Abstract
Introduction:
We sought to determine if prostatic ductal adenocarcinoma is undersampled and/or underdiagnosed at transrectal ultrasound (TRUS)-guided biopsy.
Methods:
With institutional review board approval, we searched our pathology database between 2008 and 2014 for patients with a diagnosis of ≥10% ductal adenocarcinoma on radical prostatectomy and available TRUS-guided needle biopsy specimens. Three blinded genitourinary pathologists independently examined the biopsy slides. The presence or absence of ductal adenocarcinoma was determined. Diagnostic accuracy was calculated using consensus diagnosis as the reference standard. Inter-observer agreement was assessed using Cohen’s kappa coefficient.
Results:
Based on consensus review, 66.7% (12/18) biopsy specimens demonstrated ductal adenocarcinoma and 33.3% (6/18) demonstrated conventional acinar prostatic adenocarcinoma. The sensitivity/specificity for each reader (R) was: 83/100% (R1), 100/83% (R2) and 58/83% (R3) and the inter-observer agreement was only fair (K=0.32). Only two of the original needle-biopsy reports correctly identified ductal adenocarcinoma (sensitivity = 17%). The main limitations of the study are the relatively small sample size and the potential for selection bias since we could only examine patients who underwent radical prostatectomy.
Conclusions:
Prostatic ductal adenocarcinoma may be undersampled at TRUS-guided biopsy and in this study was under-reported in routine clinical practice. This highlights the importance of increased awareness of ductal adeoncarcinoma and the need for clear diagnostic criteria. These findings have significant clinical impact especially when determining candidacy for active surveillance protocols.
Introduction
Prostatic adenocarcinoma is divided into acinar and nonacinar subtypes. Prostatic ductal adenocarcinoma is the most common of the non-acinar subtypes and is defined by characteristic morphologic features.1 Prostatic ductal adenocarcinoma is an aggressive variant of prostate cancer and its incidence varies from 0.5% to 6%.2 At radical prostatectomy (RP) ductal cancer is associated with a higher incidence of positive surgical margins, extraprostatic extension, vascular invasion, seminal vesicle invasion, and metastases.3,4 Ductal cancer detected on transrectal ultrasound (TRUS)-guided biopsy specimens warrants therapy and is considered a contraindication for active surveillance (AS).5,6
The clinical and microscopic detection of ductal cancer can be challenging. Ductal adenocarcinoma secretes less prostatic serum antigen (PSA) than acinar adenocarcinoma and it can appear occult on some magnetic resonance imaging (MRI) sequences.7,8 Most ductal adenocarcinomas arise in the peripheral zone and extend toward the urethral lumen, although occasionally ductal cancer arises in the transition zone exclusively.3,9 This creates a diagnostic challenge as many ductal cancers present with a normal digital rectal examination and are not detected on routine template TRUS-guided biopsy.1,7
On histopathology, ductal cancer can resemble high-grade prostatic intraepithelial neoplasia (HGPIN), intraductal prostatic carcinoma, the hyperplastic variant of prostate cancer, cribriform Gleason Pattern 4 acinar adenocarcinoma, and metastases (including from the colon and bladder).1,3,10 The relative rarity of ductal cancer and its frequent admixture with acinar adenocarcinoma further complicates its histological recognition.3
The purpose of this study was to determine if prostatic ductal adenocarcinoma is undersampled on routine template TRUS-guided biopsy specimens and whether it is under-reported by pathologists in routine clinical practice. A secondary objective was to determine the inter-observer agreement for the diagnosis of ductal adenocarcinoma on biopsy specimens.
Methods
Patient selection
With research ethics board approval, we searched for consecutive radical prostatectomy specimens between 2008 and 2014 from our database. The preliminary search identified 1127 specimens, of which 46 included a diagnosis of ductal cancer. The inclusion criteria for this study were: (1) ≥10% ductal component (previous studies demonstrated that <10% ductal cancer had no bearing on clinical outcome);11 (2) absence of neo-adjuvant therapy; and (3) TRUS-guided biopsy specimens available for review.
