, USA), rabbit anti-rat P75 antibody (1:5 000, Sigma, USA) was applied to basal forebrain slices, while rabbit anti-rat synaptophysin antibody (1:100, Sigma, USA) was applied to hippocampal slices. After 4°C overnight incubation, brain slices were treated with goat anti-rabbit IgG (Cwbio, China) for 2-h incubation at room temperature. Horseradish peroxidase (HRP) was then applied to tissue slices for 10-min incubation at room temperature. The signal was visualized by DAB chromogenic substrate (Maixin, China). After gentle rinsing, dehydration, and mounting, staining images were captured using a light-field microscope.
AchE staining
Five hippocampal slices in each group (n=8) were rinsed in 0.1M acetic acid buffer 3 times (5 min each). Reagents were incubated with slices for 30 min at 37°C until the occurrence of violet color. After gentle washing, brain slices were stained in 1% ammonium sulfide for 3 min, followed by rinsing in 0.1M sodium nitrate and 0.1% silver nitrate. Slices were then washed, dehydrated and mounted for images capture. The result of the process was undertaken by using double-blind counting system.
Statistical analysis
SPSS 16.0 software package was used to process all collected data, and the t test was used for comparisons between 2 groups. Multiple group comparisons were achieved by single-effect analysis of variance (one-way ANOVA) (Student-Newman-Keuls test). All data are presented as mean ± standard deviation (SD). Statistical significance was defined as p<0.05.
Results
Learning and memory of AD rats after treatment
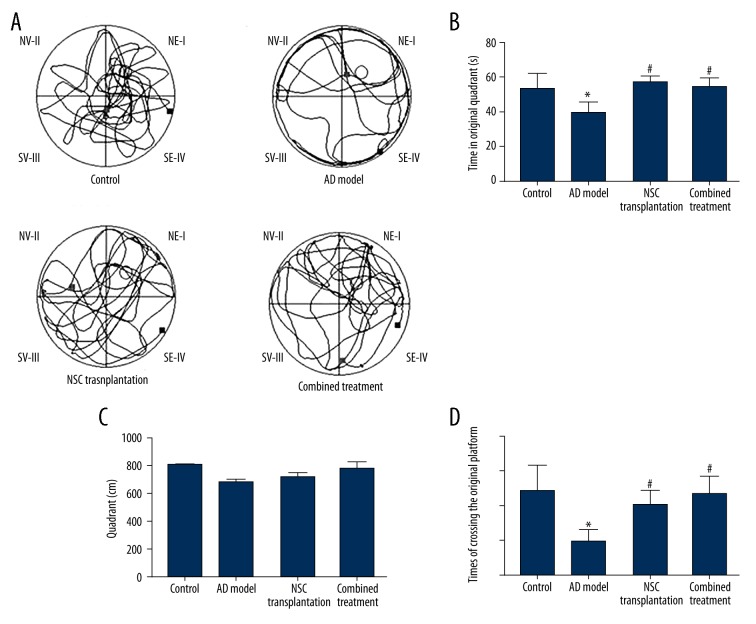
Four weeks after transplantation, the Morris water-maze test was used to describe the learning and memory function of AD rats. During the navigation task, one can observe shortening latency in locating the hidden platform with repeated trials (Figure 1). On the day 4 task, no significant difference was observed between NSC transplantation or combined treatment and control rats, all of which had shorter latencies than AD model rats (p>0.05, Figure 1). During the spatial exploration session, NSC transplantation and combined treatment rats had similar times for crossing the platform location and swimming time in the original quadrant when compared to control rats (Figure 2, p>0.05). These parameters, however, were all significantly larger than in AD model rats (Figure 2B, C, and D, p<0.05). The total distance in the original quadrant in combined treatment rats was also higher than those in the NSC transplantation group (p<0.05, Figure 2C).
Figure 1.

Morris water-maze test performance in the navigation session. (A) Showed representative movement traces from all 4 groups on D4 of the navigation session. The 2 treatment groups and control animals showed similar movement distance, while AD model rats had detouring traces before finding the platform. (B) Time latencies in locating the hidden platform on each day. All animals showed improvements in finding the platform with repeated training.
Figure 2.
