Abstract
Background
The treatment of anemia in patients with cardiorenal syndrome (CRS) is based mainly on intravenous (IV) iron therapy and/or erythropoiesis-stimulating agents (ESAs). There are concerns about the safety of ESAs due to a potentially higher risk for stroke and malignancy.
Objective
We aimed to explore whether IV iron alone is sufficient to improve anemia in CRS patients and to define the predictors of treatment response.
Methods
We retrospectively analyzed data of 81 CRS patient treated for anemia at our clinic. All patients received IV iron for 6 weeks. A subset of patients was additionally given subcutaneous ESAs. The end point was the improvement from baseline in hemoglobin (Hb) and ferritin levels at week 7.
Results
We retrieved the files of 81 patients; 34 received IV iron alone and 47 were given IV iron and ESAs (the combination group). The Hb levels significantly increased in both groups (in the IV iron alone group: 10.6 ± 1.1 to 11.9 ±1.1 g/dl, p < 0.001; in the combination group: 10.2 ± 0.9 to 12.4 ± 1.3 g/dl, p < 0.001), but more pronouncedly in the combination group (2.17 vs. 1.24 g/dl; p = 0.001). The platelet count decreased significantly in the IV iron alone group but was unchanged in the combination group. Eighty percent of patients attained a Hb target of 11 g/dl, with no significant difference between the two groups (73.5 vs. 85.1%; p = 0.197). Low baseline Hb was the only predictor of a favorable outcome to treatment.
Conclusion
Our observational study suggests that IV iron treatment without ESAs may substantially raise the Hb level to ≥11 g/dl in CRS patients. This treatment strategy may reduce the use of ESAs and hence its potential adverse effects.
Key Words: Cardiorenal syndrome, Anemia, Heart failure, Chronic kidney disease, Intravenous iron, Erythropoiesis-stimulating agents
Introduction
Anemia is common in patients with congestive heart failure (CHF) and chronic kidney disease (CKD) [1,2]. The combination and interaction of CHF and CKD comprise the cardiorenal syndrome (CRS) [3]. Anemia is an independent risk factor for cardiac mortality and morbidity both in CHF [1,2] and CKD patients [4,5]. In recent years, the attempt to treat anemia in these patients was mainly based on intravenous (IV) iron therapy and/or erythropoiesis-stimulating agents (ESAs) [6].
The value of IV iron for the treatment of anemia in CHF patient with or without CKD has been well demonstrated [6,7,8]. However, there is growing concern about the safety of ESAs. Specifically, a higher risk for hypertension [9], stroke [9,10] and malignancy [11] has been suggested. Furthermore, ESAs may aggravate iron deficiency (ID), which in turn may cause secondary thrombocytosis within several months [12,13]. It is possible that the beneficial effects of correcting anemia with ESAs in CHF or CKD are counterbalanced by some deleterious effects of the drug [13,14]. Therefore, correcting anemia by means other than ESAs may prove to yield a better cardiovascular outcome [14].
We hypothesized that anemic patients with CRS may attain the target of a hemoglobin (Hb) level >11.0 g/dl without the addition of ESAs. To evaluate this hypothesis, we retrospectively compared two groups of CRS patients treated for anemia in our clinic. One group was treated with IV iron alone, and the second group was treated with a combination of IV iron and ESAs. Our objectives were to explore whether IV iron alone is sufficient to improve anemia and to define predictors of treatment response.
Methods
Approval of the study was granted by the Ethics Committee of the Tel-Aviv Medical Center. We retrospectively analyzed data of patients treated for anemia at our heart failure (HF)/CKD clinic at the Tel-Aviv Medical Center, Tel-Aviv, Israel. Data were collected between January 2010 and January 2013. We retrieved all files of patients with a combination of CKD, defined as an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 and HF defined as symptoms of HF and evidence of diastolic or systolic dysfunction by echocardiogram. eGFR was calculated by the Modification of Diet in Renal Disease equation [15]. Anemia was defined as a serum Hb level <12 g/dl in men and <11.5 g/dl in women. ID was defined as a serum ferritin level <100 ng/l or 100-299 ng/l with a transferrin saturation <20% [16].