Specimen preparation and histopathology
The standard non-targeted extended TRUS-guided biopsy scheme at our institution uses an 18-gauge needle and yields 10 biopsy (right and left basal and middle [lateral and medial] and apical) specimens. During the study period, all biopsies were performed at a tertiary care referral centre for prostate and by experienced abdominal radiologists. Tissue is fixed overnight in 10% neutral buffered formalin. Three histological slides are prepared from each block, each with 3 serial sections cut at 3 micron thickness and stained with hematoxylin and eosin (H&E). All radical prostatectomies were performed at the same tertiary care referral centre for prostate and specimens were fixed in 40% buffered formaldehyde for 24 hours and serially sectioned to 0.3-cm thickness. All tissues were paraffin-embedded and 4 micron-thick sections were cut and stained with H&E.
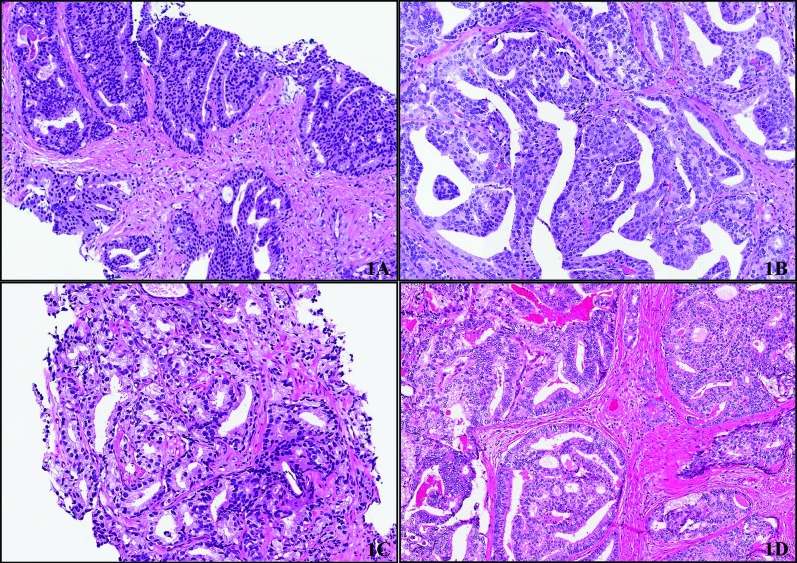
The original radical prostatectomy pathology reports were reviewed. The percentage of ductal cancer, the highest Gleason score, and the location of the ductal cancer were recorded. Location was defined as peripheral zone or non-peripheral zone. Two experienced genitourinary pathologists (TAF, SJR) reviewed the radical prostatectomy specimens to verify the diagnosis and percentage of ductal cancer. Ductal features were defined as: (1) high-grade nuclei; (2) abundant and typically amphophilic cytoplasm; and (3) tall/pseudostratified cells arranged in papillary projections, discreet glands, or cribriform structures with intervening slit like lumen. We used immunostains to demonstrate an absence of basal cells in ambiguous cases to distinguish ductal adenocarcinoma from intraductal adenocarcinoma and HGPIN (Fig. 1).10
Fig. 1.
(A) Example of consensus diagnosis for prostatic ductal adenocarcinoma on transrectal ultrasound (TRUS)-guided biopsy. Cribriform structures are lined by tall columnar cells with amphophilic cytoplasm and high grade nuclei. (B) Corresponding RP to biopsy in 1A showing similar morphologic features, including papillary and cribriform structures. (C) Conventional acinar prostatic carcinoma on TRUS guided biopsy (Gleason score 4+3 =7) showing gland fusion and irregular lumina. There are no features that are characteristic of prostatic ductal adenocarcinoma. (D) Corresponding RP to biopsy shown in 1C showing features of prostatic ducal adenocarcinoma including large glands lined by tall columnar cells with amphophilic cytoplasm and high grade nuclei. Cells are arranged in cribriform structures with slit-like spaces.
After consensus review, there were no patients who were reclassified and all 18 patients identified in the preliminary search were eligible for further analysis.
TRUS-guided biopsy specimens and visual analysis
The original TRUS-guided biopsy reports were reviewed and the overall Gleason score and the presence and percentage of ductal cancer in the report were recorded by a pathology resident (PVG). Three genitourinary pathologists with 8, 30 and 40 years of experience independently reviewed the biopsy specimens blinded to all patient information and results from the radical prostatectomy. The reviewing pathologists were also blinded to the aims and objectives of this study. Each pathologist was asked to provide a Gleason score and to identify for any histologic subtypes of prostate cancer. The presence or absence of ductal cancer was recorded by using a binary outcome (present or absent). To confirm the biopsy specimen contained ductal cancer, we used a consensus diagnosis (≥2 pathologist agreement) as the reference standard.
