Abstract
Background
Most mitochondria-mediated apoptosis has some relevance to the cell cycle, but there is still a lack of investigations about U251 cell cycle in human brain glioma cells. In this study, we aimed to clarify the correlation of mitochondria-mediated apoptosis with the U251 cell cycle and its influence on apoptosis, through observing the impact of mitochondria-mediated apoptosis in U251cell specificity cycle arrest and Caspase activation.
Material/Methods
AnnexinV/PI and API were used to label the brain glioma cells for flow cytometry analysis of U251 cell apoptosis and cell cycle. RT-PCR and Western blot were performed to detect Caspase-3 and Caspase-9 activation.
Results
Peripheral blood in stationary phase is not sensitive to apoptosis induction, but U251 cells have obvious apoptosis. Mitochondria-mediated apoptosis mainly occurs in the G1 phase of the cell cycle. Caspase-3 and Caspase-9 mRNAs and proteins expression increased significantly after the cells were treated by mitochondrial apoptosis-related gene Bax induction.
Conclusions
Mitochondria-mediated apoptosis is related to the U251 cell cycle with specific G1 stage arrest. Caspase activation occurs in the process of cell apoptosis.
MeSH Keywords: Aspartate Aminotransferase, Mitochondrial; Caspase Inhibitors; Mitochondrial ADP, ATP Translocases
Background
Cell survival and apoptosis are associated with the cell cycle, which is highly conservative. Apoptosis does not threaten the cells’ normal needs and can unnecessary regulate cells physiologically. There are potential correlations between apoptosis and cell proliferation. Many cancer genes closely associated with proliferation are closely related to cell apoptosis. With the further understanding of cell apoptosis, we found that mitochondria and membrane receptor both can mediate apoptosis. Cell apoptosis has periodic characteristics and often occurs in cell cycle arrest. Most cancer chemotherapy aims to induce cell apoptosis in the specific period to kill the tumor cells [1–4]. However, there is still little data on the relationship between mitochondrial-mediated U251 cell apoptosis and the cell cycle. We tried to clarify the mechanism of mitochondria-mediated apoptosis through cell cycle, Caspase-3, and Caspase-9.
Material and Methods
Cell culture and collection
Human brain glioma cell line U251 cells (Harbin Medical University) were maintained in DMEM medium (GIBCO, USA) containing 10% fetal bovine serum (GIBCO, USA) and penicillin streptomycin at 37°C, 5% CO2 incubator (Queue Systems, the United States). The cells were passaged when the density reached more than 4×105 cells/cm2. After removing the medium, the cells were digested by 0.02% EDTA trypsin (GIBCO, USA). Equal volume of medium was joined and collected and the cells were centrifuged at 10000 r/min for 5 min (BiofugeStratos, Germany). The cells were reseeded to the flask after discarding the supernatant. When the adherent cells achieved 4×106, mitochondrial apoptosis-related gene Bax plasmid (Santa Cruz, USA) was applied to stimulate cells for 14 h; 10 μg/ml tPA (Beyotime, Shanghai) was used to stimulate U251 cells, and after adding 12.5 μg/ml Bax for 24 h, the cells were collected.
Flow cytometry detection for cell apoptosis and cell cycle
Annexin V/PI method was adopted for apoptosis detection. 105 cells in 100 μl fluid after washing were added with Annexin V - FITC and PI. After incubating for 15 min away from light, the cells were detected by flow cytometry (BD, USA).
API method was used to test apoptosis cycle. The collected cells were added with Annexin V – FITC and incubated at room temperature away from light for 30 min. After washing twice with buffer, 1 ml formaldehyde was applied to fix the cells. PI was further used for staining and the cells were detected on flow cytometry after 1 h.
Cyclins/DNA double staining flow cytometry instrument was used to analyze the human brain glioma cell line U251 cell cycle specificity. The cells were fixed by ethanol overnight and treated with TritonX – 100 for 5 minutes twice. After washed with PBS, the cells were incubated with BSA diluted antibody at 4°C overnight. Goat anti-mouse IgG were added on the second day at room temperature for 20 min. PI and RnaseA were used for DNA staining, and the cells were detected by flow cytometry.
