Abstract
Purpose
Investigate the incidence of Parkinsonism among patients with Gaucher disease type 1 (GD1) and describe demographics, genotypes, and Gaucher disease (GD)-related characteristics for affected and non-affected patients.
Methods
Study type: Cohort study with age- and gender-matched nested case–control analysis. Calculation of event incidence, standardized morbidity ratio, and event-free survival (Kaplan–Meier). Data source: The International Collaborative Gaucher Group (ICGG) Gaucher Registry data as of June 2010. Study cohort: GD1 patients with any report of Parkinsonism. Pre-matching control group: All GD1 patients with no report of Parkinsonism.
Results
The matched study cohort comprised of 68 patients with reports of Parkinsonism and 649 patients without Parkinsonism. Demographic and clinical characteristics suggest a milder GD phenotype in patients with Parkinsonism compared to the control group. The most prevalent GD1 genotype was N370S/N370S (39% for controls; 46% for patients with Parkinsonism). Patients with Parkinsonism were diagnosed with GD1 at a mean age of 37 years compared to 31 years in control patients. The standardized morbidity ratio for the development of Parkinsonism among all GD1 patients indicated an approximately 6 to 17 fold increase over that of 2 reference populations. The mean age of reported Parkinsonism onset was 57 years compared to 60 years in the general population (Lees, Hardy, and Revesz, 2009 [1]). The probability that a patient with GD1 will develop Parkinsonism before age 70 years is 5 to 7% and 9 to 12% before age 80 years.
Conclusions
The incidence of Parkinsonism among GD1 patients is significantly increased compared to two reference populations. GD1 patients with Parkinsonism have a later median age at GD diagnosis, later age at the start of treatment, and later age at death than patients with GD1 alone. The Gaucher-related clinical profile of GD1 patients with Parkinsonism is similar to or milder than the GD1 alone group. Therefore, severity of the common GD1 clinical manifestations does not appear to be predictive for the onset of Parkinsonism.
Keywords: Parkinsonism, Gaucher disease, Glucocerebrosidase, Genotype, Genetic risk
Introduction
Gaucher disease (GD) is a hereditary metabolic disorder characterized by deficient activity of lysosomal glucocerebrosidase (GBA) that is almost always attributable to mutations in the GBA gene. The enzyme deficiency causes accumulation of its natural substrate, glucosylceramide, predominantly in tissue macrophages. The organs that are primarily affected are the spleen, liver, bone marrow, skeleton and lungs with resultant splenomegaly, hepatomegaly, hematological cytopenias and skeletal manifestations including avascular necrosis, decreased bone mineral density, lytic bone lesions and pathological fractures [2–5].
Involvement of the central nervous system in type 1 GD (GD1) is apparently much less common. In fact, GD is traditionally classified into 3 major phenotypes: type 1 (non-neuronopathic), type 2 (acute neuronopathic) and type 3 (chronic neuronopathic). GD1 is differentiated from type 2GD and type 3GD by the absence of overt neurological signs and symptoms [4,5]. However, neurological symptoms including peripheral neuropathy and Parkinsonism have increasingly been reported in systematic studies of patientswithGD1 [6–10]. Additionally, several case reports of patientswithGD1 developing Parkinsonism have been published [11–17]. Other studies in patients with known Parkinson's disease demonstrated an association with mutations in the GBA gene [15,18–22]. The GBA mutations, found in patients with Parkinson's disease and its variants, are not restricted to any specific ethnic group and include those most prevalent worldwide (N370S and L444P) and others that are less common such as R496H, V394L, D409H, RecTL, IVS2+1 and 84GG [23]. Velayati [24] asserted in a recent review, “GBA variants are currently the most common genetic risk factor associated with Parkinsonism.”
The finding of an association between Gaucher disease and Parkinsonism has been a stimulus for research into the basic mechanisms involved [25–28] and has raised questions about the traditional broad classification of GD as either neuropathic or nonneuropathic [29]. Of more immediate concern, patients and their family members are increasingly aware of the association between mutations in the GBA gene, Gaucher disease and Parkinsonism. This knowledge has led to heightened anxiety and urgent questions about the magnitude of risk and the probability that they will be affected.
Therefore, using the largest database of patients with GD, the International Collaborative Gaucher Group (ICGG) Gaucher Registry, we investigated the incidence of Parkinsonism among patients with GD1, estimated the actuarial probability that a patient with GD1 will develop Parkinsonism, and evaluated the clinical, genotypic, and phenotypic findings of the subset of GD1 patients with reported Parkinsonian manifestations.
Materials and methods
ICGG Gaucher Registry
The ICGG Gaucher Registry was launched in 1991 to track the clinical, demographic, genetic, biochemical and therapeutic characteristics of patients with GD throughout the world, irrespective of disease severity and treatment status or treatment choice [2]. The overarching goals of the Registry are to define the clinical spectrum of GD, assess its natural history through longitudinal follow-up, and assess the effect of treatment. An independent international group of physician experts in GD provide scientific direction and governance for the Registry, with logistical support from Genzyme Corporation (Cambridge, MA). With appropriate Institutional Review Board/Ethics Committee approvals, over 700 physicians from 60 countries have voluntarily submitted de-identified patient data to the Registry since 1991.
Study populations
Patients included in this study participate in the ICGG Gaucher Registry. All patients in this study were diagnosed with GD based on assay of acid β-glucosidase activity in peripheral blood leukocytes and/or genotyping of the GBA gene.
Data from the ICGG Gaucher Registry included in this study were recorded as of June 2010. We identified all patients in the Registry with GD1 who were at least 18 years of age as of their last follow-up in the Registry. We then queried the Registry to determine the subset of patients who had Parkinsonism, according to entries in the chronic disease section of the Registry case report form. The following list of search terms were used in the query: “Parkinson's,” “Parkinsonism,” “Multiple system atrophy,” “Progressive supranuclear palsy,” “Lewy body dementia,” “Shy–Drager syndrome,” “Olivopontocerebellar atrophy,” or “OPCA.” These terms were used because these disorders cause Parkinsonism-like symptoms; no patients with these disorders are included in the ICGG Gaucher Registry. We obtained the following information for all patients included in the study: date and age of GD diagnosis; imiglucerase treatment status and date of initiation of treatment (where applicable); genotype (generally not based on complete DNA sequencing); gender; splenectomy status; and dates of each patient's most recent assessment reported to the Registry. The control group consisted of all GD1 patients enrolled in the ICGG Gaucher Registry with no reports of Parkinsonism. A matched case–control analysis was conducted, whereby patients with and without Parkinsonism were matched by gender and year of birth. For each case patient, up to 10 matched control patients were randomly selected from those identified as matching on gender and year of birth (±5 years). Demographics, genotypes and GD-related characteristics were provided for both groups. For each patient identified with Parkinsonism, a survey (Supplemental Table A1) was mailed to the treating ICGG Registry physician in order to obtain additional data on Parkinsonism severity.
