Abstract
We aimed to investigate the effectiveness and safety of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) on preventing atrial fibrillation in essential hypertensive patients. Systematic literature retrieval was carried out to obtain randomized controlled trials on the effects of ACEI/ARBs on essential hypertensive patients before December, 2013. Data extraction and quality evaluation were performed. Meta-analysis was performed by Review Manager 5.2.3. Ten high quality studies (11 articles) with a total of 42,892 patients (20,491 patients in the ACEI/ARBs group and 22,401 patients in the β-blocker or the calcium antagonist group) met the inclusion criteria and were included in the meta-analysis. The results showed that ACEI/ARBs reduced the incidence of atrial fibrillation (AF) recurrence compared to calcium antagonists (RR = 0.48; 95%CI, 0.40-0.58; P<0.00001) or β-blockers (RR = 0.39; 95%CI, 0.20-0.74; P = 0.005) in long-term follow-up, respectively. Furthermore, ACEI/ARBs reduced the incidence of congestive heart failure (RR = 0.86; 95%CI, 0.77-0.96; P = 0.007). However, no significant effects were observed on the incidence of new AF, cardiac death, myocardial infarction, and stroke. Our results suggest that ACEI/ARBs may reduce the incidence of AF recurrence and congestive heart failure, with fewer serious adverse effects.
Keywords: angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, hypertension, atrial fibrillation, meta-analysis
Introduction
Hypertension is one of the most prevalent and powerful contributors to cardiovascular diseases, especially stroke, the leading cause of death all over the world[1]. Atrial fibrillation (AF), a common complication of hypertension, is associated with an increased risk of morbidity and mortality[2]. Although medication for hypertention has been well developed, a large number of well-controlled hypertensive patients still suffer from AF. Therefore, finding a more effective way of preventing AF is important for improving the prognosis of patients with essential hypertension.
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are two commonly used antihypertensives, which prevent cardiac structural remodeling and electrical remodeling. AF activates the renin-angiotensin system (RAS), which in turn promotes atrial fibrosis, atrial electrophysiological and structural remodeling, and subsequently facilitates the recurrence of AF[3]. Thus, ACEI/ARBs may theoretically attenuate deleterious cardiac remodeling and reduce the recurrence of AF[4]. However, the results of different studies are controversial. Jibrini et al.[5] found that patients with hypertension benefited from treatment of ACEI/ARBs on reducing the relative risk of AF by 23%, while the other two groups found no benefits[6,7].
To further investigate the efficacy and safety of RAS inhibitors in preventing AF, we performed a meta-analysis of randomized controlled trials. Our results may provide more powerful evidence for clinicians.
Methods
Literature search
Following the methodological guidelines in Cochrane Reviewer's Handbook (Version 5.1.0), 3 databases including PubMed (1966-2013.12), Embase (1974-2013.12) and the Cochrane Library (Issue 12, 2013) were searched with the following words: "Angiotensin-Converting Enzyme Inhibitors" [Mesh/Emtree], Angiotensin Converting Enzyme Inhibitor*, "Angiotensin II Type 1 Receptor Blockers" [Mesh/Emtree], "angiotensin receptor", ACE, ACEI, ACE-I, ACEs, captopril, enalapril, fosinopril, lisinopril, perindopril, ramipril, quinapril, benazepril, cilazapril, trandolapril, spirapril, delapril, moexipril, zofenopril, imidapril, AT 2 receptor block*, AT 2 receptor antagon*, ARB, ARBs, candesartan, eprosartan, irbesartan, losartan, olmesartan, telmisartan, valsartan, Hypertension, "Hypertension"[Mesh/Emtree], "Atrial Fibrillation" [Mesh/Emtree], and "Atrial Flutter" [Mesh/Emtree]. The process did not set limit. In addition, the references of the retrieved literature were also manually checked to filter potentially eligible studies. Last search reached December, 2013.
Criteria for considering trials for this review
Inclusion criteria
Randomized control trials (RCTs) only, detailed information about random sequence generation, allocation concealment and blinding were not considered. All patients entering the studies needed to meet the following criteria with no restriction of age suffering from essential hypertension, which was defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg. The patients should remain in sinus rhythm but with at least one electrocardiogram (ECG)-documented episode of symptomatic or paroxysmal AF during 6 months before randomization. Particularly, we also included trials in which patients suffered essential hypertension without AF. These patients were thus at risk of developing AF. The ACEI/ARB group received ACEIs or ARBs, and the control group received placebo or positive drugs, such as β-blockers and calcium antagonists. Primary endpoints: incidence of new AF or AF recurrence during follow-up. Secondary endpoints: cardiovascular events, including cardiac death, myocardial infarction, cerebral infarction, congestive heart failure, and adverse effects (bradycardia, atrial flutter, intolerable and unproductive cough, peripheral edema and dizziness) during follow-up.
Exclusion criteria
Trials in the following categories were excluded, including non-randomized controlled trials, subjects who were not treated with ACEI or ARB, and trials with no mentioning of AF prevention.