Western blot
We extracted 100 mg total protein from each group and separated it by SDS-PAGE electrophoresis. After incubation with primary antibody (1:500) at 4°C overnight and HRP-tagged secondary antibody (1:1000) at 37°C for 1 h, the membrane was detected by ECL photochemical method.
RT-PCR (reverse transcription-polymerase chain reaction)
Primer Design: Caspase-3: Upstream 5′-GTACTGGCATTG GCGGTATC-3′, Downstream 5′-GAGAATCCAGGGCATCCATT-3′; Caspase-9 Upstream 5′-GGCTTGGTAGTGTTTGCCAT-3′, Downstream 5′-GGGCAAAGAGTAAACCCACA-3′; 18sRNA Upstream 5′-CTGCCCCTACTTGTCATGGT -3′, Downstream 5′-AGATGAGCCTCACAGCCCTA -3′
Total RNA was extracted from the cells. The cDNA was synthesized using reverse transcription. The PCR cycling conditions consisted of an initial, single cycle of 5 min at 95°C, followed by 30 cycles of 30 s at 94°C, 30 s at 60°C, and 60 s at 72°C. PCR products were tested by agarose gel electrophoresis and analyzed by software; 18 s RNA was chosen as control.
Statistical analysis
All statistical analyses were performed using SPSS21.0 software (Chicago, IL). Data conformed to normal distribution were presented as means and standard deviation (χ±s). Differences between means were analyzed using the t test. P<0.05 was considered as a statistically significant difference.
Results
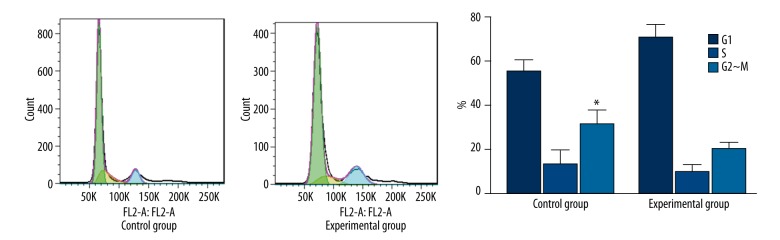
Cell cycle detection of tPA effect on human brain glioma cell line U251 cells
Cell cycle of U251 cells stimulated with tPA was detected. The results showed that after treatment with tPA, U251 cells in the stationary phase obviously entered G2-M phase (Figure 1). G2~M cells in the experimental group reached 31.27%, compared with the control group (20.24%). There is a statistically significant difference (P<0.05). (Figure 1 shows the cell cycle of human brain glioma cell line U251 after stimulation by tPA).
Figure 1.
Cell cycle of human brain glioma cell line U251 after stimulation by tPA. * P<0.05 Compared with G2~M cells in the experimental group.
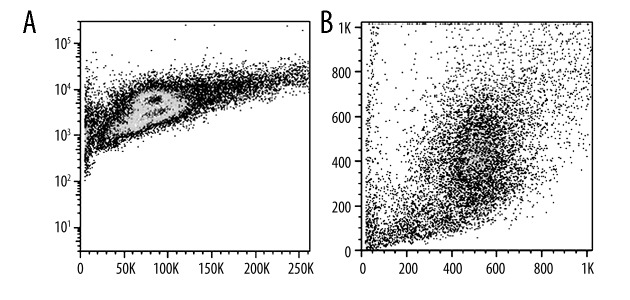
Annexin V/PI test cell apoptosis
After treated with Bax for 24 hours, the apoptotic cell rate increased for 6.8%. After stimulation with tRA and Bax for 24 h, the cell apoptotic rate up-regulated to 15.2%. tPA stimulation was more apparent in U251 cell apoptosis induction effect (Figure 2).
Figure 2.
