Abstract
There are a number of neurodegenerative diseases that principally affect humans as they age, characterized by the loss of specific groups of neurons in different brain regions. Although these are in general sporadic disorders, it is know clear that many of these diseases have a substantial genetic component. As genes are the raw material with which evolution works, we might benefit from understanding these genes in an evolutionary framework. Here, I will discuss how we can understand whether evolution has shaped genes involved in neurodegeneration and the implications for practical issues such as our choice of model systems for these diseases and more theoretical concerns such as the level of selection against these phenotypes.
Introduction
Evolutionary theory, modified to include our modern molecular views on genetics, permeates all aspects of modern biology. Understanding human biology therefore incorporates acknowledgement of our genetic heritage shaped by the evolutionary forces that have led to humans occupying our current niche. And it should not be surprising that, as a major aspect of our biology, the near universal experience of human disease can also be viewed through the evolutionist’s prism.
Here, I will discuss the relationship of evolution to age-related neurodegenerative disorders. This group of diseases is characterized by the shared property in the progressive loss of relatively specific groups of neurons. What distinguishes each is that during the aging process different groups of neurons are lost in each disease and these correlate with different clinical features. For example, neuron loss in the hippocampus and cerebral cortex underlies many of the memory problems associated with Alzheimer’s disease (AD) whereas ataxia is a consequence of loss of Purkinje cells in the cerebellum and is characteristic of the spinocerebellar ataxias. These symptoms are often profoundly disabling and sometimes fatal; loss of the neurons that innervate the diaphragm in amyotrophic lateral sclerosis (ALS) leads to an inability to breathe.
Additionally, in many but not all neurodegenerative conditions, there are other pathological events including the accumulation of specific proteins in those neurons that survive. Often these are aggregated and insoluble and, more importantly, often the genes that code for these pathological proteins either cause inherited forms of disease and/or act as genetic risk factors. Therefore, pathology, clinical phenotype and causal variation in specific genes are linked.
There are two questions to discuss here to understand the genes and proteins associated with neurodegeneration in the context of evolution. The first is in what ways can we use evolutionary views on sequences to understand aspects of protein function and dysfunction related to mutations associated with neurodegenerative diseases. This has practical implications, for example in assigning pathogenicity to specific mutations in genes or for understanding how far we can extrapolate from model systems to human diseases. The second, consideration is whether evolutionary forces have shaped these degenerative diseases. To provide a detailed example, I will first cover Parkinson’s disease (PD), a disorder that illustrates many of the key points under discussion here. I admit this is biased by my own research interests, so refer the interested reader to other review articles about the genetics of neurodegenerative diseases more generally [1, 2].
Genes associated with Parkinson’s disease
Parkinson’s disease conforms to the general description of neurodegeneration outlined above of cell loss with accumulation of pathological proteins, but there are specific events that define this disease. First, there is loss of neurons that project from the substantia nigra pars compacta to the striatum and produce the neurotransmitter dopamine (Figure 1). There are other brain areas involved in PD but it is the death of dopamine neurons that are responsible for some of the prominent problems with movement seen in people living with PD; tremor, slowness of movement and problems with posture. Loss of dopamine neurons is probably fairly well established by the time clinical signs are noticed and replacement of dopamine has dramatic effects on symptoms, at least early in the disease course [3].
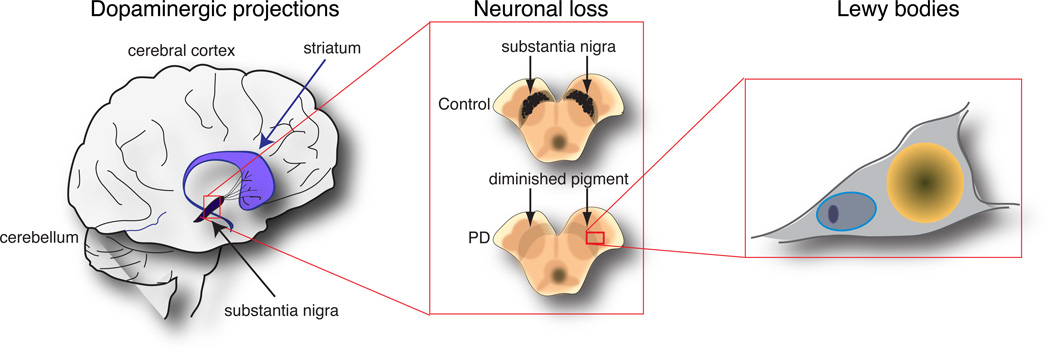
Figure 1. The pathology of Parkinson’s disease.
The cartoon shows a simplified view of the main neuropathological events in Parkinson’s disease at three levels from left to right. At the level of the brain, a major pathway is degeneration of the dopaminergic projections from the substantia nigra (in black) to the striatum (in purple), both of which are in the midbrain underneath the cerebral cortex. At the level of substantia nigra, the neurons that form the presynaptic portion of this pathway are normally melanized and are easily identified by this pigment in control brains (upper panel). In contrast, the loss of neurons in this region is so substantial that the whole area becomes depigmented in Parkinson’s disease cases (lower panel). Of the few remaining cells, many show pathological changes including the accumulation of proteins and lipids in Lewy bodies. A characteristic protein in Lewy bodies is α-synuclein, which is encoded by the SNCA gene that increases the risk of PD.
A second pathological characteristic of PD and related disorders is the presence of Lewy bodies and Lewy neurites [4]. These are accumulations of proteins and lipids, in many of the neurons that survive to the end of the disease. Of the protein components, one of the most reliable markers is α–synuclein (Fig. 2). Other reviews have covered the genetics of PD [5, 6] so here I will outline some of the key discoveries that allow for discussion of the nature of the disease in an evolutionary context.