Demographic and clinical characteristics of patients
Baseline demographic characteristics included: gender, ethnicity (as indicated by the reporting physician), GBA genotype, and country of origin. Clinical characteristics included: hemoglobin concentration, platelet count, spleen volume, liver volume, and skeletal manifestations of GD, including bone mineral density, reports of bone pain, and/or bone crisis. Dates of assessment for clinical characteristics vary as patients can enroll into the Registry at any time point.
For imiglucerase-treated patients, we used data around the date of first infusion. For untreated patients, we used each patient's most recent date for which a data point was available.
Data analysis
Demographic data and clinical characteristics were analyzed with descriptive statistics. Proportions were calculated for categorical variables (e.g., gender, genotype, ethnicity). Summary statistics (mean, standard deviation, percentiles) were calculated for continuous measures (e.g., age). For those continuous measures, a Student's t-test was used to test the null hypothesis of equal distributions between the 2 groups. For categorical variables, a Chi Square goodness of fit test was used when comparing the distributions of 2×2 contingency tables. For variables with more than 2 categories, a Cochran–Mantel–Haenszel test of general association was used when comparing distributions.
The incidence rates of Parkinsonism were calculated using standard statistical methodology as follows:
| (1) |
Person-years of follow-up for each GD1 patient began at birth and continued until: (1) the date of reported onset of Parkinsonism (for case patients), or (2) the date of their last recorded assessment in the Registry (for control patients). If a patient had Parkinsonism reported without an onset date, we inputted their onset date to be midway between their first and last reported assessments to the Registry. To compare the number of observed to expected Parkinsonism cases, a standardized morbidity ratio (SMR) [30] was calculated using published data from 2 reference populations [31,32]. The SMR is interpretable as the relative risk of developing Parkinsonism. Parkinsonism-free survival probability was calculated and presented in a Kaplan–Meier curve. Differences in survival proportion between males and females were compared using a log-rank test.
All analyses were conducted in SAS 9.1 (SAS Institute Inc., Cary, NC, USA) in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines (http://www.strobe-statement.org).
Results
As of June 2010, the ICGG Gaucher Registry database included 68 patients with GD1 who were 18 years of age or older at last Registry assessment, and who had reports of Parkinsonism (Table 1). The control group before matching consisted of 3983 patients with GD1 who were 18 years of age or older at last Registry assessment, and who had no reports of Parkinsonism. The matched control group comprised 649 patients without Parkinsonism. As shown in Table 1A before matching, the Parkinsonism group had proportionately more males (56%) than the control group (45%) and an age distribution that was shifted towards older patients. After matching (Table 1B), the years of birth and gender distributions of both groups were nearly identical.
Table 1.
Matching characteristics of GD1 patients with and without Parkinsonism.
| A. Before matching | |||
|---|---|---|---|
| Before matching | p-value | ||
| Patients without Parkinsonism reported |
Patients with Parkinsonism reported |
||
| Patients enrolled | 3983 | 68 | |
| Sex, n (%) | n = 3983 | n = 68 | 0.0627 |
| Males | 1775 (44.6) | 38 (55.9) | |
| Females | 2208 (55.4) | 30 (44.1) | |
| Year of birth, n (%) | n = 3983 | n = 68 | <0.0001 |
| 1900–1909 | 3 (0.1) | 0 | |
| 1910–1919 | 41 (1.0) | 1 (1.5) | |
| 1920–1929 | 149 (3.7) | 9 (13.2) | |
| 1930–1939 | 279 (7.0) | 14 (20.6) | |
| 1940–1949 | 500 (12.6) | 23 (33.8) | |
| 1950–1959 | 689 (17.3) | 16 (23.5) | |
| 1960–1969 | 756 (19.0) | 4 (5.9) | |
| 1970–1979 | 818 (20.5) | 1 (1.5) | |
| 1980–1989 | 655 (16.4) | 0 | |
| 1990–1999 | 93 (2.3) | 0 | |
| B. After matching | |||
|---|---|---|---|
| After matching | p-value | ||
| Patients without Parkinsonism reported |
Patients with Parkinsonism reported |
||
| Patients enrolled | 649 | 68 | |
| Sex, n (%) | n = 649 | n = 68 | 0.9869 |
| Males | 362 (55.8) | 38 (55.9) | |
| Females | 287 (44.2) | 30 (44.1) | |
| Year of birth, n (%) | n = 649 | n = 68 | 0.9583 |
| 1900–1909 | 0 | 0 | |
| 1910–1919 | 17 (2.6) | 1 (1.5) | |
| 1920–1929 | 79 (12.2) | 9 (13.2) | |
| 1930–1939 | 141 (21.7) | 14 (20.6) | |
| 1940–1949 | 193 (29.7) | 23 (33.8) | |
| 1950–1959 | 151 (23.3) | 16 (23.5) | |
| 1960–1969 | 58 (8.9) | 4 (5.9) | |
| 1970–1979 | 6 (0.9) | 1 (1.5) | |
| 1980–1989 | 4 (0.6) | 0 | |
| 1990–1999 | 0 | 0 | |
The demographic and clinical characteristics of the study groups are shown in Table 2. In both groups of patients with and without Parkinsonism, the majority of patients were Ashkenazi Jews (approximately 65%) and non-Jewish Caucasians (26% to 30%, p = 0.4591). Patients with Parkinsonism were distinguished by a later mean age at GD1 diagnosis (37 years) compared to controls (31 years, p = 0.0208). Accordingly, patients with Parkinsonism were significantly older (mean age 54 years) when they received their first infusion of imiglucerase compared to the controls (48 years, p = 0.0028). Ninety percent of patients with Parkinsonism were diagnosed with Gaucher disease prior to the onset of Parkinsonism, which was first reported at a mean age of 57 years. Among deceased patients, those with Parkinsonism died at an older mean age (72 years) than the controls (63 years, p = 0.0018).
Table 2.
Demographic and clinical characteristics of GD1 patients with and without Parkinsonism, after matching.