Annexin V/PI test cell apoptosis. (A) Mitochondria-mediated human brain glioma cell line U251 cell apoptosis scatter plot; (B) Mitochondria-mediated tPA stimulated human brain glioma cell line U251 cell apoptosis scatter plot.
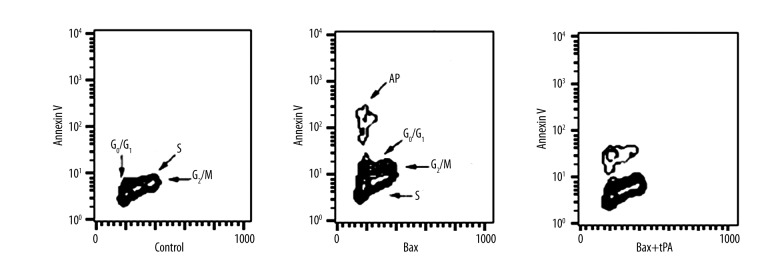
API detect cell cycle specificity
Bax induced U251 apoptosis appeared in G1 stage. Bax induction and tPA stimulation U251 apoptosis occurred in G1 stage. Cells in the control group with no tPA stimulation showed no significant cell apoptosis (Figure 3).
Figure 3.
API detect cell cycle specificity.
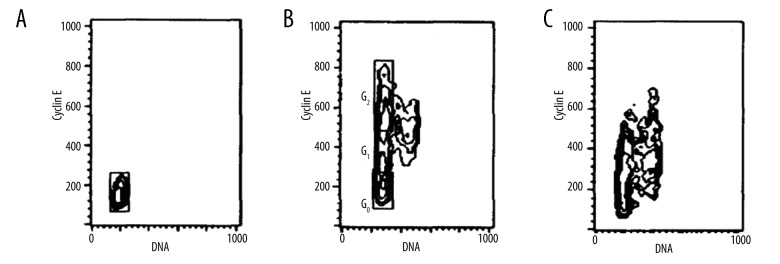
Cyclin E/DNA double staining flow cytometry analysis on cell cycle
Human brain glioma cell line U251 cells stayed in G0 stage. After stimulation with tPA, cells appeared in G0, early/late G1 phase, S2, G2, and M stages. Cyclin E expression decreased after addition of Bax and cells were arrested in G1 stage (Figure 4).
Figure 4.
Cyclin E/DNA double staining flow cytometry analysis on cell cycle. (A) Control; (B) tPA; (C) Bax+tPA.
Caspase-3 and Caspase-9 protein expression
After being induced by the mitochondrial apoptosis-related gene Bax, U251 cells showed obviously elevated Caspase-3 and Caspase-9 protein expression (Figure 5).
Figure 5.
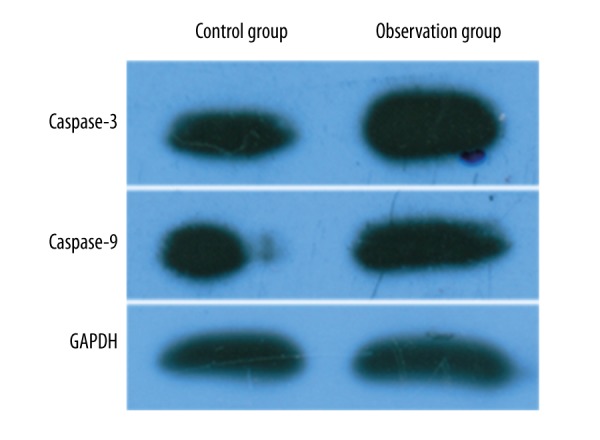
Caspase-3 and −9 protein expression.
Caspase-3 and Caspase-9 mRNA expression
After induction by mitochondrial apoptosis-related gene Bax, Caspase-3 and Caspase-9 mRNA expression significantly increased (P<0.05) (Table 1).
Table 1.
Caspase-3 and Caspase-9 mRNA expression comparison.
P<0.05 compared with control.