In our HF/CKD clinic, we use two main strategies to treat ID: combining IV iron with ESAs, or using IV iron alone. The decision to adopt a specific strategy is at the discretion of the senior physician. In the present analysis, we defined two groups of patients according to the treatment strategy used: group A (treatment with IV iron alone) included patients who received IV iron sucrose (200 mg/week) for 6 consecutive weeks. Group B (combined therapy) included patients who received the same IV iron regiment as group A, with the addition of subcutaneous epoetin-β (10,000 units/week) during the indicated time. We gathered the clinical and laboratory data from each patient at the beginning and at the end of a 6-week course of treatment. Our end point was the improvement from baseline in the Hb, platelet and ferritin levels at week 7.
Statistical Analysis
All data are presented as mean (± standard deviation) for continuous variables, and as the number (percentage) of patients in each group for categorical variables. Student's t test and the χ2 test were used to evaluate the statistical significance between continuous and categorical variables, respectively. The Mann-Whitney U test was used for the continuous variables that were not normally distributed. In order to check the association between the baseline variables and the change in the Hb level, we used linear regression with the variable ‘delta Hb’ as the dependent variable and tested the potential associations of all baseline variables using the stepwise method. All of the analyses were considered significant at a 2-tailed p value <0.05. The SPSS statistical package 21 was used to perform all statistical evaluation (SSPS, Chicago, Ill., USA).
Results
Patient Data
The files of 81 anemic patients with CRS were retrieved and investigated. Thirty-four patients received IV iron alone (group A), and 47 patients were given the combined therapy of IV iron and ESAs (group B). The baseline characteristics of the patients in both groups are summarized in table 1. In the comparison of the two groups, baseline eGFR was significantly higher in group A (30.8 ± 8.1 vs. 23.5 ± 16.1 ml/min/1.73 m2; p = 0.018). The mean corpuscular volume (MCV; 85.6 ± 4.5 vs. 88.7 ± 7.2 fl; p = 0.034) and mean corpuscular Hb (MCH; 28.6 ± 2.2 vs. 29.8 ± 2.5 pg/cell; p = 0.030) were significantly lower in group A compared to group B.
Table 1.
Baseline characteristics of the study population
| Variable | Group A: IV iron alone (n = 34) | Group B: combined therapy (n = 47) | p value |
|---|---|---|---|
| Baseline clinical characteristics | |||
| Age, years | 75.4 ± 9.3 | 76 ± 10.2 | 0.766 |
| Male | 19 (56) | 37 (79) | 0.032 |
| Diabetes mellitus | 23 (67) | 25 (53) | 0.253 |
| Hypertension | 29 (85) | 39 (83) | 0.779 |
| eGFRa, ml/min/1.73 m2 | 30.8 ± 8.1 | 23.5 ± 16.1 | 0.018 |
| Ejection fraction, % | 38.5 ± 13.9 | 36.5 ± 16.2 | 0.685 |
| ß-Blockers | 27 (84) | 35 (76) | 0.410 |
| RAAS inhibitors | 24 (70) | 25 (53) | 0.779 |
| Baseline laboratory characteristics | |||
| Hemoglobin, g/dl | 10.6 ± 1.1 | 10.2 ± 0.9 | 0.350 |
| Hematocrit, % | 31.9 ± 3.4 | 31.4 ± 6.7 | 0.704 |
| MCV, fl | 85.6 ± 4.5 | 88.7 ± 7.2 | 0.034 |
| MCH, pg/cell | 28.6 ± 2.2 | 29.8 ± 2.5 | 0.030 |
| Platelets, ×109/l | 225 ± 69 | 219 ± 63 | 0.714 |
| Ferritin, ng/ml | 146 ± 112 | 148 ± 156 | 0.560 |
| Serum iron, µg/dl | 51.1 ± 16 | 49.7 ± 21 | 0.772 |
| Transferrin, Rg/dl | 246 ± 62 | 247 ± 61 | 0.910 |
| Transferrin saturation, % | 21.0 ± 9.7 | 19.8 ± 10.5 | 0.682 |
| ID indices | |||
| Ferritin level <100 ng/ml | 13 (39) | 20 (45) | 0.647 |
| Transferrin saturation <20% | 18 (58) | 26 (62) | 0.811 |
| IDb | 21 (64) | 32 (71) | 0.485 |
Data are expressed as number (%) or as the mean ± SD. RAAS = Renin angiotensin aldosterone system.
