Abstract
This study developed probabilistic models to determine the initiation time of growth of Pseudomonas spp. in combinations with NaNO2 and NaCl concentrations during storage at different temperatures. The combination of 8 NaCl concentrations (0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, and 1.75%) and 9 NaNO2 concentrations (0, 15, 30, 45, 60, 75, 90, 105, and 120 ppm) were prepared in a nutrient broth. The medium was placed in the wells of 96-well microtiter plates, followed by inoculation of a five-strain mixture of Pseudomonas in each well. All microtiter plates were incubated at 4, 7, 10, 12, and 15℃ for 528, 504, 504, 360 and 144 h, respectively. Growth (growth initiation; GI) or no growth was then determined by turbidity every 24 h. These growth response data were analyzed by a logistic regression to produce growth/no growth interface of Pseudomonas spp. and to calculate GI time. NaCl and NaNO2 were significantly effective (p<0.05) on inhibiting Pseudomonas spp. growth when stored at 4-12℃. The developed model showed that at lower NaCl concentration, higher NaNO2 level was required to inhibit Pseudomonas growth at 4-12℃. However, at 15℃, there was no significant effect of NaCl and NaNO2. The model overestimated GI times by 58.2±17.5 to 79.4±11%. These results indicate that the probabilistic models developed in this study should be useful in calculating the GI times of Pseudomonas spp. in combination with NaCl and NaNO2 concentrations, considering the over-prediction percentage.
Keywords: Pseudomonas aeruginosa, Pseudomonas fluorescens, NaCl, NaNO2, processed meats
Introduction
Pseudomonas spp. are psychrotrophic bacteria, and they are the main cause for milk spoilage (Reddy et al., 1969), chicken (Pittard et al., 1982), fish (Miller et al., 1973), and meat especially at chill temperatures (Nychas et al., 2008). In food, they produce special fluorescent green, yellow or bluish compounds (Brown et al., 1958). Moreover, they generate off-odors in the meats by producing prolyitc and lipolyic enzymes (Champagne et al., 1994; Sorhaug and Stepaniak, 1997). Although Pseudomonas spp. causes physicochemical changes, microbiological criteria for the bacteria are not established because they are not pathogenic bacteria.
In processed meat products, NaNO2 plays an important role in developing of cured meat color and flavor, retarding lipid autoxidation, and preventing Clostridium botulinum germination in anaerobic condition (Pegg and Shahidi, 2006). However, NaNO2 has the potential to produce N-nitroso compounds under acidic conditions in stomach (Sugimura, 2000). N-nitroso compounds have been found to cause carcinogenic activity in many animal models (Cassens, 1995). Hence, consumers have low acceptance level for processed meat products. Even though processed meat products formulated with low concentrations of NaNO2 have been developed to avoid potential side effects, but the concern for microbial safety due to lowered concentrations of NaNO2 has now increased (Sinedlar et al., 2007).
NaCl has been used to improve water-holding capacity, fat binding properties, flavor and the inhibition of microbial growth in processed meat products (Guàrdia et al., 2006; Rhee and Zipirin, 2001). However, the high level of NaCl intake is related to hypertension (Tobian et al., 1979), cardiac failure (Frolich, 1999), and stroke (Perry and Beevers, 1992). Thus, consumers are willing to have the low concentration of NaCl in processed meat products, but this low NaCl concentration may not inhibit bacterial growth. Therefore, the minimum concentrations of NaNO2 and NaCl need to be determined to inhibit bacterial growth and also to meet consumers’ requirement. Thus, the interactive responses for these two ingredients should be considered in order to determine the minimum concentrations. The probabilistic model should be appropriate to achieve this goal. Probabilistic model using logistic regression can estimate the probabilities of bacterial growth and interface between growth and no growth of bacteria under various conditions (López-Malo et al., 2000; Tienungoon et al., 2000). This mathematical technique can be applied to estimate the GI time of foodrelated bacteria.
Most studies on the relationship between Pseudomonas spp. and NaNO2 have focused on the antimicrobial effect of NaNO2 on Pseudomonas spp. (Henry and Bessieres, 1984; Nicke et al., 2013), but the combination effect of NaNO2 and NaCl on Pseudomonas spp. in processed meats has not been fully studied yet.