| Patients without Parkinsonism reported |
Patients with Parkinsonism reported |
p-value | |
|---|---|---|---|
| Patients enrolled | 649 | 68 | |
| Age at Gaucher diagnosisa (years) | n = 598 | n = 65 | 0.0208 |
| Median (25th, 75th) | 29 (14, 46) | 41 (19, 49) | |
| Mean (SD) | 31 (20) | 37 (20) | |
| Min, max | 0, 85 | 3, 80 | |
| Age at Gaucher diagnosisa, n (%) | n = 598 | n = 65 | 0.0269 |
| Prenatal to <10 years | 109 (18.2) | 10 (15.4) | |
| 10 to <20 years | 95 (15.9) | 7 (10.3) | |
| 20 to <30 years | 110 (18.4) | 6 (8.8) | |
| 30 to <40 years | 89 (14.9) | 8 (11.8) | |
| 40 to <50 years | 75 (12.5) | 18 (26.5) | |
| 50 to <60 years | 62 (10.4) | 7 (10.3) | |
| 60 to <70 years | 37 (6.2) | 7 (10.3) | |
| 70 years or more | 21 (3.5) | 2 (2.9) | |
| Treatment status, n (%) | n = 649 | n = 68 | 0.0808 |
| Ever on imiglucerase | 565 (87.1) | 54 (79.4) | |
| Never on imiglucerase | 84 (12.9) | 14 (20.6) | |
| Age at first infusion (years) | n = 564 | n = 54 | 0.0028 |
| Median (25th, 75th) | 47 (38, 58) | 54 (47, 63) | |
| Mean (SD) | 48 (14) | 54 (13) | |
| Min, max | 9, 87 | 15, 80 | |
| Deceased, n (%) | n = 649 | n = 68 | 0.0573 |
| Yes | 88 (13.6) | 15 (22.1) | |
| No | 561 (86.4) | 53 (77.9) | |
| Age at death (years) | n = 83 | n = 13 | 0.0018 |
| Median (25th, 75th) | 62 (50, 75) | 73 (71, 75) | |
| Mean (SD) | 63 (15) | 72 (8) | |
| Min, max | 20, 89 | 50, 85 | |
| Ethnicity, n (%) | n = 368 | n = 57 | 0.4591 |
| Caucasian, non-Jewish | 109 (29.6) | 15 (26.3) | |
| Jewish, Ashkenazi | 241 (65.5) | 37 (64.9) | |
| All others | 18 (4.9) | 5 (8.8) | |
| Splenectomy status, n (%) | n = 649 | n = 68 | 0.0128 |
| Never splenectomized | 356 (54.9) | 48 (70.6) | |
| Ever splenectomized | 293 (45.1) | 20 (29.4) | |
| Age at onset of Parkinsonism (years) | n = 61 | ||
| Median (25th,75th) | 56 (49, 64) | ||
| Mean (SD) | 57 (10) | ||
| Min, max | 30, 80 |
Patients with no diagnosis date or with diagnosis date earlier than 1 year prior to their birth were excluded from the analysis.
GBA genotypes were reported for approximately 87% of the GD1 patients with Parkinsonism and 68% of patients in the control group (Table 3). There were no significant differences in genotype between the 2 groups, with the most prevalent GBA genotype in both groups being N370S/N370S (Parkinsonism 46%; controls 39%).
Table 3.
Frequency of genotypes for GD1 patients with and without Parkinsonism, after matching.
| Patients without Parkinsonism reported |
Patients with Parkinsonism reported |
p-value | |
|---|---|---|---|
| Patients enrolled | 649 | 68 | |
| Patients reporting genotype, n (%) | n = 438 (67.5) | n = 59 (86.8) | 0.4149 |
| Genotypec, n(%) | |||
| N370S/N370S | 172 (39.2) | 27 (45.8) | |
| N370S/L444P | 71 (16.2) | 9 (15.3) | |
| N370S/84GG | 58 (13.2) | 4 (6.8) | |
| N370S/?a | 58 (13.2) | 8 (13.6) | |
| N370S/rare alleleb | 40 (9.1) | 5 (8.5) | |
| N370S/IVS2+1 | 13 (3.0) | 2 (3.4) | |
| L444P/rare alleleb | 5 (1.1) | 2 (3.4) | |
| Rare alleleb/rare alleleb | 5 (1.1) | – | |
| L444P/?a | 4 (0.9) | – | |
| 84GG/rare alleleb | 3 (0.7) | – | |
| Rare alleleb/?a | 3 (0.7) | – | |
| N370S/D409H | 2 (0.5) | – | |
| 84GG/?a | 1 (0.2) | – | |
| IVS2+1/rare alleleb | 1 (0.2) | – | |
| IVS2+1/?a | 1 (0.2) | – | |
| ?a/?a | 1 (0.2) | 2 (3.4) |
Results of genotype test did not match any tested mutations.
Rare allele is defined as known allele which is not N370S, L444P, IVS2+1, D409H or 84GG.
Calculations based on the number of patients reporting genotype.
The baseline hematological, visceral, and bone characteristics of GD1 patients with and without Parkinsonism are shown in Tables 4 and 5. Significantly fewer GD1 patients with Parkinsonism had anemia (18%) compared to controls (40%, p = 0.0041). No significant differences were observed between the 2 groups for thrombocytopenia or other organomegaly measures, though there did appear to be a trend toward significance for thrombocytopenia (p = 0.0596) and hepatomegaly (p = 0.0897). In GD1 patients with Parkinsonism, 79% had platelet counts less than 150,000/mm3 at baseline; 71% had spleen volumes >5 multiples of normal; and 48% had liver volumes >1.25 multiples of normal. Bone pain at baseline was reported in 60% of patients with Parkinsonism and low bone mineral density (DXA t-score ≤−1) in 64%. Six (19%) of these patients with Parkinsonism experienced bone crises at some point during the course of their GD1. For comparison, among GD1 patients without Parkinsonism 90% had platelet counts less than 150,000/mm3; 75% had spleen volumes >5 multiples of normal; and 58% had liver volumes >1.25 multiples of normal. Bone pain was reported in 63% of all patients and low bone mineral density in 55%.
Table 4.
Hematological and visceral manifestations at baselinea for GD1 patients with and without Parkinsonism, after matching.
| Patients without Parkinsonism reported |
Patients with Parkinsonism reported |
p-value | |
|---|---|---|---|
| Patients enrolled | 649 | 68 | |
| Anemiab, n (%) | n = 519 | n = 44 | 0.0041 |
| Yes | 208 (40.1) | 8 (18.2) | |
| No | 311 (59.9) | 36 (81.8) | |
| Thrombocytopeniac (platelet count, × 103/mm3) [non-splenectomized patients only], n (%) | n = 293 | n = 33 | 0.0596 |
| Present (<150) | 263 (89.8) | 26 (78.8) | |
| Absent (≥150) | 30 (10.2) | 7 (21.2) | |
| Splenomegaly (spleen volume in multiples of normal), n (%) | n = 186 | n = 21 | 0.5997 |
| Mild or none (≤5) | 47 (25.3) | 6 (28.6) | |
| Moderate (>5 to ≤15) | 84 (45.2) | 11 (52.4) | |
| Severe (>15) | 55 (29.6) | 4 (19.0) | |
| Hepatomegaly (liver volume in multiples of normal), n (%) | n = 282 | n = 25 | 0.0897 |
| Mild or none (≤1.25) | 118 (41.8) | 13 (52.0) | |
| Moderate (>1.25 to ≤2.5) | 118 (41.8) | 12 (48.0) | |
| Severe (>2.5) | 46 (16.3) | 0 (0.0) |
For ‘never on imiglucerase’ patients, baseline is defined as the most recent date for which a data point for a given variable was available. Therefore different variables may have different assessment dates. For ‘ever on imiglucerase patients,’ baseline is defined as the data point closest to the first infusion date, no more than −3 months/+4 weeks (inclusive) from first infusion for hemoglobin/platelet, and −6 months/+6 weeks (inclusive) from first infusion for liver/spleen. Treated patients with no infusion date were excluded from the analysis for each hematological and visceral assessment.
