Abstract
Objectives
Many older cancer survivors are overweight or obese, with additional illness burden increasing functional decline, which may affect their ability to engage in lifestyle interventions. This study examined how overweight long-term survivors’ symptom severity associated with comorbidity prior to a diet and exercise intervention was associated with post-intervention function and examined symptoms’ effects on function through change in physical activity, diet quality, and weight status.
Materials and Methods
This is a secondary data analysis of 514 breast, prostate, and colorectal cancer survivors who participated in the one-year home-based diet and exercise intervention Reach-Out to Enhance Wellness trial. Measures included symptoms, weight, physical activity, diet quality, overall physical function (PF), and basic and advanced lower extremity function (BLEF and ALEF). Simple and serial mediation analyses were conducted to examine direct effects of symptom severity on PF, BLEF and ALEF and indirect effects of symptom severity through changes in diet quality, physical activity, and weight.
Results
Symptom severity was directly associated with lower functioning scores for PF (b=−0.63 p<0.001), BLEF (b=−0.33, p<0.001) and ALEF (b=−0.22, p<0.001). Indirect effects of symptom severity through weight loss, physical activity and diet were not significant. Weight loss and physical activity were associated with higher PF and ALEF and diet quality was associated with higher BLEF.
Conclusion
Symptom severity of older, overweight cancer survivors negatively affects physical function. However, greater weight loss and more physical activity were associated with higher functioning scores, regardless of symptom severity.
Keywords: survivors, prostate, breast, symptoms, physical activity, weight loss, functioning
Introduction
Older cancer survivors (≥65 years) experience more rapid functional decline compared to younger cancer survivors, as well as age-matched counterparts in the general population.[1,2] Declines in physical function are concerning because of the consistent associations with inability to perform activities of daily living,[3] therefore threatening the propensity for independent living among older adults.[4] Functional decline may be compounded by obesity – an increasingly prevalent problem among cancer survivors and the elderly.[5,6] Physical activity and dietary interventions have had positive effects on preventing or slowing functional decline in obese and overweight cancer survivors.[7,8] One mechanism through which interventions may prevent functional decline is through physical activity’s positive effects on muscular strength and balance.[9] Alternatively, physical activity and diet may influence function through weight loss in those who are overweight and obese.[9,10]
Similar to their non-cancer counterparts, aging and overweight cancer survivors are also more likely to be “sicker” than normal weight survivors (e.g., comorbidities and associated symptoms, such as muscle weakness, chest pain or balance issues [11]) which may influence behavior change, such as improving physical activity[12] or dietary habits. The relationship between symptom severity, physical activity and diet quality, weight loss, and the impact on function is not straightforward. Among overweight survivors, symptoms may have independent effects on functional limitations as well as through their relationship with physical activity,[12,13] diet, and weight loss. Physical activity and diet quality may improve symptoms,[14–17] but changes in these behaviors, whether positive or negative, may also be indicative of the baseline health state of survivors who had activity-limiting symptom severity at the time activity and diet was measured.
Understanding the influence of older overweight cancer survivors’ health states on their ability to successfully change health behaviors, lose weight, and ultimately retain function is critical for intervention development and evaluation. Examining this issue is timely given the American Society of Clinical Oncology’s recent position on weight management and recognition that comorbidity is exceptionally prevalent in this population.[18] The Reach-Out to Enhance Wellness (RENEW) trial provides a unique data source to examine the relationship between symptom severity, behavioral intervention (exercise and diet quality), weight loss and lower extremity function in overweight long-term cancer survivors.[7] The trial used a multicomponent approach of both diet and exercise to purposefully initiate gradual weight loss among older obese and overweight breast, prostate, and colorectal cancer survivors to positively impact physical functioning.
Our objective was to examine how pre-intervention symptom severity was associated with post-intervention overall physical functioning and lower extremity functioning through its effects on survivors’ weight loss via physical activity and diet quality changes. To accomplish this objective, we examined the mediating effects of physical activity and diet quality changes and weight loss on the relationship between survivors’ pre-intervention symptom severity and post-intervention function (Appendix A). We hypothesized that baseline symptom severity would have negative direct effects on post-intervention function. We also hypothesized that symptom severity would have negative effects weight loss through lower changes in physical activity and diet quality, and subsequently lead to lower function.