Morris water-maze test performances during the spatial exploration task. (A) Representative movement traces from all 4 groups on the day of exploration. The 2 treatment groups and control animals showed similar movement distance, while AD model rats had more dispersed paths, suggesting memory impairments. (B) Time in the original quadrant; (C) Distance in the original quadrant; and (D) Times of crossing the original platform during the exploration task. The 2 treatment groups had similar performance as in controls, while AD model rats had impaired activity in the original quadrant, suggesting memory deficits. In addition, the combined treatment group had longer distances than in the NSC transplantation group. * p<0.05 compared to controls; # p<0.05 compared to AD model rats, & p<0.05 compared to NSC transplantation rats.
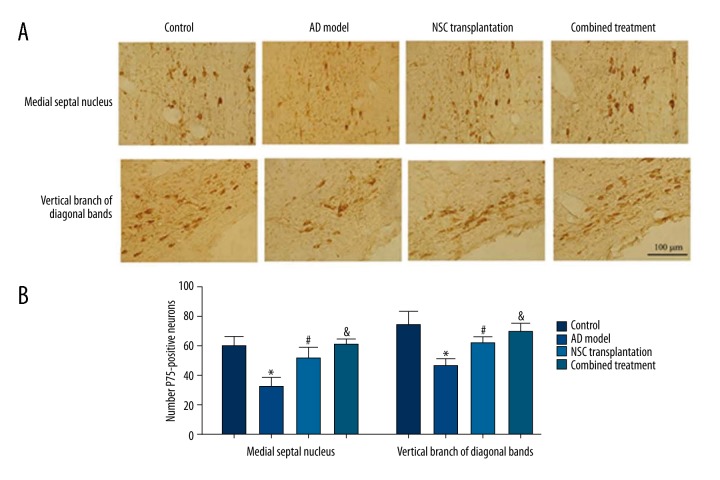
Basal forebrain cholinergic neurons
After the water-maze test, rats were sacrificed for IHC staining of the basal forebrain region. Results (Figure 3A) showed significantly more P75-positive neurons in medial septal nucleus and vertical branches of diagonal bands in the control, NSC transplantation, and combined treatment group, compared to AD model rats, which had irregularly arranged cells with shorter and fewer processes (Figure 3A). Five randomly selected fields were counted for cholinergic neurons and no significant difference was found between NSC transplantation and combined treatment group (p>0.05), both of which had more cholinergic neurons compared to those in the AD model group (Figure 3B, p<0.05). The number of such neurons in combined treatment animals was also higher than that in NSC transplantation animals (Figure 3B).
Figure 3.
P75 staining of basal forebrain neurons. (A) Representative images of neurons in medial septal nucleus (upper panels) and vertical branch of diagonal bands (lower panels). (B) Quantitative data of P75-positve neuron numbers in each high-magnification field (X200) from all groups. Scale bar in (A), 100 μm. * p<0.05 compared to controls; # p<0.05 compared to AD model rats; & p<0.05 compared to NSC transplantation group.
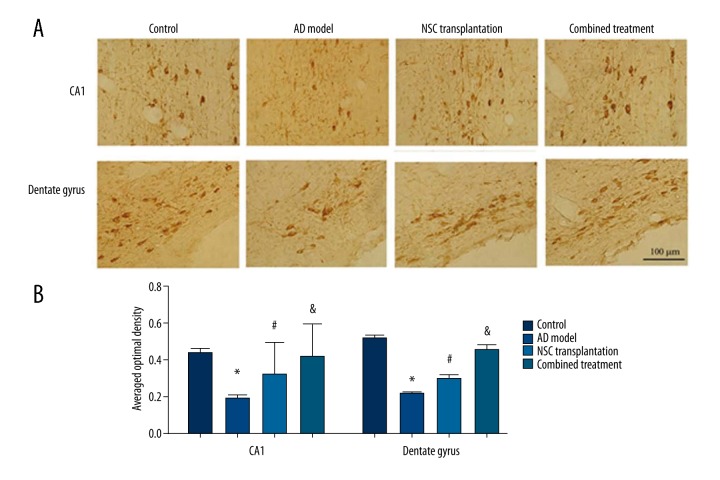
Synaptophysin expression in hippocampal neurons on AD rats
A further IHC staining used synaptophysin to describe the expressional profile of hippocampal neurons. As shown in Figure 4A, control, NSC transplantation, and combined treatment groups had weaker staining compared to the AD model group, in addition to irregular arrangement of neurons. Five randomly selected filed were measured for optical density (OD) values of synaptophysin (Figure 4B). Results showed significantly higher OD values in control, NSC transplantation, and combined treatment groups compared to the AD model group (p<0.05). Further comparison revealed elevated averaged OD values in the combined treatment group compared to NSC transplantation animals (p<0.05).
Figure 4.