Calculated by the Modification of Diet in Renal Disease equation.
Defined as a serum ferritin level <100 ng/l or 100–299 ng/l with a transferrin saturation <20%.
All the patients in our cohort had anemia. The mean baseline Hb level was similar in both groups (10.6 ± 1.1 vs. 10.2 ± 0.9 g/dl; p = 0.350). There were no differences between groups with regard to baseline ferritin, serum iron levels and transferrin saturation. ID rates were similar between the groups (64 vs. 71%; p = 0.485). All the patients in the cohort had ferritin levels <800 ng/ml.
Response to Treatment
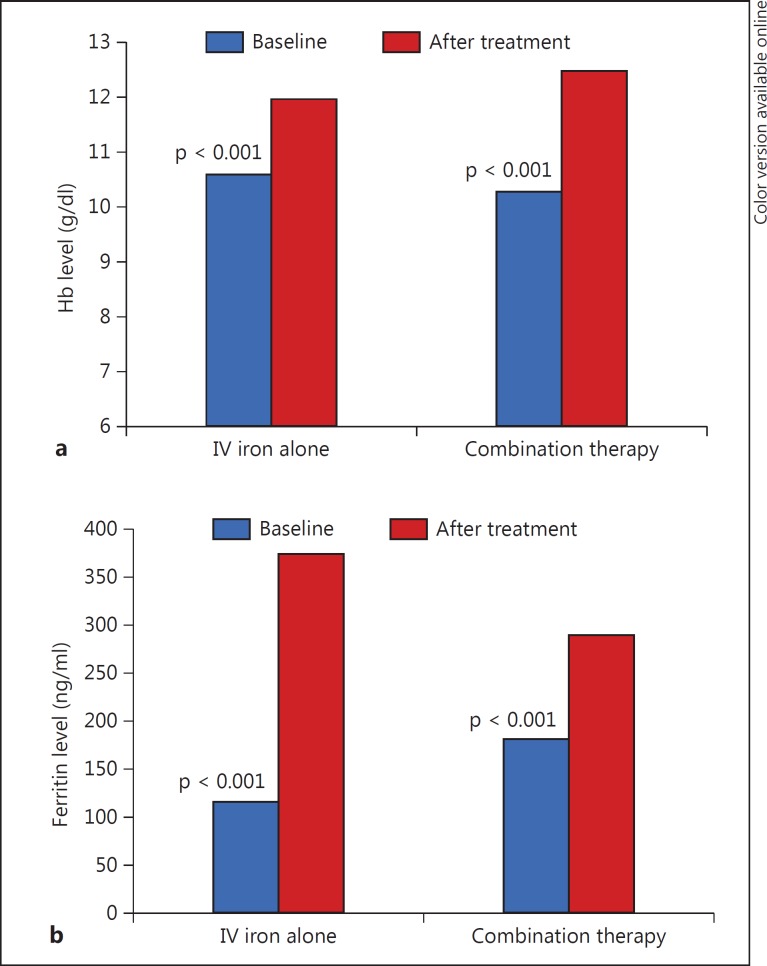
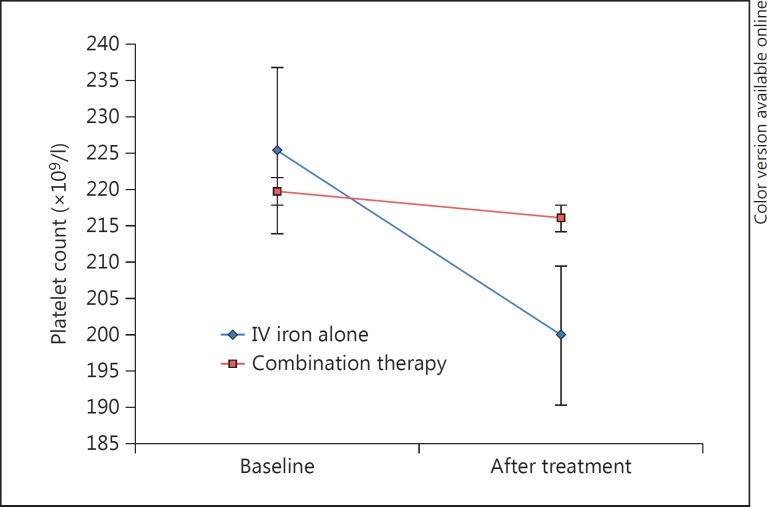
The Hb levels were significantly increased in both groups (10.6 ± 1.1 to 11.9 ± 1.1 g/dl, p < 0.001, and 10.2 ± 0.9 to 12.4 ± 1.3 g/dl, p < 0.001, respectively; table 2;fig. 1a), but more pronouncedly in group B (net Hb increase of 1.24 ± 0.9 vs. 2.17 ± 1.2 g/dl; p = 0.001). Ferritin levels increased significantly in both groups (table 2;fig. 1b), but the net ferritin increment was greater in group A (308 ± 219 vs. 161 ± 133 ng/ml; p = 0.002). The platelet count was significantly decreased in group A (225 ± 69 to 199 ± 51 × 109/l; p = 0.002) while it remained unchanged in group B (219 ± 63 to 216 ± 54 × 109/l; p = 0.54; table 2; fig. 2). At the end of the treatment course, Hb levels were increased to >11 g/dl in 80.2% of our cohort, with no significant difference between the groups (73.5 vs. 85.1%; p = 0.197).