Materials and Methods
Study Design and population
This is a secondary data analysis of the RENEW trial which has been described in detail elsewhere.[9,19] Briefly, RENEW was a 12-month home-based diet-exercise intervention delivered to overweight or obese (body mass index (BMI) ≥25 kg/m2) and inactive (<150 minutes of exercise/week) survivors of breast, prostate, and colorectal cancer. Survivors were at least 5 years from diagnosis with no evidence of progressive disease. Survivors with health conditions that precluded them from unsupervised exercise were excluded. Tailored mailed-print materials and telephone counseling were delivered to 641 survivors in a cross-over design with the primary goals of increasing physical functioning and quality-of-life by improving diet quality and physical activity. The mailed material included personalized workbooks as well as exercise and diet “tools” (i.e., resistance bands, tableware to enhance portion control) designed to assist survivors in meeting national guidelines. Telephone counseling was provided to help survivors overcome barriers to change and monitor change. Intervention details are described in Snyder et al, 2009.[19] All surveys were delivered each of the three measurement periods. The current study focuses on survivors with complete pre- and post-intervention data (n=514; Appendix B). The study was approved by the Duke University Health System and the North Carolina Cancer Registry Institutional Review Boards.
Measures
Overall physical function
The physical functioning (PF) subscale of the Medical Outcomes Study Short Form-36 (MOS SF-36)[20] served as the primary outcome of the RENEW trial and the present analysis. This 10-item subscale assesses a range of function, from basic activities (i.e., self-care) to participation in vigorous activities and is scored on a 0 to 100 scale. The RENEW intervention prevented the significant decline in PF.[9] Therefore, our primary outcome of interest was the post-intervention PF subscale score.
Lower extremity function
We also used the Basic and Advanced Lower Extremity Function subscales of the Late-Life Function and Disability Index to create two other primary outcome measures.[21] These included: basic lower extremity function (BLEF) and advanced lower extremity function (ALEF). Questions ask how much difficulty the respondent has doing a particular activity without the help of others or assistive devices. Responses range from “none” to “cannot do.” Responses are summed and scored as 0–100 with higher scores indicating higher functioning. The BLEF assesses the ability to perform activities such as stooping and walking without assistance while the ALEF assesses the ability to perform physical activities that require a significant degree of ability and endurance. Similar to PF, we used post-intervention BLEF and ALEF scores.
Symptom severity
Symptom severity was measured using items from a modified version of the Duke Older American Resources and Services (OARS) physical health items.[22] Survivors were asked to what degree 22 different symptoms limited normal activities (e.g., chest pain, shortness of breath; Appendix C) on a scale of 1–4 with 1=not all and 4=quite a bit. The OARS symptom items are designed to measure common symptoms associated with general aging, not specifically for cancer treatment side-effects, given that these are long-term survivors. Total scores of baseline measures were calculated where higher scores indicate worse symptoms.
Change in physical activity
The Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity (PA) scale[23] is a 41-item measure to assess PA in adults 50 years and older. We used the change in total minutes of PA and energy expended weekly in all physical activities of moderate or higher intensity from pre- to post-intervention. Higher values indicated an increase in PA.
Change in diet quality
The Healthy Eating Index (HEI) measured diet quality (DQ). The HEI uses dietary intake data averaged from two unannounced 24-hour recalls using the Nutrition Data System for Research software.[24] The Healthy Eating Index 2005 criterion, in practice during the time of the RENEW trial, was used to create scores pre- and post-intervention.[25,26] Change in DQ was calculated by subtracting the baseline HEI score from the follow-up HEI score. Higher values indicated DQ improvement.
Weight loss
Self-reported height and weight were used to calculate BMI pre- and post-intervention. We examined the change in BMI [BMIchange=BMIbaseline–BMIfollow-up]. Higher values (positive) indicated a greater weight loss.
Demographic and clinical characteristics
Age, race, gender, income, stage at diagnosis, treatment received, years since diagnosis, and comorbidities were all self-reported at baseline. Comorbidities were measured using the Duke OARS comorbidity scale. The OARS is a “yes or no” checklist of 32 comorbid conditions.[22] To reduce survey administration burden, the RENEW study assessed six of the most common comorbid conditions (arthritis or rheumatism, high blood pressure, heart trouble, circulation trouble in arms or legs, osteoporosis, cataracts). Scores were summed to create a total number of comorbidities with a range of 0 to 6 conditions.