Patients were further evaluated for eligibility of AS, as defined by the Royal Marsden Hospital criteria (2008), which includes: (1) Gleason score ≤3+4=7; (2) clinical stage ≤T2a; (3) PSA ≤15 ng/mL; (4) total positive cores ≤50%;12 and (5) the University of Toronto criteria (Gleason score ≤3+4=7, PSA ≤15 ng/mL, clinical stage T1/T2, ≤3 positive biopsies, ≤50% single core involvement).13
Statistical analysis
The sensitivity for detection of ductal cancer from TRUS-guided needle biopsy specimens was calculated for each reader and from the original reports. The consensus diagnosis of TRUS-guided biopsy specimens was used as the reference standard to determine if ductal cancer was present in the biopsy specimens. The inter-observer agreement for the diagnosis of ductal cancer based on the TRUS-guided needle biopsy specimens was calculated using Cohen’s Kappa statistic. Kappa values were defined as: <0 less than chance agreement, 0.01–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement; and 0.81–0.99 for almost perfect agreement. Statistical analysis was performed using STATA v12.0 (Statacorp, College Station, TX).
Results
In total, 18 patients were identified once the inclusion and exclusion criteria were applied. We tallied age, PSA level at diagnosis, Gleason score from needle-biopsy and radical prostatectomy, and location of the ductal cancer (Table 1). The mean age at the time of TRUS biopsy was 63 (range: 36–75). Pathological stage for the resection specimens was pT2a in 2 (11%), pT2c in 7 (39%), pT3a in 7 (39%), and pT3b in 2 (11%). The mean preoperative (resection) PSA was 12.9 ng/mL (range: 1.63–90). Only 1 patient was lost to follow-up. The remaining 17 are alive and currently disease-free. On review of the location of ductal cancer, 95% (17/18) involved at least the peripheral zone (11/18 exclusively peripheral zone, 6/18 both peripheral zone and non-peripheral zone), while 5% (1/18) exclusively involved the transitional zone (Table 1).
Table 1.
Patient age, serum PSA level at time of first diagnosis, Gleason score on TRUS biopsy and RP, and location of prostatic ductal adenocarcinoma on resection
| Patient | Age | PSA | Gleason score on TRUS biopsy | Gleason score on RP | Location on RP (PZ or non-PZ) |
|---|---|---|---|---|---|
| 1 | 70 | 9.94 | 4 + 4 = 8 | 4 + 4 = 8 | Non-PZ |
| 2 | 66 | 4.62 | 3 + 4 = 7 | 4 + 3 = 7 | PZ |
| 3 | 65 | 3.1 | 4 + 3 = 7 | 4 + 3 = 7 | Both |
| 4 | 54 | 90 | 4 + 5 = 9 | 4 + 5 = 9 | Both |
| 5 | 63 | 3.14 | 3 + 4 = 7 | 4 + 4 = 8 | PZ |
| 6 | 69 | 7.5 | 3 + 3 = 6 | 3 + 4 = 7 | PZ |
| 7 | 66 | 18 | 4 + 4 = 8 | 4 + 5 = 9 | PZ |
| 8 | 58 | 13 | 4 + 4 = 8 | 4 + 5 = 9 | Both |
| 9 | 74 | 8.8 | 4 + 3 = 7 | 3 + 4 = 7 | PZ |
| 10 | 52 | 13 | 4 + 3 = 7 | 4 + 3 = 7 | PZ |
| 11 | 65 | 8.9 | 3 + 3 = 6 | 3 + 5 = 8 | Both |
| 12 | 70 | 4.65 | 4 + 4 = 8 | 4 + 3 = 7 | PZ |
| 13 | 58 | 4.41 | 3 + 4 = 7 | 4 + 3 = 7 | Both |
| 14 | 61 | 5.16 | 4 + 5 = 9 | 4 + 5 = 9 | Both |
| 15 | 75 | 1.63 | 4 + 5 = 9 | 4 + 4 = 8 | PZ |
| 16 | 59 | 8.64 | 4 + 5 = 9 | 4 + 5 = 9 | PZ |
| 17 | 67 | 6.64 | 3 + 4 = 7 | 3 + 4 = 7 | PZ |
| 18 | 74 | 22.39 | 4 + 4 = 8 | 4 + 3 = 7 | PZ |
PSA: prostate-specific antigen; TRUS: transrectal ultrasound; RP: radical prostatectomy; PZ: peripheral zone.
