Abstract
Cardiorenal syndrome type 1 (CRS1) pathophysiology is complex, and immune-mediated damage, including alterations in the immune response with monocyte apoptosis and cytokine release, has been reported as a potential mechanism. In this study, we examined the putative role of renal tubular epithelial cell (RTC) apoptosis as a pathogenic mechanism in CRS1. In particular, we investigated the caspase pathways involved in induced apoptosis. We enrolled 29 patients with acute heart failure (AHF), 11 patients with CRS1, and 15 controls (CTR) without AHF or acute kidney injury (AKI). Patients who had AKI prior to the episode of AHF or who had any other potential causes of AKI were excluded. Plasma from different groups was incubated with RTCs for 24 h. Subsequently, cell apoptosis, DNA fragmentation, and caspase-3, −8, and −9 activities were investigated in RTCs incubated with AHF, CRS1, and CTR plasma. A p value <0.5 was considered statistically significant. A quantitative analysis of apoptosis showed significantly higher apoptosis rates in CRS1 patients compared to AHF patients and CTR (p < 0.01). This increase in apoptosis was strongly confirmed by caspase-3 levels (ρ = 0.73). Caspase-8 and −9 were significantly higher in CRS1 patients compared to AHF patients and CTR (p < 0.01). Furthermore, caspase-3 levels showed a significantly positive correlation with caspase-8 (ρ = 0.57) and −9 (ρ = 0.47; p < 0.001). This study demonstrated the significantly heightened presence of dual apoptotic disequilibrium in CRS1. Our findings indicated that apoptosis may have a central role in the mechanism of CRS1, and it could be a potential therapeutic target in this syndrome.
Key Words: Cardiorenal syndromes, Caspase, Acute kidney injury, Apoptosis, Renal tubular cells
Introduction
Organ systems are closely interconnected, performing a larger task, which is leading to ‘organ crosstalk’; in particular, heart performance and kidney function are intimately related by a synergistic relationship. Furthermore, recent studies have demonstrated that cardiac disease can directly contribute to worsening kidney function and vice versa [1,2,3,4]. The Acute Dialysis Quality Initiative (ADQI) proposed the cardiorenal syndrome (CRS) definition and classification, a comprehensive characterization of this complex heart-kidney crosstalk [5]. The intersection of cardiac and renal dysfunction has important therapeutic and prognostic implications, so this new classification represents a first step forward to a better understanding of the pathobiology, pathophysiology, and management strategies of the reciprocal heart-kidney interactions.
CRS type 1 (CRS1) is a multifactorial syndrome involving different mechanisms and is characterized by a rapid worsening of cardiac function leading to acute kidney injury (AKI) [1,6,7,8]. Activation of the immune and cellular response, inflammation, oxidative stress, and activation of apoptotic pathways are involved in the pathogenesis of CRS1 [7,9,10,11,12,13].
Approximately one third of patients with acute heart failure (AHF) develop AKI defined by an increase in serum creatinine (sCr) of ≥0.3 mg/dl [14]. Furthermore, patients who develop AKI after an acute cardiac event have a significantly higher mortality risk [15]. Moreover, AKI has been associated with a higher risk of prolonged/re-hospitalization, cardiovascular events, and all-cause mortality and with a faster progression to chronic kidney disease (CDK) [16,17].
A number of pathobiological processes contribute to initiating and perpetuating AKI, including endothelial and epithelial cell death as well as immunological and inflammatory processes. In addition, experimental evidence supports a pathogenic role for apoptosis in AKI [18]. Several common renal insults, such as ischemia, toxic injury, radiation, and ureteral obstruction, cause apoptosis in the kidney [18].
The kidney epithelium is particularly susceptible to injury that results in cell death due to apoptosis and necrosis with the loss of renal epithelial cells [19]. In fact, renal tubular epithelium is a major site of cell injury and cell death during AKI [20,21], and all experimental models of AKI, such as ischemia-reperfusion, sepsis-endotoxemia, and toxin exposure, demonstrated a strong association between inflammatory activity and renal cell apoptosis [18,20].