Statistical approach
Data were analyzed using SAS statistical software Version 9.3 (Cary, NC).[27] Frequencies were calculated for categorical variables, and means and standard deviations were calculated for continuous variables. We first tested bivariate relationships between the hypothesized mediating variables, covariates, and functioning outcomes (PF, BLEF, ALEF). We used the PROCESS macro developed by Hayes[28] to examine a series of multiple regression models examine the relationship between pre-intervention symptom severity and post-intervention function, both directly and through the intervention[29,30].
We used linear regression to evaluate the bivariate relationships between the three dependent variables and pre-intervention symptoms, PA change, DQ change, weight loss, age, race, gender, income, stage at diagnosis, time since diagnosis, number of comorbidities, and treatment received. We did not require statistically significant relationships between all hypothesized mediators, dependent variables, and primary independent variable, but required a significant relationship between the primary independent variable (symptom severity) and each dependent variable (function) for the mediation model. This approach is tested and supported by MacKinnon and colleagues and is valid with bootstrapping and resampling techniques in the Hayes’ PROCESS macro.[28–30]
Multiple regression analyses were used to test for mediation. We conducted both simple (Appendix A, Model A) and serial mediation (Appendix A, Model B) adjusting each model for pre-intervention values of function, DQ, and PA as well as age, race, gender, income, stage at diagnosis, time since diagnosis, treatment received, comorbidity, and randomization level.
We examined the direct effect (e.g., associations between two variables) of pre-intervention symptoms on post-intervention function outcomes (PF, BLEF, ALEF) and the effect of symptoms on function through weight loss (indirect effects). Indirect effects refer to the amount of mediation introduced by including additional relationships between the primary independent variable and the dependent variable (Y) in to the model (e.g., the association of symptoms with post-intervention function score through weight loss). Using PROCESS, weight loss (M) is estimated from symptom severity (X) controlling for covariates (Appendix A). Then, the indirect effect of X on Y through M is estimated as the product of coefficients linking X to M and X to Y.[30,31]
We then examined the associations of symptom severity on post-intervention function through weight loss via the effect of change in DQ and PA on weight loss. For serial mediation, estimation of direct and indirect effects requires coefficients from up to 4 equations: one for each mediator and one for function (Appendix C). Indirect effects are the product of coefficients for variables linking symptom severity to function through one or more mediators.
The significance of indirect effects of symptom severity through mediators was determined using a bootstrap procedure[32] which uses non-parametric resampling that provides empirical approximation of the sampling distributions of indirect effects.[31] The procedure does not require distributional assumptions of the shape of variables nor sampling distribution of the statistic.[33] We report bias-corrected and accelerated 95% CIs for the significance of the mean indirect effects from the 5,000 bootstrap resampling results.[34]
Results
Study characteristics
The sample was mostly white (89%) and female (55%) (Table 1). The mean age of participants was 79 years and almost half were breast cancer survivors (44%). At baseline, participants were on average 8.6 years post-diagnosis and had two comorbid conditions. Table 2 shows the mean PF, BLEF and ALEF post-intervention scores were 74, 78 and 52, respectively.
Table 1.
Sample characteristics (n=514)
| N | %a | |
|---|---|---|
| Age | ||
| Mean (SD) | 72.97 (5.1) | |
| Range | (65–87) | |
| Race | ||
| White | 459 | 89.3 |
| Gender | ||
| Male | 233 | 45.3 |
| Income | ||
| <$50,000 | 180 | 35.0 |
| >$50,000 | 334 | 65.0 |
| Education | ||
| Less than high school | 29 | 5.6 |
| High school | 160 | 31.2 |
| Some college | 125 | 24.4 |
| College graduate or higher | 198 | 38.6 |
| Missing | 2 | 0.39 |
| Cancer type | ||
| Colon/rectal cancer | 80 | 15.6 |
| Breast cancer | 229 | 44.6 |
| Prostate | 205 | 39.9 |
| Stage at diagnosis | ||
| In situ | 4 | 0.8 |
| Localized | 350 | 68.1 |
| Regional | 141 | 27.4 |
| Unknown/unstaged | 10 | 2.0 |
| Missing | 8 | 1.56 |
| Number of conditionsb | ||
| Mean (SD) | 1.99 (1.28) | |
| (range) | (0–6) | |
| Time from diagnosis to baseline (years) | ||
| Mean (SD) | 8.61 (2.6) | |
| (Range) | (5–26) | |
| Received surgery | ||
| Yes | 460 | 89.5 |
| Received chemotherapy | ||
| Yes | 131 | 25.5 |
| Received radiation | ||
| Yes | 229 | 44.6 |
| Received hormone therapy | ||
| Yes | 216 | 42.0 |
| BMI | ||
| Pre-intervention | 28.9 (3.5) (22–46) | |
| Post-intervention | 28.2 (3.5) (21–44) | |
Variables with no missing category had no missing data.
