Abstract
Introduction:
Metastasis of prostate cancer (PC) to bone (metastatic bone disease, MBD) increases morbidity, but Canadian data are lacking on the associated healthcare resource utilization (HCRU) and costs. We quantified MBD-related HCRU and associated costs in this population, and assessed skeletal-related events (SREs), such as pathologic fracture, spinal cord compression, bone radiotherapy, and bone surgery.
Methods:
We conducted a retrospective, population-based cohort study using the Québec health insurance agency database. Prescription drug and medical services data were retrieved for patients with ≥1 healthcare claim in 2001 with a PC diagnosis (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code of 185.xx). Patients with ≥2 MBD-related claims or an SRE were compared with a matched-control group of PC patients without MBD. Patients were followed until death, loss to follow-up, or the end of available data (August 31, 2010). Costs (in 2012 Canadian dollars) were adjusted for age, year of MBD diagnosis, general health status, and baseline resource utilization.
Results:
Compared with controls (n = 1671), MBD patients (n = 626) had significantly higher HCRU. Adjusted mean (95% confidence interval) all-cause healthcare costs were $11 820 (7248–16 058) higher, and MBD-related costs were $3 091 (1267–4861) higher in MBD patients than in controls. Nearly 50% of MBD patients received radiotherapy within 2.5 years of their MBD diagnosis, but most exited the study without experiencing other SREs.
Conclusion:
MBD imposes a heavy HCRU and cost burden among patients with PC in Canada. Effective therapy is needed to reduce the clinical and economic impact of MBD in this population.
Introduction
Prostate cancer (PC) is the most common cancer and the second leading cause of cancer death among males in Canada.1 One in 8 men in Canada are expected to face a PC diagnosis in their lifetimes and 23 600 new cases are predicted for 2014; PC accounts for 24% of male cancers.1 Of all solid tumours, PC has the highest incidence of metastatic spread to bone, which occurs in 65% to 75% of advanced cases.2 More than 70% of PC patients are diagnosed between the ages of 60 and 80; therefore age-related change in bone structure is an important element in the pathophysiology of metastatic bone disease (MBD).3 Nearly all patients who die of PC have skeletal involvement,4 and MBD increases the risk of death nearly 7-fold in PC patients.5
Bone metastases in PC patients frequently lead not only to intractable pain, but also to burdensome complications known collectively as skeletal-related events (SREs).2 SREs include pathological fracture, spinal cord compression, bone surgery, and radiation to bone.2 Compared with other cancer types, the rate of SREs is elevated in advanced PC.3 PC patients with SREs and MBD have a 10 times higher mortality risk compared to those without skeletal involvement.5
Available evidence, mainly from Europe and the United States, reveals that MBD and SREs substantially increase healthcare resource utilization (HCRU) and healthcare costs in cancer patients.6–15 The cost burden due to MBD among PC patients in the United States was estimated at US$1.9 billion in 2004.13 However, few studies have evaluated the HCRU and costs associated with MBD in Canada, and none have done so comprehensively for the PC population. In a retrospective analysis of patient records from 4 hospitals in Québec and Ontario, Habib and colleagues quantified the HCRU attributable to SREs, but did not assess costs or the complete healthcare burden of MBD.16 Dragomir and colleagues used a simulation approach to estimate drug costs associated with metastatic castration-resistant PC in Canada.17 While they reported drug costs for therapy intended to prevent SREs, they did not evaluate resources other than drugs used in the management of MBD and its consequences.17
To address the knowledge gap related to the burden of illness of MBD in patients with PC in Canada, we undertook a retrospective healthcare-claims database study in PC patients who had received prior androgen deprivation therapy (ADT). The primary objective was to quantify HCRU and associated costs directly related to MBD in this population. Secondary objectives were to compare HCRU and associated costs between patients who developed MBD and those who did not, and to describe the incidence and time course of SREs.