Therefore, the objective of this study was to develop probabilistic models to determine the GI time of Pseudomonas spp. in combinations with NaNO2 and NaCl concentrations.
Materials and Methods
Inoculum preparation
The isolated colonies of Pseudomonas aeruginosa strains (NCCP10338, NCCP10250, and NCCP11229) and Pseudomonas fluorescens strains (KACC10326 and KACC 10323) in Cetrimide agar (Becton Dickinson and Company, USA) were cultured in a nutrient broth (NB; Becton Dickinson and Company) at 35℃ for 24 h. One hundred microliter fractions of the cultures were transferred into 10 mL NB for subculture at 35℃ for 24 h. After incubation, five strains were mixed and centrifuged at 1,912 g and 4℃ for 15 min, and cell pellet was washed twice with phosphate-buffered saline (PBS; pH 7.4; 0.2 g of KH2PO4, 1.5 g of Na2HPO4·7H2O, 8.0 of NaCl, and 0.2 g of KCl in 1 L of distilled water). The cell suspension was diluted with PBS to 5 Log CFU/mL.
Growth/no growth response
The combination of 8 levels (0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, and 1.75%) of NaCl (Samchun pure chemical Co. Ltd., Korea) and 9 levels (0, 15, 30, 45, 60, 75, 90, 105, and 120 ppm) of NaNO2 (Duksan pure chemicals Co. Ltd., Korea) were prepared in NB. Two hundred and twenty five microliters of the medium were placed in wells of 96-well microtiter plates, and 25 μL of inoculum was inoculated into each well. All microtiter plate wells were then incubated at 4, 7, 10, 12, and 15℃ for 528, 504, 504, 360, and 144 h, respectively. Growth (growth initiation; GI) or no growth was then determined by turbidity every 24 h. The combinations that became turbid, were considered growth, while the unturbid combination was considered no growth. The growth response was regarded as ‘1’ and no growth response was assigned as ‘0’ (Koutsoumanis et al., 2004).
Probabilistic model development
The growth response data were analyzed with the SAS version 9.2 logistic regression analysis (SAS Institute Inc., USA) to estimate the growth probabilities of Pseudomonas spp. Significant parameters were selected through a stepwise selection method (p<0.05).
Logit (P) = a0 + a1·NaCl + a2·NaNO2 + a3·Time + a4·NaCl2 + a5·NaNO2 2 + a6·Time2 + a7·NaCl·NaNO2 + a8·NaCl·Time + a9·NaNO2·Time
Where Logit (P) is an abbreviation of ln[P/(1−P)], P is the probability of growth within the range of 0 to 1, ai is the estimates, NaCl is NaCl concentrations, NaNO2 is NaNO2 concentrations, and Time is storage time.
Evaluation of developed model
Observed data for Pseudomonas spp. growth were obtained from commercial frankfurters and bacon. The frankfurters and bacon were cut into 7 g and placed into plastic bags (Food Saver®, Rollpack, Korea). The 0.1 mL portions of the inoculum were inoculated on one side of the sample surface. The inoculated samples were massaged 15 times in order to spread the bacteria and then sealed using a packager (Food Guard®; Rollpack, Korea). The samples were then aerobically stored at 4, 7, 12, and 15℃ for 336, 312, 192, and 120 h, respectively. To quantify bacterial populations, the samples were analyzed at appropriate intervals. The 30 mL of 0.1% buffered peptone water (BPW; Becton Dickinson and Company) was added into the sample bag and homogenized using a pummeler (BagMixer®, Interscience, France) for 60 s. The homogenates were serially diluted with 0.1% BPW, and 0.1 mL of the diluents was surface-plated on Cetrimide agar. The plates were incubated at 35℃ for 24 h, and the typical colonies were manually counted to determine GI time. A growth greater than 1-log considered growth (Koutsomanis et al., 2004; Lee et al., 2013). The observed GI times were then compared to the predicted GI times of Pseudomonas spp. estimated under the developed model.