Discussion
Studies [5,6] have shown that the cell cycle plays an important role in the process of cell proliferation and apoptosis. The cell cycle is mainly regulated by signaling pathways. Signal blockage or strong stimulation can cause cell cycle imbalance and lead to tumor occurrence. A tumor is a kind of disease characterized as abnormal cell proliferation. Cell proliferation and apoptosis regulation pathways were destroyed in this process. One kind is the activation of signal transduction pathway, while the other is excessive cell proliferation [7]. Signaling pathways includes CDKs, Cyclins abnormal activation and expression, and gene mutation. However, single-gene locus mutation is unlikely to cause cancer, and multiple genes combined are required to for the occurrence of cancer cells [8]. Under the abnormal proliferation condition, cell apoptosis is often an obstacle. Cancer treatment is also a process of tumor cell apoptosis initiation, and its core mechanism is the activation of Caspase and promoting cells disintegration [9]. Phosphorylated Akt mutation, Bcl-2, and Bax overexpression both can activate apoptosis through mitochondria. There is still lack of related research about the role of mitochondria-mediated apoptosis in human brain glioma cells. This study revealed the relationship between mitochondria-mediated human brain glioma cell apoptosis and the cell cycle through selecting the mitochondria-mediated apoptosis inducer protein Bax, applying API technology for cell apoptosis and cell cycle analysis, and exploring the impact of mitochondria-mediated apoptosis on brain glioma cell cycle specificity and apoptosis related proteins Caspase-3 and Caspase-9 activation.
U251 cells proliferate quickly during inflammation or trauma, and its status becomes stable again when the inflammatory lesions fade away [10–14]. Human brain glioma cells stay in G0 phase without stimulus. When the cells were induced by mitochondrial apoptosis-related gene Bax, apoptotic cells were fewer and cells did not enter the cell cycle. On this basis, the tectotype of plasminogen activator tPA stimulus can activate the U251 cells into the cell cycle, promote cell proliferation, and lets cells enter the S and G2/M phases. At the same time, Bax induction greatly increased cell apoptosis rate [14–17]. Thus, apoptosis can only be detected after the cells enter the metaphase, which is similar with clinical chemoradiotherapy treatment. Chemoradiotherapy made the enter the cell cycle, and the cell apoptosis rate is much higher after treatment than in stationary phase [18].
To test whether cell apoptosis has cycle specificity, we tested mitochondria-mediated apoptosis by API method. Membrane eversion phenomenon appeared in the early phase of apoptosis, and API method used Annexin V combined with this phenomenon to judge apoptosis cycle specificity. The results suggested that mitochondria-mediated apoptosis and cycle specificity apoptosis mainly occurred in the G1 phase. Cell cycle protein consists of various types of proteins specifically expressed in the cell cycle that can make cells have a specific cycle manifestation by stimulating the cell cycle-dependent protein kinase. The major characteristic of cell cycle protein is the time phase. CyclinE/DNA double-stain flow cytometry combined cell place and CyclinE expression to test the detailed information [19,20]. CyclinE is completely synthesized in G1 phase and will not appear in G0 phase. Thus, it can separate G1 phase from G0. Our results show that the mitochondria-mediated apoptosis decreased CyclinE expression in brain glioma cells, and cells were arrested in G1 phase; this process also complied with apoptosis-related proteins Caspase-3 and −9 overexpression and activation.
Conclusions
The human brain glioma cells did not start the cycle process in stationary stage and the apoptosis became sensitive only after entering the cell cycle. Mitochondria-mediated apoptosis is closely related to the brain glioma cell cycle and occurs in a particular phase. It also explained the relationship between apoptosis and cycle specificity. It further revealed the role of apoptosis period in malignant tumor. This study investigated mitochondria-mediated apoptosis and has clinical significance in inducing tumor apoptosis and treatment.