Anemia is defined according to age and gender norms for hemoglobin concentrations as follows: <12 g/dL for males older than 12 years; <11 g/dL for females older than 12 years; <10.5 g/dL for children ages >2 to 12 years; <9.5 g/dL for children ages 6 months to 2 years; and <10.1 g/dL for children younger than 6 months of age.
For patients without Parkinsonism, among the 235 partial or total splenectomy patients, thrombocytopenia was classified as present in 65 (28%) and absent in 170 (72%). For patients with Parkinsonism, among the 11 partial or total splenectomy patients, thrombocytopenia was classified as present in 2 (18%) and absent in 9 (82%).
Table 5.
Bone manifestations at baselinea for GD1 patients with and without Parkinsonism, after matching.
| Patients without Parkinsonism reported |
Patients with Parkinsonism reported |
p-value | |
|---|---|---|---|
| Patients enrolled | 649 | 68 | |
| Bone pain, n (%) | n = 320 | n = 42 | 0.6500 |
| Absent | 118 (36.9) | 17 (40.5) | |
| Present | 202 (63.1) | 25 (59.5) | |
| Bone crisis, n (%) | n = 273 | n = 31 | 0.5496 |
| Absent | 207 (75.8) | 25 (80.6) | |
| Present | 66 (24.2) | 6 (19.4) | |
| Radiological bone disease, n (%) | n = 303 | n = 37 | 0.7065 |
| Evidence of any bone disease | |||
| Absent | 12 (4.0) | 1 (2.7) | |
| Present | 291 (96.0) | 36 (97.3) |
| Type of bone disease reported |
Any data available, n |
Abnormality present, n (%) |
Any data available, n |
Abnormality present, n (%) |
|
|---|---|---|---|---|---|
| Avascular necrosis | 155 | 77 (49.7) | 19 | 5 (26.3) | 0.0542 |
| Erlenmeyer flask deformity | 150 | 120 (80.0) | 24 | 18 (75.0) | 0.5745 |
| Fractures | 95 | 18 (18.9) | 19 | 4 (21.1) | 0.8319 |
| Infarction | 147 | 98 (66.7) | 20 | 8 (40.0) | 0.0201 |
| Lytic lesions | 78 | 31 (39.7) | 13 | 6 (46.2) | 0.6631 |
| Marrow infiltration | 163 | 153 (93.9) | 18 | 18 (100.0) | 0.2796 |
| Osteopenia | 149 | 127 (85.2) | 20 | 17 (85.0) | 0.9778 |
| Decreased bone mineral density (lumbar spine DXA t-scoreb), n (%) | n = 49 | n = 14 | 0.2309 | ||
| Mild or none (> −1) | 22 (44.9) | 5 (35.7) | |||
| Moderate (>−2.5 to ≤−1) | 10 (20.4) | 6 (42.9) | |||
| Severe (≤ −2.5) | 17 (34.7) | 3 (21.4) |
For ‘never on imiglucerase’ patients, baseline is defined as the most recent date for which a data point for a given variable was available. Therefore different variables may have different assessment dates. For ‘ever on imiglucerase’ patients, baseline is defined as the data point closest to the first infusion date, no more than −2 years/+6 weeks (inclusive) from first infusion. Treated patients with no infusion date were excluded from the analysis for each bone assessment.
Standard deviations of age and gender-adjusted norms.
Table 6A and B show the age- and gender-specific incidence rates of Parkinsonism in 2 reference populations, and the corresponding number of observed cases among our GD1 patients. Applying the incidence rates in the reference populations, we calculated the expected numbers of cases of Parkinsonism in our GD1 population. We then calculated the standardized morbidity ratio of observed to expected cases for men of all ages, women of all ages and the overall group. The SMR (95% CI) for Parkinsonism for all GD1 patients over 18 years of age in the ICGG Gaucher Registry, relative to the first reference population [31], was 16.96 (13.27,21.37), p<0.0001. Relative to the second reference population [32], the risk was 6.34 (4.96, 7.98), p<0.0001. The SMRs for men were 15.08 (10.82, 20.48), p<0.0001 in the first reference population, and 5.82 (4.18, 7.91), p<0.0001 in the second reference population. The SMRs for Parkinsonism in women with GD1 were even greater for the first and second populations, respectively: 20.13 (13.83, 28.38), p<0.0001 and 7.14 (4.91,10.07), p<0.0001.
Table 6.
Parkinson's incidence rates calculated with 2 reference populations: (A) Winter [31] and (B) Linder [32].
| A. | ||||||
|---|---|---|---|---|---|---|
| Gender | Age group (years) |
Person-years of follow-up (Gaucher Registry) |
Incidence rate per 100,000 person-years (Winter [31]) |
Number of expected cases |
Number of observed cases |
Standardized morbidity ratio (95% CI) |
| Men | 0–44 | 67,854.30 | 0.00 | 0.00 | 1 | |
| 45–49 | 3901.41 | 1.42 | 0.06 | 7 | ||
| 50–54 | 3098.50 | 3.49 | 0.11 | 4 | ||
| 55–59 | 2340.04 | 7.32 | 0.17 | 7 | ||
| 60–64 | 1689.87 | 24.88 | 0.42 | 7 | ||
| 65–69 | 1185.05 | 28.90 | 0.34 | 6 | ||
| 70–74 | 762.67 | 109.29 | 0.83 | 4 | ||
| 75–79 | 401.51 | 85.03 | 0.34 | 2 | ||
| 80+ | 246.46 | 99.90 | 0.25 | 0 | ||
| All ages | 81,479.81 | 8.17a | 2.52 | 38 | 15.08b (10.82, 20.48) | |
| Women | 0–44 | 83,479.89 | 0.00 | 0.00 | 4 | |
| 45–49 | 4263.09 | 2.77 | 0.12 | 7 | ||
| 50–54 | 3338.91 | 3.85 | 0.13 | 5 | ||
| 55–59 | 2435.04 | 4.28 | 0.10 | 8 | ||
| 60–64 | 1651.40 | 12.83 | 0.21 | 2 | ||
| 65–69 | 1096.90 | 23.09 | 0.25 | 2 | ||
| 70–74 | 637.74 | 62.90 | 0.40 | 0 | ||
| 75–79 | 306.35 | 61.56 | 0.19 | 2 | ||
| 80+ | 175.67 | 50.37 | 0.09 | 0 | ||
| All ages | 97,384.99 | 9.40a | 1.49 | 30 | 20.13b (13.83, 28.38) | |
| Total | All ages | 178,864.80 | 9.03a | 4.01 | 68 | 16.96b (13.27, 21.37) |
| B. | ||||||
|---|---|---|---|---|---|---|
| Gender | Age group (years) |
Person-years of follow-up (Gaucher Registry) |
Incidence rate per 100,000 person-years (Linder [32]) |
Number of expected cases |
Number of observed cases |
Standardized morbidity ratio (95% CI) |
| Men | 0–29 | 51,370.19 | 0.00 | 0.00 | 0 | |
| 30–39 | 11,831.47 | 2.50 | 0.30 | 0 | ||
| 40–49 | 8554.05 | 2.80 | 0.24 | 8 | ||
| 50–59 | 5438.54 | 27.90 | 1.52 | 11 | ||
| 60–69 | 2874.92 | 61.60 | 1.77 | 13 | ||
| 70–79 | 1164.18 | 198.50 | 2.31 | 6 | ||
| 80–89 | 243.24 | 161.60 | 0.39 | 0 | ||
| 90+ | 3.22 | 106.40 | 0.00 | 0 | ||
| All ages | 81,479.81 | 26.80a | 6.53 | 38 | 5.82b (4.18, 7.91) | |
| Women | 0–29 | 63,892.20 | 0.00 | 0.00 | 1 | |
| 30–39 | 14,232.97 | 0.00 | 0.00 | 0 | ||
| 40–49 | 9617.82 | 5.80 | 0.56 | 10 | ||
| 50–59 | 5773.95 | 16.90 | 0.98 | 13 | ||
| 60–69 | 2748.31 | 42.60 | 1.17 | 4 | ||
| 70–79 | 944.09 | 138.60 | 1.31 | 2 | ||
| 80–89 | 171.87 | 106.90 | 0.18 | 0 | ||
| 90+ | 3.80 | 0.00 | 0.00 | 0 | ||
| All ages | 97,385.01 | 21.80a | 4.20 | 30 | 7.14b (4.91, 10.07) | |
| Total | All ages | 178,864.82 | 27.50a | 10.73 | 68 | 6.34b (4.96, 7.98) |
Age-adjusted incidence per 100,000 person-years.