Recent studies have specifically shown that apoptosis is implicated in CRS1 pathogenesis [11,12]. These findings suggest the presence of proinflammatory cytokines and proapoptotic factors in CRS1 plasma that induce a defective regulation of monocyte apoptosis in such patients [11,12]. Unfortunately, the authors of these studies did not elucidate the precise signaling pathway involved in this induced apoptosis: there are two main intracellular pathways for apoptosis (intrinsic and extrinsic) characterized by activation of different activator caspases [22]. The two pathways are linked and converged by caspase-3 [23].
In this study, we examined the effect of CRS1 plasma on renal tubular epithelial cell (RTC) apoptosis as a pathogenic mechanism in this syndrome. In particular, we investigated the caspase pathway involved in this induced apoptosis.
Materials and Methods
Study Population
Patients admitted to the Department of Internal Medicine of San Bortolo Hospital in Vicenza, Italy, between September 2011 and December 2011 were screened. A total of 40 patients with AHF were further examined for inclusion into the study. Echocardiograms were performed within 6 h of admission into the Internal Medicine ward. AHF was defined according to the European Society of Cardiology guidelines [24], while AKI was defined according to the Acute Kidney Injury Network (AKIN) criteria [16]. Applying AKIN criteria, the strict definition was used for increments of sCr within 48-hour intervals. We compared daily sCr values during the first 7 days of hospitalization. CRS1 was defined according to the current classification system [1,6], sCr was measured by the Jaffe method, and the estimated glomerular filtration rate (eGFR) was calculated with the 4-variable standardized Modification of Diet in Renal Disease (MDRD) study equation.
Patients with AKI prior to the episode of AHF, patients with other potential causes of AKI or with CKD (eGFR <45 ml/min/1.73 m2, CKD stage 3a), and patients with previous kidney transplantation were excluded. Septic and hypotensive patients who required inotropic support prior to the diagnosis of AKI were not included into the study. We considered the creatinine level of the 3 months before admission of all patients enrolled into the study as the baseline value.
Forty patients were finally enrolled. Subsequently, 11 patients who exhibited AKI at the time of admission for AHF or who developed AKI during the course of hospitalization were classified as CRS1. AKI was presumed to be related to cardiac dysfunction after exclusion of other possible causes of renal damage based on the review of the clinical course of the patients. The 29 patients with AHF who did not develop AKI during hospitalization were analyzed to better understand the contribution of cardiac dysfunction to renal apoptosis. In addition, 15 healthy volunteers without AHF or AKI and other comorbidities were recruited as a control (CTR) group for this study. Patients were recruited at the Blood Center of San Bortolo Hospital, and plasma from these subjects was kindly provided by the blood bank of San Bortolo Hospital.
Clinical data, blood pressure, sCr, blood urea, hemoglobin, serum albumin, and brain natriuretic peptide (BNP) were evaluated and collected at admission. All patients were informed about the experimental protocol and the objectives of the study before providing informed consent and blood samples. All procedures were in accordance with the Helsinki Declaration. The protocol and consent form were approved by the Ethics Committee of San Bortolo Hospital.
Sample Collection
Peripheral venous blood samples were collected from all 40 patients within 8 h of admission into the Internal Medicine ward; for those who developed CRS1, we also collected a blood sample within 24 h of AKI. Blood samples were collected in EDTA tubes and subsequently centrifuged for 10 min at 3,500 rpm. Following centrifugation, plasma was immediately separated from blood cells and stored at −80°C until use. All samples were processed within 4 h after collection. Collection and processing of control samples from healthy volunteers followed an identical protocol.
RTC Culture
Primary cultures of human proximal RTCs were obtained from kidneys removed by surgical procedures from patients affected by renal carcinomas. An immortalized human proximal RTC line was generated by infection with a hybrid Adeno5/SV40 virus. The purity of the primary cultures was assessed on the basis of cell characterization, according to published criteria [25,26]. RTCs were cultured in completed liquid-phase medium (RPMI-1640, PBI International, Milan, Italy) supplemented with 10% heat-inactivated fetal bovine serum for 30 min at 56°C, 2 mM L-glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin (Sigma Chemical Co., St. Louis, Mo., USA). These cells were maintained in a controlled atmosphere (5% CO2) at 37°C and passaged at 80% confluence checked by an inverted microscope.