Arthritis or rheumatism, high blood pressure, heart trouble, circulation trouble in the arms or legs, osteoporosis, cataracts.
Table 2.
Mean scores and standard deviations for function scores, symptom severity, change in PA, change in DQ, and weight loss.
| Mean (SD) | Range | |
|---|---|---|
|
| ||
| Physical functioning | ||
| Pre-intervention | 71.7 (23.1) | 5–100 |
| Post-interventiona | 73.5 (20.4) | 5–100 |
| Basic lower extremity function | ||
| Pre-intervention | 77.7 (14.8) | 34.5–100 |
| Post-interventiona | 78.1 (15.5) | 0–100 |
| Advanced lower extremity function | ||
| Pre-intervention | 52.4 (15.7) | 0–100 |
| Post-interventiona | 52.3 (17.1) | 0–100 |
| Symptom severityb | 8.1 (7.9) | 0–52 |
| Change in PA score (minutes per week exercise)c,d | 45.8 (288.77) | −2265–1720 |
| Change in DQ (HEI) c,d | 6.2 (14.3) | −37.7–54.7 |
| Weight lost by BMI (kg/m2) d | 0.69 (1.39) | −4–11 |
Post-intervention score was used as the outcome for mediation models.
Pre-intervention score.
Significant change from pre- to post-
Higher (positive) values indicate more PA, better DQ, and more weight lost
Bivariate regression
Table 3 shows that higher symptom severity was significantly associated with lower post-intervention PF, BLEF, and ALEF scores (B=−1.49, p<0.001; B=−0.89, p<0.001; B=−0.96, p<0.001, respectively). Symptom severity explained about one-quarter of the variance in all three function outcomes. Increased PA was significantly associated with PF, BLEF, and ALEF, but explained <1% of the variance. Increased DQ was significantly associated with BLEF and ALEF. Older age was associated with lower BLEF and ALEF. Female gender, lower income, more comorbidities, and hormonal therapy were significantly negatively associated with all three outcomes.
Table 3.
Bivariate relationship between physical functioning, mediators, and demographic and clinical characteristics
| SF-36 physical functioninga | Basic lower extremity functiona | Advanced lower extremity functiona | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (SD) | p-value | Adj R2 | B (SD) | p-value | Adj R2 | B (SD) | p-value | Adj R2 | |
| Symptom severityb | −1.49 (0.11) | <0.001 | 0.26 | −0.89 (0.11) | <0.001 | 0.28 | −0.96 (0.07) | <0.001 | 0.26 |
| Change in physical activityc | 0.01 (0.004) | 0.001 | 0.01 | 0.01 (0.002) | 0.01 | 0.01 | 0.01 (0.003) | 0.011 | 0.01 |
| Change in diet qualityc | 0.10 (0.07) | 0.144 | <0.01 | 0.12 (0.05) | 0.024 | 0.01 | 0.15 (0.06) | 0.011 | 0.01 |