Results and Discussion
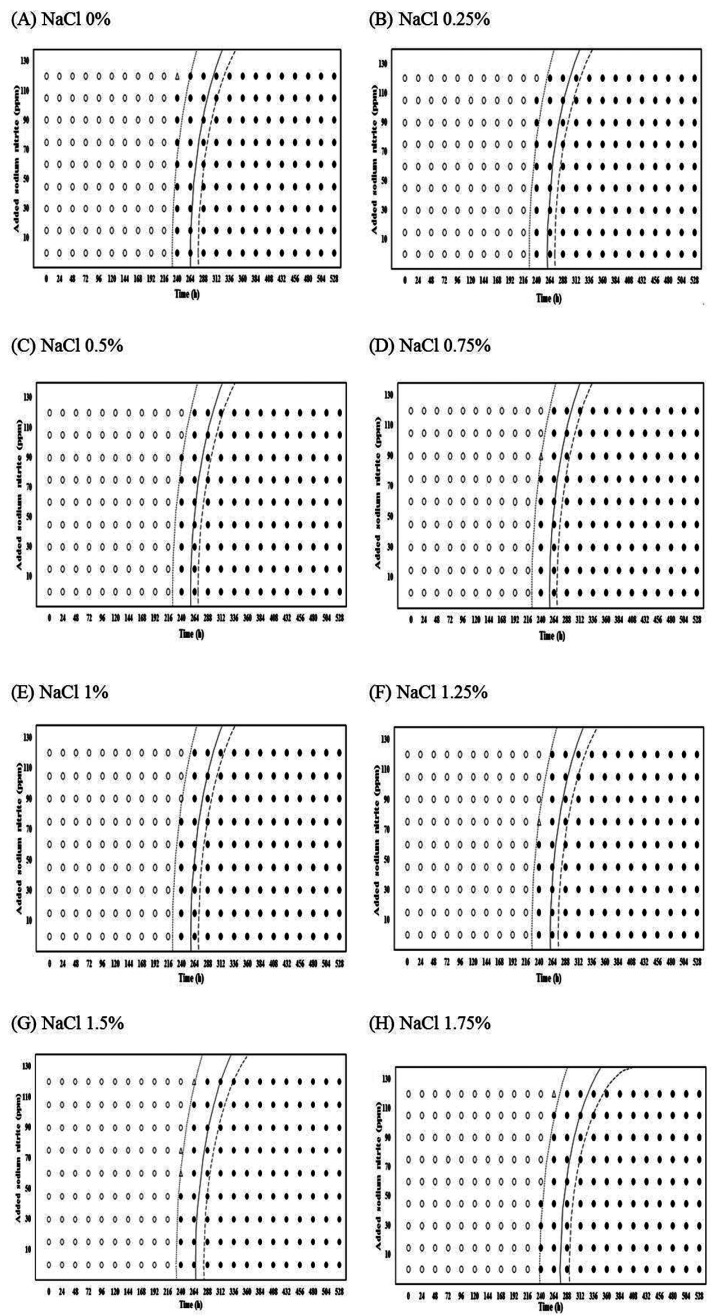
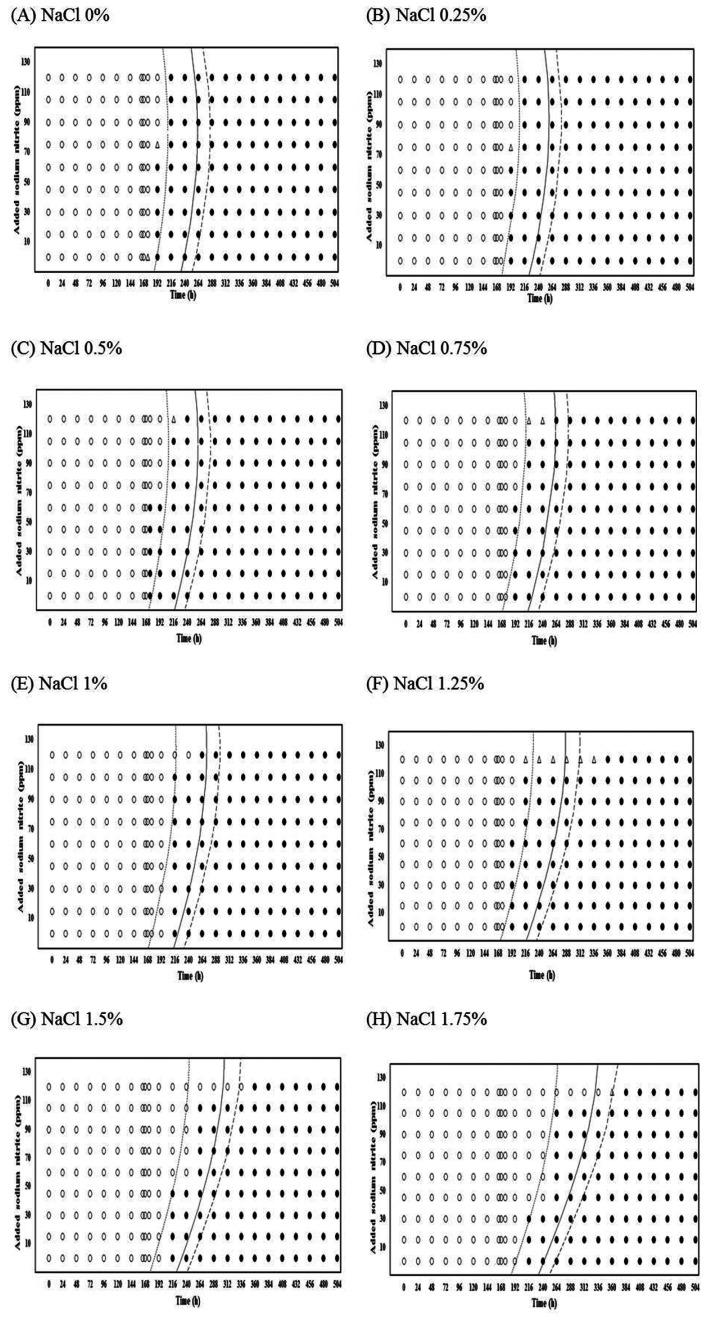
The estimates of coefficients selected from the logistic regression analysis, using an automatic variable selection option with a stepwise selection method, are shown in Table 1. The estimates were then used to produce interfaces between growth and no growth of Pseudomonas spp. at 0.1, 0.5, and 0.9 of probabilities with the combination for NaNO2 and NaCl level for each storage temperature (Figs. 1-2). This result can also be used to determine the GI time of Pseudomonas spp. NaCl, NaNO2, and storage time were generally significant (p<0.05) factors for inhibiting Pseudomonas spp. growth during storage at 4-12℃ (Table 1). However, NaNO2 and NaCl did not have any significant effects on the growth of the bacteria at 15℃. Moreover, a square function for NaCl and NaNO2 was not observed at 12 and 15℃ (Table 1).
Table 1. Estimates of the parameters selected from the logistic regression analysis by a stepwise selection method to calculate the growth probability of Pseudomonas spp. at 4-15℃.
| Variable | 4℃ | 7℃ | 10℃ | 12℃ | 15℃ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SEa | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Intercept | −19.4190 | 0.77 | <0.001 | −9.1128 | 0.43 | <0.001 | −7.0291 | 0.34 | <0.001 | 10.5739 | 0.45 | <0.001 | 25.3165 | 2.24 | <0.001 |