After consensus review, it was determined that 67% (12/18) of biopsy specimens demonstrated ductal cancer (Fig. 1), while the remaining 33% (6/18) showed acinar adenocarcinoma. Only 2 of the original pathology reports described the presence of ductal cancer (sensitivity 17%).
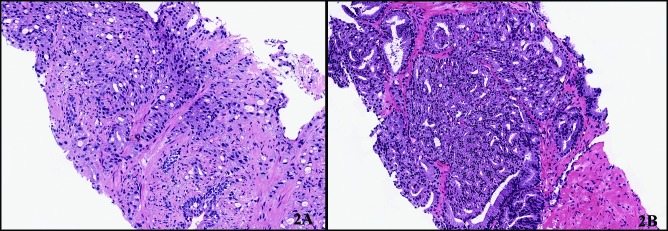
The diagnostic accuracy for each reader and the inter-observer agreement for detection of ductal cancer are presented in Table 2. All 3 readers outperformed the original TRUS-guided biopsy report with sensitivities ranging from 58% to 100%. The specificity among the readers ranged from 83% to 100% (Table 3). There was a false positive diagnosis for Reader 2 and 3 (Fig. 2).
Table 2.
Original report and re-review diagnoses of all three readers for each case and the total number of ductal carcinoma diagnoses
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original report | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Reader 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
| Reader 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| Reader 3 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 8 |
| Total | 2 | 2 | 3 | 3 | 2 | 0 | 1 | 0 | 0 | 4 | 0 | 1 | 3 | 3 | 3 | 2 | 2 | 2 | |
| Consensus | Y | Y | Y | Y | Y | N | N | N | N | Y | N | N | Y | Y | Y | Y | Y | Y |
1: ductal carcinoma; 0 : no ductal carcinoma; Y : consensus ductal carcinoma; N : consensus non-ductal carcinoma.
Table 3.
Sensitivities and specificities for diagnosis of prostate ductal adenocarcinoma at needle-biopsy for the original biopsy report and for each reader
| Original report | Reader 1 | Reader 2 | Reader 3 | |
|---|---|---|---|---|
| Sensitivity (CI) | 16.7% (3.5%–16.7%) | 83.3% (63.2%–88.3%) | 100% (81%–100%) | 58.3% (38.1%–66.2%) |
| Specificity (CI) | 100% (73.7%–100%) | 100% (59.8%–100%) | 83.3% (45.4%–83.3%) | 83.3% (42.9%–99.1%) |
CI: confidence interval.
Fig. 2.
The two false positive biopsies from readers 2 and 3, respectively. Figure 2A shows fused glands with numerous intracytoplasmic vacuoles and amphophilic cytoplasm. There is no cribriform or papillary rchitecture and the cells are not tall columnar although there are some elongated nuclei. High grade are absent. Consensus for prostatic ductal adenocarcinoma for this case was not achieved and was instead this was called conventional acinar prostate carcinoma (Gleason 4+4=8) by the other two readers. Figure 2B shows multiple fused glands with some slit-like spaces, but no true cribriform or papillary structures. There are some tall columnar cells but the nuclei are generally round and low grade. This did not reach consensus diagnosis for prostatic adenocarcinoma and was called conventional acinar prostatic adenocarcinoma (Gleason 4+4=8) by the other two readers.
The inter-observer agreement for the diagnosis of ductal cancer was only fair (K = 0.32). Of the patients with prostatic ductal adenocarcinoma, 33% were not demonstrated on initial TRUS-guided biopsy specimens. Additionally, 55.6% (10/18) were not recognized on the initial biopsy specimen pathology report.
Applying the Royal Marsden Hospital and the University of Toronto criteria, we found that 3/18 patients (17%) would have been eligible for active surveillance (3 included under the Royal Marsden Hospital criteria and of these, only 2 would have been eligible by the University of Toronto guidelines) (Table 4).
Table 4.