Data extraction
According to previously defined data-extraction form, 2 investigators (D-Z and Z-MW) independently read the titles, abstracts and full texts, using the following steps: (1) examining titles and abstracts to remove obviously irrelevant studies, (2) retrieving the full texts of potentially relevant trials, (3) examining full texts for compliance of studies with eligibility criteria, and (4) making final decisions on study inclusion and proceeding to data collection. Baseline information of patients and detailed methods of study designs were extracted from included studies. Disagreement was solved by discussion with others (D-Z and Z-MW).
Quality evaluation
Evaluation of methodological quality was based on criteria described in Cochrane Reviewer's Handbook 5.1.0. It contains random sequence generation, allocation concealment, blinding, and incomplete outcome data. Each study was subjected to quality assessment by 2 investigators (D-Z and Z-MW). For unclear information for study design or data, investigator contacted the author by E-mail.
Statistical analysis
Differences were expressed as risk ratios (RRs) and odd ratio (ORs) with 95% confidence intervals (95%CIs) for dichotomous outcomes and standardized mean differences (SMDs) with 95%CIs for continuous outcomes. Heterogeneity across studies was tested by using the I2 statistic, which is a quantitative measure of inconsistency across studies. Studies with an I2 statistic of 25%-50% were considered as low heterogeneity, those with an I2 statistic of 50%-75% had moderate heterogeneity, and those with an I2 statistic of >75% had a high degree of heterogeneity[8]. An I2 value >50% indicated significant heterogeneity. A fixed-effects model was used, and a random-effects model was used in the case of significant heterogeneity (I2>50%)[9]. We further conducted sensitivity analyses to explore possible explanations for heterogeneity on the overall pooled estimate and to examine influence of various exclusion criteria on the overall pooled estimate. Differences were considered statistically significant at P<0.05. All statistical analyses were performed by Review Manager Software (Version 5.2.3, Cochrane Collaboration).
Results
Process for included trials
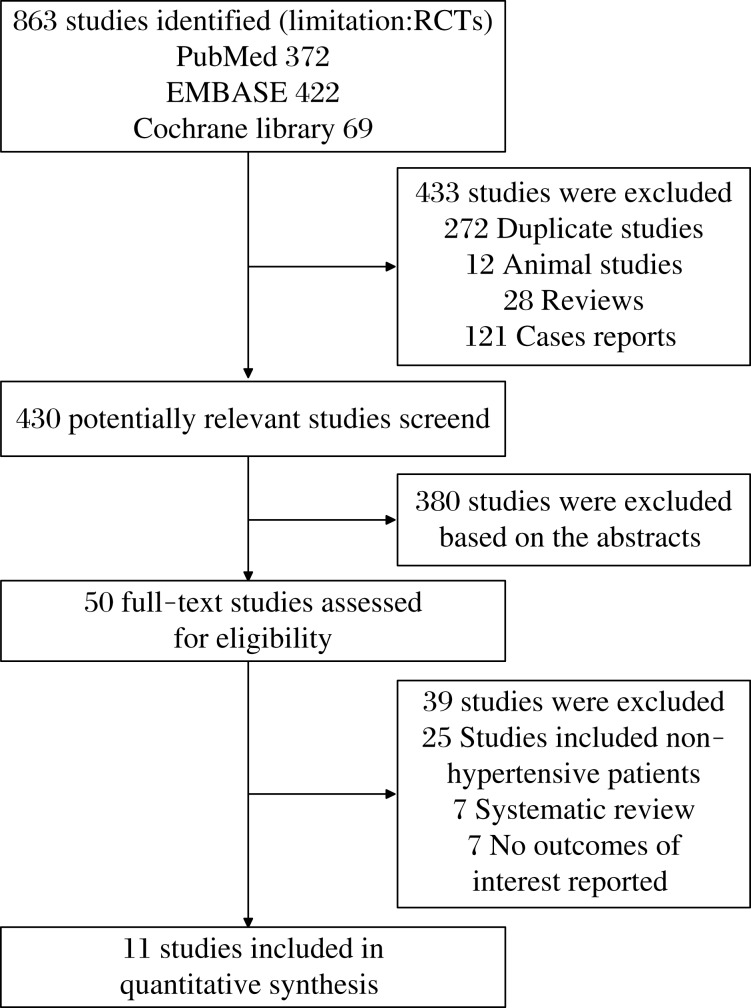
As shown in Fig. 1, a total of 863 potentially relevant studies were identified and screened for retrieval. After reading titles and abstracts, 433 studies were excluded due to duplications, reviews, case reports and animal experiments. Then, 380 studies were excluded after reading the abstracts in more detail. Among the remaining 50 studies, 39 studies were excluded because they included non-hypertensive patients or did not report interesting outcomes. Finally, 11 studies[10-20] were included in our review. As the studies of Julius et al.[18] and Schmieder et al.[20] are the same trial in different time, we included them as one study.
Fig. 1. Flowchart of studies included in the meta-analysis.
Characteristics of included trials and quality evaluation
Main characteristics of the trials included in our meta-analysis are shown in Table 1. There were 20,491 hypertensive patients in the ACEI/ARBs group, and 22,401 patients in the β-blocker or calcium antagonist group. Six studies[11-15,20] included outpatients with mild essential hypertension and at least one ECG-documented episode of symptomatic or paroxysmal AF in the previous 6 months before randomization. Four studies[10,17-19] included hypertensive patients without a history of AF. Seven studies[11-15,18,19] compared the efficiency between ACEI/ARBs and calcium antagonists, and 4 studies[10,16,17,20] compared the efficiency between ACEI/ARBs and β-blockers. Duration of follow-up varied from 3 months to 73.2 months.