Induction of Apoptosis
RTCs were treated with participants' plasma from the CRS1, AHF, and CTR groups. For CRS1 patients, we used plasma within 24 h of the AKI event. The ability of plasma to induce apoptosis was evaluated at 24 h. Untreated cells were maintained in the same manner and used as an internal control.
RTCs were plated at 2 × 106 cells per well in 6-well plates and incubated with 90% RPMI-1640 medium (with 2 mM L-glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin) and 10% of EDTA plasma from the CRS1, AHF, and CTR groups in the standard condition (at 37°C in 5% CO2 for 24 h). Prior to use, RTCs were washed twice in Dulbecco's phosphate-buffered saline (PBS; without calcium and magnesium), pH 7.4. Each incubation was performed in triplicate.
Evaluation of Apoptosis
RTC apoptosis was evaluated 24 h following treatment with CRS1, AHF, and CTR plasma in accordance with published protocols [9,27,28].
Trypan Blue Exclusion Cell Viability Assay
50 μl of cell culture was added to 50 μl of trypan blue (Sigma Chemical Co.) exclusion dye and examined under the microscope to verify membrane integrity. Cell viability is calculated by the number of viable cells divided by the number of total cells in percent at a magnification of ×20.
Detection of DNA Fragmentation
Apoptosis is characterized by DNA fragmentation, showing a ladder-like pattern, and nuclear fragmentation in several small fragments.
Untreated and plasma-treated RTCs (1 × 106 cells) were harvested and washed with Dulbecco's PBS. The DNA fragmentation assay was performed using an Apoptotic DNA Ladder Extraction Kit (BioVision Inc., Milpitas, Calif., USA) according to the manufacturer's protocol.
DNA ladder fragmentation was detected by electrophoresis on 1.5% agarose gel staining with Syber Safe (Life Technologies, Monza, Italy). The bands were visualized under ultraviolet light.
Cytofluorometric Assay
The Annexin V-FITC kit (Beckman Coulter, Brea, Calif., USA) is an apoptotic detection kit based on the binding properties of annexin V to phosphatidylserine and on the DNA-intercalating capabilities of propidium iodide (PI). Cells were washed twice with cold Dulbecco's PBS and resuspended in 500 μl of PBS at a concentration of 1 × 106 cells/ml. 100 μl of this solution was incubated by 5 μl of FITC-conjugated annexin V and 2.5 μl PI (Beckman Coulter). The cells were gently vortexed and incubated for 15 min at room temperature (25°C) in the dark. Then, 400 μl of 1× binding buffer was added to each tube. Analysis was performed by Navios Flow Cytometer (Beckman Coulter) to identify the subpopulations of the apoptotic cells within 1 h. Apoptotic cells were gated and enumerated by identifying those cells that exhibited FITC and PI staining. Annexin V-FITC labeling was used to quantitatively determine the percentage of cells that were undergoing apoptosis. PI was used to distinguish necrotic from nonnecrotic cells. The biparametric analysis showed three distinct populations: viable cells which had low FITC and low PI signals, apoptotic cells which had high FITC and low PI signals, and necrotic cells which had high FITC and high PI signals. A minimum of 20,000 events were collected on each sample.
Determination of Caspase-3, −8, and −9 Activity
RTCs were assayed for activation of caspase-3, −8, and −9. Caspase-3 concentration was measured by human caspase-3 instant enzyme-linked immuno-sorbent assay (ELISA) kit (eBioscience, San Diego, Calif., USA) with a fluorometric assay, while caspase-8 and −9 concentration was measured, respectively, by human caspase-8 and human caspase-9 platinum ELISA kit (eBioscience) with a fluorometric assay. RTCs incubated with plasma for 24 h were processed according to the manufacturer's instructions. Caspase-3, −8, and −9 levels were measured in cell lysates at 450 nm by VICTOR X4 multilabel plate reader (Perkin Elmer Life Sciences, Waltham, Mass., USA). Caspase-3, −8, and −9 concentrations (ng/ml) were calculated from the standard curve according to the manufacturer's protocol. Standard samples ranged from 0.16 to 10.0 ng/ml for caspase-3 and −8, and from 1.6 to 100 ng/ml for caspase-9. Human caspase-3 instant ELISA kit sensitivity is 0.12 ng/ml, human caspase-8 platinum ELISA kit sensitivity is 0.10 ng/ml, and human caspase-9 platinum ELISA kit sensitivity is 0.4 ng/ml.