Clinicopathologic features of patients eligible for enrolment into AS
| Patient no. | Age | Gleason score on TRUS Biopsy | PSA | T stage | % of core involved | No. positive cores on TRUS biopsy | Percentage of Gleason pattern 4 | PDCa reported on TRUS biopsy | PDCa after consensus review | AS eligible (Royal Marsden criteria) | AS eligible (University of Toronto criteria) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 69 | 3+3=6 | 7.5 | <T2a | 5% | 2 of 10 | Not Applicable | No | No | Yes | Yes |
| 11 | 65 | 3+3=6 | 8.8 | T2c | 25% | 4 of 10 | Not Applicable | No | No | Yes | No |
| 13 | 58 | 3+4=7 | 4.0 | T1c | 50% | 2 of 12 | 10% | No | Yes | Yes | Yes |
TRUS: transrectal ultrasound; PSA: prostate-specific antigen; PDCa: Prostatic ductal adenocarcinoma; AS: active surveillance.
Discussion
In our study, prostatic ductal adenocarcinoma was not sampled in up to one-third of template TRUS-guided biopsy specimens. Furthermore, even when ductal cancer was present on needle biopsy, it was often not reported by the pathologist. Even on review by expert genitourinary pathologists, ductal cancer was often not identified. Our results suggested that ductal cancer is both undersampled and under-reported on biopsy in routine clinical practice.
These findings are important for patients with ductal adenocarcinoma because this is an aggressive subtype that warrants definitive treatment in most cases.2–6 A failure to sample or identify ductal cancer on needle biopsy could result in the inappropriate management of patients with active surveillance when they require definitive management. In this study, 3/18 patients could have erroneously been considered candidates for active surveillance based on the University of Toronto and/or Royal Marsden Hospital eligibility criteria. The biopsies of two of these patients failed to sample ductal carcinoma. However, the third patient’s biopsy confirmed the presence of ductal adenocarcinoma after consensus review, although this finding was not reported at the time of initial biopsy. All 3 patients showed Gleason score upgrading after radical prostatectomy.
Prostatic ductal adenocarcinoma was under-reported in this study and there are several potential explanations for this finding. Ductal adenocarcinoma is relatively rare, is frequently associated and inter-mixed with acinar adenocarcinoma, and can be confused with microscopic mimickers.1,3,10 The absence of clear diagnostic criteria and a potential lack of awareness among non-genitourinary pathologists may further compound the problem.14 In our study, the inter-observer agreement for the diagnosis of ductal cancer on TRUS-guided biopsy was only fair and consistent with a previous study.14 Future studies and guidelines would be helpful to improve detection and inter-reader agreement of this pathologic entity.
There are also several possible explanations why ductal cancer is undersampled at routine template TRUS biopsy. The limitations of template TRUS-guided needle biopsy are well-known.15 Due to the non-targeted nature of TRUS guidance and because ductal adenocarcinoma typically only represents a small proportion of the tumour composed mainly of acinar adenocarcinoma, areas of ductal cancer can easily be missed. Ductal cancer’s occasional central (non-peripheral zone) location may also result in undersampling.6,16 In our study, 1/18 tumours were located exclusively in the transition zone, which was undersampled or not sampled at all during routine template TRUS-guided biopsies.14 These results are consistent with a previous study of 86 ductal adenocarcinomas, in which 71 (82.5%) were in the peripheral zone, and 2 (2.3%) in the transition zone.3 Extended biopsy sets with additional cores may have resulted in more ductal tumours being sampled. Targeted biopsies may also be problematic in these tumours because ductal cancer is underestimated or occult on some MRI sequences, although it may be more apparent on functional imaging techniques.8,17
Our study has its limitations. One is that the patient cohort only included patients who underwent radical prostatectomy. This creates the potential for selection bias, where patients with more advanced ductal cancer are included. The absence of a large control group of radical prostatectomy patients without ductal cancer for comparison is also a limitation. This study also had a relatively small sample of patients, although we feel that this is a necessary limitation given the relative rarity of ductal adenocarcinoma. Our sample size compares well to recent reports.14
Conclusion
This study demonstrates that prostatic ductal adenocarcinoma is undersampled at routine template TRUS-guided biopsy and under-recognized during routine microscopic examination in clinical practice. The undersampling and underdiagnosis of this aggressive variant can have clinical implications resulting in potential under-treatment of patients, especially in an era of active surveillance. Our study confirmed low inter-observer agreement in the detection of ductal cancer indicating a need for clear diagnostic criteria. An increased awareness and understanding of the importance of ductal cancer is required by pathologists when analyzing prostate needle biopsy specimens and urologists when interpreting needle-biopsy reports to ensure that patients receive appropriate management.