Table 1. Characteristics of included trials.
| Study | Drugs and Dose | No. of patients | Female | Mean Age (years) | Definition of Hypertension | Study Population | How AF was Diagnosed | Follow-Up (Months) |
| Hansson 1999 (STOP-2) | ||||||||
| ACEI/ARB | Enalapril 10 mg/day, Lisinopril 10 mg/day | 2205 | 1462 | 76.1 | 60 | |||
| Conventional drugs | Atenolol 50 mg/day, Metoprolol 100 mg/day, Pindolol 5 mg/day | 2213 | 1505 | 76.0 | SBP≥180 mmHg, DBP≥105 mmHg, or both | Patients who had treated or untreated primary hypertension | Yearly ECG and if symptoms | |
| Calcium antagonists | Felodipine 2.5 mg/day, Isradipine 2.5 mg/day | 2196 | 1449 | 75.9 | ||||
| Yamashita 2011 | ||||||||
| ACEI/ARB | Candesartan 8.0±2.7 mg/day | 158 | 49 | 66.0 | SBP≥140 mmHg and/or DBP≥90 mmHg, or requiring any treatment at enrolment | Patients with a history of paroxysmal AF within 6 months and hypertension or requiring any hypertension treatment at enrolment | A transtelephonic monitoring device which was requested to transmit ECG records and any arrhythmia-related symptoms every day to a central service | 12 |
| Calcium antagonists | Amlodipine 4.3±1.7 mg/day | 160 | 50 | 65.1 | ||||
| Hansson 1999 (CAPPP) | ||||||||
| ACEI/ARB | Captopril 50 mg/day | 5492 | 2476 | 52.4 | 73.2 | |||
| Conventional drugs | Atenolol and Metoprolol 50-100 mg/day | 5493 | 2635 | 52.7 | DBP≥100 mmHg | Patients who had treated or untreated primary hypertension | ECG during follow-up visits | |
| Fogari 2006 | ||||||||
| ACEI/ARB | Losartan 50 mg/day | 111 | 48 | 63.5 | SBP>140 mmHg and/or 90 mmHg<DBP<100 mmHg | Outpatients with mild essential hypertension and at least 2 ECG-documented episodes of symptomatic AF in the previous 6 months and in treatment with a maintenance dose of amiodarone for at least 8 weeks | To identify a symptomatic AF episodes, 24-hour ambulatory ECG monitoring was performed every 4 weeks by using a Syneflash Holter recorder by ElaMedical Inc. | 3 |
| Calcium antagonists | Amlodipine 5 mg/day | 111 | 50 | 63.2 | ||||
| Wachtell 2005 | ||||||||
| ACEI/ARB | Losartan 50 mg/day | 4298 | 2125 | 57.6 | 160 mmHg<SBP< 200 mmHg and/or 95 mmHg<DBP<115 mmHg | Patients with previously treated or untreated hypertension and ECG signs of left ventricular hypertrophy, without a history of AF | New-onset AF was identified from annual in-study ECGs that underwent Minnesota coding for AF at a single ECG core center | 57.6 |
| Conventional drugs | Atenolol 50 mg/day | 4182 | 2084 | 57.6 | ||||
| Fogari 2012 | ||||||||
| ACEI/ARB | Telmisartan 80-160 mg/day | 188 | 101 | 68.5 | 140 mmHg<SBP< 160 mmHg and/or 90 mmHg<DBP<100 mmHg | Outpatients with stage I hypertension, in sinus rhythm, but with ≥2 ECG-documented episodes of symptomatic AF in the previous 6 months, each lasting >60 minutes but <7days and terminating spontaneously | 24-hour ambulatory ECG monitoring was performed using a Syneflash Holter recorder (ELA Medical, Paris, France) to detect asymptomatic AF episodes | 3 |
| Calcium antagonists | Amlodipine 5-10 mg/day | 190 | 103 | 67.9 | ||||
| Galzerano 2012 | ||||||||
| ACEI/ARB | Telmisartan 80 mg/day | 70 | 21 | 56.2 | 140 mmHg<SBP< 160 mmHg and/or 90 mmHg<DBP<100 mmHg | Mild hypertensive outpatients in sinus rhythm with 1 or more ECG-documented episodes of AF in the previous 6 months | When to palpitations and new symptoms, patients were asked to report any episodes of symptomatic AF and to have ECG evaluations performed as soon as possible | 12 |
| Conventional drugs | Carvedilol 25 mg/day | 62 | 19 | 55.4 | ||||
| Du 2013 | ||||||||