Statistical Analysis
Statistical analysis was performed using the STATA software package. Categorical variables were expressed as percentages; continuous variables were expressed as means ± standard deviations (parametric variables) or medians and interquartile ranges (IQRs; nonparametric variables). The Mann-Whitney U test or t test were used for comparison of two groups, as appropriate. The Kruskal-Wallis test for multiple comparisons was applied to compare the groups. A p value of <0.05 was considered statistically significant.
Results
Subjects' Baseline Characteristics
Causes of admission for AHF included non-ST segment elevation myocardial infarction (2%), excessive salt and fluid intake (29%), hypertensive crisis (14%), and other causes (45%). The other 10% of patients did not have a recognizable cause of AHF.
The mean age of the 11 patients with CRS1 was 74.0 ± 13.1 years, and 45% of these patients were male. The median baseline sCr of CRS1 patients was 0.96 mg/dl (IQR 0.88-1.02), and the median eGFR was 62 ml/min/1.73 m2 (IQR 55-75). Seven CRS1 subjects (63%) had diabetes and 10 (90%) had hypertension.
Three patients (27%) exhibited AKI at the time of admission (caused by AHF and other potential excluded sources of AKI), 6 patients (55%) exhibited AKI during the second day, and 2 patients (18%) exhibited AKI during the third day. All CRS1 patients were classified as AKIN stage 1.
The mean age of the 29 patients with AHF was 73.6 ± 9.5 years, and 58% of these patients were male. The median baseline sCr of AHF subjects was 0.98 mg/dl (IQR 0.87-1.15), and the median eGFR was 67 ml/min/1.73 m2 (IQR 53-82). Twelve subjects (41%) of the AHF group had diabetes and 27 AHF subjects (93%) had hypertension. The characteristics of CRS1 and AHF patients are described in table 1.
Table 1.
Baseline characteristics of CRS1 and AHF patients and clinical parameters
| CRS1 | AHF | p | |
|---|---|---|---|
| Age, years | 74.0 ± 13.1 | 73.6 ± 9.5 | n.s. |
| Weight, kg | 77 (67–85) | 75 (64–88) | n.s. |
| Diabetes, % | 63 | 41 | n.s. |
| Hypertension, % | 90 | 93 | n.s. |
| Peripheral vascular disease, % | 42 | 38 | n.s. |
| Cardiovascular disease, % | 19 | 16 | n.s. |
| Obesity, % | 23 | 20 | n.s. |
| Dyslipidemia, % | 43 | 37 | n.s. |
| Creatinine, mg/dl | 0.96 (0.88–1.02) | 0.98 (0.87–1.15) | n.s. |
| eGFR, ml/min/1.73 m2 | 62 (55–75) | 67 (53–82) | n.s. |
| Lowest systolic blood pressure, mm Hg | 140 (l17–159) | 150 (135–163) | n.s. |
| Lowest diastolic blood pressure, mm Hg | 80 (75–95) | 80 (75–96) | n.s. |
| Mean arterial pressure, mm Hg | 100 (89–116) | 103 (93–121) | n.s. |
| Ejection fraction, % | 35 (25–51) | 35 (24–48) | n.s. |
| BNP, pg/ml | 695 (409–1,837) | 632 (398–946) | n.s. |
| Troponin I, ng/ml | 0.07 (0.04–0.26) | 0.07 (0.04–0.27) | n.s. |
| Hemoglobin, g/dl | 11.4 (9.7–13.0) | 11.1 (13.6–14.2) | n.s. |
| Albumin, g/l | 4.3 (4.0–4.4) | 4.0 (4.2–4.4) | n.s. |
| Urea, mg/dl | 73 (55–109) | 66 (41–91) | n.s. |
Values are means ± standard deviations or medians (IQRs), unless otherwise specified. n.s. = Not significant.
The mean age of the 15 healthy volunteers was 52.0 ± 7.7 years, and 47% of these subjects were male.
No patients had exposure to radiocontrast media in the 72 h preceding AKI. No patients developed a need for mechanical ventilation and renal replacement therapy. Urea, hemoglobin, albumin, BNP, and troponin I were not significantly different at admission in CRS1 and AHF patients.