| NaNO2 | 0.00320 | 0.01 | 0.623 | -0.0165 | 0.00 | 0.001 | 0.0180 | 0.01 | 0.001 | 0.00645 | 0.00 | 0.018 | 0.00212 | 0.00 | 0.573 |
| NaCl | 1.4815 | 0.36 | <0.001 | 1.3652 | 0.32 | <0.001 | −0.0798 | 0.15 | 0.587 | −0.9429 | 0.26 | 0.001 | −0.4328 | 0.25 | 0.083 |
| Time | 0.1149 | 0.01 | <0.001 | 0.0592 | 0.00 | <0.001 | 0.0517 | 0.00 | <0.001 | 0.1223 | 0.00 | <0.001 | 0.4673 | 0.04 | <0.001 |
| NaNO22 | −0.00011 | 0.00 | 0.005 | 0.000099 | 0.00 | 0.004 | 0.00024 | 0.00 | <0.001 | - | - | - | - | - | - |
| NaCl2 | −0.8441 | 0.19 | <0.001 | −0.8103 | 0.16 | <0.001 | - | - | - | - | - | - | - | - | - |
| Time2 | −0.00014 | 0.00 | <0.001 | −0.0006 | 0.00 | <0.001 | 0.00006 | 0.00 | <0.001 | 0.00023 | 0.00 | <0.001 | 0.00132 | 0.00 | <0.001 |
| NaNO2×NaCl | −0.00689 | 0.00 | 0.003 | −0.00783 | 0.00 | 0.001 | −0.00535 | 0.00 | 0.009 | 0.00902 | 0.00 | 0.001 | −0.0244 | 0.00 | <0.001 |
| NaNO2×Time | 0.00004 | 0.00 | 0.001 | - | - | - | 0.00004 | 0.00 | 0.003 | - | - | - | - | - | - |
| NaCl×Time | - | - | - | - | - | - | - | - | - | 0.00333 | 0.00 | 0.017 | - | - | - |
a Standard error
Fig. 1. Growth/no-growth interfaces of Pseudomonas spp. at 4℃ with respect to NaNO2 concentration and storage time as a function of NaCl levels at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △.