| ACEI/ARB | Telmisartan 80 mg/day | 74 | 31 | 61.5 | 140 mmHg<SBP< 180 mmHg and/or 90 mmHg<DBP<110 mmHg | All hypertensive patients with paroxysmal AF | The development of persistent AF implied AF had continued for>7 days but was terminated after pharmacological and electric conversion | 24 |
| Calcium antagonists | Nifedipine 30 mg/day | 75 | 26 | 62.0 | ||||
| Julius 2004 Schmieder 2008 | ||||||||
| ACEI/ARB | Valsartan 80-160 mg/day | 7649 | 3240 | 67.2 | 160 mmHg<SBP< 210 mmHg and/or DBP<115 mmHg | Patients with treated or untreated hypertension | ECG during follow-up visits | 50.4 |
| Calcium antagonists | Amlodipine 5-10mg/day | 7596 | 3228 | 67.3 | ||||
| Fogari 2008 | ||||||||
| ARB | Valsartan 160 mg/day | 122 | 65 | 66.0 | 140 mmHg<SBP< 160 mmHg and/or 90 mmHg<DBP<100 mmHg | Outpatients with mild essential hypertension, in sinus rhythm but with at least 2 ECG documented episodes of symptomatic AF in the previous 6 months, and without any treatment | 24-h ambulatory ECG monitoring was performed every 4 weeks using a Syneflash Holter recorder (ElaMedical, Paris, France) to identify asymptomatic AF episodes | 3 |
| ACEI | Ramipril 5 mg/day | 124 | 67 | 64.0 | ||||
| Calcium antagonists | Amlodipine 5 mg/day | 123 | 68 | 65.0 | ||||
Table 2 shows that the quality of studies in this meta-analysis was good. Five studies[11,12,17-19] reported random sequence generation, which was from computerized randomization, and the rest were randomized controlled trials. Allocation concealment in detail was only reported in one study[18]. Six studies[10-12,16,17,19] had open-label design. Six studies[10,11,13-16,18] used the double-blind method, 1 study the single-blind method, and 2 studies[17,19] applied masked-endpoint for evaluation. A total of 428 patients were lost to follow-up in 9 studies[11-17,19,20].
Table 2. Quality evaluation of the studies in this meta-analysis.
| Study | Random sequence generation | Allocation concealment | Blinding | Completeness of data | Intention to treat analysis |
| Hansson 1999(STOP-2) | Computerized randomization | Open-label | Masked-endpoint | No patient was lost | Yes |
| Yamashita 2011 | Computerized randomization | Open-label | Double-blind | 8 patients withdrew | Yes |
| Hansson 1999(CAPPP) | Computerized randomization | Open-label | Masked-endpoint | 27 patients were lost | Yes |
| Fogari 2006 | Unclear | Unclear | Double-blind | 8 patients withdrew | Yes |
| Wachtell 2005 | Unclear | Open-label | Double-blind | Unclear | Yes |
| Fogari 2012 | Unclear | Unclear | Double-blind | 27 patients were lost | Yes |
| Galzerano 2012 | Unclear | Open-label | Single-blind | 27 patients were lost | No |
| Du 2013 | Computerized randomization | Open-label | Unclear | No patient was lost | No |
| Julius 2004 Schmieder 2008 |
Computerized randomization | List was prepared centrally by the sponsor with appropriate blocks | Double-blind | 251 patients were lost | Yes |
| Fogari 2008 | Unclear | Unclear | Double-blind | 80 patients were lost | Yes |
Meta-analysis results
Primary endpoints
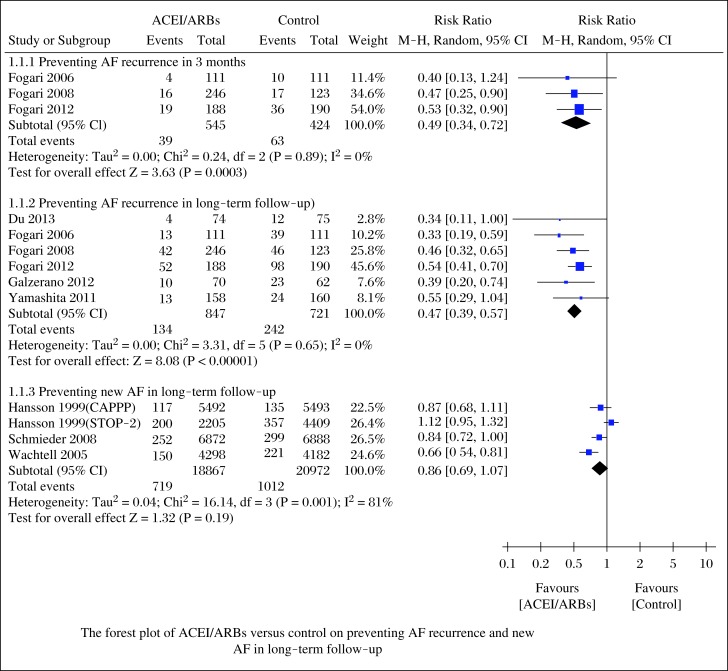
As shown in Fig. 2, ACEI/ARBs decreased the incidence of AF recurrence at 3 months (RR = 0.49; 95%CI, 0.34–0.72; P = 0.0003) and long-term follow-up (RR = 0.47; 95%CI, 0.39–0.47; P<0.00001), and the tests for heterogeneity in those subgroups were I2 = 0%, P = 0.89 and I2 = 0%, P = 0.65, respectively. However, ACEI/ARBs did not change the incidence of new AF in long-term follow-up (RR = 0.86; 95%CI, 0.69-1.07; P = 0.19), with a high heterogeneity (I2 = 81%, P = 0.001).