Table 2.
Response to treatment in CRS anemic patients
| Variable | Group A: IV iron alone (n = 34) |
Group B: combined therapy (n = 47) |
||||
|---|---|---|---|---|---|---|
| before | after | p value | before | after | p value | |
| Hb, g/dl | 10.6 ± 1.1 | 11.9 ± 1.1 | >0.001 | 10.2 ± 0.9 | 12.4 ± 1.3 | >0.001 |
| Hematocrit, % | 31.9 ± 3.4 | 35.2 ± 3.9 | >0.001 | 31.4 ± 6.7 | 37.1 ± 4.2 | >0.001 |
| MCV, fl | 85.6 ± 4.5 | 87.5 ± 4.3 | 0.001 | 88.7 ± 7.2 | 90.3 ± 9.6 | 0.021 |
| MCH, pg/cell | 28.6 ± 2.2 | 29.7 ± 1.9 | >0.001 | 29.8 ± 2.5 | 30.2 ± 2.5 | 0.043 |
| MCHC, g/dl | 33.3 ± 1.1 | 33.7 ± 0.9 | 0.066 | 33.5 ± 1.1 | 33.5 ± 0.9 | 0.979 |
| Platelets, ×109/l | 225 ± 69 | 199 ± 51 | 0.002 | 219 ± 63 | 216 ± 54 | 0.540 |
| Ferritin, ng/ml | 146 ± 112 | 454 ± 271 | >0.001 | 148 ± 156 | 310 ± 203 | >0.001 |
| Serum iron, µg/dl | 51.1 ± 16 | 73.6 ± 23 | >0.001 | 49.7 ± 21 | 60.1 ± 18 | 0.004 |
| Transferrin, µg/dl | 246 ± 62 | 222 ± 51 | 0.015 | 248 ± 61 | 218 ± 48 | >0.001 |
| Transferrin saturation, % | 21.0 ± 9.7 | 31.2 ± 9.3 | >0.001 | 19.8 ± 10.5 | 26.1 ± 10.5 | >0.001 |
Data are expressed as the mean ± SD. MCHC = MCH concentration.
Fig. 1.
Response to therapy with IV iron alone or a combination of IV iron and ESAs in anemic patients with CRS. a Hb response. b Ferritin response.
Fig. 2.
Effect of treatment on platelet counts. Platelet counts before and after treatment with IV iron alone or a combination of IV iron and ESAs in anemic patients with CRS.
Predictors of Treatment Response
The only independent predictor of a favorable increase in Hb in both groups was a low baseline Hb level (p = 0.001). Baseline levels of serum iron, ferritin, transferrin, MCV and MCH did not predict a favorable increase in Hb.