Synaptophysin expression in hippocampus. (A) Representative images of neurons in CA1 region (upper panels) and dentate gyrus (lower panels). (B) Averaged optical density of all cells. Scale bar in (A), 100 μm. * p<0.05 compared to controls; # p<0.05 compared to AD model rats; & p<0.05 compared to the NSC transplantation group.
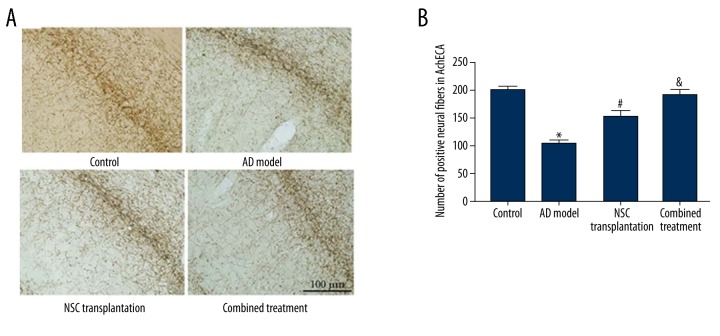
The effects of combined therapy on AchE fibers of AD rats
We further stained AchE in hippocampal neurons. As shown in Figure 5A, control group, NSC transplantation group, and combined treatment group had significantly more AchE-positive fibers compared to AD model animals. A grid measurement slide under the microscope was used to quantify the number of crossings, which is defined as the number of AchE-positive fibers. Our results showed that the NSC transplantation and combined therapy groups had significantly more AchE-positive fibers compared to the AD group (p<0.05, Figure 5B). Moreover, the combined treatment group had no difference when compared to control ones (p>0.05). Further analysis showed that combined treatment rats had even more AchE-positive fibers compared to those in the NSC transplantation group (Figure 5B).
Figure 5.
AchE-positive neural fibers in hippocampal regions. (A) Representative staining images of hippocampal CA1 regions. (B) Number of positive neural fibers. Scale bar in (A), 100 μm. * p<0.05 compared to controls; # p<0.05 compared to AD model rats; & p<0.05 compared to NSC transplantation group.
Discussion
Early symptoms of AD include cognitive failures, in addition to loss of attention and memory. In this study we injected 192IgG-saproin into the lateral ventricle of AD rats and found elongated latencies during the navigation task, and decreased times of crossing the platform and time or distance in the original quadrant in the spatial exploration session of the water-maze test. Studies have reported improved learning ability in AD rats by NSC transplantation [15]. Another study using NSC transplantation in the hippocampal region also showed significant improvements of spatial learning and memory in a water-maze test on AD rats after 2 months [16]. Similar transplantation of NSCs in treating PP and PS1 transgenic AD rats showed similar improvements [17,18]. This study found consistent results that NSC transplantation significantly improved AD rats’ learning and memory function, as did the combined treatment of both NGF-PEG-PLGA-NPs and NSC transplantation, the former of which had more significant improvements. This may be due to the long-term local release of NGF after the introduction of complex. NSCs with over-expression of NGF have been transplanted into the hippocampus of AD rats, which had significantly improved learning and memory and improved survival and differentiation of NSCs [5]. Our study obtained consistent results because NSC transplants combined with de novo NGF production may have better treatment efficacy.
There is a close relationship between basal forebrain cholinergic neurons and learning-related brain cortex via the formation of the septo-hippocampal pathway, thereby participating in various higher brain functions, including spatial memory and cognitive activities. The septo-hippocampal pathway is the major projection pathway from cholinergic neurons in medial septal nucleus and diagonal band to the hippocampus. We previously have generated AD rat models using unilateral fimbria-fornix transection and confirmed the above-mentioned model [19]. As cholinergic neurons in both basal forebrain septal region and diagonal bands simultaneously express ChAT and NGFR, we selected P75 as specific markers for describing cholinergic neurons in basal forebrain. P75 IHC staining results showed that both NSC transplantation and combined treatment had replenished and protective roles on those neurons, with more satisfactory effects in animals receiving combined treatment. Such results further support that the long-term release of NGF can protect basal forebrain cholinergic neurons from immune injuries. It can also facilitate the survival, growth, and differentiation into cholinergic neurons of those transplanted NSCs. All these effects contribute to the protection and replenishment of basal forebrain neurons in AD rats. Another study utilized the over-expression of NGF in transplanted NSCs and obtained similar results [20], suggesting that NGF-PEG-PLGA-NPs may mimic the endogenous NGF release to maintain the phenotype of basal forebrain cholinergic neurons.