Fig. 2. Forest plot of ACEI/ARBs versus control on preventing AF recurrence and new AF in long-term follow-up.
We further performed sensitivity analyses to explore the stability of our results. After removal of 2 studies[12,16] with modest sample sizes (n≤150), we still found that ACEI/ARBs decreased the incidence of AF recurrence in long-term follow-up (RR = 0.49; 95%CI, 0.40-0.59; P<0.00001) with low heterogeneity (I2 = 0%, P = 0.48). Changing effect size did not influence the pooled results substantially: AF recurrence at 3 months (OR = 0.45; 95%CI, 0.29-0.69; P = 0.0003), AF recurrence in long-term follow-up (OR = 0.34; 95%CI, 0.27-0.44; P<0.00001) and new AF in long-term follow-up (OR = 0.86; 95%CI, 0.68-1.08; P = 0.19), and the heterogeneity was (I2 = 0%, P = 0.93), (I2 = 0%, P = 0.80), and (I2 = 81%, P = 0.001), respectively.
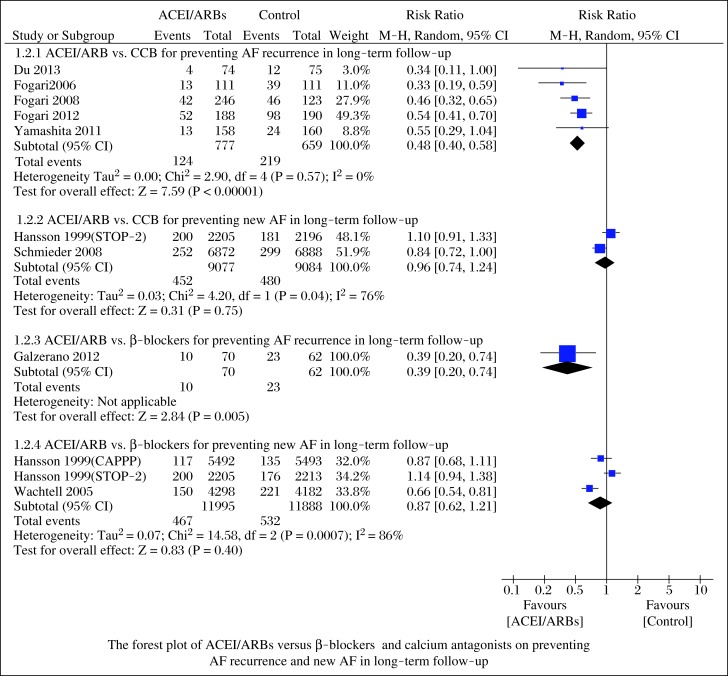
When compared to the different control groups, the incidence of AF recurrence was lower in patients receiving ACEI/ARBs than in those receiving calcium antagonists in long-term follow-up (RR = 0.48; 95%CI, 0.40-0.58; P<0.00001; Fig. 3) with low heterogeneity (I2 = 0%, P = 0.57). However, ACEI/ARBs did not reduce new AF in long-term follow-up (RR = 0.96; 95%CI, 0.74-1.24; P = 0.75; Fig. 3) with high heterogeneity (I2 = 76%, P = 0.04). Similarly, ACEI/ARBs reduced the incidence of AF recurrence (RR = 0.39; 95%CI, 0.20-0.74; P = 0.005; Fig. 3), but not new AF (RR = 0.87; 95%CI, 0.62-1.21; P = 0.40; Fig. 3) with high heterogeneity (I2 = 86%, P = 0.0007), when compared to β-blockers.
Fig. 3. Forest plot of ACEI/ARBs versus β-blockers and calcium antagonists on preventing AF recurrence and new AF in long-term follow-up.
Median time of AF recurrence was reported only in 4 studies[12-15]. Du et al.[12] reported that median time of AF recurrence had no significant differences between the nifedipine group and the telmisartan group. However, the other 3 studies reported that ARBs postponed AF recurrence. Therefore, preliminary comparison of these data without statistics did not reveal tendency that ACEI/ARBs could postpone AF recurrence.