Fig. 2. Growth/no-growth interfaces of Pseudomonas spp. at 7℃ with respect to NaNO2 concentration and storage time as a function of NaCl levels at growth probabilities of 0.1 (left line), 0.5 (middle line), and 0.9 (right line); no growth: ○, growth: ●, 50% growth: △.
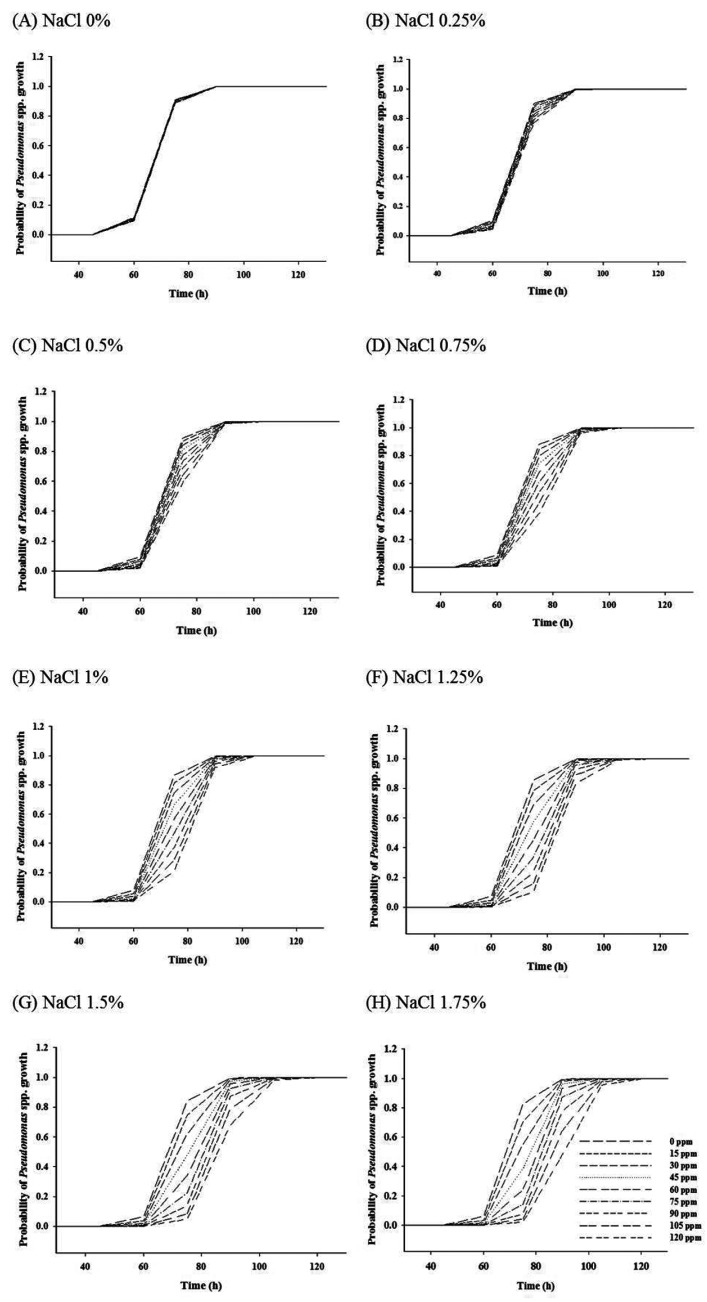
For 4 and 7℃, the antimicrobial effect of NaNO2 on Pseudomonas spp. growth slightly increased to 1% NaCl, but the antimicrobial effect dramatically increased to 1.25% NaCl (Figs. 1 and 2). This result indicates that the obvious antimicrobial effect of NaNO2 to inhibit Pseudomonas spp. growth can be found in high NaCl concentration (>1.25% NaCl). In addition, the combination effect of NaCl and NaNO2 on the inhibition of Pseudomonas spp. growth was also observed at 10, 12, and 15℃ (data not shown). According to these results, it is suggested that NaCl concentration of ready-to-eat meat products should be at a certain level to have the obvious antimicrobial effect of NaNO2 on Pseudomonas spp. growth. This is proven by the result from Fig. 3, showing that the difference of growth probability among NaNO2 concentrations became more obvious as NaCl concentration increased. Similarly, a study by Pelroy et al. (1994) also showed that the concentration-dependent antimicrobial effect of NaNO2 on L. monocytogenes in cold-processed salmon in high NaCl concentrations when stored at 5 and 10℃. Shahamat et al. (1980) and Buchanan et al. (1989) examined the antimicrobial effects of NaNO2 on L. monocytogenes and suggested that the antilisterial effect is improved with NaCl and other factor such as pH, and temperature. Hence, Allaker et al. (2001) suggested that even though the specific inhibitory modes of nitrite are not well clarified, its antimicrobial effectiveness depends on several factors including salt concentration, pH, reductants, iron content, and others.