Methods
Study design
This was a retrospective, observational, non-interventional, open-cohort study using records of a population-based pharmaceutical and medical services administrative database in Québec. As in other Canadian provinces, all Québec residents are eligible for public insurance coverage of physician visits and medical services provided by clinics and hospitals. In Québec, these services are covered by the Régie de l’Assurance Maladie du Québec (RAMQ), which also covers the cost of prescription drugs purchased in retail (but not hospital) pharmacies by residents ≥65 years of age, welfare recipients, and those without access to private drug plan coverage, such as self-employed individuals. Data in the RAMQ database have been validated previously and are accurate and reliable for identifying drug prescriptions and estimating inpatient length of stay and number of hospitalizations.18–22
Demographic, prescription drug, and medical services data were extracted for a sample of randomly selected patients who had ≥1 claim with a PC diagnosis between January 1, 2001 and December 31, 2001. To allow sufficient time before this ascertainment window to capture initial diagnoses and enough time afterwards to record outcomes, we set the study period from January 1, 1996 to August 31, 2010. An earlier start date was not feasible due to a change in the RAMQ database structure, which complicates data requests before 1996. For each patient, the PC index date was defined as the date of the first PC-related claim recorded within the study period, which was used as a proxy for the diagnosis date. The MBD index date was defined as the first claim associated with a diagnosis of MBD starting on or following the PC index date – this served as the proxy for the initial MBD diagnosis date.
Patients
In 2001, the RAMQ database included 7 291 001 admissible patients covered by health services, and 3 159 781 covered by the provincial drug plan, in which most (90%) of the elderly population was enrolled. From this source population, patients with a PC diagnosis were ascertained using a method developed and validated by the Institut national de santé publique du Québec.23 Patients were identified based on a claim associated with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic code for PC (ICD-9-CM code 185.XX) or a procedure code for physician diagnostic and therapeutic claims related to PC. Patients were identified as new PC cases if they had ≥2 additional claims dated on different occasions, in <731 days following the first claim related to PC. Newly diagnosed PC patients were excluded if they met any of the following criteria: younger than 50 years; first claim not followed by ≥2 other claims within 2 years; medical coverage <2 years prior to PC index date; pharmaceutical coverage <1 year prior to PC index date; no claim for ADT; a claim for ADT prior to PC index date; or an osteoporosis-related claim (ICD-9-CM code 733.0x or bisphosphonate use) prior to ADT. In addition to capturing demographic and clinical information, we estimated a validated index of disease and health status, hospitalization, and mortality (the chronic disease score [CDS])24 for all patients in the PC cohort.
MBD cases were identified using a combination of ICD-9-CM diagnostic codes and a variety of procedure codes. Patients were considered to have MBD if they had ≥2 MBD-related claims within a 1-year period of each other, with the first of these occurring on or after the PC index date. Diagnoses or procedure claims considered MBD-related were those associated with the following: pathologic fracture (ICD-9-CM code 733.1x); spinal cord decompression or compression (ICD-9-CM code 336.9x); radiotherapy related to bone metastasis (to eliminate radiotherapy intended as curative therapy for PC, only radiotherapy claims occurring after the MBD index date and ≥6 months after the start of ADT were included); MBD or a malignant bone neoplasm (ICD-9-CM codes 198.5x or 170.x); or bone surgery. Patients with <1 month of follow-up data after the MBD index date and those who received their first prescription of ADT after the MBD index date were excluded from the MBD cohort.
Matched-control patients were drawn from the pool of patients in the PC cohort who received ADT, but otherwise did not meet the criteria for an MBD diagnosis at any time during the follow-up period. From 1 to a maximum of 4 control patients were matched by an algorithm to each MBD patient for age, CDS score (as a proxy for disease severity), date of PC diagnosis, and date of ADT initiation. Any MBD patients for whom the matching algorithm did not yield ≥1 control patient were excluded from the study.
While unique identifier codes permitted matching of patients’ demographic information with their medical and drug use information for analysis, these were encrypted by the RAMQ before providing the data for use in this study to protect patient confidentiality.
Study outcomes
The main outcomes were MBD-related HCRU and associated costs. MBD-related healthcare resources were identified by ICD-9-CM and/or procedure codes. These included resources related to management of pathologic fracture, cord compression, and hypercalcemia, and procedures of radiotherapy, bone surgeries, hormonal implants or orchiectomy, scintigraphy, magnetic resonance imaging (MRI), computed tomography (CT), radiology, and biopsy. MBD-related drugs included antiandrogens, luteinizing hormone releasing hormone (LHRH) agonists, bisphosphonates, and selected opioids. While these resources and drugs were related to MBD, they could also be used by some patients without MBD (e.g., drugs prescribed with intent to prevent MBD, and diagnostic imaging in patients with suspected MBD), thus resulting in “MBD-related” HCRU and costs in the control group.