Secondary endpoints
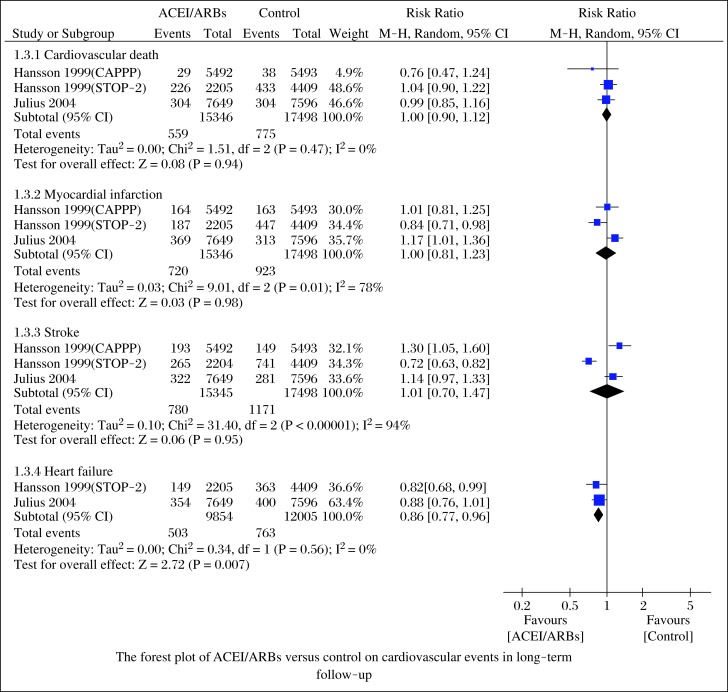
We also compared the cardiovascular events in the follow-up, which included cardiac death, myocardial infarction, stroke, and congestive heart failure. Cardiovascular events were reported in three large-scale studies[17-19]. When compared to β-blockers and calcium antagonists, ACEI/ARBs did not reduce cardiac death (RR = 1.00; 95%CI, 0.90-1.12; P = 0.94), myocardial infarction (RR = 1.00; 95%CI, 0.81-1.23; P = 0.98), and stroke (RR = 1.01; 95%CI, 0.70-1.47; P = 0.94; Fig. 4). Heterogeneities were (I2 = 0%, P = 0.47), (I2 = 78%, P = 0.001), and (I2 = 94%, P<0.00001), respectively. ACEI/ARBs reduced the incidence of congestive heart failure (RR = 0.86; 95%CI, 0.77-0.96; P = 0.007; Fig. 4), with low heterogeneity (I2 = 0%, P = 0.56).
Fig. 4. Forest plot of ACEI/ARBs versus control on cardiovascular events in long-term follow-up.
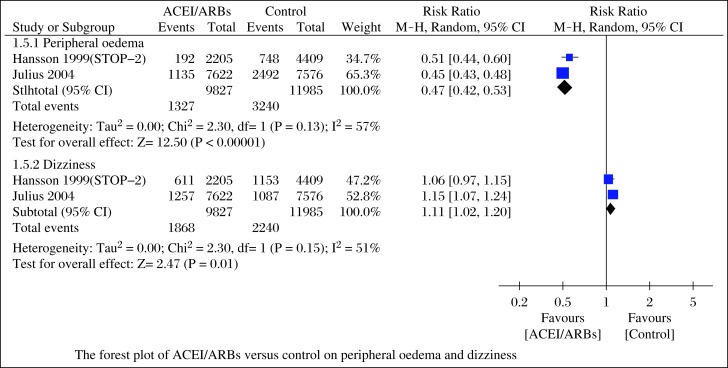
Data of adverse effects (bradycardia, atrial flutter, intolerable and unproductive cough, peripheral edema and dizziness) during follow-up were reported in 6 studies[12-15,17,18]. Four studies[12-15] reported adverse effects requiring discontinuation due to bradycardia, atrial flutter, intolerable and unproductive cough, and the aggregated results of these studies suggested that ACEI/ARBs could decrease these adverse effects (RR = 0.44; 95%CI, 0.21-0.89; P = 0.02; Fig. 5) with low heterogeneity (I2 = 0%, P = 0.63). In the studies of Hansson et al. (STOP-2)[17] and Julius et al.[18], they compared the incidence of peripheral edema and dizziness, the pooled outcomes showed that ACEI/ARBs reduced peripheral edema (RR = 0.47; 95%CI, 0.42-0.53; P<0.00001) with high heterogeneity (I2 = 57%, P = 0.13), but increased the risk of dizziness (RR = 1.11; 95%CI, 1.02-1.20; P = 0.01; Fig. 6) with high heterogeneity (I2 = 51%, P = 0.15).
Fig. 5. Forest plot of ACEI/ARBs versus control on adverse effects requiring discontinuation.
Fig. 6. Forest plot of ACEI/ARBs versus control on peripheral oedema and dizziness.
Publication bias
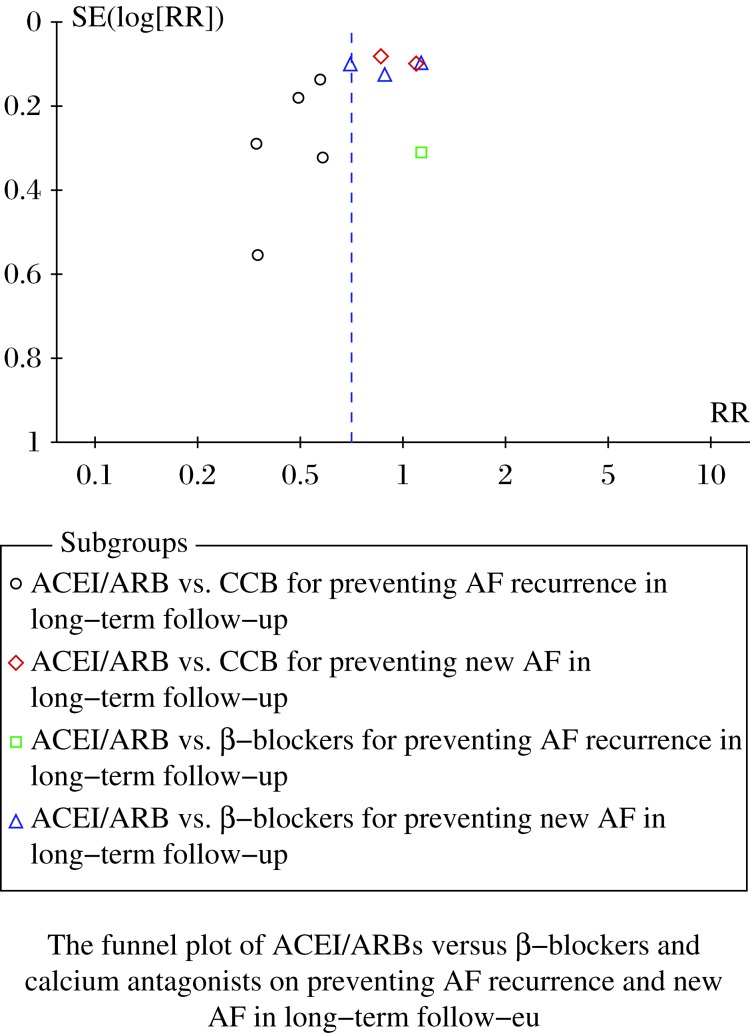
Publication bias was assessed, even though only 10 studies were included in this analysis. The results illustrated that the probability of publication bias was possible due to asymmetry (Fig. 7).