Apoptotic Effect of CRS1 Plasma on RTCs
Since cell growth inhibition and formation of apoptotic bodies were indicative of apoptosis induction, we examined the induction of DNA ladder formation and other characteristic features of apoptosis by the methods described above. RTC viability was 95%, as assessed by trypan blue exclusion.
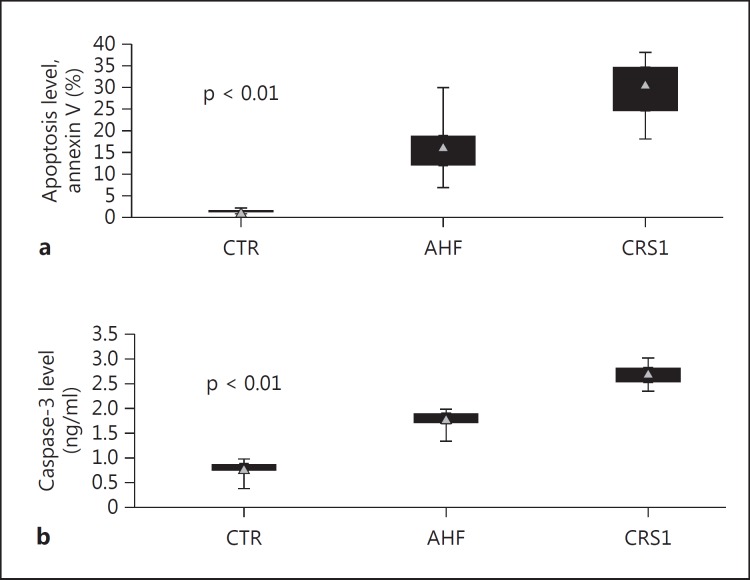
In the RTC line treated for 24 h with CRS1 and AHF plasma, the results showed DNA ladder formation with different molecular weight fractions, suggesting the presence of apoptotic events. The quantitative analysis of apoptosis by flow cytometry using the Annexin V/PI cytofluorometric assay confirmed that RTCs incubated with plasma from CRS1 patients had significantly higher apoptosis rates compared with those incubated with plasma from AHF patients and CTR (p < 0.001). The level of apoptosis detected after incubation was 31.0% (IQR 24.5-34.5) for CRS1 patients, while it was 16.4% (IQR 12.0-18.9) in the AHF group and 1.3% (IQR 1.2-1.6) in CTR (fig. 1a).
Fig. 1.
Quantitative analysis of apoptosis in RTCs. In a quantitative analysis of apoptosis, RTCs incubated with plasma from CRS1 patients showed significantly higher apoptosis rates compared with those incubated with plasma from AHF patients and CTR (a). In concordance with the apoptosis rate, RTCs incubated with plasma from CRS1 patients demonstrated a significantly higher caspase-3 concentration (b).
The increase in apoptosis indicated by flow cytometry was further confirmed by the caspase-3 concentration. In accordance with the apoptosis rate, RTCs incubated with plasma from CRS1 patients demonstrated a significantly higher caspase-3 activity compared to the AHF and CTR groups (p < 0.01). The level of caspase-3 detected after 24 h of incubation was 2.69 ng/ml (IQR 2.51-2.80) for CRS1 patients, while it was 1.77 ng/ml (IQR 1.68-1.87) in the AHF group versus 0.76 ng/ml (IQR 0.74-0.86) in CTR (fig. 1b). A strong correlation between caspase-3 and cytofluorometric apoptotic levels was observed (ρ = 0.73; p < 0.05).
The activity of caspase-8 detected after 24 h of incubation was 0.71 ng/ml (IQR 0.70-0.78) for CRS1 patients, while it was 0.18 ng/ml (IQR 0.15-0.28) in the AHF group versus 0.14 ng/ml (IQR 0.12-0.17) in CTR. The concentration of caspase-9 detected after 24 h of incubation was 37.86 ng/ml (IQR 32.99-52.38) for CRS1 patients, while it was 15.89 ng/ml (IQR 11.49-19.48) in the AHF group versus 7.41 ng/ml (IQR 6.29-9.14) in CTR. Caspase-8 and −9 were significantly higher in the CRS1 group compared to the AHF group and CTR (p < 0.01; table 2). Furthermore, caspase-3 levels showed a significantly positive correlation with caspase-8 (ρ = 0.57) and caspase-9 (ρ = 0.47) (both, p < 0.001).