Footnotes
Source of support: Departmental sources
References
- 1.Wang C, Chen X, Zou H, et al. The roles of mitoferrin-2 in the process of arsenic trioxide-induced cell damage in human gliomas. Eur J Med Res. 2014;19:49. doi: 10.1186/s40001-014-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx J. Nobel Prize in physiology or medicine. Tiny worm takes a star turn. Science. 2002;298:526. doi: 10.1126/science.298.5593.526. [DOI] [PubMed] [Google Scholar]
- 3.Balter M, Vogel G. Nobel prize in physiology or medicine. Cycling toward Stockholm. Science. 2001;294:502–3. doi: 10.1126/science.294.5542.502. [DOI] [PubMed] [Google Scholar]
- 4.Yan YY, Bai JP, Xie Y, et al. The triterpenoid pristimerin induces U87 glioma cell apoptosis through reactive oxygen species-mediated mitochondrial dysfunction. Oncol Lett. 2013;5:242–48. doi: 10.3892/ol.2012.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Xu X, Feng X, et al. Adenovirus-mediated delivery of bFGF small interfering RNA reduces STAT3 phosphorylation and induces the depolarization of mitochondria and apoptosis in glioma cells U251. J Exp Clin Cancer Res. 2011;30:80. doi: 10.1186/1756-9966-30-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao X, Deeb D, Jiang H, et al. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of proCaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol. 2005;5:39–48. [PubMed] [Google Scholar]
- 7.Lin CJ, Lee CC, Shih YL, et al. Inhibition of mitochondria- and endoplasmic reticulum stress-mediated autophagy augments temozolomide-induced apoptosis in glioma cells. PLoS One. 2012;7:e38706. doi: 10.1371/journal.pone.0038706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiou SM, Chiu CH, Yang ST, et al. Danthron triggers ROS and mitochondria-mediated apoptotic death in C6 rat glioma cells through Caspase cascades, apoptosis-inducing factor and endonuclease G multiple signaling. Neurochem Res. 2012;37:1790–800. doi: 10.1007/s11064-012-0792-3. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Gil M, Tozzi MG, Varani S, et al. The combination of adenosine deaminase inhibition and deoxyadenosine induces apoptosis in a human astrocytoma cell line. Neurochem Int. 2015;80:14–22. doi: 10.1016/j.neuint.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Tao D, Wu J, Feng Y, et al. New method for the analysis of cell cycle-specific apoptosis. Cytometry A. 2004;57:70–74. doi: 10.1002/cyto.a.10117. [DOI] [PubMed] [Google Scholar]
- 11.Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–67. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 12.Gong J, Li X, Darzynkiewicz Z. Different patterns of apoptosis of HL-60 cells induced by cycloheximide and camptothecin. J Cell Physiol. 1993;157:263–70. doi: 10.1002/jcp.1041570208. [DOI] [PubMed] [Google Scholar]
- 13.King KL, Cidlowski JA. Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem. 1995;58:175–80. doi: 10.1002/jcb.240580206. [DOI] [PubMed] [Google Scholar]
- 14.Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992;71:543–46. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- 15.Bhuyan BK, Groppi VE. Cell cycle specific inhibitors. Pharmacol Ther. 1989;42:307–48. doi: 10.1016/0163-7258(89)90029-6. [DOI] [PubMed] [Google Scholar]
- 16.Gong J, Traganos F, Darzynkiewicz Z. Growth imbalance and altered expression of cyclins B1, A, E, and D3 in MOLT-4 cells synchronized in the cell cycle by inhibitors of DNA replication. Cell Growth Differ. 1995;6:1485–93. [PubMed] [Google Scholar]
- 17.Dulic V, Lees E, Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–61. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 18.Koff A, Giordano A, Desai D, et al. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–94. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 19.Pasupuleti N, Leon L, Carraway KL, III, Gorin F. 5-Benzylglycinyl-amiloride kills proliferating and nonproliferating malignant glioma cells through Caspase-independent necroptosis mediated by apoptosis-inducing factor. J Pharmacol Exp Ther. 2013;344:600–15. doi: 10.1124/jpet.112.200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F, Tu J, Pan JQ, et al. Light-controlled inhibition of malignant glioma by opsin gene transfer. Cell Death Dis. 2013;4:e893. doi: 10.1038/cddis.2013.425. [DOI] [PMC free article] [PubMed] [Google Scholar]