Discussion
In this small observational study, we found that treating anemic CRS patients with IV iron alone can significantly raise Hb levels, attaining a target Hb level of 11.0 g/dl in >80% of the patients. It implies that elevating Hb levels in the majority of anemic CRS patients may be achieved without the use of ESAs. To the best of our knowledge, this is the first description of similar effects on Hb levels of IV iron therapy alone or a combination of IV iron with ESAs in anemic CRS patients. Similar findings were briefly described by Terrovitis et al. [17] in anemic patients with advanced CHF and ID. However, the eGFR levels were not given by the authors, and therefore, the proportion of CRS patients in this study is unknown [17]. We have previously shown that the combination of IV iron and low-dose ESAs was more effective in elevating hematocrit levels in comparison with IV iron alone in predialysis CKD patients with anemia [18]. Yet, in that study, the percentage of patients attaining the target hematocrit of 35% was similar in the group treated with the combination of IV iron and ESAs to the group treated with IV iron alone [18].
Importantly, the rate of ID did not differ between the patients treated with IV iron alone and those treated with IV iron and ESAs. It suggests that the similar Hb elevation in both groups was not due to a higher rate of ID in the patients on IV iron alone.
Baseline serum iron, ferritin, transferrin, MCV and MCH levels were all poor indicators of Hb increase with either IV iron alone or IV iron in combination with ESAs. In CKD, there is disagreement about the value of these parameters as predictors of response to IV iron alone or combined with ESAs [18,19]. The results of our study suggest that iron status parameters should only have a minor influence on our decision to initiate IV iron therapy in anemic CRS patients.
The platelet count decreased significantly in the group treated with IV iron alone but was unchanged in the group treated with both IV iron and ESAs (table 2; fig. 2). Possible explanations for these phenomena include: (1) ID can cause an elevated platelet count and even thrombocytosis, which may lead to increased thrombosis, atherosclerosis and increased mortality [12,13]. Indeed, ID correction with IV iron in ESA-treated dialysis patients was shown to significantly reduce the platelet count [13]. (2) High doses of ESAs in CKD are associated with higher ID and more severe thrombocytosis [13]. (3) ESAs may increase the platelet count through amplification of the thrombopoietin effect on thrombopoiesis [20]. In this study, it is therefore possible that ESA therapy counterbalanced a potential decline in platelet counts by IV iron therapy. The different effect on platelet counts may well be important, since thrombocytosis, or at least an elevation of platelet counts with ESA therapy, may be one of the missing links in causing the increased incidence of cardiovascular events in CKD [12,13] and cancer [14].
This study has several limitations. First and foremost, it is a small nonrandomized, retrospective, observational study in a single medical center. Importantly, the baseline eGFR was significantly higher in the group on IV iron alone as compared to the group on IV iron and ESAs (table 1). It is therefore possible that the similar effects on Hb in both groups are due to the fact that the patients on IV iron and ESAs had worse renal function with lower levels of endogenous erythropoietin. Nevertheless, similar effects of therapy were found in both groups even after adjusting for eGFR. The results here still suggest that IV iron alone will be sufficient to elevate Hb towards a level of 11.0 g/dl in a large proportion of anemic CRS patients.
Recently, the safety as well as the benefit of ESAs in anemic CKD or CHF patients has come into question. Specifically, a higher risk of stroke and malignancy has been suggested with the use of ESAs [10,20]. Furthermore, a recent large, long-term, double-blind study with the ESA darbepoetin-α did not meet its primary end point of reducing the composite end point of time to death from any cause or first hospital admission for worsening HF in patients with systolic CHF [21]. A higher rate of thromboembolic adverse events with a nonsignificantly higher stroke rate but no cancer was also reported in this study [21]. A meta-analysis showed that ESA treatment in HF patients had no significant impact on mortality or morbidity [22]. These results led the American College of Physicians to recommend against ESA treatment in patients with mild to moderate anemia and CHF [23]. Recent guidelines of anemia management in CKD patients suggest reducing the dose of ESAs to a minimum and targeting Hb to levels of ≤11.5 g/dl [24]. It is also recommended to use ESA therapy with great caution, if at all, in CKD patients with active malignancy (in particular when cure is the anticipated outcome), a history of stroke or a history of malignancy [24]. These recommendations put the focus once again on IV iron as a potential beneficial therapy for anemia in CHF and/or CKD patients. IV iron therapy improves many ‘soft’ outcome measures in patients with CHF or CKD and ID [7,8,16]. However, concerns have also been raised about the safety of IV iron with its possible worsening of oxidative stress and reduced immunological function with a possible increased tendency to infections [25,26]. It is, in our opinion, the time for large, randomized, double-blind studies to test the safety and efficacy of IV iron in anemic CRS with an emphasis on ‘hard’ outcome measures such as cardiovascular morbidity and mortality.