Table 2.
Caspase levels in RTCs incubated with CRS1, AHF and CTR groups for 24 h
| CRS1 | AHF | CTR | p | |
|---|---|---|---|---|
| Caspase–3, ng/ml | 2.69 (2.51–2.80) | 1.77 (1.68–1.87) | 0.76 (0.74–0.86) | >0.01 |
| Caspase–8, ng/ml | 0.71 (o.70–0.78) | 0.18 (0.15–0.28) | 0.14 (0.12–0.17) | >0.01 |
| Caspase–9, ng/ml | 37.86 (32.99–52.38) | 15.89 (11.49–19.48) | 7.41 (6.29–9.14) | >0.01 |
Values are medians (IQRs).
Discussion
The pathological mechanisms that cause AKI after AHF are multiple and very complex, including renal endothelial and epithelial cell death, inflammation, and immunological processes. Recent studies have demonstrated a pathogenic role of apoptosis in the development of AKI, in the development of AHF, and in the CRS1 initiation [7,11,18]. Renal apoptosis is supposed to have a central role in the mechanism of CRS1, and it could be a potential therapeutic target in this syndrome.
Two main intracellular pathways for apoptosis have been recognized: ligation of plasma membrane death receptors (extrinsic pathway) and perturbation of the intracellular homeostasis (intrinsic pathway). In the extrinsic pathway, the Fas/FasL system transmits apoptotic signals from the surrounding environment into the cell; the binding of FasL with Fas initiates receptor oligomerization, which recruits Fas-associated death domain (FADD) and activator caspase-8 and −10 [29,30,31]. These caspases are activated upon oligomerization and then cleave protein substrates to activate downstream effector caspases. In contrast, the intrinsic pathway involves intracellular organelles, the most important being mitochondria [32,33,34]. Caspases are widely expressed in an inactive monomeric proenzyme form in most cells and require dimerization and cleavage for activation; once they are activated, other procaspases are often initiated, allowing the initiation of a protease cascade. This proteolytic cascade, in which one caspase can activate other caspases, amplifies the apoptotic signaling pathway leading to an accelerated feedback loop of caspase activation and to rapid cell death [22,35].
In this study, we reported a marked proapoptotic activity, highlighted by phosphatidylserine translocation to the external portion of the cell membrane, DNA fragmentation, and caspase-8, −9, and −3 activation in the RTCs incubated with plasma from CRS1 patients compared to AHF patients and CTR. We included an AHF population to better understand the contribution of cardiac dysfunction to renal apoptosis. Importantly, the activation of both intrinsic and extrinsic pathways leads to cellular apoptosis, as observed in RTCs treated with CRS1 plasma.
In fact, apoptosis occurred via the external apoptotic pathway and caspase-8 activation and via the loss of mitochondrial potential, release of factors from the mitochondria, and activation of caspase-9. In RTCs, CRS1 induces dual apoptotic pathway activation; in fact, both caspase-8 and −9 lead to activation of caspase-3. Caspase-3 is an effector caspase; it plays a direct role in the proteolytic cleavage of the cellular proteins responsible for progression to apoptosis [23].
This study provides an initial characterization of the apoptotic program in CRS1. We found that different apoptotic stimuli activate dual apoptotic pathways, one mediated by the extrinsic pathway and the other by the intrinsic pathway. The extrinsic and intrinsic pathways converge on the same execution pathway and on caspase-3 activation. The cleavage of caspase-3 and its activation causes DNA fragmentation, demolition of key structural cytoskeletal and nuclear proteins, cross-linking of proteins, and formation of apoptosis bodies [22]. Our results showed apoptotic DNA ladder formation with different molecular-weight fractions, suggesting the presence of apoptotic events. Fragmentation of the genomic DNA is a biochemical hallmark of apoptosis, an irreversible event that commits the cell to die. In many systems, this DNA fragmentation has been shown to result from activation of an endogenous nuclear endonuclease. This enzyme selectively cleaves DNA at sites located between nucleosomal units generating mono- and oligonucleosomal DNA fragments. In the final stage of apoptosis, the cell disintegrates into apoptotic bodies that are rapidly phagocytosed and destroyed by phagocytes avoiding inflammation, tissue damage, and damage to surrounding cells [36].