Footnotes
Competing interests: The authors all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- 2.Meeks JJ, Zhao LC, Cashy J, et al. Incidence and outcomes of ductal carcinoma of the prostate in the USA: Analysis of data from the Surveillance, Epidemiology, and End Results program. BJU Int. 2012;109:831–4. doi: 10.1111/j.1464-410X.2011.10520.x. [DOI] [PubMed] [Google Scholar]
- 3.Seipel AH, Wiklund F, Wiklund NP, et al. Histopathological features of ductal adenocarcinoma of the prostate in 1,051 radical prostatectomy specimens. Virchows Arch. 2013;462:429–36. doi: 10.1007/s00428-013-1385-5. [DOI] [PubMed] [Google Scholar]
- 4.Amin A, Epstein JI. Pathologic stage of prostatic ductal adenocarcinoma at radical prostatectomy: Effect of percentage of the ductal component and associated grade of acinar adenocarcinoma. Am J Surg Pathol. 2011;35:615–9. doi: 10.1097/PAS.0b013e31820eb25b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzucchelli R, Lopez-Beltran A, Cheng L, et al. Rare and unusual histological variants of prostatic carcinoma: Clinical significance. BJU Int. 2008;102:1369–74. doi: 10.1111/j.1464-410x.2008.08074.x. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Lin DW, Gore JL, et al. The critical role of the pathologist in determining eligibility for active surveillance as a management option in patients with prostate cancer: Consensus statement with recommendations supported by the College of American Pathologists, International Society of Urological Pathology, Association of Directors of Anatomic and Surgical Pathology, the New Zealand Society of Pathologists, and the Prostate Cancer Foundation. Arch Pathol Lab Med. 2014;138:1387–405. doi: 10.5858/arpa.2014-0219-SA. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TM, Welty CJ, Vakar-Lopez F, et al. Ductal adenocarcinoma of the prostate: Increased mortality risk and decreased serum prostate specific antigen. J Urol. 2010;184:2303–7. doi: 10.1016/j.juro.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schieda N, Coffey N, Gulavita P, et al. Prostatic ductal adenocarcinoma: An aggressive tumour variant unrecognized on T2 weighted magnetic resonance imaging (MRI) Eur Radiol. 2014;24:1349–56. doi: 10.1007/s00330-014-3150-9. [DOI] [PubMed] [Google Scholar]
- 9.Bock BJ, Bostwick DG. Does prostatic ductal adenocarcinoma exist? Am J Surg Pathol. 1999;23:781–5. doi: 10.1097/00000478-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI. Prostatic ductal adenocarcinoma: A mini review. Med Princ Pract. 2010;19:82–5. doi: 10.1159/000252842. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 12.van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008;54:1297–305. doi: 10.1016/j.eururo.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Klotz L. Active surveillance: The Canadian experience with an “inclusive approach”. J Natl Cancer Inst Monogr. 2012;2012:234–41. doi: 10.1093/jncimonographs/lgs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seipel AH, Delahunt B, Samaratunga H, et al. Diagnostic criteria for ductal adenocarcinoma of the prostate: Interobserver variability among 20 expert uropathologists. Histopathology. 2014;65:216–27. doi: 10.1111/his.12382. [DOI] [PubMed] [Google Scholar]
- 15.Rothwax JT, George AK, Wood BJ, et al. Multiparametric MRI in biopsy guidance for prostate cancer: Fusion-guided. Biomed Res Int. 2014;2014:439171. doi: 10.1155/2014/439171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol. 2004;17:316–27. doi: 10.1038/modpathol.3800052. [DOI] [PubMed] [Google Scholar]
- 17.Coffey N, Schieda N, Cron G, et al. Multi-parametric (mp) MRI of prostatic ductal adenocarcinoma. J Magn Reson Imaging. 2015;41:1639–45. doi: 10.1002/jmri.24694. [DOI] [PubMed] [Google Scholar]