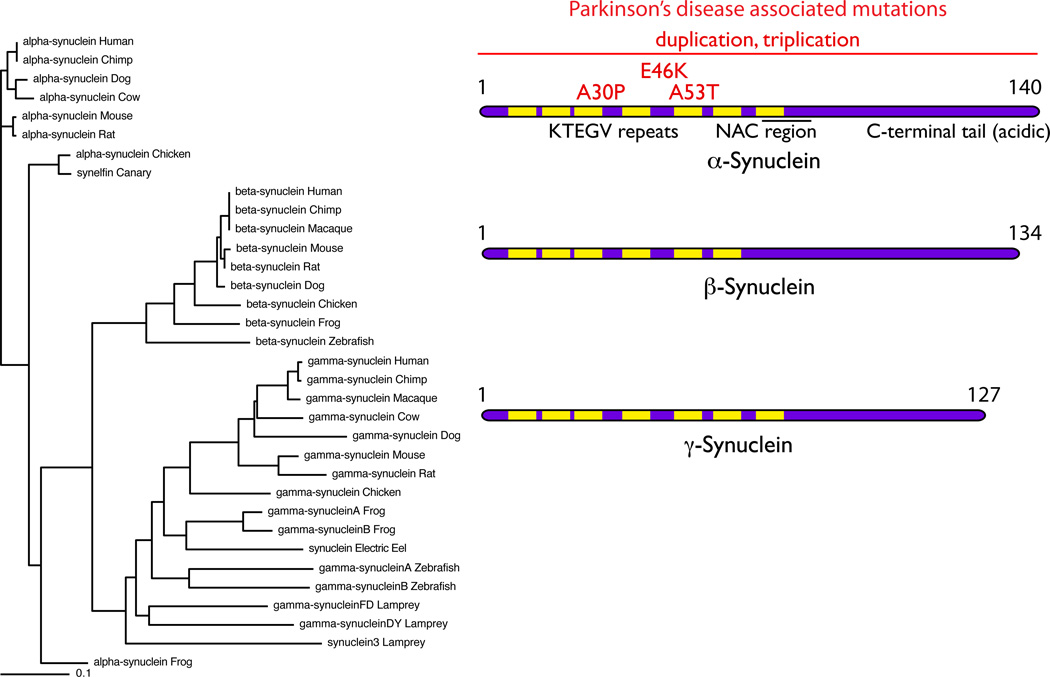
Figure 2. The Synuclein family.
The tree on the left of the figure shows that there are several synuclein homologues in many vertebrate species. These separate into three groups, identified as α-, β- and γ- synuclein and, interestingly, most species have one of each homologue. Exceptions to this general rule include species such as the lamprey and zebrafish, which appear to have evolved multiple γ- synuclein homologues but lack an α-synuclein homologue. On the right are ideograms of the proteins. The characteristic KTEGV repeats, the number of which vary between homologues are indicated in yellow. Mutations in α-synuclein, shown in red, are associated with autosomal dominant Parkinson’s disease and include three point mutations and multiplications of the whole SNCA locus (indicated by the horizontal line).
α-synuclein
Critically for understanding the pathophysiology of PD, there are a series of point mutations in the gene that codes for α–synuclein, SNCA, which are associated with dominantly inherited PD. The first reported was Ala53Thr in a series of families from Greek and Italian descent [7]. Additional amino acid changing variants include A30P [8] and E46K [9].
Furthermore, there are mutations with a tandem triplication [10] or duplication [11] of the normal SNCA locus. These genetic observations show that the normal protein has the capacity to cause damage to the brain at higher than normal expression levels. Additionally, common variants at the same locus are risk factors for PD [12]. These risk variants are not strong enough to produce Mendelian inheritance, but instead increase the chances of developing PD by about 20–40% over the lifetime of an individual. It is likely that these common variants around the SNCA locus will also produce higher expression of α–synuclein, but not quite as high as the multiplication cases, although this is not proven [13]. Therefore, α-synuclein shows that inherited and sporadic forms of PD might have some common etiopathological mechanisms.
How α-synuclein mutations lead to Parkinson’s disease is not quite clear. In the test tube, α-synuclein is prone to protein aggregation and it is aggregated forms of synuclein are found in Lewy bodies [14]. Specifically, α-synuclein aggregates into large, insoluble assemblies with a beta-sheet-like structure, similar to other aggregating proteins called amyloid. However, it is not clear if it is fully aggregated fibrils of α-synuclein, as deposited in Lewy bodies, that cause neuronal death or whether some other biophysical species is more important. Attempts to answer this question have generally found a correlation between the amount of small oligomeric assemblies of α–synuclein and toxic effects on neurons [15, 16]. Even within the range of oligomers there may be species that are especially toxic, as has been shown recently using single molecule techniques to identify specific types of oligomers that can generate high levels of oxidative stress when applied to primary neurons [17].
A53T is more prone to form all types of aggregates (from oligomers to fibrils) than normal human α–synuclein. This enhanced aggregation is also seen with several alanine to threonine mutations in other amyloidogenic proteins that cause human diseases [18]. Similar enhancement in aggregation is seen with the E46K mutation, which is at a more conserved residue [19, 20]. However, the A30P mutation readily makes small soluble oligomers but forms fibrils more slowly than wild type α–synuclein in vitro [21]. If A30P were genuinely a pathogenic mutation, then this would support the notion that oligomers are the toxic species.
Interestingly, one case with this mutation has been shown recently to have rather extensive Lewy pathology that, by extension, represents fibrillar α–synuclein [22]. This is surprising if A30P forms fibrils less readily than wild type synuclein. However, one thing to consider is that the material deposited in this case might include wild type protein. In many assays, A30P α–synuclein behaves as a loss of function protein: for example, it lacks the ability to bind lipids that characterizes all synucleins [23]. It is therefore possible that the wild type allele is up regulated as a compensation for a loss of function protein. There is also evidence that the wild type allele is expressed at relatively higher levels then the A53T variant [24, 25]. I acknowledge that this is provocative, and data on the relative expression of E46K is not available, but it might imply that mutations at the SNCA locus all include an element of increased expression of the normal human protein.
At a cellular level, the toxicity of α-synuclein has been ascribed to effects on any number of different subcellular functions, including effects on protein-turnover systems and energy generation via mitochondria [26]. One class of genes that were initially identified from studies in yeast is that toxic α-synuclein may impact trafficking of lipid vesicles through the cell [27], which is particularly interesting as α-synuclein is normally a lipid-binding protein. The mechanism involved may involve disruption of Rabs, small GTPases that regulate crucial aspects of lipid membrane trafficking [28]. Furthermore, several experiments have shown that manipulating the level of expression of different Rab homologues, can overcome the toxic effects of synuclein in models in several different species [29, 30]. Therefore, the toxicity of α-synuclein may be intimately tied to its normal cellular role of binding lipids. Whether this is related to aggregation is unclear.
Overall, these data show that there is at least one protein in our brain, that at levels similar to those normally expressed, can be toxic and that toxicity may relate to normal function. Are there other proteins with the same properties?
LRRK2 and MAPT are additional genes for PD
A second gene for PD is Leucine-rich repeat kinase 2 (LRRK2) [31, 32]. LRRK2 is, as the name implies, one of two homologous kinases in the human genome that contain leucine-rich repeats (Fig. 3). LRRK1/2 are also part of the larger family of ROCO proteins with tandem ROC (ras of complex proteins) and COR (C-terminal of ROC) bidomain [33]. The function of the ROC:COR region is to bind and hydrolyze GTP, possibly through dimerization that is mediated by the COR domain [34]. The domain structure of LRRK2 is probably relevant for PD because most of the convincing mutations are found within the ROC:COR and kinase domains. In fact, it appears likely that there is co-regulation of these two portions of the molecule [35], so mutations may have slightly different biochemical effects but all presumably impact the overall function of the protein.