The dysregulation of cell death by excessive or defective apoptosis has been implicated in a variety of disease states [37]. These findings suggest that there is a significantly heightened presence of dual apoptotic disequilibrium in RTCs incubated with CRS1 plasma compared to RTCs treated with AHF patients' and CTR plasma. We demonstrate evidence for the delineation of two distinct apoptotic pathway activations in CRS1, and complex interactions between different intracellular pathways determine the cellular responses to the extracellular environment or to intracellular damage. Finally, apoptotic mechanisms and caspase activations are implicated in the CRS1 pathophysiology; furthermore, these findings strengthened our previous results [6,11].
Over the last few years, many studies have demonstrated that survival factors and anti-cytokine strategies, such as TNF-α, FasL, and TWEAK, can prevent apoptosis in vivo [38,39,40,41,42,43,44,45,46,47]. In vivo caspase inhibitors protect against ischemic injury in different organs, such as the brain, heart, and kidney [48]. These interventions might be beneficial in AHF patients with CRS1 based on this evidence. However, an understanding of the exact mechanisms involved in CRS1 apoptosis in RTCs is very important and fundamental for exploring the utility of antiapoptotic therapies to prevent or cure kidney apoptosis in vivo. Although accumulating evidence suggests a role for apoptotic pathways in the CRS1 pathobiology, actually, there is an incomplete understanding of the molecular regulation of apoptosis in the renal cell. In particular, stimulus- and cell-specific apoptotic pathways activated during the course of CRS1 should be better characterized and explored.
This study has some limitations which should be taken into account when interpreting the results. It is limited by the restricted number of patients and by the in vitro experimental evaluations (it can be very challenging to extrapolate the results of an in vitro model to the biology of the intact organism as a whole). Furthermore, for our experiments, we used a human renal tubular cell line immortalized by the Adeno5/SV40 virus. In vivo, the response of these cells could be partially different because of the presence of cell-cell interactions, extracellular matrix interactions, and the real biological context.
Further investigations are therefore necessary to better understand this pathophysiological process and the therapeutic and prognostic implications for CRS1. Future research should focus on defining the cellular and molecular targets, the optimal time frame, and the specific strategies for therapeutic intervention in this syndrome.
References
- 1.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O'Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–338. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 4.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. 2012;60:1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 7.Virzi GM, Clementi A, Brocca A, de Cal M, Vescovo G, Granata A, Ronco C. The hemodynamic and nonhemodynamic crosstalk in cardiorenal syndrome type 1. Cardiorenal Med. 2014;4:103–112. doi: 10.1159/000362650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz DN. Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Adv Chronic Kidney Dis. 2013;20:56–66. doi: 10.1053/j.ackd.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Virzi GM, de Cal M, Cruz DN, Bolin C, Vescovo G, Ronco C. Type 1 cardiorenal syndrome and its possible pathophysiological mechanisms (in Italian) G Ital Nefrol. 2012;29:690–698. [PubMed] [Google Scholar]
- 10.Virzi G, Day S, de Cal M, Vescovo G, Ronco C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014;18:201. doi: 10.1186/cc13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virzi GM, Torregrossa R, Cruz DN, Chionh CY, de Cal M, Soni SS, Dominici M, Vescovo G, Rosner MH, Ronco C. Cardiorenal syndrome type 1 may be immunologically mediated: a pilot evaluation of monocyte apoptosis. Cardiorenal Med. 2012;2:33–42. doi: 10.1159/000335499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastori S, Virzi GM, Brocca A, de Cal M, Clementi A, Vescovo G, Ronco C. Cardiorenal syndrome type 1: a defective regulation of monocyte apoptosis induced by proinflammatory and proapoptotic factors. Cardiorenal Med. 2015;5:105–115. doi: 10.1159/000371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virzi GM, Clementi A, de Cal M, Brocca A, Day S, Pastori S, Bolin C, Vescovo G, Ronco C. Oxidative stress: dual pathway induction in cardiorenal syndrome type 1 pathogenesis. Oxid Med Cell Longev. 2015;2015:391790. doi: 10.1155/2015/391790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough PA. Cardiorenal syndromes: pathophysiology to prevention. Int J Nephrol. 