p<0.0001.
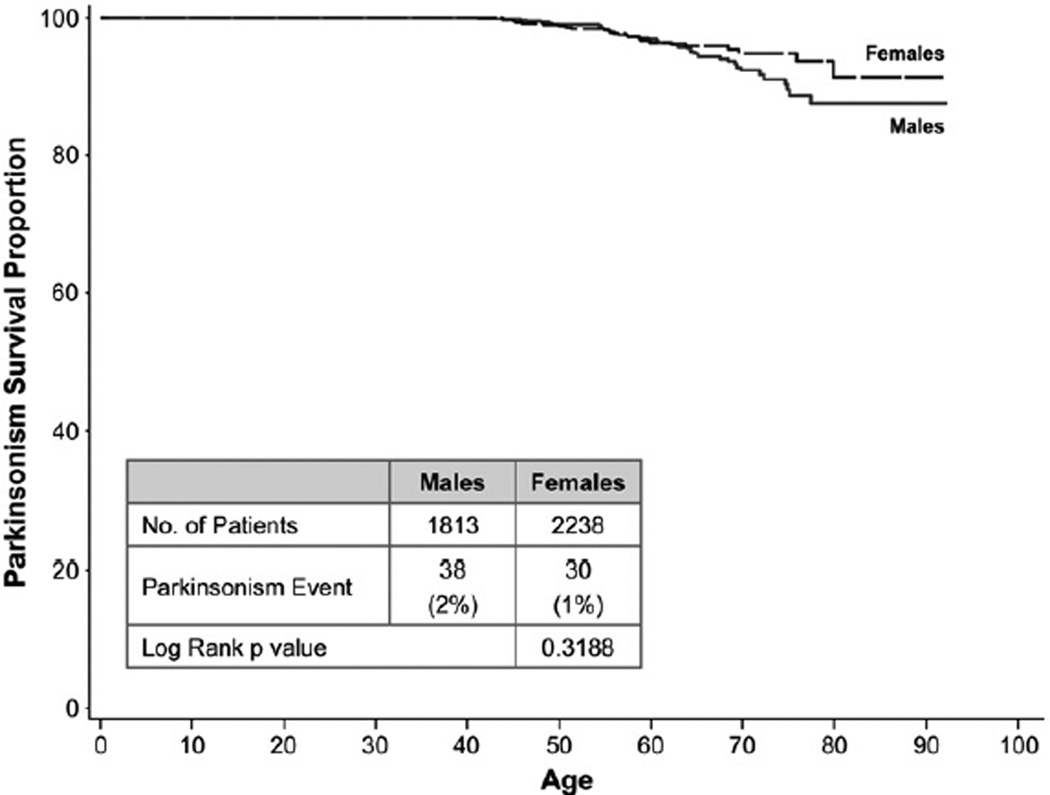
Despite this increase in relative risk, the Kaplan–Meier event-free survival curves (Fig. 1) indicate that the probability of developing Parkinsonism by 70 years of age is approximately 7% in men and in women, approximately 5%. By 80 years of age, the probability is approximately 12% in men and 9% in women.
Fig. 1.
Kaplan–Meier curve of Parkinsonism for adult GD1 patients in the ICGG Gaucher Registry.
Table 7 reviews the Parkinsonism-related symptoms of GD1 patients with Parkinsonism (survey data). Of the 68 GD1 patients with Parkinsonism surveyed, 31 surveys were returned. Of the 31 patients in this group, the symptoms of Parkinsonism typically reported were resting tremor, rigidity, and gait disturbance. Other atypical features in our patients included early falls, frequent action tremor, and in general, only a mild response to levodopa (survey data).
Table 7.
Symptoms of GD1 patients with Parkinsonism (survey data).