| Change in BMI (kg/m2)c | 1.37 (0.73) | 0.061 | <0.01 | 0.95 (0.49) | 0.050 | 0.01 | 0.83 (0.54) | 0.123 | <0.01 |
| Age (years) | −0.39 (0.20) | 0.056 | 0.01 | −0.38 (0.13) | 0.005 | 0.01 | −0.63 (0.15) | <0.001 | 0.03 |
| Gender (ref*=female) | |||||||||
| Male | 7.03 (2.02) | <0.001 | 0.02 | 5.66 (1.35) | <0.001 | 0.03 | 9.54 (1.46) | <0.001 | 0.08 |
| Race (ref=Non-minority) | |||||||||
| Minority | 4.36 (3.29) | 0.186 | 0.002 | 3.88 (2.20) | 0.078 | <0.01 | 2.56 (2.44) | 0.295 | <0.01 |
| Income (ref=>$50,000) | |||||||||
| <$50,000 | −7.62 (2.11) | <0.001 | 0.02 | −4.89 (1.42) | 0.001 | 0.02 | −6.92 (1.56) | <0.0001 | 0.04 |
| Stage at diagnosis (ref=Stage 0/I) | |||||||||
| Stage II–III | −0.54 (2.23) | 0.808 | <0.01 | −0.84 (1.50) | 0.576 | 0 | −0.12 (1.65) | 0.943 | 0 |
| Number of comorbiditiesb | −5.23 (0.75) | <0.001 | 0.08 | −4.09 (0.51) | <0.001 | 0.11 | −5.48 (0.55) | <0.001 | 0.16 |
| Surgery (ref=no) | |||||||||
| Yes | −1.29 (3.32) | 0.697 | <0.01 | −4.23 (2.22) | 0.057 | 0.01 | −3.00 (2.46) | 0.224 | <0.01 |
| Chemotherapy (ref=no) | |||||||||
| Yes | −3.12 (2.33) | 0.182 | <0.01 | −2.35 (1.56) | 0.133 | <0.01 | −3.78 (1.73) | 0.029 | 0.01 |
| Radiation (ref=no) | |||||||||
| Yes | −5.88 (2.03) | 0.004 | 0.01 | −2.63 (1.37) | 0.055 | 0.01 | −4.12 (1.51) | 0.007 | 0.01 |
| Hormone therapy (ref=no) | |||||||||
| Yes | −5.99 (2.05) | 0.004 | 0.01 | −4.69 (1.37) | 0.001 | 0.02 | −5.59 (1.51) | <0.001 | 0.02 |
B=unstandardized coefficients; SD=standard deviation. Ref=reference group.
Post-intervention.
Pre-intervention.
Change scores; higher (positive) values indicate improvement, i.e., more physical activity.
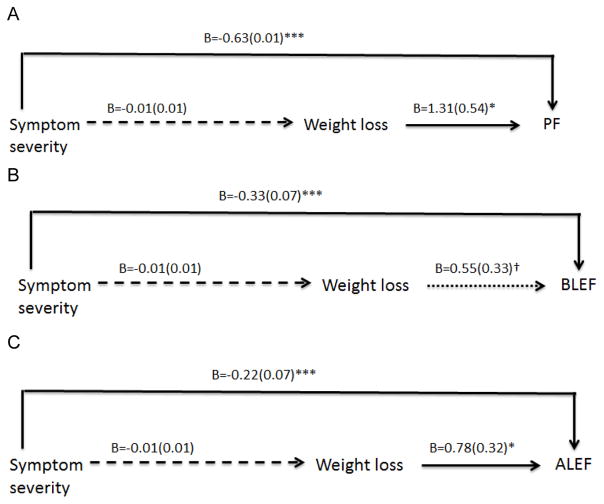
Simple mediation (Figure 1)
Figure 1.
Simple mediation models: physical functioning (PF), basic (BLEF) and advanced (ALEF) lower extremity functioning. Unstandardized coefficients and standard deviations are presented. All Models adjusted for pre-intervention values, comorbidities, age, gender, race, income, and diagnosis stage, treatment, and years since diagnosis. PA and DQ change remain as covariates. Solid lines indicate statistically significant relationships. *p<0.001; **p<0.01; ***p<0.05; †p<0.10.
Symptom severity was directly associated with lower PF, BLEF, and ALEF (Figure 1A–C). Conversely, weight loss was directly associated with higher PF and ALEF scores. The specific indirect effect of symptom severity on function through weight loss was not significant (Table 4).
Table 4.