2011;2011:762590. doi: 10.4061/2011/762590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, Kapeliovich M, Agmon Y, Markiewicz W, Aronson D. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337. doi: 10.1016/j.ahj.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 16.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 18.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 20.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 25.Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Galimi F, Camboni MG, Deriu E, Conaldi P, Bottelli A, Orlandi V, Herrera MB, Pacitti A, Segoloni GP, Camussi G. Macrophage stimulating protein may promote tubular regeneration after acute injury. J Am Soc Nephrol. 2008;19:1904–1918. doi: 10.1681/ASN.2007111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conaldi PG, Biancone L, Bottelli A, Wade-Evans A, Racusen LC, Boccellino M, Orlandi V, Serra C, Camussi G, Toniolo A. HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Invest. 1998;102:2041–2049. doi: 10.1172/JCI3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bordoni V, Piroddi M, Galli F, de Cal M, Bonello M, Dimitri P, Salvatori G, Ranishta R, Levin N, Tetta C, Ronco C. Oxidant and carbonyl stress-related apoptosis in end-stage kidney disease: impact of membrane flux. Blood Purif. 2006;24:149–156. doi: 10.1159/000089452. [DOI] [PubMed] [Google Scholar]
- 28.de Cal M, Cruz DN, Corradi V, Nalesso F, Polanco N, Lentini P, Brendolan A, Tetta C, Ronco C. HLA-DR expression and apoptosis: a cross-sectional controlled study in hemodialysis and peritoneal dialysis patients. Blood Purif. 2008;26:249–254. doi: 10.1159/000122110. [DOI] [PubMed] [Google Scholar]
- 29.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 30.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 31.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Sanz AB, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol. 2008;19:1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 33.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 34.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 35.Kaushal GP. Role of caspases in renal tubular epithelial cell injury. Semin Nephrol. 2003;23:425–431. doi: 10.1016/s0270-9295(03)00086-x. [DOI] [PubMed] [Google Scholar]
- 36.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341:1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- 38.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M, Thiemermann C, Yaqoob MM. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 39.Vijayan A, Martin DR, Sadow JL, Kissane J, Miller SB. Hepatocyte growth factor inhibits apoptosis after ischemic renal injury in rats. Am J Kidney Dis. 2001;38:274–278. doi: 10.1053/ajkd.2001.26087. [DOI] [PubMed] [Google Scholar]
- 40.Hirschberg R, Kopple J, Lipsett P, Benjamin E, Minei J, Albertson T, Munger M, Metzler M, Zaloga G, Murray M, Lowry S, Conger J, McKeown W, O'Shea M, Baughman R, Wood K, Haupt M, Kaiser R, Simms H, Warnock D, Summer W, Hintz R, Myers B, Haenftling K, Capra W, et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 1999;55:2423–2432. doi: 10.1046/j.1523-1755.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- 41.Hladunewich MA, Corrigan G, Derby GC, Ramaswamy D, Kambham N, Scandling JD, Myers BD. A randomized, placebo-controlled trial of IGF-1 for delayed graft function: a human model to study postischemic ARF. Kidney Int. 2003;64:593–602. doi: 10.1046/j.1523-1755.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 42.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA. Tumor necrosis factor α-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller-Werdan U, Schumann H, Fuchs R, Reithmann C, Loppnow H, Koch S, Zimny-Arndt U, He C, Darmer D, Jungblut P, Stadler J, Holtz J, Werdan K. Tumor necrosis factor α (TNFα) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(NO)-synthase (iNOS) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–2923. doi: 10.1006/jmcc.1997.0526. [DOI] [PubMed] [Google Scholar]
- 44.Patten M, Kramer E, Bunemann J, Wenck C, Thoenes M, Wieland T, Long C. Endotoxin and cytokines alter contractile protein expression in cardiac myocytes in vivo. Pflugers Arch. 2001;442:920–927. doi: 10.1007/s004240100612. [DOI] [PubMed] [Google Scholar]
- 45.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2004;101:14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK. TNF-α mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol. 2005;288:F406–F411. doi: 10.1152/ajprenal.00099.2004. [DOI] [PubMed] [Google Scholar]
- 48.Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:269–287. doi: 10.2174/1568008054863754. [DOI] [PubMed] [Google Scholar]