| Patient number |
Age of onset of Parkinsonism |
Years of Parkinsonism reported to the registry |
Cognition | Rigidity | Tremor at rest |
Tremor with active motion |
Motion deficit |
Voice | Writing | Gait | Falls | Treatment response to L-DOPA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | <1 | Mild | Mild | Mild | Mild | Mild | Mild | Mild | Mild | ||
| 2 | 63 | 2 | Mild | Mild | Mild | Mild | Mild | Mild | Mild | Moderate | ||
| 3 | 46 | 7 | Moderate | Mild | Mild | Mild | Moderate | Moderate | Severe | Moderate | ||
| 4 | 69 | 6 | Mild | Mild | Mild | None | Moderate | Moderate | Mild | Moderate | Mild | |
| 5 | 59 | 11 | Mild | Severe | Severe | Severe | Mild | Mild | Severe | Severe | Severe | |
| 6 | 80 | 2 | Mild | Mild | Moderate | Mild | Mild | Moderate | Mild | |||
| 7 | 61 | 8 | Mild | Mild | Mild | Moderate | ||||||
| 8 | 55 | 6 | Moderate | Moderate | Moderate | Mild | Moderate | Mild | Moderate | Moderate | Mild | |
| 9 | 46 | 11 | Moderate | Severe | Severe | Severe | Mild | Severe | Severe | Severe | Mild | |
| 10 | 60 | 2 | Mild | Mild | Mild | Mild | Mild | Mild | Mild | Moderate | Mild | |
| 11 | 58 | 3 | Mild | Mild | Mild | None | ||||||
| 12 | 56 | 12 | Severe | Severe | Severe | Severe | Moderate | Moderate | Moderate | Severe | Mild | Mild |
| 13 | 60 | 4 | None | Mild | Mild | None | Mild | None | Mild | None | None | Not Tested |
| 14 | 62 | Severe | Severe | Severe | Moderate | Moderate | Moderate | Mild | Severe | None | Moderate | |
| 15 | 70 | 6 | None | Moderate | Moderate | None | Mild | None | None | Mild | None | Moderate |
| 16 | 48 | 13 | Moderate | Severe | Severe | Severe | Moderate | Moderate | Moderate | Severe | None | Moderate |
| 17 | 51 | 1 | None | Mild | Mild | None | Mild | None | None | None | None | Mild |
| 18 | 72 | 7 | Mild | Mild | Mild | None | Moderate | None | Mild | None | None | Mild |
| 19 | 55 | 11 | None | Moderate | Moderate | None | Moderate | None | Mild | None | None | Moderate |
| 20 | 49 | 10 | Moderate | Severe | Severe | Severe | Moderate | Moderate | Moderate | Severe | None | Mild |
| 21 | 57 | 9 | Mild | Mild | Mild | None | None | Mild | None | None | Mild | Not treated |
| 22 | 61 | 2 | Mild | Moderate | Mild | None | None | None | None | Mild | Mild | Mild |
| 23 | 46 | <1 | None | Mild | Mild | Moderate | Mild | None | Mild | Moderate | None | Mild |
| 24 | 47 | 1 | None | Severe | Moderate | Severe | Severe | None | Mild | Mild | None | Mild |
| 25 | 65 | 4 | Mild | Mild | None | None | None | Mild | None | None | Mild | |
| 26 | 72 | 3 | None | Moderate | Severe | Severe | Mild | None | Severe | Severe | Mild | None |
| 27 | 51 | <1 | Mild | Mild | None | None | Mild | None | None | Mild | None | Mild |
| 28 | 48 | 5 | None | Moderate | None | Mild | None | Mild | None | Mild | None | Mild |
| 29 | 49 | 1 | None | Mild | Mild | None | Mild | None | None | Mild | None | Mild |
| 30 | 55 | 11 | None | Moderate | Moderate | None | Moderate | None | Mild | None | None | Moderate |
| 31 | 49 | 10 | Moderate | Severe | Severe | Severe | Moderate | Moderate | Moderate | Severe | None | Mild |
Discussion
This is the first analysis to use data from the ICGG Gaucher Registry, a global database of nearly 6000 patients with GD, to investigate the occurrence of Parkinsonism among patients with GD1. By comparing GD1 patients with and without Parkinsonism, we found the incidence of Parkinsonism is 6 to 17 times higher in GD1 patients than in carefully analyzed reference populations from Sweden [32] and Russia [31].
The finding of relative is risk essentially in agreement with a recent 444 patient New York metropolitan area study that estimated the adjusted lifetime relative risk of GD1 patients developing Parkinson's disease compared to the general population as 21.4 [9]. We have extended these findings to indicate that the likelihood of an individual patient with GD1 to develop Parkinsonism before age 70 years is relatively small, only 5% to 7%. For comparison, the incidence of Parkinsonism in the general population is estimated at 0.3% in the entire population and 1% in those over 60 years of age [17].
The probability of GD1 patients developing Parkinsonism is contextually important because the life expectancy from birth for all patients with GD1 was recently estimated at 68 years (72 years in non-splenectomized patients) [33]. However, these life expectancy calculations relied primarily on outcomes from patients who did not have enzyme therapy with alglucerase or imiglucerase for most of their lives. It is possible that patients who start enzyme therapy with imiglucerase earlier in life would live longer than the subjects in our control group. In addition, these life expectancy calculations used the US population as a reference via standard life tables and global calculations may be different. Regardless of the global life expectancy, the likelihood that a patient with GD1 will develop Parkinsonism before age 80 years is only 9% to 12%.
This study found phenotypic differences among GD1 patients with and without Parkinsonism. After matching and comparing Gaucher Registry patients with Parkinsonism to control GD1 patients without Parkinsonism, we found that, on average, the clinical phenotype of GD1 patients with Parkinsonism is milder than GD1 patients without Parkinsonism, as evidenced by a significantly lower prevalence of anemia, and a trend toward lesser degrees of thrombocytopenia and hepatomegaly. GD1 patients with Parkinsonism were often diagnosed later in life than the matched control patients, a finding that may be attributable to clinically milder disease. With respect to skeletal disease, bone manifestations in GD1 patients with Parkinsonism were similar to those in control patients. Among deceased patients, those with Parkinsonism died at an older mean age (72 years) than the controls (63 years), an observation that is consistent with clinically milder GD in the Parkinsonism group. In any event, unlike Bultron [9], we found no evidence that GD patients with Parkinsonism have more severe GD1.
How do GD1 patients with Parkinsonism compare to Parkinsonian patients in general? The median age of onset of Parkinsonism in affected ICGG Gaucher Registry patients is 56 years compared to 60 years in the general population [1]. In Parkinsonism, the ratio of males to females is usually 2:1. In our study, although male GD1 patients with Parkinsonism outnumbered females, the ratio was only 1.3:1. This finding suggests that whatever pathophysiology is responsible for the emergence of Parkinsonism in patients with GD1, it is sufficiently strong to overcome any “protective” effect associated with female gender.
As expected, the most common Parkinsonian symptoms in ICGG Gaucher Registry patients were resting tremor and rigidity. There were, however, other less typical features including early falls, frequent action tremor, dementia, and in general, only a mild response to levodopa. Refractoriness to L-dopa in GD1 patients with Parkinsonism has been previously reported [17,34]. Other features of Parkinsonism in GD1 patients described in the literature and found in some of the ICGG Gaucher Registry patients include bradykinesia and aggressive progression [34].
Due to the rarity of GD1, our study was feasible only because of the existence of the ICGG Gaucher Registry, which provided longitudinal data from a patient population that is sufficiently large for statistical sub-group and matching analyses. Nevertheless, there are limitations associated with our study that are common to most observational (non-randomized) research studies. Unlike clinical trials, registry data is often not submitted at well-defined time points, and the severity at baseline and treatment effects are allowed to vary by patient. All ICGG Gaucher Registry data are retrospective and unaudited. Patients followed in the ICGG Gaucher Registry are not randomized to treatment with imiglucerase and treated patients are known to have more severe disease than untreated patients. Other potential confounders not considered in this analysis are genetic polymorphisms other than GBA genotype, epigenetic factors, and environmental factors such as concurrent illnesses, alcohol use, smoking and the use of other medications.
The pathophysiology that results in GD1 patients developing Parkinsonism is not well understood. One hypothesis is that a mutation in the GBA gene may itself be a susceptibility [6] or risk [19,35] factor for the development of Parkinsonism. However, repeated studies as reviewed by Velayati [24] show there is no specific GBA mutation that is associated with Parkinsonism. This was also true in our study. The N370S homoallelic genotype, which is associated with a milder GD phenotype, was the most prevalent mutation in both Parkinsonism and control groups (27/59 in patients with Parkinsonism and 172/438 in control patients), followed by the N370S/L444P mutation (9/59 in GD1 patients with Parkinsonism and 71/438 in GD1 patients). A similar distribution of common GBA mutations was reported by Kraoua [17] and Bultron [9].