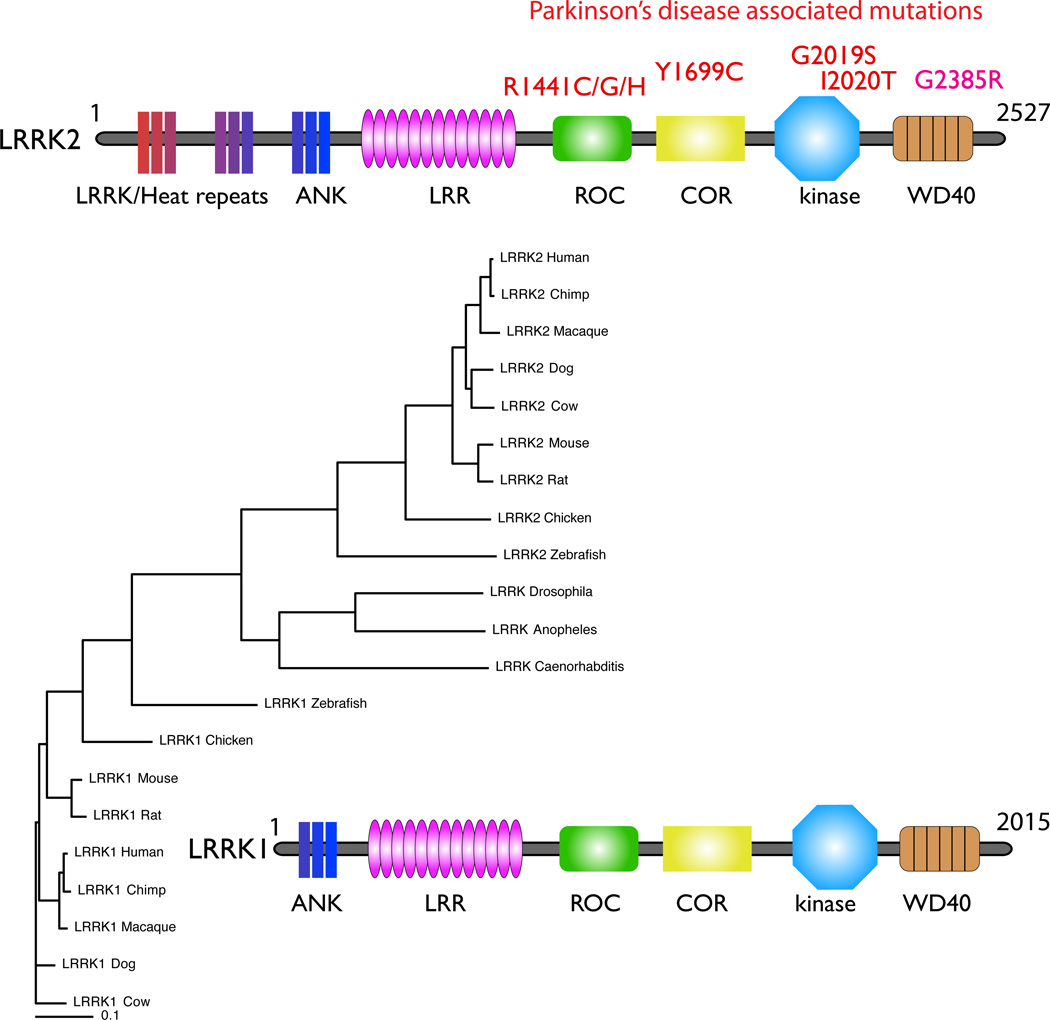
Figure 3. The LRRK family.
Like the synucleins, there are at least two distinct branches of the LRRK family represented by LRRK1 (lower part of the tree) and LRRK2 (upper part of the tree) in vertebrate species. The invertebrate LRRKs form a group that sits between the two vertebrate LRRK homologues (see text). The distinction between the LRRK1 and LRRK2 orthologues includes differences at the N-terminal, where LRRK2 (upper ideogram) includes a series of repeat sequences that LRRK1 (lower ideogram) lacks. Other domains include the anykrin-like repeats (ANK), leucine-rich repeats (LRR), Ras of complex proteins (Roc) and C-terminal of ROC (COR), kinase and WD40 domains.
What the function(s) of LRRK2 is that is relevant to PD is not yet clear, although clues may be gleaned from understanding where the protein is expressed. LRRK2 appears to be associated with several different vesicular compartments in cells, including in neurons. Membrane association of LRRK2 has been shown in several studies [36, 37], and it has been suggested that it is the kinase active form of LRRK2 that is found in these fractions [38]. Of the many membrane-bound structures in cells, there is strong evidence for LRRK2 association with vesicles of the endosomal system involved in autophagy [39] and with synaptic vesicles [40, 41]. Like α-synuclein, mutant LRRK2 is toxic when overexpressed directly in neurons [42–47]. Toxicity can be limited by mutations that impact normal functions like kinase and GTP binding [42–47], suggesting that, as with α–synuclein, the normal function of LRRK2 in neurons may be relevant for toxicity.
LRRK2 is also expressed in microglia [48–50], resident immunologically active cells of the brain that have been shown to contribute to neurodegeneration in other diseases including ALS and tauopathies. Whether this contributes to disease is not clear, but it has been suggested that LRRK2 mutant microglia might release cytokines that are neurotoxic [51]. Therefore, in the case of LRRK2 there may be cell-autonomous and non cell-autonomous mechanisms at play.
Perhaps more importantly, knocking out LRRK2 mitigates the toxicity associated with A53T α-synuclein [52] suggesting that LRRK2 and α-synuclein are in the same pathway. A caveat to this result is that pathology associated with expression of A53T mutant α-synuclein with alternative promoters does not respond to LRRK2 deletion [53, 54]. However, the pathology of most human LRRK2 cases includes α-synuclein positive Lewy bodies, although some cases do not fit to this general pattern [55, 56]. The suggestion of this is that LRRK2 is genetically ‘upstream’ of α-synuclein but either (1) not all cases go through the same α-synuclein pathway or (2) that deposition of α-synuclein into Lewy bodies is not a required event in disease pathogenesis.
As discussed above, a supportive argument for a role of α-synuclein in sporadic disease is the existence of variants around the SNCA locus that are associated with PD. The same appears to be true for variants around the LRRK2 locus [57]. If we follow the logic outlined for the effects of the wild-type α-synuclein protein, and further assume that there aren’t rare amino acid changing variants of LRRK2 causing the association, then this implies that the normal human LRRK2 sequence can contribute to disease risk. The mechanistic basis of the genetic association is unclear; the effect could be due to increased LRRK2 expression but other mechanisms (altered splicing, changes in RNA localization) cannot yet be ruled out. But, when stacked together with the observation that LRRK2 mutants have a disease that is clinically similar to sporadic PD and often have α-synuclein positive pathology, these genetic observations suggest that more than one normal protein, when accumulated or misregulated, can lead to neurodegenerative disease.