MBD-related costs were calculated by multiplying MBD-specific HCRU by unit costs. All costs used were from the RAMQ database except for hospitalization and medical imaging, for which costs were based on Canadian Institute for Health Information (CIHI) 2010 costs. Costs were adjusted for inflation to December 31, 2012 using the All-items Consumer Price Index for Québec for medical costs and the Prescribed Medicine Consumer Price Index for Québec for pharmaceutical costs.25 Chemotherapy costs were not included, as these may have been for treatment of PC rather than MBD.
SREs were identified with an adaptation of an algorithm developed by Delea and colleagues.6 An SRE was identified based on the presence of ≥1 claim associated with either radiotherapy or pathological fracture ≥6 months after ADT initiation, spinal cord compression, or bone surgery.
Statistical analysis
Patient age, ADT use, and bisphosphonate use were assessed at the MBD index date. Comorbidities, CDS, and all HCRU were estimated over the 1-year period prior to the MBD index date.
Mean per-patient per-year unadjusted HCRU and costs post-MBD diagnosis were calculated over total person-years at risk. Costs were also calculated with adjustment for age, year of the MBD index date (reference year: 1998–1999), MBD status, and both CDS and baseline resource utilization in the year prior to the MBD index date.
The nonparametric Wilcoxon-Mann-Whitney test for continuous variables and the Chi-square test for dichotomous variables were used to compare MBD patients and their matched comparators. Multivariable analyses exploring the association between HCRU and the presence of MBD used general linear models (GLMs) with a negative binomial distribution and a log link function using an offset term to control for the length of follow-up, with the same covariates used to adjust HCRU (i.e., age, year of MBD index date, MBD status, CDS, baseline resource utilization in the year prior to MBD index date). MBD-related drug use was compared as a binomial variable (yes/no) using logistic regression with the same covariates. A confidence interval (CI) for each estimate was computed using the percentile bootstrap method. Adjusted costs were not disaggregated within medical costs or pharmacy costs due to insufficient data to reliably construct bootstrapped estimates.
Cumulative incidence of SREs was calculated from the PC index date to the date of the first SRE. SRE incidence was computed annually. Time to the first SRE was estimated using the Kaplan–Meier method, from the beginning of ADT to the first SRE, with censoring due to loss to follow-up or the end of available data.
Data analyses were performed with SAS Statistical Software v9.3 (SAS Institute Inc., Cary, NC).
Results
Patients
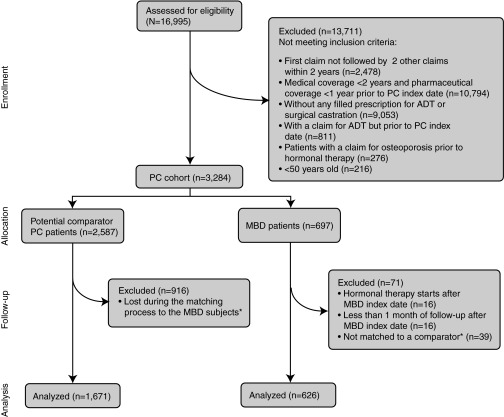
After applying the exclusion criteria, we had a total of 2297 patients for analysis, including 626 patients in the MBD cohort and 1671 control patients (Fig. 1). The first PC index date was in 1998 and the last in 2006; MBD index dates ranged from 1998 to 2010. The mean age was similar in the MBD and control patients (Table 1), although a statistically significant difference was observed (p = 0.0007) despite matching for age because the number of control patients matched to each control patient was not constant, ranging from 1 to 4. The poorer health status of MBD patients in the 1-year period preceding the MBD index date was revealed by their significantly higher CDS score (6.22 vs. 5.42, p < 0.0001).
Fig. 1.
Study cohort selection. *Patients were not matched (n=39) if they had ≥1 of the following criteria: <50 years old, first claim not followed by ≥2 other claims within 2 years, medical coverage <2 years prior to PC index date, pharmaceutical coverage <1 year prior to PC index date, no claim for hormonal therapy, a claim for hormonal therapy prior to PC index date, and an osteoporosis-related claim prior to hormonal therapy. MBD: metastatic bone disease; PC: prostate cancer.
Table 1.