Other biochemical defects that could increase the likelihood of GD1 patients developing Parkinsonism are lysosomal dysfunction and/or malformation of glucocerebrosidase. Decreased glucocerebrosidase activity that is common to all symptomatic GBA mutations may cause local concentrations of lipids [26] such as ceramide [36] and glucosylceramide [25] in critical focal brain regions such as the hippocampus. Lysosomal storage of glucosylceramide and glucosylsphingosine may disrupt normal protein degradation through the autophagy pathway [37], leading to the deposition of protein aggregates and subsequent apoptosis and dopaminergic neuronal loss. Dysfunction of lysosomal autophagy has been implicated in the pathogenesis of Parkinsonism [24,38]. Since GBA mutations lead to impaired autophagy, it is possible that the slow course of GD1 in certain patients gives enough time for the development of synuclein pathology to form in the central nervous system. This is supported by our finding that GD1 patients who develop Parkinsonism are diagnosed with GD1 later in life and often have mild GD1 phenotypes.
An additional/alternative hypothesis is that Parkinsonism in GD1 may result from the misfolding of mutant glucocerebrosidase, leading to the disruption of the endoplasmic reticulum-associated degradation pathway mediated by the E3 ubiquitin ligase parkin, and the subsequent toxic aggresome accumulation [28]. Yet a higher prevalence of Parkinson's disease was reported among carriers of the null allele 84GG than in carriers of the N370S mutation, 13.6% compared to 2.2%, respectively [23], thus misfolding of the mutant enzyme could possibly explain only some of the cases. A recent neuropathologic study revealed the presence of glucocerebrosidase (some ubiquitinated; some non-ubiquitinated) in 32% to 90% of α-synuclein containing Lewy bodies in specimens from 7 patients with Parkinson's disease and Gaucher mutations (3 GD1 patients and 4 GD carriers) [39].
Which, if either, of the above hypotheses is correct has direct bearing on whether Gaucher disease treatments that are substrate-oriented (enzyme replacement or substrate reduction) are likely to influence the occurrence of Parkinsonism in GD1. If accumulation of substrate or substrate metabolites is a key, such treatments, if properly targeted to the appropriate tissues, should be preventative. If aggregation of mutant glucocerebrosidase is not the dominant cause, neither enzyme treatment nor substrate reduction would likely alter GD pathophysiology that leads to Parkinsonism. Indeed, few publications have reported a lack of impact of optimal imiglucerase therapy on Parkinsonian manifestations in patients with GD [14,40]. As regards enzyme therapy, almost all of the GD1 Parkinsonism patients in our study were started on imiglucerase treatment relatively late in life. It will likely be many years before we can determine whether GD1 patients who have started enzyme replacement treatment with imiglucerase or substrate reduction treatment early in their lives will experience a decrease in the incidence of Parkinsonism.
Conclusion
Although the large majority of patients with GD1 are not likely to manifest Parkinsonism during their expected lifetime, the incidence of Parkinsonism among GD1 patients is nonetheless significantly increased. GD1 patients with Parkinsonism have an older median age at GD diagnosis than patients with GD1 alone, and a clinical profile that is similar or milder than that of the control patients. Therefore, severity of the common GD1 clinical manifestations is not predictive for onset of Parkinsonism.
Supplementary Material
Acknowledgments
We would like to thank the patients with type 1 (non-neuronopathic) Gaucher disease and their physicians and health care personnel who submit data to the Gaucher Registry, the Gaucher Registry support team at Genzyme Corporation, Robert Brown, Sarah Kulke, MD and Radhika Tripuraneni, MD, MPH. This manuscript was supported, in part, by Genzyme Corporation.
Role of the funding source
Logistical support for this manuscript was provided by Genzyme Corporation. The database for the International Collaborative Gaucher Group (ICGG) Gaucher Registry is supported by Genzyme Corporation.
Conflict of interest
Barry Rosenbloom, Manisha Balwani, Edwin Kolodny and Ari Zimran receive honoraria and expense reimbursement for serving on a Board of Advisors of the ICGG Gaucher Registry. Barry Rosenbloom receives research grants from Genzyme Corporation. Neal Weinreb receives travel reimbursements and/or honoraria and/or research support from Genzyme Corporation, Shire Pharmaceuticals, Amicus Therapeutics and Actelion. Edwin Kolodny receives research grants and/or honoraria from Genzyme Corporation and Shire. Ari Zimran receives consultancy fees from Shire Human Genetic Therapies and Protalix Biotherapeutics; participates in the Speakers' Bureau for Actelion Pharmaceuticals; has options in Protalix Biotherapeutics, and sits on the Scientific Advisory Board of Protalix Biotherapeutics. John Taylor and J. Alexander Cole are employees of Genzyme Corporation. Andrea Gwosdow is a medical writer contracted by Genzyme Corporation.
Abbreviations
- CI
confidence interval
- GBA
glucocerebrosidase
- GD
Gaucher disease
- GD1
type 1 Gaucher disease
- ICGG
International Collaborative Gaucher Group
- SD
standard deviation
- SMR
standardized morbidity ratio
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.bcmd.2010.10.006.
Contributor Information
Barry Rosenbloom, Email: rosenbloomb@toweroncology.com.
Manisha Balwani, Email: manisha.balwani@mssm.edu.
Jeff M. Bronstein, Email: jbronste@ucla.edu.
Edwin Kolodny, Email: edwin.kolodny@nyumc.org.
Swati Sathe, Email: swati.sathe@nyumc.org.
Andrea R. Gwosdow, Email: andrea.gwosdow@genzyme.com.
John S. Taylor, Email: john.taylor@genzyme.com.
J. Alexander Cole, Email: alexander.cole@genzyme.com.
Ari Zimran, Email: azimran@gmail.com.