There are almost certainly other genes in the same category. The power of genome-wide association studies (GWAS), performed by looking for statistical over-representation of genetic variants comparing many thousands of PD cases and controls, is to identify in a relatively unbiased way some of the underlying genetic architecture of sporadic PD. Recent large scale studies [58–60] suggest that there are many variants that influence lifetime risk of PD. Although each variant has a small effect size, cumulatively these small effects explain a significant proportion of the overall risk for PD to the general population.
Going through each of the PD risk loci is outside of the scope of this article, but there is one additional risk factor gene, MAPT, that is worth discussing. Variation at the MAPT locus influences lifetime risk of PD to almost as great an extent as α-synuclein [58–60]. The MAPT gene encodes tau [61] which, in contrast to α-synuclein, is not a deposited protein in PD (see below). This shows that proteins can be involved in neurodegeneration without being deposited and, by extrapolation, that α-synuclein might still be involved in the LRRK2 cases without Lewy bodies. Tau is part of the family of microtubule binding proteins (MAPs) whose normal function is to bind to and stabilize microbules, a major cytoskeletal element composed of tubulins. This normal function is tightly regulated by modification of the MAPs; for example MAPT function is controlled by alternative splicing and by a number of phosphorylation events.
It is possible that the role of tau in PD is related to LRRK2 and/or α-synuclein. There is evidence that LRRK2 may influence tau including some biochemical effects via altered phosphorylation [62–64] and the accumulation of phosphorylated tau in LRRK2 mouse models [65, 66]. This may be related to the ability of LRRK2 to bind to microtubules to which tau binds, a property that several mutations in LRRK2 exaggerate [67]. However, tau expression is not required for neurodegeneration in models with expression of α-synuclein [68]. Overall, the data hints that there might be relationships between these different genes but the precise nature of the underlying pathways is obscure.
What is clear is that mutations in these different genes are only part of the spectrum of changes and that normal proteins may play an important role in the same processes by acting as risk factors. Furthermore, the pathological roles are potentially related to normal roles and expression in the nervous system. We might therefore ask whether this is specific to PD or whether some of the same principles, perhaps even for the same proteins, apply to other neurodegenerative conditions. Again, other reviews on neurodegeneration are available that cover more examples than I can here, so I will take the example of MAPT/tau in more depth as this brings in additional diseases.
Alzheimer’s and Frontotemporal dementias; additional roles of tau
The MAPT gene was introduced above as a risk factor gene for PD. What wasn’t discussed is that pathological involvement of Tau is much more characteristic of Alzheimer’s disease (AD). In AD, but not PD, Tau protein is heavily phosphorylated and deposited as insoluble forms in neurofibrillary tangles [69]. Tangles and similar pathologies are found in a number of diseases collectively termed tauopathies. Like α-synuclein, tau is prone to aggregate. What distinguishes AD from other tauopathies is that in AD there are also accumulations of another protein fragment, Aβ, in extracellular deposits called amyloid plaques. A general concept of AD pathogenesis is that Aβ generation drives Tau pathology and it is the latter that is most strongly associated with neuronal damage and cell death [70, 71]. The proteins that generate Aβ are APP and Presenilins, which are the precursor of Aβ and the catalytic subunits of the enzymes that act on APP respectively, each contain mutations that cause familial early onset AD [72]. All the mutations seem to map to an increase in the generation of Aβ and because Aβ containing plaques define the pathology of sporadic AD, the Mendelian forms of this disease have helped tie together genetics and neuropathology in what seems to be a straightforward manner.
In contrast, point mutations in MAPT don’t cause AD but instead cause frontotemporal dementia (FTD), sometimes with accompanying parkinsonism [73]. There may be a boundary issue as to whether we include AD and FTD in a single category of “dementias”, but to my mind these are distinct. FTD, as its name implies, primarily affects the frontal portion of the cerebral cortex whereas Alzheimer’s affects other cortical and subcortical regions (such as the entorhinal cortex and hippocampus) earlier in the disease course. Additionally, FTD is not a plaque disease. It is also important to note there are other pathologies that can be associated with FTD, particularly FTD associated with ALS, but here I will focus on tau pathologies.
In contrast to PD, common variation around the MAPT locus is not a risk factor for sporadic AD, although it does contribute to risk of progressive supranuclear palsy (PSP) [74], a disease with deposition of tau and Parkinson’s-like symptoms. Therefore, the contribution of MAPT/tau to different neurodegenerative conditions is surprisingly complex. Causal mutations are found in one condition (FTD), but common variation around this locus contributes to lifetime risk of others (PD and PSP) and the protein is deposited in yet others (including AD). This then leads to the question of where proteins like α-synuclein and tau came from in evolution and why they would be present in our brain.
The varied evolutionary history of genes that cause neurodegeneration
What is known about the evolutionary history of these proteins? To start with α-synuclein, Homo sapiens has two additional homologues, β- and γ-, with distinct sequences but overall high homology (Fig. 2). Most vertebrate species, including teleost and cartilaginous fish, lizards, birds and mammals have at least one homologue although the number varies. Zebrafish, for example, have no α- homologue but two γ- synucleins [75]. Lampreys have at least two γ- synuclein genes [76] but it is not known if older vertebrate groups do. In contrast, the synucleins are apparently absent from bacteria, plants and invertebrates including important model organisms Drosophila melanogaster and Cenorhabditis elegans. Therefore, the presence of the synucleins seems to mark the separation of vertebrate from invertebrate animals (Fig. 2).
This evolutionary history is interesting because it implies that none of the synucleins are actually required to make a functioning neuron: flies and worms have nervous systems that do quite fine without these genes. Supporting this, mouse knockouts are viable and have working brains although there is controversy as to whether neurodegeneration occurs in α–/β–/γ– triple knockouts [77–79]. There is also a report of subtle phenotypes, including changes in vocalization, in α–synuclein knockout mice [80]. But overall, the brains of vertebrates seem to spend a lot of energy generating three proteins that are not essential to make or maintain a nervous system.
It is also interesting that Ala53, one of the amino acids where mutations are found, is not a particularly well-conserved residue. In fact, the same residue is a threonine in rodent homologues of α–synuclein and therefore A53T is a revertant mutation. The alteration from Thr to Ala specifically distinguishes new world from old world primates [81], a separation that is estimated to have occurred ~35 mya. Conservation is often used as a supportive argument for pathogenicity in human genetics study as it is used to suggest that the residue must be ‘important’, but here this is clearly not the case. Mice (or most other species) are not known to suffer from PD in the wild so simply possessing a 53Thr variant is not sufficient to cause Lewy body disease. But, A53T is pathogenic in the context of the human protein and human brain.