Fig. 7. Funnel plot of ACEI/ARBs versus β-blockers and calcium antagonists on preventing AF recurrence and new AF in long-term follow-up.
Discussion
Regarding the effects of ACEI/ARBs on hypertensive patients and AF, the results of individual trials are conflicting. Here, we performed a meta-analysis of available data to define the conditions and circumstances in which ACEI/ARBs may be a promising preventive therapy. The pooled results from 10 RCTs using a random effects model suggested that ACEI/ARBs decreased AF recurrence rate by 7% in 3 months, and 17% in long-term follow-up. In subgroups, ACEI/ARBs reduced more AF recurrence rate by 17% than calcium antagonists and 23% than β-blockers. However, ACEI/ARBs did not decrease the rate of new AF. Compared to the control group, ACEI/ARBs did not reduce cardiac death, myocardial infarction or stroke, excepting congestive heart failure. ACEI/ARBs cut down adverse effects, but may increase dizziness.
Our meta-analysis indicated that ACEI/ARBs could decrease the incidence of AF recurrence at 3 months and in long-term follow-up. However, ACEI/ARBs could not reduce the incidence of new AF in long-term follow-up. The heterogeneities were great in subgroups analyses. We found that heterogeneities come from Hansson et al. (STOP-2)[17]. The blood pressures of patients in this study were higher than those in other studies, with SBP≥180 mmHg and/or DBP≥105 mmHg. Diuretics, amiloride and fixed-ratio hydrochlorothiazide were used in the β-blocker group, which may also contribute to heterogeneity. When compared to the different control groups, the incidence of AF recurrence was lower in patients receiving ACEI/ARBs than in those receiving calcium antagonists or β-blockers in long-term follow-up; however, ACEI/ARBs did not reduce new AF in long-term follow-up when compared to calcium antagonists and β-blockers. Median time to AF recurrence was described without pooled data, which did not reveal tendency that ACEI/ARBs could postpone AF recurrence.
Cardiovascular events were assessed, and the results showed that ACEI/ARBs could reduce the incidence of congestive heart failure, but not cardiac death, myocardial infarction, or stroke, comparing to β-blockers and calcium antagonists. Although ACEI/ARBs are generally regarded as safe and well tolerated drugs in most populations, it should be careful that ACEIs may induce non-productive cough and peripheral edema.
Our results are partly similar to the last 2 meta-analyses[21,22]. Huang et al.[21] reported that ACEIs/ARBs were effective for new AF and AF recurrence. Han et al.[22] also demonstrated that ACEI/ARBs prevented AF recurrence. In our present analysis, considering the close relation between hypertension and AF, we specifically included hypertensive patients for review. We found that ACEI/ARBs did not prevent new AF in hypertensive patients. The results are different from Huang et al.[21], which may result from different included patients. In their study, patients were included as follows: myocardial infarction, coronary heart disease, hypertension and chronic heart failure, without any subgroup analysis. Furthermore, our study also investigated the role of ACEI/ARBs in cardiovascular events and adverse effects, which may provide more powerful evidence for clinicians.
Our meta-analysis has several potential limitations that should be taken into account. First, even though we analyzed calcium antagonists and β-blockers in subgroups, their characteristics are different, and the effect may be unequal. In the randomized controlled trials, the characteristics of hypertensive patients were not based on a unified level, which varies in the range of SBP≥140 mmHg and DBP≥90 mmHg. These factors may have potential impact on our results. Second, follow-up varies from 3 months to 73.2 months. Finally, as many ACEI/ARBs drugs, involving enalapril, lisinopril, ramipril, captopril, candesartan, losartan, valsartan and telmisartan, were used in our included studies, and we are not sure to assess the impact of ACEI/ARBs basing on meaningful endpoints.
In conclusion, our results suggest that ACEI/ARBs may reduce the incidence of AF recurrence, heart failure, with less serious adverse effects. Further unified protocol and well-designed randomized controlled trials on this topic are still needed.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81270255 to L-SW).