Patient baseline characteristics at the MBD index date
| MBD patients | Control patients | p value | |
|---|---|---|---|
|
| |||
| (n = 626) | (n = 1671) | ||
| Age, years | |||
| Mean | 72.24 | 73.23 | |
| SD | 6.25 | 5.91 | 0.0007 |
| Min, Max | 52.5, 87.5 | 52.5, 87.5 | |
| Time since PC index date, years | |||
| Mean | 3.23 | 3.08 | 0.5042 |
| SD | 2.84 | 2.69 | |
| Min, Max | 0.0, 11.9 | 0.0, 11.9 | |
| Comorbidities, n (%) | |||
| CVD | 243 (38.8%) | 744 (44.5%) | 0.0139 |
| Diabetes | 94 (15.0%) | 261 (15.6%) | 0.7217 |
| Chronic disease score | |||
| Mean | 6.22 | 5.42 | |
| SD | 3.50 | 3.60 | <0.0001 |
| Min, Max | 0, 18 | 0, 17 | |
CVD: cardiovascular disease; MBD: metastatic bone disease; PC: prostate cancer; SD: standard deviation; Min: minimum; Max: maximum.
Healthcare resource use
With the exception of some diagnostic procedures that had few occurrences, MBD patients consumed significantly more healthcare resources in the year prior to the MBD index date compared with controls (Table 2). Similar patterns were seen for HCRU following the MBD diagnosis. The risk of consuming healthcare resources was significantly increased in patients with MBD compared with controls for all healthcare resources, except MRIs, bone density tests, and biopsies.
Table 2.
Healthcare resource use
| Resource | N in year prior to MBD index date | Annual rate in follow-up | Regression analysis; adjusted annual rate in follow-up* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||||||
| MBD patients (n = 626) | Control patients (n = 1671) | p value | MBD patients (n = 626) | Control patients (n = 1671) | p value | Coefficient (95% CI) | p value | RR or OR* (95% CI) | |
| Hospitalizations | |||||||||
| N | 1.06 (1.41) | 0.67 (1.15) | <0.0001 | 2.82 (4.93) | 1.06 (2.25) | <0.0001 | 0.50 (0.37, 0.62) | <0.0001 | 1.64 (1.44, 1.87) |
| Days in hospital | 5.26 (11.72) | 3.36 (9.42) | <0.0001 | 24.39 (47.57) | 8.62 (21.35) | <0.0001 | 0.67 (0.49, 0.85) | <0.0001 | 1.96 (1.64, 2.34) |
| Emergency room visits, n | 1.73 (2.66) | 0.99 (1.98) | <0.0001 | 3.07 (5.35) | 1.68 (2.90) | <0.0001 | 0.26 (0.14, 0.38) | <0.0001 | 1.29 (1.15, 1.46) |
| Outpatient physician visits | |||||||||
| GP visits | 5.33 (4.46) | 4.77 (4.31) | 0.0082 | 4.04 (5.89) | 3.50 (4.43) | 0.8546 | 0.16 (0.05, 0.26) | 0.0027 | 1.17 (1.06, 1.29) |
| Specialist visits | 10.47 (7.56) | 8.24 (7.05) | <0.0001 | 11.08 (9.09) | 6.05 (7.00) | <0.0001 | 0.39 (0.32, 0.46) | <0.0001 | 1.47 (1.37, 1.58) |
| Total (GP + specialist) | 15.80 (8.45) | 13.01 (8.53) | <0.0001 | 15.13 (11.04) | 9.55 (8.55) | <0.0001 | 0.31 (0.25, 0.37) | <0.0001 | 1.36 (1.28, 1.44) |
| Diagnostic procedures | |||||||||
| MRI tests | 0.09 (0.33) | 0.02 (0.18) | <0.0001 | 0.20 (0.93) | 0.03 (0.17) | <0.0001 | 0.90 (0.57, 1.24) | <0.0001 | 2.47 (1.77, 3.44) |
| Bone density tests | 0.03 (0.17) | 0.02 (0.14) | 0.1604 | 0.03 (0.12) | 0.04 (0.12) | 0.0572 | −0.22 (−0.53, 0.09) | 0.1619 | 0.80 (0.59, 1.09) |
| Biopsies | 0.0016 (0.04) | 0.0006 (0.02) | 0.4700 | 0.0014 (0.03) | 0.0004 (0.01) | 0.3067 | 1.03 (−1.25, 3.31) | 0.3753 | 2.80 (0.29, 7.33) |