LRRK1 and 2 are also part of a larger protein family, the ROCO proteins, representatives of which are found in many species including evolutionarily old species including cyanobacteria and slime molds [33]. Because the ROCO domain is found in a large number of structurally diverse multidomain proteins, it is likely that this region is more ancient than other portions of LRRK2, including the kinase domain for which it is named, which were acquired later. Interestingly, in some anemone species there are four LRRK paralogues, and it has been suggested that different homologues were lost in the vertebrate and invertebrate lineages. Therefore, the LRRK proteins in important model organisms such as Drosophila and C elegans are not strict orthologues of LRRK2 but a slightly different protein (Fig. 3). Functional conservation between the different LRRK proteins is not well understood, although LRRK1 and LRRK2 certainly have different biochemical properties [82].
The various mutations in LRRK2 are relatively well conserved in different species and homologues. For example, the most common mutation is G2019S, found in the kinase domain, specifically in the Mg2+ binding motif at the N-terminal portion of the activation loop. This sequence is DYG or DFG in all known protein kinases. However, activating mutations in this motif were reported in many cancers and in some cases the invariant glycine residue was replaced by a serine as in LRRK2 [83]. G2019S is activating in LRRK2 [35]. Mutations in the ROC:COR domains are also found at residues that are reasonably well conserved between homologues and species, where the mutant forms tend to have lower GTP hydrolysis activity compared to the wild type protein. Therefore, the mutations in LRRK2 generally modify residues that are likely to be important in the control of catalysis and are conserved, presumably due to evolutionary constraints on which amino acids are tolerated.
Because tau is one of many microtubule-binding proteins it should also be considered part of a gene family (Fig. 4). However, sequence analysis suggests that MAPT genes in different species are close only to one additional brain protein (MAP2) and to MAP4, which is expressed outside of the central nervous system [83]. Even within this group there is likely functional specification, with tau being found in axons and MAP2 proteins in dendrites. There are some evolutionarily old microtubule binding proteins, but close homologues of MAPT are found only in animals, where they show reasonable levels of conservation.
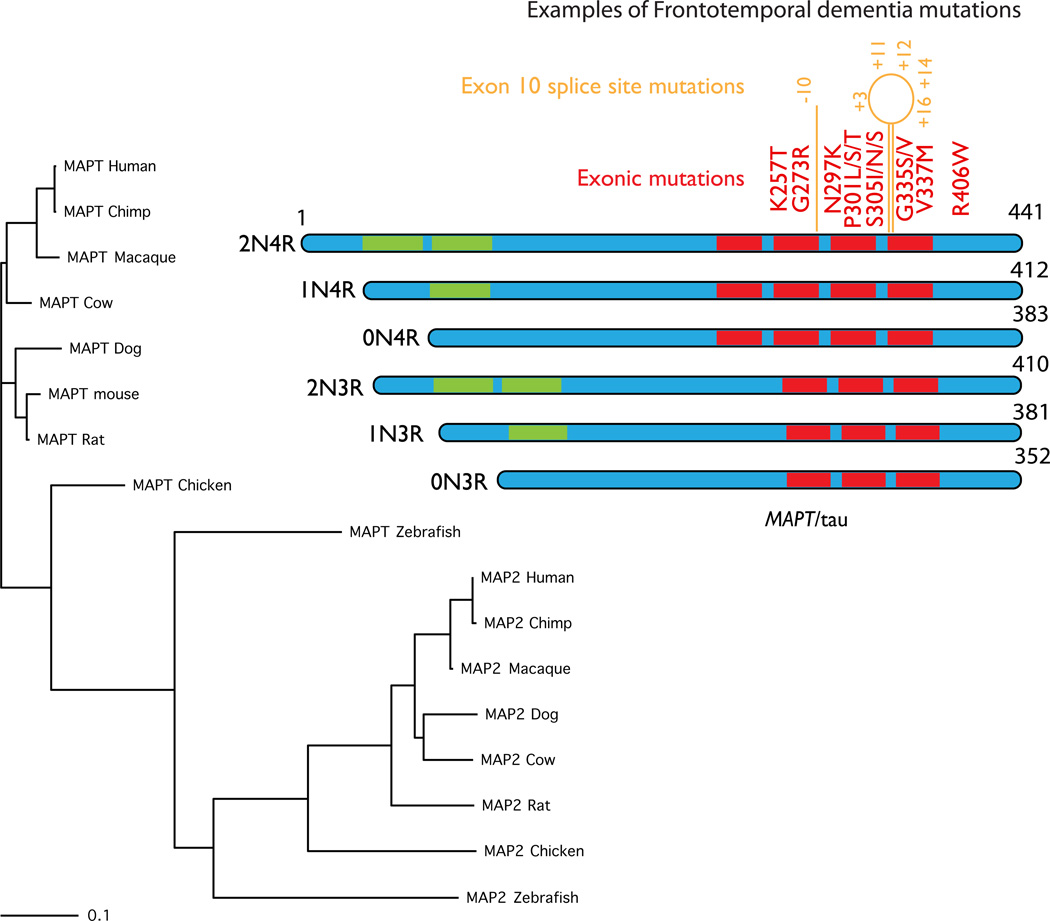
Figure 4. MAPT.
MAPT, coding for the axonal microtubule binding protein tau, is found in most vertebrates and conservation generally follows the standard phylogenetic tree for these organisms, as shown on the left of this figure. There are many other microtubule binding proteins but for clarity, here I have shown the nearest group, which includes the dendritic microtubule binding protein MAP2. These are a separate group of proteins but again these follow the same phylogenetic pattern across species. The protein structure of the six known tau isoforms is shown on the left. These isoforms are generated by alternate splicing of exons 2 and 3 (in green in the ideograms) generating different numbers of amino terminal inserts (2N, 1N or 0N) and by splicing of exon 10, which generates one extra microtubule binding repeat (4R or 3R). A selection of the known exonic FTD mutations is shown in red. In orange are intronic mutations that change the splicing but not the amino acid sequence, numbered by nucleotide position relative to exon 10.
All genes in the MAP2/tau family show extensive splicing and, interestingly, there are differences between splicing in different species. For example, in mice exon 10 of tau, which encodes tau protein that has four microtubule binding repeats (4R tau in contrast to 3R tau), has low levels of inclusion in mRNA in embryonic mice but rises during development to near complete inclusion in adults, i.e., the tau in adult mice is predominantly 4R-tau [84]. In contrast, the normal human adult brain contains both 4R and 3R tau at apparently equimolar amounts [85].
The mutations in tau associated with FTD include both point mutations and mutations in splice sites [86]. Therefore, both the sequence of the tau protein and the relative inclusion of specific exons is important in determining the risk of dementias. The role of splicing in tau is further emphasized by the fact that there is variation in the deposited tau species in different tauopathies. For example, in AD there is deposition of both 3R and 4R tau whereas in PSP 4R tau predominates [87]. This demonstrates that for a protein such as tau, the underlying protein structure may be important in understanding how it may play roles in different diseases. I suspect that tau has the general capacity to be neurotoxic but there are multiple ways in which it can be detrimental to neurons, by changing the balance of splicing, by overall net expression levels or by accumulation of modifications such as phosphorylation.