Fig. 3. Probabilities of Pseudomonas spp. at different levels of NaCl and NaNO2 when stored at 15℃.
The concordance index was used in order to measure the goodness of fit in the developed probabilistic model. The concordance index indicated the degree of agreement between the observations and calculated probabilities. In this study, the concordance index was 94.5-98.1%, while the discordance was 1.9-5.3%, depending on the storage temperature (data not shown).
To evaluate the performance of the developed probabilistic models in this study, the model performance was assessed with the observed data. The predicted GI times calculated by the estimates of the parameters listed in Table 1 at the probability level of 0.5 were then compared to the predicted GI times (Table 2). A growth more than 1-log scale was considered ‘growth’. The developments of the growth/no growth model were compared with the observed growth data. The predicted GI times were generally overestimated when compared to the observed values by 58.2±17.5% to 79.4±11%. This result indicates that Pseudomonas spp. initiated to grow earlier in frankfurter and bacon than in broth media by 58.2-79.4%. Over-prediction percentages were 79.4±11% (4℃), 66.4±14.6% (7℃), 58.2±17.5% (12℃), and 68.2±2.1% (15℃) (Table 2). In our study, the broth media became turbid, when Pseudomonas spp. grew up to approximately 5-6 Log CFU/mL, and at the point, Pseudomonas spp. growth was determined. However, data from ready-to-eat meats were considered as “growth” if a growth greater than 1-log was observed. Because of this reason, there was a difference between the predicted data and the observed data. Therefore, decreased GI time by 58.2 to 79.4 % compared to the predicted GI time from developed probabilistic model should be applied for real processed meat products such as frankfurters and bacon.