Conclusions
This observational study suggests that IV iron treatment without ESAs can raise the Hb level to ≥11.0 g/dl. In addition, IV iron treatment without ESAs can reduce a potentially hazardous secondary thrombocytosis. This treatment strategy may elevate Hb levels in anemic CRS patients while reducing the use of ESAs and hence its possible adverse effects. Further studies should be conducted to evaluate the potential of treating CRS anemic patients with IV iron alone.
Disclosure Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients: a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg DS, Wexler D, Palazzuoli A, Iaina A, Schwartz D. The anemia of heart failure. Acta Haematol. 2009;122:109–119. doi: 10.1159/000243795. [DOI] [PubMed] [Google Scholar]
- 3.Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9:99–111. doi: 10.1038/nrneph.2012.279. [DOI] [PubMed] [Google Scholar]
- 4.Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community-based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919–2925. doi: 10.1097/01.asn.0000092138.65211.71. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg DS. The role of erythropoiesis stimulating agents and intravenous (IV) iron in the cardio renal anemia syndrome. Heart Fail Rev. 2011;16:609–614. doi: 10.1007/s10741-010-9194-2. [DOI] [PubMed] [Google Scholar]
- 7.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 8.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 11.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 12.Besarab A, Horl WH, Silverberg D. Iron metabolism, iron deficiency, thrombocytosis, and the cardiorenal anemia syndrome. Oncologist. 2009;14(suppl 1):22–33. doi: 10.1634/theoncologist.2009-S1-22. [DOI] [PubMed] [Google Scholar]
- 13.Streja E, Kovesdy CP, Greenland S, Kopple JD, McAllister CJ, Nissenson AR, et al. Erythropoietin, iron depletion, and relative thrombocytosis: a possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52:727–736. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am J Hematol. 2012;87:308–310. doi: 10.1002/ajh.22262. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–829. doi: 10.1093/eurheartj/ehs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrovitis JV, Kaldara E, Ntalianis A, Sventzouri S, Kapelios C, Barbarousi D, et al. Intravenous iron alone is equally effective with the combination of iron and erythropoietin for the treatment of iron-deficiency anemia in advanced heart failure. J Am Coll Cardiol. 2012;60:2255–2256. doi: 10.1016/j.jacc.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg DS, Blum M, Agbaria Z, Deutsch V, Irony M, Schwartz D, et al. The effect of IV iron alone or in combination with low-dose erythropoietin in the rapid correction of anemia of chronic renal failure in the predialysis period. Clin Nephrol. 2001;55:212–219. [PubMed] [Google Scholar]
- 19.Sunder-Plassmann G, Spitzauer S, Horl WH. The dilemma of evaluating iron status in dialysis patients – limitations of available diagnostic procedures. Nephrol Dial Transplant. 1997;12:1575–1580. doi: 10.1093/ndt/12.8.1575. [DOI] [PubMed] [Google Scholar]
- 20.Del Vecchio L, Locatelli F. Safety issues related to erythropoiesis-stimulating agents used to treat anemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2012;11:923–931. doi: 10.1517/14740338.2012.712680. [DOI] [PubMed] [Google Scholar]
- 21.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 22.Kansagara D, Dyer E, Englander H, Fu R, Freeman M, Kagen D. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med. 2013;159:746–757. doi: 10.7326/0003-4819-159-11-201312030-00007. [DOI] [PubMed] [Google Scholar]
- 23.Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:770–779. doi: 10.7326/0003-4819-159-11-201312030-00009. [DOI] [PubMed] [Google Scholar]
- 24.KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 25.Van Buren P, Velez RL, Vaziri ND, Zhou XJ. Iron overdose: a contributor to adverse outcomes in randomized trials of anemia correction in CKD. Int Urol Nephrol. 2012;44:499–507. doi: 10.1007/s11255-011-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaziri ND. Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis. 2013;61:992–1000. doi: 10.1053/j.ajkd.2012.10.027. [DOI] [PubMed] [Google Scholar]