One interesting aspect of the evolutionary history of MAPT is that in the locus on Chromosome 17 in which this gene is situated appears to have undergone inversion at several times in different species [88]. In humans, the two inverted version of the locus are named H1 and H2; H1 is the more frequent version but is more recent evolutionarily [89]. Some authorities have speculated that the retention of H2 seen in some human populations may have come from interbreeding with Homo neanderthalensis [90] although full support for this idea would require sequencing the MAPT region in samples from Neanderthals. H2 is protective against lifetime risk of PD and PSP, but is absent from Asian populations which explains why signals were seen in GWAS studies performed in European [74, 91] but not Japanese [92] ancestry.
The overall message from the history of these genes is that they appear to have relatively little in common with each other, apart from being expressed in the brain. However, in two cases humans and primates have difference in sequence (for SCNA) or splicing (MAPT) that distinguishes them from other organisms. Many of these organisms are used as model systems, each with their own advantages and disadvantages, but of course none are human. Where model systems lack a close homologue, e.g., for α-synuclein in yeast flies and worms, it is important to validate results in species that do [30]. Even within vertebrate species, it is interesting that removal of endogenous murine homologues of SNCA [93] or MAPT [94] produces exaggerated effects of expression of the mutant human proteins. Therefore, in at least some circumstances our most convenient models may need to be ‘humanized’ to provide better models.
Is neurodegeneration subject to evolutionary constraints?
Why would we have in our brains proteins such as α-synuclein or tau that, without substantive modification appears to be able to accumulate and cause some rather distressing diseases? One argument is that evolutionary forces will be generally neutral as neurodegeneration occurs in a period of life that, for most people throughout most of human history, is post-reproductive. I would concur that the presence of neurodegenerative diseases at reasonable population frequencies in people over 50 years old does imply that there is not strong evolutionary selection against those diseases.
But this doesn’t mean that there isn’t selection at the same loci for beneficial properties of the same protein. Overall we see that, although neurodegeneration is not likely to have been selected for and may not have been selected against, we have proteins that are apparently dispensable for CNS function but that are expressed at levels close to those needed to trigger neurodegeneration.
For example, that expression of α-synuclein is relatively high, and close to the threshold needed to cause disease, suggests that even if not necessary to CNS function, it likely plays an important role in the human brain. In this context it is interesting that the known roles of synuclein relate to plasticity [95] and/or stress [96]. Therefore, in a long-lived species like humans, where chronic stress may have a cumulative effect, the role of α-synuclein might be especially important in keeping the brain functioning to its highest level. Therefore, the levels of α-synuclein protein may have been selected for, and there may be a delicate balancing act in keeping within an appropriate concentration range.
Loci for other neurodegenerative diseases are also subjected to evolutionary forces. For example, the MAPT inversion has been shown to be under purifying selection in some populations [89]. The reasons for this selection are not clear although there appears to be an association with the more recent allele and women having more children. Another observation is that several infectious diseases that affect the brain cause tauopathy [96] and it is therefore possible that certain brain diseases may contribute some of the selection, although again this is difficult to test. Perhaps most dramatically tau is required for the normal development of the human brain – deletions at the locus are associated with severe developmental problems in children [97]. It is likely therefore that the structural role that tau plays in strengthening axons is beneficial for the development of the human brain.
Part of what makes human tau different from other species is the more complex mix of isoforms discussed above. The relevance of this is uncertain, although the interestingly the presence of 3R can inhibit the aggregation of 4R tau in mixtures of the two in vitro [98]. The human brain is relatively large compared to other species, and I wonder if there is adaptation for higher levels of tau protein to support longer axons of neurons in the brain that, in turn, might require the presence of 3R tau to prevent 4R protein constantly aggregating.
There is also evidence of purifying selection at the LRRK2 locus, acting to remove rare deleterious alleles [99]. Again, whether this has anything to do with the PD, or even the brain, is unclear. The LRRK2 locus is a risk factor for Crohn’s disease and leprosy. The molecular basis of this association is unclear [100], but may be related to the expression of LRRK2 in immune cells discussed above.
The main point is that many, perhaps all, of the genes that are associated with neurodegeneration have been selected for because of properties that are beneficial in the context of the human brain or other tissues. Following from this, those proteins are evolutionarily advantageous, even if redundancy of other proteins in the same family masks the effects of loss of function mutations in model organisms. The generally post-reproductive phenotypes of late-onset neurodegeneration are not strong enough for purifying selection to remove the specific alleles associated with Parkinson’s disease even if those phenotypes are unpleasant. Therefore, pathogenic alleles persist in the population and this may, in part, be due to their subtle effects where disease processes are initiated in the context of a specific organ in a long-lived species where chronic stress and other stochastic processes abound.