NGF can be synthesized and released from forebrain cortex and hippocampus, up-taken by axons of basal forebrain cholinergic neurons, and retrogradely transported to the soma, where it exerts its critical roles in the survival of neurons and axonal growth [21,22]. A previous study reported significantly more ChAT-positive neurons in human embryonic stem cell-derived NSCs after treatment with 50 ng/mL NGF, accompanied by a 1.7-fold increase in ChAT mRNA expression and consistent patterns of basal forebrain specific transcriptional factor Lhx-8 [7]. All these results suggest the critical role of NGF in facilitating basal forebrain cholinergic neuron development.
There was a correlation between loss of hippocampal synapse or decrease of AchE fibers and learning/memory function of AD rats [23–25]. The statistical analysis revealed no significant difference of hippocampal synaptophysin or AchE fiber counts in NSC transplantation or combined therapy animals compared to normal ones, suggesting the effective synaptic genesis and AchE fiber regeneration after either treatment. On the other hand, the combined treatment group had significantly higher averaged OD values and higher AchE fibers, suggesting better regenerative effects. Endogenous NGF has been reported to maintain the number of cholinergic synapse in neocortex. The NGF-PEG-PLGA-NPs utilized in the present study can mimic the continuous release of endogenous NGF, thereby facilitating NSC differentiation into cholinergic neurons and maintaining hippocampal projecting nerve and synapse numbers. In this study, we injected drugs in both septal and basal hippocampal regions, thus simultaneously replenishing basal forebrain cholinergic neurons and elevating hippocampal NGF levels. This can stimulate cholinergic hippocampal projecting axonal terminals to uptake NGF, which can be retrogradely transported to the cell body and further exerts protective roles of basal forebrain cholinergic neurons and facilitates reconstruction of hippocampal synapse. These beneficial effects may elevate the regeneration of AchE fibers and facilitate long-term potentiation (LTP), thus improving learning and memory functions of AD rats.
In summary, our study demonstrated that both NSC transplantation and NGF-PEG-PLGA-NPs combined with NSC significantly improved learning and memory function in 192IgG-saporin-induced AD rats. The combined treatment obtained better efficacy in terms of generating cholinergic neurons in basal forebrain regions, hippocampal synaptic formation, and generating AchE-positive nerve fibers. This can be explained by the chronic release of NGF mimicking the endogenous production in vivo, thereby maintaining the phenotype of basal forebrain neurons. This study, however, did not investigate whether multiple injections had better efficacy compared to single-site injection, or detailed mechanisms underlying such protective functions. These questions warrant further studies.
Conclusions
Our study on treatment of Alzheimer’s disease in a rat model showed that NGF-PEG-PLGA-NPs plus NSC transplantation can significantly improve learning and memory functions of AD rats, by supplementing basal forebrain cholinergic neurons and forming hippocampal synapses and AchE-positive fibers.
Footnotes
Source of support: Research supported by the Youth Fund Project of Guangzhou Medical University (NO: 2011A17), Science Foundation of Education Bureau of Guangzhou City (2012C042), Science and Technology Foundation of Guangzhou City (2014J4100065), and Characteristic Innovation Foundation of Innovative and Strong University Project of Guangdong Province (Education and Science Missive of Guangdong Province 2014 65)
References
- 1.Taupin P. Adult neurogenesis, neural stem cells and Alzheimer’s disease: developments, limitations, problems and promises. Curr Alzheimer Res. 2009;6(6):461–70. doi: 10.2174/156720509790147151. [DOI] [PubMed] [Google Scholar]
- 2.Kim SU, de Vellis J. Stem cell-based cell therapy in neuroligical diseases: a review. J Neurosci Res. 2009;87(10):2183–200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- 3.Deng X, Liang Y, Lu H, et al. Co-transplantation of GDNF-over expressing neural stem cells and fetal dopaminergic neurons mitigates motor symptoms in a rat model of Parkinson’s disease. PLoS One. 2013;8(12):e80880. doi: 10.1371/journal.pone.0080880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer’s disease. Exp Neurol. 2012;237(1):142–46. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HJ, Lim IJ, Park SW, et al. Human neural stem cells genetically modified to expresshuman nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012;21(11):2487–96. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- 6.Kuo YC, Shih KH, Yang JT. Capillary electrophoresis of bone marrow stromal cells with uptake of heparin-functionalized poly (lactide-co-lycolide) nanoparticles during differentiation towards neurons. Electrophoresis. 2010;31(2):315–23. doi: 10.1002/elps.200900336. [DOI] [PubMed] [Google Scholar]