Neal J. Weinreb, Email: boneal@winning.com.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Charrow J, Andersson HC, Kaplan P, et al. The Gaucher registry: demographics and disease characteristics of 1698 patients with Gaucher disease. Arch. Intern. Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb NJ. The bone in Gaucher disease. Touch Briefings: European Musculoskeletal Review 2007 Issue. 2007;2:3–6. [Google Scholar]
- 4.Grabowski GA, Petsko G, Kolodny EH. Gaucher disease. In: Scriver C, Beaudet A, Valle D, Slye W, editors. The Online Metabolic and Molecular Basis of Inherited Metabolic Disease. McGraw-Hill Publishers; 2010. [Google Scholar]
- 5.Zimran A, Elstein D. Lipid storage diseases. In: Lichtman M, Kipps T, Seligsohn U, Kaushansky K, Prchal J, editors. Williams Hematology. Eighth Ed. New York: McGraw-Hill Publishers; 2011. pp. 1065–1071. [Google Scholar]
- 6.Halperin A, Elstein D, Zimran A. Are symptoms of peripheral neuropathy more prevalent in patients with Gaucher disease? Acta Neurol. Scand. 2007;115:275–278. doi: 10.1111/j.1600-0404.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 7.Capablo JL, Saenz de Cabezon A, Fraile J, et al. Neurological evaluation of patients with Gaucher disease diagnosed as type 1. J. Neurol. Neurosurg. Psychiatry. 2008;79:219–222. doi: 10.1136/jnnp.2006.111518. [DOI] [PubMed] [Google Scholar]
- 8.Biegstraaten M, Mengel E, Marodi L, et al. Peripheral neuropathy in adult type 1 Gaucher disease: a 2-year prospective observational study. Brain. 2010 doi: 10.1093/brain/awq198. (Epub) [DOI] [PubMed] [Google Scholar]
- 9.Bultron G, Kacena K, Pearson D, et al. The risk of Parkinson's disease in type 1 Gaucher disease. J. Inherit. Metab. Dis. 2010;33:167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherin P, Rose C, de Roux-Serratrice C, et al. The neurological manifestations of Gaucher disease type 1: the French Observatoire on Gaucher disease (FROG) J. Inherit. Metab. Dis. 2010;33:331–338. doi: 10.1007/s10545-010-9095-5. [DOI] [PubMed] [Google Scholar]
- 11.McKeran RO, Bradbury P, Taylor D, Stern G. Neurological involvement in type 1 (adult) Gaucher's disease. J. Neurol. Neurosurg. Psychiatry. 1985;48:172–175. doi: 10.1136/jnnp.48.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turpin J, Dubois G, Brice A, et al. Parkinsonism symptomatology in a patient with Type 1 (Adult) Gaucher's Disease. In: Salvayre R, Douste-Blazy L, Gatt S, editors. Lipid Storage Disorders. Biological and Medical Aspect. New York: Plenum Publishing; 1987. pp. 103–104. [Google Scholar]
- 13.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 14.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol. Genet. Metab. 2001;73:313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 15.Varkonyi J, Rosenbaum H, Baumann N, et al. Gaucher disease associated with parkinsonism: four further case reports. Am. J. Med. Genet. A. 2003;116A:348–351. doi: 10.1002/ajmg.a.10028. [DOI] [PubMed] [Google Scholar]
- 16.Itokawa K, Tamura N, Kawai N, Shimazu K, Ishii K. Parkinsonism in type I Gaucher's disease. Intern. Med. 2006;45:1165–1167. doi: 10.2169/internalmedicine.45.1790. [DOI] [PubMed] [Google Scholar]
- 17.Kraoua I, Stirnemann J, Ribeiro MJ, et al. Parkinsonism in Gaucher's disease type 1: ten new cases and a review of the literature. Mov. Disord. 2009;24:1524–1530. doi: 10.1002/mds.22593. [DOI] [PubMed] [Google Scholar]
- 18.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 19.Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E. Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Clark LN, Nicolai A, Afridi S, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson's disease in subjects of Jewish ethnicity. Mov. Disord. 2005;20:100–103. doi: 10.1002/mds.20320. [DOI] [PubMed] [Google Scholar]
- 21.Sato C, Morgan A, Lang AE, et al. Analysis of the glucocerebrosidase gene in Parkinson's disease. Mov. Disord. 2005;20:367–370. doi: 10.1002/mds.20319. [DOI] [PubMed] [Google Scholar]
- 22.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype–phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70:2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 24.Velayati A, Yu WH, Sidransky E. The role of glucocerebrosidase mutations in Parkinson disease and Lewy body disorders. Curr. Neurol. Neurosci. Rep. 2010;10:190–198. doi: 10.1007/s11910-010-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning-Bog AB, Schule B, Langston JW. Alpha-synuclein–glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology. 2009;30:1127–1132. doi: 10.1016/j.neuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Goldin E. Gaucher disease and parkinsonism, a molecular link theory. Mol. Genet. Metab. 2010 doi: 10.1016/j.ymgme.2010.08.004. (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kono S, Ouchi Y, Terada T, et al. Functional brain imaging in glucocerebrosidase mutation carriers with and without parkinsonism. Mov. Disord. 2010;25:1823–1829. doi: 10.1002/mds.23213. [DOI] [PubMed] [Google Scholar]
- 28.Ron I, Rapaport D, Horowitz M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum. Mol. Genet. 2010;19:3771–3781. doi: 10.1093/hmg/ddq292. [DOI] [PubMed] [Google Scholar]
- 29.Cherin P, Sedel F, Mignot C, et al. Neurological manifestations of type 1 Gaucher's disease: is a revision of disease classification needed? Rev. Neurol. (Paris) 2006;162:1076–1083. doi: 10.1016/s0035-3787(06)75120-7. [DOI] [PubMed] [Google Scholar]
- 30.Rothman K, Greenland S, Poole C, Lash T. Causation and causal inference. In: Rothman K, Greenland S, Lash T, editors. Modern Epidemiology. Third Ed. Philadelphia: Lippincott-Williams-Wilkins; 2008. [Google Scholar]
- 31.Winter Y, Bezdolnyy Y, Katunina E, et al. Incidence of Parkinson's disease and atypical parkinsonism: Russian population-based study. Mov. Disord. 2010;25:349–356. doi: 10.1002/mds.22966. [DOI] [PubMed] [Google Scholar]
- 32.Linder J, Stenlund H, Forsgren L. Incidence of Parkinson's disease and parkinsonism in northern Sweden: a population-based study. Mov. Disord. 2010;25:341–348. doi: 10.1002/mds.22987. [DOI] [PubMed] [Google Scholar]
- 33.Weinreb NJ, Deegan P, Kacena KA, et al. Life expectancy in Gaucher disease type 1. Am. J. Hematol. 2008;83:896–900. doi: 10.1002/ajh.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bembi B, Zambito Marsala S, Sidransky E, et al. Gaucher's disease with Parkinson's disease: clinical and pathological aspects. Neurology. 2003;61:99–101. doi: 10.1212/01.wnl.0000072482.70963.d7. [DOI] [PubMed] [Google Scholar]
- 35.Bras JM, Singleton A. Genetic susceptibility in Parkinson's disease. Biochim. Biophys. Acta. 2009;1792:597–603. doi: 10.1016/j.bbadis.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bras J, Singleton A, Cookson MR, Hardy J. Emerging pathways in genetic Parkinson's disease: potential role of ceramide metabolism in Lewy body disease. FEBS J. 2008;275:5767–5773. doi: 10.1111/j.1742-4658.2008.06709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Grabowski GA. Impaired autophagosomes and lysosomes in neuronopathic Gaucher disease. Autophagy. 2010;6:648–649. doi: 10.4161/auto.6.5.12047. [DOI] [PubMed] [Google Scholar]
- 38.García-Arencibia M, Hochfeld WE, Toh PPC, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin. Cell Dev. Biol. 2010;21:691–698. doi: 10.1016/j.semcdb.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010 doi: 10.1007/s00401-010-0741-7. (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobstyl M, Zabek M, Koziara H, Dzierzecki S. A patient with parkinsonism related to Gaucher's disease type I successfully treated by unilateral pallidotomy — a 3-year follow-up. Neurol. Neurochir. Pol. 2009;43:293–297. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.