Total and Indirect effects and 95% confidence intervals
| Mediation model | SF-36 PFa | BLEFa | ALEFa |
|---|---|---|---|
| Figure 1 | B 95% CI | B 95% CI | B 95% CI |
| Symptomb–weight lossc–function | −0.018(−0.061, 0.004) | −0.005(−0.026, 0.003) | −0.010(−0.036, 0.002) |
| Total effects | −0.649(−0.869, −0.428) | −0.330(−0.463, −0.198) | −0.232(−0.361, −0.102) |
| Adjusted R2 | 0.53 | 0.62 | 0.69 |
| Figure 2 | |||
| Symptom–weight loss–function | −0.018(−0.061, 0.004) | −0.005(−0.026, 0.003) | −0.0102(−0.036, 0.002) |
| Symptom–DQ changec–function | 0.0003(−0.011, 0.018) | −0.004(−0.029, 0.011) | 0.0004(−0.013, 0.018) |
| Symptom–PA changec–function | −0.010(−0.055, 0.027) | −0.015(−0.039, 0.005) | −0.01(−0.039, 0.0134) |
| Symptom–PA change–weight loss–function | −0.0002(−0.004, 0.001) | −0.0002(−0.002, 0.0003) | −0.0001(−0.002, 0.0003) |
| Symptom–DQ change–weight loss–function | 0.0001(−0.002, 0.005) | −0.0003(−0.003, 0.0004) | 0.0001(−0.002, 0.002) |
| Symptom–DQ change–PA change–function | 0.0004(−0.008, 0.010) | −0.002(−0.007, 0.004) | 0.0002(−0.006, 0.006) |
| Symptom–DQ change–PA change–weight loss–function | 0.00001(−0.0003, 0.0006) | −0.0001(−0.0007, 0.0001) | 0.0001(−0.0002, 0.0002) |
| Total indirect | −0.028(−0.093, 0.020) | −0.026(−0.061, 0.003) | −0.019(−0.057, 0.015) |
| Total effects | −0.658(−0.883, −0.434) | −0.351(−0.487, −0.215) | −0.241(−0.375, −0.107) |
| Adjusted R2 | 0.51 | 0.59 | 0.67 |
B=unstandardized coefficients;
Post-intervention score.
Pre-intervention score.
Change scores; higher (positive) values indicate improvement, i.e., more physical activity.
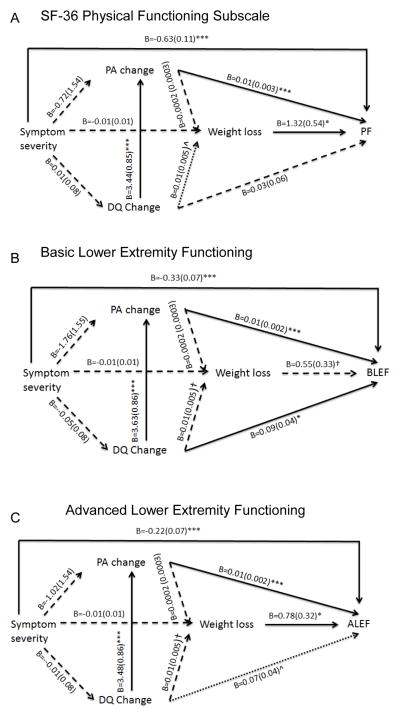
Serial mediation (Figure 2)
Figure 2.
Serial mediation models. Unstandardized coefficients and standard deviations are presented. All models adjusted for pre-intervention values for change scores and function, comorbidities, age, gender, race, income, and stage at diagnosis, and treatment. → statistically significant relationships; *p<0.001; **p<0.01; ***p<0.05. ⇢ marginal significance at ^p=0.05. †p<0.10
In Figure 2, symptom severity remained directly associated with lower PF, BLEF and ALEF scores, with a similar magnitude of effect within each function outcome. Greater weight loss remained significantly and directly associated with higher PF and ALEF scores. Similar to Figure 1A–C, the indirect effects of symptom severity were not significant. A greater increase in PA was also directly associated with higher PF, BLEF, and ALEF scores. Symptom severity and weight loss remained directly associated with PF and ALEF while change in DQ was significantly associated with BLEF, and marginally significantly associated with ALEF (B=0.07, p=0.05).
Discussion
While physical functioning has been an important target for improvement among older cancer survivors, obesity is a new concern, and oncologists may be reluctant to advise weight loss, especially among older, sicker survivors. However, findings from this secondary analysis suggest that despite the symptom burden experienced by older survivors, purposeful weight loss may indeed be a key factor for overall physical functioning and advanced lower extremity function. Our sample of “at-risk” cancer survivors, i.e., overweight and obese with an average of two comorbid conditions, provides additional evidence to the few previous studies,[35] that overweight long-term cancer survivors stand to improve their functioning through purposeful weight loss. We were also able to examine whether common symptoms influenced diet and exercise changes aimed at improving function. While some of our findings were anticipated, we also uncovered some that were unexpected.