References
- [1].Kotwani P, Kwarisiima D, Clark TD, et al. Epidemiology and awareness of hypertension in a rural Ugandan community: a cross-sectional study[J] BMC Public Health. 2013;13(1):1151. doi: 10.1186/1471-2458-13-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study[J] Am J Med. 1995;98(5):476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- [3].New approaches to antiarrhythmic therapy, part II, emerging therapeutic applications of the cell biology of cardiac arrhythmias[J] Circulation. 2001;104(24):2990–2994. doi: 10.1161/hc4901.099493. [DOI] [PubMed] [Google Scholar]
- [4].Makkar KM, Sanoski CA, Spinler SA. Role of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and aldosterone antagonists in the prevention of atrial and ventricular arrhythmias[J] Pharmacotherapy. 2009;29(1):31–48. doi: 10.1592/phco.29.1.31. [DOI] [PubMed] [Google Scholar]
- [5].Jibrini MB, Molnar J, Arora RR. Prevention of atrial fibrillation by way of abrogation of the renin-angiotensin system: a systematic review and meta-analysis[J] Am J Therb. 2008;15(1):36–43. doi: 10.1097/MJT.0b013e31804beb59. [DOI] [PubMed] [Google Scholar]
- [6].Anand K, Mooss AN, Hee TT, et al. Meta-analysis: inhibition of renin-angiotensin system prevents new-onset atrial fibrillation[J] Am Heart J. 2006;152(2):217–222. doi: 10.1016/j.ahj.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [7].Healey JS, Baranchuk A, Crystal E, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis[J] J Am Coll Cardiol. 2005;45(11):1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- [8].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses[J] BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Armitage P, Berry G, Matthews JNS. Analysing Means and Proportions. Statistical Methods in Medical Research[J] Blackwell Science Ltd. 2008:83–146. [Google Scholar]
- [10].Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study[J] J Am Coll Cardiol. 2005;45(5):712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- [11].Yamashita T, Inoue H, Okumura K, et al. Randomized trial of angiotensin II-receptor blocker vs. dihydropiridine calcium channel blocker in the treatment of paroxysmal atrial fibrillation with hypertension (J-RHYTHM II study)[J] Europace. 2011;13(4):473–479. doi: 10.1093/europace/euq439. [DOI] [PubMed] [Google Scholar]
- [12].Du H, Fan J, Ling Z, et al. Effect of nifedipine versus telmisartan on prevention of atrial fibrillation recurrence in hypertensive patients[J] Hypertension. 2013;61(4):786–792. doi: 10.1161/HYPERTENSIONAHA.111.202309. [DOI] [PubMed] [Google Scholar]
- [13].Fogari R, Mugellini A, Destro M, et al. Losartan and prevention of atrial fibrillation recurrence in hypertensive patients[J] J Cardiovasc Pharmacol. 2006;47(1):46–50. doi: 10.1097/01.fjc.0000193808.99773.28. [DOI] [PubMed] [Google Scholar]
- [14].Fogari R, Derosa G, Ferrari I, et al. Effect of valsartan and ramipril on atrial fibrillation recurrence and P-wave dispersion in hypertensive patients with recurrent symptomatic lone atrial fibrillation[J] Am J Hypertens. 2008;21(9):1034–1039. doi: 10.1038/ajh.2008.217. [DOI] [PubMed] [Google Scholar]
- [15].Fogari R, Zoppi A, Maffioli P, et al. Effect of telmisartan on paroxysmal atrial fibrillation recurrence in hypertensive patients with normal or increased left atrial size[J] Clin Cardiol. 2012;35(6):359–364. doi: 10.1002/clc.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Galzerano D, Di Michele S, Paolisso G, et al. A multicentre, randomized study of telmisartan versus carvedilol for prevention of atrial fibrillation recurrence in hypertensive patients[J] J Renin Angiotensin Aldosterone Syst. 2012;13(4):496–503. doi: 10.1177/1470320312443909. [DOI] [PubMed] [Google Scholar]
- [17].Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study[J] Lancet. 1999;354(9192):1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- [18].Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial[J] Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- [19].Hansson L, Lindholm LH, Niskanen L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial[J] Lancet. 1999;353(9153):611–616. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- [20].Schmieder RE, Kjeldsen SE, Julius S, et al. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial[J] J Hypertens. 2008;26(3):403–411. doi: 10.1097/HJH.0b013e3282f35c67. [DOI] [PubMed] [Google Scholar]
- [21].Huang G, Xu JB, Liu JX, et al. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers decrease the incidence of atrial fibrillation: a meta‐analysis[J] Eur J Clin Invest. 2011;41(7):719–733. doi: 10.1111/j.1365-2362.2010.02460.x. [DOI] [PubMed] [Google Scholar]
- [22].Han M, Zhang Y, Sun S, et al. Renin-Angiotensin System Inhibitors Prevent the Recurrence of Atrial Fibrillation: A Meta-Analysis of Randomized Controlled Trials[J] J Cardiovasc Pharmacol. 2013;62(4):405–415. doi: 10.1097/FJC.0b013e3182a094a1. [DOI] [PubMed] [Google Scholar]