- 7.Wicklund L, Leão RN, Strömberg A-M, et al. α-Amyloid 1-42 oligomers impair function of human embryonic stem cell-derived forebrain cholinergic neurons. PLoSONE. 2010;5(12):e15600. doi: 10.1371/journal.pone.0015600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Gao X, Su L, et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–20. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Cheng KK, Yeung CF, Ho SW, et al. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–36. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Han L, Qin J, et al. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG-PLGA nanoparticles to the brain. Pharm Res. 2013;30(10):2560–72. doi: 10.1007/s11095-013-1055-y. [DOI] [PubMed] [Google Scholar]
- 11.Rocha S. Targeted drug delivery across the blood brain barrier in Alzheimer’s disease. Curr Pharm Des. 2013;19(37):6635–46. doi: 10.2174/13816128113199990613. [DOI] [PubMed] [Google Scholar]
- 12.Bao G, Long D, Chen Y, et al. Pharmacodynamics effect of nerve growth factor-loaded nanoparticles to induce PC12 cells. Chinese Journal of Tissue Engineering Research. 2013;17(16):2891–98. [Google Scholar]
- 13.Juliandi B, Abematsu M, Nakashima K. Epigenetic regulation in neural stem cell differentiation. Develop Growth Differ. 2010;52(6):493–504. doi: 10.1111/j.1440-169X.2010.01175.x. [DOI] [PubMed] [Google Scholar]
- 14.Zeitschel U, Schliebs R, Rossner S, et al. Changes in activity and expression of phosphofructokinase in different rat brain regions after basal forebrain cholinergic lesion. J Neurochem. 2002;83(2):371–80. doi: 10.1046/j.1471-4159.2002.01127.x. [DOI] [PubMed] [Google Scholar]
- 15.Park D, Lee HJ, Joo SS, et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol. 2012;234(2):521–26. doi: 10.1016/j.expneurol.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Chen SQ, Cai Q, Shen YY, et al. Neural stem cell transplantation improves spatial learning and memory via neuronal regeneration in amyloid-β precursor protein/presenilin 1/tau triple transgenic mice. Am J Alzheimers Dis Other Demen. 2014;29(2):142–49. doi: 10.1177/1533317513506776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Wang GM, Wang PJ, et al. Effects of neural stem cells on synaptic proteins and memory in a mouse model of Alzheimer’s disease. J Neurosci Res. 2014;92(2):185–94. doi: 10.1002/jnr.23299. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Wang PJ, Gu GJ, et al. Effects of neural stem cells transplanted into an animal model of Alzheimer disease on Aβ plaques. Zhonghua Yi Xue Za Zhi. 2013;93(45):3636–39. [PubMed] [Google Scholar]
- 19.Gu HG, Long DH, Zhang GP, et al. The effects of neural stem cell transplantation on cholinergic neurons of the basal forebrain and abilities of learning and memory of the rat model of Alzheimer’s disease. Chinese Journal of Clinical Anatomy. 2009;27(1):85–89. [Google Scholar]
- 20.Kim S, Chang KA, Kim JA, et al. The preventive and therapeutic effects of intravenoushuman adipose-derived stem cells in Alzheimer’s disease mice. PLoS One. 2012;7(9):e45757. doi: 10.1371/journal.pone.0045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conner JM, Franks KM, Titterness AK, et al. NGF is essential for hippocampal plasticity and learning. J Neurosci. 2009;29(35):10883–89. doi: 10.1523/JNEUROSCI.2594-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Jiang H, Hu Z. Concentration-dependent effect of nerve growth factor on cell fate determination of neural progenitors. Stem Cells Dev. 2011;20(10):1723–31. doi: 10.1089/scd.2010.0370. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Tang Y, Feng B, et al. Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbeneglucoside in aged rats. Neuroscience. 2007;149(4):739–46. doi: 10.1016/j.neuroscience.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 24.Tannenberg RK, Scott HL, Westphalen RI, et al. The identification and characterization of excitotoxic nerve-endings in Alzheimer disease. Curr Alzheimer Res. 2004;1(1):11–25. doi: 10.2174/1567205043480591. [DOI] [PubMed] [Google Scholar]
- 25.Kirvell SL, Esiri M, Francis PT. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease. J Neurochem. 2006;98(3):939–50. doi: 10.1111/j.1471-4159.2006.03935.x. [DOI] [PubMed] [Google Scholar]