We had hypothesized that the relationship between symptom severity, weight loss, and function would be partially due to the effects of symptoms on the ability to change behaviors. However, our findings indicate that symptom severity did not deter survivors’ behavior change. This is in contrast to other studies where symptoms, including fatigue, psychological distress, or lymphedema, were associated with less physical activity,[36] and better diet quality was associated with decreased fatigue.[37] It is likely that factors in the fully adjusted model, particularly comorbidities, explained some of the variance in the relationship between symptoms and weight loss in our models. We considered whether cancer type may have an effect on the relationship between symptoms, weight loss, and function, but cancer type did not affect the results and was not significant. The type of treatment received was more strongly associated with both symptoms and function.
Thus, this study provides unique findings regarding the effect of symptom severity on function via impact on lifestyle intervention “engagement” and associated weight loss; moreover, it does so in an understudied patient population, i.e., long-term cancer survivors age 65 and older.[11] These older survivors are in need of lifestyle intervention studies as much, if not more than, their younger counterparts. Targeting this group of older survivors, as opposed to excluding, for functional improvement and purposeful weight loss when needed will require innovative intervention approaches to deal with complex illness burden associated with aging. Future studies should determine whether symptom alleviation actually result in improvement in physical function, as is suggested in our preliminary study, and identify which symptoms are critical barriers to function.
Despite these strengths and implications, limitations should be considered. First, the sample was largely homogenous based on sample characteristics and there was 23% loss-to-follow-up thus limiting generalizability to other populations. Second, we relied on self-reported measures of diet, weight and physical activity and function. Finally, the primary RENEW intervention trial was not designed to achieve adequate power to detect indirect effects from this secondary analysis, therefore results are preliminary.
To conclude, while symptom severity among older, overweight cancer survivors negatively affected all aspects of physical functioning, it did not significantly influence diet or physical activity changes, or weight loss. Findings are especially relevant given the increasing number of aging cancer survivors and the health care burden associated with functional problems that limit independence.[38] Furthermore, our findings build from the recent emphasis on the negative effects of obesity on survivor outcomes to highlight weight loss as an important factor in maintaining function in older cancer survivors.[39] Weight loss and physical activity change interventions can successfully influence functioning despite the underlying symptom severity of overweight survivors.
Supplementary Material
Acknowledgments
Funding: RENEW was supported by grants no. CA106919, P30AG028716 from the National Institutes of Health(MCM, HJC, RS) and from Veterans Affairs Research & Development E3386R(MCM); grant 2 T32 HS013852 from the Agency for Healthcare Research and Quality(KK); UAB Comprehensive Cancer Center P30 CA13148-40(WDW);
Abbreviations
- PF
physical functioning (SF-36 domain)
- BLEF
basic lower extremity functioning
- ALEF
advanced lower extremity functioning
- PA
physical activity
- DQ
diet quality
- BMI
body mass index
- CI
confidence interval
Footnotes
Conflict of interest: All authors confirm that they have no financial disclosures to report and no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellizzi KM, Rowland JH. Role of comorbidity, symptoms and age in the health of older survivors following treatment for cancer. Aging Health. 2007;3:625–635. [Google Scholar]
- 2.Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of Older, Long-Term Cancer Survivors: The Role of Cancer and Comorbidities. J Am Geriatr Soc. 2009;57:S289–S292. doi: 10.1111/j.1532-5415.2009.02515.x. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33. 2318-11–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR. Functional Limitations in Elderly Female Cancer Survivors. J Natl Cancer Inst. 2006;98:521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 5.Bennet J, Winters-Stone K, Dobek J, Nail L. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013;40:E126–E134. doi: 10.1188/13.ONF.E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J Am Med Dir Assoc. 2014;15:240–250. doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, et al. Reach Out to Enhance Wellness Home-Based Diet-Exercise Intervention Promotes Reproducible and Sustainable Long-Term Improvements in Health Behaviors, Body Weight, and Physical Functioning in Older, Overweight/Obese Cancer Survivors. J Clin Oncol. 