Conclusions
The human brain expresses a number of genes that when mutated or expressed at higher levels contribute to the risk of neurodegenerative diseases. It seems likely that the expression of these genes and their regulation has been selected for over our evolutionary history, even as the specific alleles that endow an increased risk of disease are not strongly selected against. It is not clear that these considerations help cure diseases and in some cases simple arguments about conservation of residues isn’t helpful even in understanding pathogenicity, but understanding the evolutionary history of genes like SNCA, MAPT and LRRK2 helps place their function into context and should influence our views about model organisms. Some of these considerations suggest why alleles persist in the human population and why, especially as the population ages, neurodegenerative diseases are going to remain a significant medical problem.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Hardy J, Orr H. The genetics of neurodegenerative diseases. J. Neurochem. 2006;97:1690–1699. doi: 10.1111/j.1471-4159.2006.03979.x. [DOI] [PubMed] [Google Scholar]
- 2.Lill CM, Bertram L. Towards unveiling the genetics of neurodegenerative diseases. Semin Neurol. 2011;31:531–541. doi: 10.1055/s-0031-1299791. [DOI] [PubMed] [Google Scholar]
- 3.Halliday G, Lees A, Stern M. Milestones in Parkinson’s disease--clinical and pathologic features. Mov. Disord. 2011;26:1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- 4.Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 5.Gasser T, Hardy J, Mizuno Y. Milestones in PD genetics. Mov. Disord. 2011;26:1042–1048. doi: 10.1002/mds.23637. [DOI] [PubMed] [Google Scholar]
- 6.Saiki S, Sato S, Hattori N. Molecular pathogenesis of Parkinson’s disease: update. J. Neurol. Neurosurg. Psychiatr. 2012;83:430–436. doi: 10.1136/jnnp-2011-301205. [DOI] [PubMed] [Google Scholar]
- 7.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 8.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 9.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 10.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 11.Chartier-Harlin M-C, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 12.Spencer CCA, Plagnol V, Strange A, Gardner M, Paisan-Ruiz C, Band G, Barker RA, Bellenguez C, Bhatia K, Blackburn H, et al. Dissection of the genetics of Parkinson’s disease identifies an additional association 5’ of SNCA and multiple associated haplotypes at 17q21. Hum. Mol. Genet. 2011;20:345–353. doi: 10.1093/hmg/ddq469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devine MJ, Gwinn K, Singleton A, Hardy J. Parkinson’s disease and α-synuclein expression. Mov. Disord. 2011;26:2160–2168. doi: 10.1002/mds.23948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cookson MR. The biochemistry of Parkinson’s disease. Annu. Rev. Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- 15.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J. Neurosci. 2012;32:3301–3305. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cremades N, Cohen SIA, Deas E, Abramov AY, Chen AY, Orte A, Sandal M, Clarke RW, Dunne P, Aprile FA, et al. Direct Observation of the Interconversion of Normal and Toxic Forms of α-Synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podoly E, Hanin G, Soreq H. Alanine-to-threonine substitutions and amyloid diseases: butyrylcholinesterase as a case study. Chem. Biol. Interact. 2010;187:64–71. doi: 10.1016/j.cbi.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J. Biol. Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 21.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U.S.A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidel K, Schöls L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, Dickson D, Gai WP, Bornemann A, Riess O, et al. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann. Neurol. 2010;67:684–689. doi: 10.1002/ana.21966. [DOI] [PubMed] [Google Scholar]
- 23.Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J. Biol. Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 24.Markopoulou K, Wszolek ZK, Pfeiffer RF, Chase BA. Reduced expression of the G209A alpha-synuclein allele in familial Parkinsonism. Ann. Neurol. 1999;46:374–381. doi: 10.1002/1531-8249(199909)46:3<374::aid-ana13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Krüger R, Markopoulou K, Wszolek Z, Chase B, Taka H, Mineki R, Murayama K, Riess O, Mizuno Y, et al. Haploinsufficiency at the alpha-synuclein gene underlies phenotypic severity in familial Parkinson’s disease. Brain. 2003;126:32–42. doi: 10.1093/brain/awg010. [DOI] [PubMed] [Google Scholar]
- 26.Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science. 2003;302:1769–1772. doi: 10.1126/science.1090389. [DOI] [PubMed] [Google Scholar]
- 28.Sancenon V, Lee S-A, Patrick C, Griffith J, Paulino A, Outeiro TF, Reggiori F, Masliah E, Muchowski PJ. Suppression of α-synuclein toxicity and vesicle trafficking defects by phosphorylation at S129 in yeast depends on genetic context. Human Molecular Genetics. 2012;21:2432–2449. doi: 10.1093/hmg/dds058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet J-C, McCaffery JM, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM, Khan N, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Marín I, van Egmond WN, van Haastert PJM. The Roco protein family: a functional perspective. FASEB J. 2008;22:3103–3110. doi: 10.1096/fj.08-111310. [DOI] [PubMed] [Google Scholar]
- 34.Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: regulation of G proteins by dimerization. Nat. Rev. Mol. Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- 35.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu S-W, Savitt JM, Waldvogel HJ, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 37.Hatano T, Kubo S-I, Imai S, Maeda M, Ishikawa K, Mizuno Y, Hattori N. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum. Mol. Genet. 2007;16:678–690. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- 38.Berger Z, Smith KA, Lavoie MJ. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry. 2010;49:5511–5523. doi: 10.1021/bi100157u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alegre-Abarrategui J, Christian H, Lufino MMP, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum. Mol. Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, Kim C-H, Han BS, Tong Y, Shen J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp. Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Piccoli G, Condliffe SB, Bauer M, Giesert F, Boldt K, De Astis S, Meixner A, Sarioglu H, Vogt-Weisenhorn DM, Wurst W, et al. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J. Neurosci. 2011;31:2225–2237. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. NeuroBiol. Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 44.Iaccarino C, Crosio C, Vitale C, Sanna G, Carrì MT, Barone P. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum. Mol. Genet. 2007;16:1319–1326. doi: 10.1093/hmg/ddm080. [DOI] [PubMed] [Google Scholar]
- 45.Jorgensen ND, Peng Y, Ho CC-Y, Rideout HJ, Petrey D, Liu P, Dauer WT. The WD40 domain is required for LRRK2 neurotoxicity. PLoS ONE. 2009;4:e8463. doi: 10.1371/journal.pone.0008463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee BD, Shin J-H, VanKampen J, Petrucelli L, West AB, Ko HS, Lee Y-I, Maguire-Zeiss KA, Bowers WJ, Federoff HJ, et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat. Med. 2010;16:998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dusonchet J, Kochubey O, Stafa K, Young SM, Jr, Zufferey R, Moore DJ, Schneider BL, Aebischer P. A rat model of progressive nigral neurodegeneration induced by the Parkinson’s disease-associated G2019S mutation in LRRK2. J. Neurosci. 2011;31:907–912. doi: 10.1523/JNEUROSCI.5092-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hakimi M, Selvanantham T, Swinton E, Padmore RF, Tong Y, Kabbach G, Venderova K, Girardin SE, Bulman DE, Scherzer CR, et al. Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J Neural Transm. 2011;118:795–808. doi: 10.1007/s00702-011-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim B, Yang M-S, Choi D, Kim J-H, Kim H-S, Seol W, Choi S, Jou I, Kim E-Y, Joe E-H. Impaired inflammatory responses in murine lrrk2-knockdown brain microglia. PLoS ONE. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillardon F, Schmid R, Draheim H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Lin X, Parisiadou L, Gu X-L, Wang L, Shim H, Sun L, Xie C, Long C-X, Yang W-J, Ding J, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daher JPL, Pletnikova O, Biskup S, Musso A, Gellhaar S, Galter D, Troncoso JC, Lee MK, Dawson TM, Dawson VL, et al. Neurodegenerative phenotypes in an A53T α-synuclein transgenic mouse model are independent of LRRK2. Human Molecular Genetics. 2012;21:2420–2431. doi: 10.1093/hmg/dds057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, Hafner T, Stemmelen C, Troxler TJ, Schmid P, Danner S, et al. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whaley NR, Uitti RJ, Dickson DW, Farrer MJ, Wszolek ZK. Clinical and pathologic features of families with LRRK2-associated Parkinson’s disease. J. Neural Transm. 2006;(Suppl.):221–229. doi: 10.1007/978-3-211-45295-0_34. [DOI] [PubMed] [Google Scholar]