Table 2. Comparison of observed and predicted growth initiation (GI) time of Pseudomonas spp. at various concentrations of NaCl and NaNO2.
| Temperature (℃) | Product | Conditions |
Predicted GI time (h) | Observed GI time (h) | Over-prediction percentage (%)b | |
|---|---|---|---|---|---|---|
| NaCl (%) | NaNO2 (ppm) | |||||
| 4 | Frankfurt A | 1.4 | 5a | 230 | 168 | 79.4±11 |
| Frankfurt B | 1.3 | 0 | 228 | 168 | ||
| Bacon | 1.6 | 26 | 234.6 | 216 | ||
| 7 | Frankfurt A | 1.4 | 5 | 186 | 96 | 66.4±14.6 |
| Frankfurt B | 1.3 | 0 | 180 | 120 | ||
| Bacon | 1.6 | 26 | 208 | 168 | ||
| 12 | Frankfurt A | 1.4 | 5 | 121 | 48 | 58.2±17.5 |
| Frankfurt B | 1.3 | 0 | 119 | 72 | ||
| Bacon | 1.6 | 26 | 129 | 96 | ||
| 15 | Frankfurt A | 1.4 | 5 | 69.6 | 48 | 68.2±2.1 |
| Frankfurt B | 1.3 | 0 | 68.8 | 48 | ||
| Bacon | 1.6 | 26 | 73 | 48 | ||
aadded NaNO2 level
bPercentage (%) = (the observed data/the predicted data)×100; values are means±standard errors.
In conclusion, the probabilistic models developed in this study can be used to calculate the GI times of Pseudomonas spp. in frankfurters and bacon as a function of NaCl and NaNO2 concentrations, considering the over-prediction percentage, and thus, the probabilistic models can be useful in controlling bacterial spoilage in the processed meats by Pseudomonas spp.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009237)” Rural Development Administration, Republic of Korea.
References
- 1.Allaker R. P., Silva Mendez L. S., Hardie J. M., Benjamin N. Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol. Immunol. (2001);16:253–256. doi: 10.1034/j.1399-302X.2001.160410.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown A. D., Weidemann J. F. The taxonomy of the psychrophilic meat-spoilage bacteria: a reassessment. J.Appl. Bacteriol. (1958);21:11–17. doi: 10.1111/j.1365-2672.1958.tb00108.x. [DOI] [Google Scholar]
- 3.Buchanan R. L., Stahl H. G., Whiting R. C. Effects and interactions of temperature, pH, atmosphere, sodium chloride, and sodium nitrite on the growth of Listeria monocytogenes. J. Food Prot. (1989);52:844–851. doi: 10.4315/0362-028X-52.12.844. [DOI] [PubMed] [Google Scholar]
- 4.Cassens R. G. Use of sodium nitrite in cured meats today. Food Technol. (1995);49:72–80. [Google Scholar]
- 5.Champagne C. P., Laing R. R., Roy D., Mafu A. A. Psychrotrophs in dairy products: Their effects and their control. Crit. Rev. Food Sci. Nutr. (1994);34:1–30. doi: 10.1080/10408399409527648. [DOI] [PubMed] [Google Scholar]
- 6.Frolich E. D. Risk mechanisms in hypertensive heart disease. Hypertension. (1999);34:782–789. doi: 10.1161/01.HYP.34.4.782. [DOI] [PubMed] [Google Scholar]
- 7.Guàrdia M. D., Guerrero L., Gelabert J., Gou P., Arnau J. Consumer attitude towards sodium reduction in meat products and acceptability of fermented sausages with reduced sodium content. Meat Sci. (2006);73:484–490. doi: 10.1016/j.meatsci.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Henry Y., Bessieres P. Denitrification and nitrite reduction: Pseudomonas aeruginosa nitrite-reductase. Biochimie. (1984);66:259–289. doi: 10.1016/0300-9084(84)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.Koutsoumanis K. P., Kendall P. A., Sofos J. N. Modeling the boundaries of growth of Salmonella Typhimurium in broth as a function of temperature, water activity, and pH. J. Food Prot. (2004);67:53–59. doi: 10.4315/0362-028x-67.1.53. [DOI] [PubMed] [Google Scholar]
- 10.Lee S., Lee H., Lee J. Y., Skandamis P., Park B. Y., Oh M. H., Yoon Y. Mathematical models to predict kinetic behavior and growth probabilities of Listeria monocytogenes on pork skin at constant and dynamic temperatures temperatures. J. Food Prot. (2013);76:1868–1872. doi: 10.4315/0362-028X.JFP-13-197. [DOI] [PubMed] [Google Scholar]