2012;30:2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller PE, Morey MC, Hartman TJ, Snyder DC, Sloane R, Cohen HJ, et al. Dietary Patterns Differ between Urban and Rural Older, Long-Term Survivors of Breast, Prostate, and Colorectal Cancer and Are Associated with Body Mass Index. J Acad Nutr Diet. 2012;112:824–831. e1. doi: 10.1016/j.jand.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomey KM, Sowers MR, Crandall C, Johnston J, Jannausch M, Yosef M. Dietary Intake Related to Prevalent Functional Limitations in Midlife Women. Am J Epidemiol. 2008;167:935–943. doi: 10.1093/aje/kwm397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sehl M, Lu X, Silliman R, Ganz PA. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv. 2013;7:20–31. doi: 10.1007/s11764-012-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. Factors associated with the development of arm lymphedema following breast cancer treatment: a match pair case-control study. Lymphology. 2002;35:59–71. [PubMed] [Google Scholar]
- 13.Cohen HJ, Lan L, Archer L, Kornblith AB. Impact of age, comorbidity and symptoms on physical function in long-term breast cancer survivors (CALGB 70803) J Geriatr Oncol. 2012;3:82–89. doi: 10.1016/j.jgo.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin ML, Ainsworth BE. Physical activity interventions following cancer diagnosis: methodologic challenges to delivery and assessment. Cancer Invest. 2004;22:30–50. doi: 10.1081/cnv-120027579. [DOI] [PubMed] [Google Scholar]
- 15.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 17.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. Journal of the National Cancer Institute. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder DC, Morey MC, Sloane R, Stull V, Cohen HJ, Peterson B, et al. Reach Out to Enhance Wellness in Older Cancer Survivors (RENEW): Design, Methods and Recruitment Challenges of a Home-based Exercise and Diet Intervention to Improve Physical Function among Long-term Survivors of Breast, Prostate, and Colorectal Cancer. Psychooncology. 2009;18:429–439. doi: 10.1002/pon.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 21.Sayers SP, Jette AM, Haley SM, Heeren TC, Guralnik JM, Fielding RA. Validation of the Late-Life Function and Disability Instrument. J Am Geriatr Soc. 2004;52:1554–1559. doi: 10.1111/j.1532-5415.2004.52422.x. [DOI] [PubMed] [Google Scholar]
- 22.Fillenbaum GG. Multidimensional Functional Assessment of Older Adults: The Duke Older American Resources and Service Procedures. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 23.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Jonnalagadda SS, Mitchell DC, Smiciklas-Wright H, Meaker KB, Van Heel N, Karmally W, et al. Accuracy of energy intake data estimated by a multiple-pass, 24-hour dietary recall technique. J Am Diet Assoc. 2000;100:303–8. doi: 10.1016/s0002-8223(00)00095-x. quiz 309–11. [DOI] [PubMed] [Google Scholar]
- 25.Guenther PM, Reedy J, Krebs-Smith SM, et al. Development and Evaluation of the Healthy Eating Index–2005. Technical Report [Google Scholar]
- 26.Miller PE, Mitchell DC, Harala PL, Pettit JM, Smiciklas-Wright H, Hartman TJ. Development and evaluation of a method for calculating the Healthy Eating Index-2005 using the Nutrition Data System for Research. Public Health Nutr. 2011;14:306–313. doi: 10.1017/S1368980010001655. [DOI] [PubMed] [Google Scholar]
- 27.SAS Institute. SAS 93. 2012. p. 9.3. [Google Scholar]
- 28.Hayes AF. Introduction to meidation, moderation, and conditional process analysis: a regression based approach. New York: The Guilford Press; [Google Scholar]
- 29.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 32.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 33.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 34.Effron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82:171–200. [Google Scholar]
- 35.Young A, Weltzien E, Kwan M, Castillo A, Caan B, Kroenke CH. Pre- to post-diagnosis weight change and associations with physical functional limitations in breast cancer survivors. J Cancer Surviv. 2014 doi: 10.1007/s11764-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3:215–226. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 37.George SM, Alfano CM, Neuhouser ML, Smith AW, Baumgartner RN, Baumgartner KB, et al. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J Cancer Surviv. 2014 doi: 10.1007/s11764-014-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowland JH, Bellizzi KM. Cancer Survivorship Issues: Life After Treatment and Implications for an Aging Population. J Clin Oncol. 2014:32. doi: 10.1200/JCO.2014.55.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cancer Policy Forum, Board on Health Care Services, Institute of Medicine. The Role of Obesity in Cancer Survival and Recurrence: Workshop Summary. Washington (DC): National Academices Press; 2012. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.