- 56.Cookson MR, Hardy J, Lewis PA. Genetic neuropathology of Parkinson’s disease. Int J Clin Exp Pathol. 2008;1:217–231. [PMC free article] [PubMed] [Google Scholar]
- 57.Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide B-MM, Schjeide LM, Meissner E, Zauft U, Allen NC, et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS Genet. 2012;8:e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, et al. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.International Parkinson’s Disease Genomics Consortium (IPDGC), and Wellcome Trust Case Control Consortium 2 (WTCCC2) A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet. 2011;7:e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pankratz N, Beecham GW, DeStefano AL, Dawson TM, Doheny KF, Factor SA, Hamza TH, Hung AY, Hyman BT, Ivinson AJ, et al. Meta-analysis of Parkinson’s disease: identification of a novel locus, RIT2. Ann. Neurol. 2012;71:370–384. doi: 10.1002/ana.22687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004;84:361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 62.Lin C-H, Tsai P-I, Wu R-M, Chien C-T. LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3β. J. Neurosci. 2010;30:13138–13149. doi: 10.1523/JNEUROSCI.1737-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zach S, Felk S, Gillardon F. Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity. PLoS ONE. 2010;5:e13191. doi: 10.1371/journal.pone.0013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawakami F, Yabata T, Ohta E, Maekawa T, Shimada N, Suzuki M, Maruyama H, Ichikawa T, Obata F. LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS ONE. 2012;7:e30834. doi: 10.1371/journal.pone.0030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melrose HL, Dächsel JC, Behrouz B, Lincoln SJ, Yue M, Hinkle KM, Kent CB, Korvatska E, Taylor JP, Witten L, et al. Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. NeuroBiol. Dis. 2010;40:503–517. doi: 10.1016/j.nbd.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kett LR, Boassa D, Ho CC-Y, Rideout HJ, Hu J, Terada M, Ellisman M, Dauer WT. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum. Mol. Genet. 2012;21:890–899. doi: 10.1093/hmg/ddr526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris M, Koyama A, Masliah E, Mucke L. Tau reduction does not prevent motor deficits in two mouse models of Parkinson’s disease. PLoS ONE. 2011;6:e29257. doi: 10.1371/journal.pone.0029257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 70.Golde TE, Petrucelli L, Lewis J. Targeting Abeta and tau in Alzheimer’s disease, an early interim report. Exp. Neurol. 2010;223:252–266. doi: 10.1016/j.expneurol.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.St George-Hyslop PH. Molecular genetics of Alzheimer’s disease. Biol. Psychiatry. 2000;47:183–199. doi: 10.1016/s0006-3223(99)00301-7. [DOI] [PubMed] [Google Scholar]
- 73.Boeve BF, Hutton M. Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN) Arch. Neurol. 2008;65:460–464. doi: 10.1001/archneur.65.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Höglinger GU, Melhem NM, Dickson DW, Sleiman PMA, Wang L-S, Klei L, Rademakers R, de Silva R, Litvan I, Riley DE, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milanese C, Sager JJ, Bai Q, Farrell TC, Cannon JR, Greenamyre JT, Burton EA. Hypokinesia and reduced dopamine levels in zebrafish lacking β- and γ1-synucleins. J. Biol. Chem. 2012;287:2971–2983. doi: 10.1074/jbc.M111.308312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busch DJ, Morgan JR. Synuclein accumulation is associated with cell-specific neuronal death after spinal cord injury. The Journal of Comparative Neurology. 2011;520:1751–1771. doi: 10.1002/cne.23011. [DOI] [PubMed] [Google Scholar]
- 77.Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, et al. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, Threlfell S, Kooner G, Deacon RM, Bannerman DM, et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurz A, Wöhr M, Walter M, Bonin M, Auburger G, Gispert S, Schwarting RKW. Alpha-synuclein deficiency affects brain Foxp1 expression and ultrasonic vocalization. Neuroscience. 2010;166:785–795. doi: 10.1016/j.neuroscience.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 81.Hamilton BA. alpha-Synuclein A53T substitution associated with Parkinson disease also marks the divergence of Old World and New World primates. Genomics. 2004;83:739–742. doi: 10.1016/j.ygeno.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 82.Greggio E, Lewis PA, van der Brug MP, Ahmad R, Kaganovich A, Ding J, Beilina A, Baker AK, Cookson MR. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J. Neurochem. 2007;102:93–102. doi: 10.1111/j.1471-4159.2007.04523.x. [DOI] [PubMed] [Google Scholar]
- 83.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D’Souza I. Tau isoform regulation is region- and cell-specific in mouse brain. J. Comp. Neurol. 2008;511:788–803. doi: 10.1002/cne.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nature reviews. [Accessed June 30, 2012];Neurology. 2012 doi: 10.1038/nrneurol.2012.117. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22732773. [DOI] [PMC free article] [PubMed]
- 87.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau) J. Mol. Neurosci. 2011;45:384–389. doi: 10.1007/s12031-011-9589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zody MC, Jiang Z, Fung H-C, Antonacci F, Hillier LW, Cardone MF, Graves TA, Kidd JM, Cheng Z, Abouelleil A, et al. Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat. Genet. 2008;40:1076–1083. doi: 10.1038/ng.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stefansson H, Helgason A, Thorleifsson G, Steinthorsdottir V, Masson G, Barnard J, Baker A, Jonasdottir A, Ingason A, Gudnadottir VG, et al. A common inversion under selection in Europeans. Nat. Genet. 2005;37:129–137. doi: 10.1038/ng1508. [DOI] [PubMed] [Google Scholar]
- 90.Hardy J, Pittman A, Myers A, Gwinn-Hardy K, Fung HC, de Silva R, Hutton M, Duckworth J. Evidence suggesting that Homo neanderthalensis contributed the H2 MAPT haplotype to Homo sapiens. Biochem. Soc. Trans. 2005;33:582–585. doi: 10.1042/BST0330582. [DOI] [PubMed] [Google Scholar]
- 91.Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 93.Cabin DE, Gispert-Sanchez S, Murphy D, Auburger G, Myers RR, Nussbaum RL. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neuro Biol. Aging. 2005;26:25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 94.Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde Y-A, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- 95.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 96.Quilty MC, King AE, Gai W-P, Pountney DL, West AK, Vickers JC, Dickson TC. Alpha-synuclein is upregulated in neurones in response to chronic oxidative stress and is associated with neuroprotection. Exp. Neurol. 2006;199:249–256. doi: 10.1016/j.expneurol.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, et al. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat. Genet. 2006;38:1032–1037. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- 98.Adams SJ, DeTure MA, McBride M, Dickson DW, Petrucelli L. Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS ONE. 2010;5:e10810. doi: 10.1371/journal.pone.0010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rubio JP, Topp S, Warren L, St Jean PL, Wegmann D, Kessner D, Novembre J, Shen J, Fraser D, Aponte J, et al. Deep sequencing of the LRRK2 gene in 14,002 individuals reveals evidence of purifying selection and independent origin of the p.Arg1628Pro mutation in Europe. Human mutation. 2012;33:1087–1098. doi: 10.1002/humu.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis PA, Manzoni C. LRRK2 and human disease: a complicated question or a question of complexes? Sci Signal. 2012;5:pe2. doi: 10.1126/scisignal.2002680. [DOI] [PubMed] [Google Scholar]