- 11.López-Malo A., Guerrero S., Alzamora S. M. Probabilistic modeling of Saccharomyces cerevisiae inhibition under the effects of water activity, pH, and potassium sorbate concentration. J. Food Prot. (2000);63:91–95. doi: 10.4315/0362-028x-63.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Miller A., III, Scanlan R. A., Lee J. S., Libbey L. M. Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas putrefaciens, Pseudomonas fluorescens, and an Achromobacter species. Appl. Microbiol. (1973);26:18–21. doi: 10.1128/am.26.1.18-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicke T., Schitzer T., Munch K., Adamczack J., Hqufschildt K., Buchmeier S., Kucklick M., Felgentrager U., Jansch L., Riedel K., Layer G. Maturation of the cytochrome cd1 nitrite reductase NirS from Pseudomonasaeruginosa requires transient interactions between the three proteins NirS, NirN and NirF. Biosci. Rep. (2013);33:529–539. doi: 10.1042/BSR20130043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nychas G. J. E., Skandamis P. N., Tassou C. C., Koutsoumanis K. P. Meat spoilage during distribution. Meat Sci. (2008);78:77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Pegg R. B., Shahidi F. Processing of nitrite-free cured meats In: L. Nollet M. L., Todra F., editors. Advanced Technologies for Meat Processing. Taylor & Francis Group, CRC Press, LLC; Boca Raton, FL: (2006). [Google Scholar]
- 16.Pelroy G., Peterson M., Paranjpye R., Almond J., Eklund M. Inhibition of Listeria monocytogenes in coldprocess (smoked) salmon by sodium nitrite and packaging method. J. Food Prot. (1994);57:114–119. doi: 10.4315/0362-028X-57.2.114. [DOI] [PubMed] [Google Scholar]
- 17.Perry I. J., Beevers D. G. Salt intake and stroke:a possible direct effect. J. Hum. Hypertens. (1992);6:23–25. [PubMed] [Google Scholar]
- 18.Pittard B. T., Freeman L. R., Later D. W., Lee M. L. Identification of volatile organic compounds produced by fluorescent pseudomonads on chicken breast muscle. Appl. Environ. Microb. (1982);43:1504–1506. doi: 10.1128/aem.43.6.1504-1506.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy M. C., Bills D. D., Lindsay R. C. Ester production by Pseudomonas fragi. II. Factors influencing ester levels in milk cultures. Appl. Microbiol. (1969);17:779–782. doi: 10.1128/am.17.6.779-782.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee K. S., Zipirin Y. A. Pro-oxidative effects of NaCl in microbial growth-controlled and uncontrolled beef and chicken. Meat Sci. (2001);57:105–112. doi: 10.1016/S0309-1740(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 21.Shahamat M., Seaman A., Woodbine M. Influence of sodium chloride, pH and temperature on the inhibitory activity of sodium nitrite on Listeria monocytogenes. Soc. Appl. Bacteriol. (1980);15:227–237. [Google Scholar]
- 22.Sindelar J. J., Cordray J. C., Olson D. G., Sebranek J. G., Love J. A. Investigating quality attributes and consumer acceptance of uncured, no-nitrate/nitrite-added commercial hams, bacons, and frankfurters. J. Food Sci. (2007);72:551–559. doi: 10.1111/j.1750-3841.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 23.Sorhaug T., tepaniak L. Psychrotrophs and their enzymes in milk and dairy products: quality aspects. Trends Food Sci. Technol. (1997);8:35–40. doi: 10.1016/S0924-2244(97)01006-6. [DOI] [Google Scholar]
- 24.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. (2000);21:387–395. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 25.Tienungoon S., Ratkowsky D. A., McMeekin T. A., Ross T. Growth limits of Listeria monocytogenes as a function of temperature, pH, NaCl, and lactic acid. Appl. Environ. Microb. (2000);66:4979–4987. doi: 10.1128/AEM.66.11.4979-4987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobian L., Lange J., Iwai J., Hiller K., Johnson M. A., Goossens P. Prevention with thiazide of NaCl-induced hypertension in Dahl “S” rats. Evidence for a Na-retaining humoral agent in “S” rats. J. Am. Heart Assoc. (1979);1:316–323. doi: 10.1161/01.hyp.1.3.316. [DOI] [PubMed] [Google Scholar]