| Diagnostic radiology tests | 0.28 (0.83) | 0.20 (0.64) | 0.1510 | 0.16 (0.45) | 0.20 (0.54) | <0.0001 | −0.25 (−0.46, −0.04) | 0.0222 | 0.78 (0.63, 0.97) |
| CT tests | 1.05 (1.56) | 0.41 (0.86) | <0.0001 | 1.37 (2.38) | 0.60 (1.21) | <0.0001 | 0.48 (0.34, 0.62) | <0.0001 | 1.61 (1.40, 1.85) |
| Scintigraphy tests | 0.79 (0.80) | 0.35 (0.59) | <0.0001 | 0.38 (0.76) | 0.22 (0.61) | <0.0001 | 0.41 (0.23, 0.59) | <0.0001 | 1.51 (1.26, 1.81) |
| Medication use, n (%) | |||||||||
| ADT | 418 (66.8%) | 854 (51.1%) | <0.0001 | 282 (45.1%) | 712 (42.6%) | 0.2935 | −0.26 (−0.49, −0.03) | 0.0298 | 0.77 (0.61, 0.98) |
| LHRH agonists | 484 (77.3%) | 988 (59.1%) | <0.0001 | 347 (55.4%) | 824 (49.3%) | 0.0090 | −0.18 (−0.42, 0.06) | 0.1347 | 0.83 (0.66, 1.06) |
| Bisphosphonates | 49 (7.8%) | 69 (4.1%) | 0.0004 | 130 (20.8%) | 245 (14.7%) | 0.0004 | 0.10 (−0.19, 0.39) | 0.4971 | 1.11 (0.83, 1.48) |
| Opioids | 164 (26.2%) | 127 (7.6%) | <0.0001 | 348 (55.6%) | 547 (32.7%) | <0.0001 | 0.73 (0.52, 0.94) | <0.0001 | 2.07 (1.68, 2.55) |
RR calculated using general linear models for hospitalizations, physician visits, and diagnostic procedures; OR calculated using logistic regression for medication use; adjusted for age, CDS in year prior to MBD index date, year of MBD index date (reference: 1998–1999), MBD status, and baseline resource utilization in year prior to MBD index date. ADT: androgen deprivation therapy; CDS: chronic disease score; CI: confidence interval; CT: computed tomography; GP: general practitioner; LHRH: luteinizing hormone releasing hormone; MBD: metastatic bone disease; OR: odds ratio; RR: relative risk; SD: standard deviation; MRI: magnetic resonance imaging.
Healthcare costs
MBD patients incurred significantly higher mean costs overall and in each category of resource use, except ADT and LHRH agonists (Table 3). Adjusted differences between MBD patients and controls in all-cause or MBD-related pharmacy costs were less than $250. All-cause and MBD-related medical costs were $12 597 and $2835 higher, respectively, in MBD patients compared with controls.
Table 3.
Annual healthcare costs in follow-up
| Unadjusted annual costs, $ | Adjusted annual costs,* mean (95% CI), $ | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean (SD) | ||||||
|
|
|
|||||
| Cost component | MBD patients (n = 626) | Control patients (n =1671) | p value | MBD patients (n = 626) | Control patients (n = 1671) | Difference |
| Medical | ||||||
| All-cause | 31 905 (58 215) | 11 719 (26 417) | <0.0001 | 27 346 (23 063; 32 557) | 14 749 (12 912; 17 442) | 12 597 (7812; 17 518) |
| MBD-related† | 9079 (25 815) | 3219 (12 333) | <0.0001 | 7314 (5919; 9367) | 4479 (3710; 5752) | 2835 (1095; 4847) |
| Hospitalizations‡ | ||||||
| All-cause | 28 957 (56 480) | 10 230 (25 355) | <0.0001 | |||
| MBD-related† | 7403 (24 681) | 2583 (11 719) | <0.0001 | |||
| Emergency room visits | ||||||
| All-cause | 626 (1091) | 342 (591) | <0.0001 | |||
| MBD-related† | 33 (189) | 0.08 (2.41) | <0.0001 | |||
| Outpatient physician visits | ||||||
| All-cause | 873 (737) | 517 (470) | <0.0001 | |||
| MBD-related† | 194 (327) | 5.00 (34.00) | <0.0001 | |||
| Diagnostic procedures | 1449 (2434) | 630 (1212) | <0.0001 | |||
| Pharmacy | ||||||
| All-cause | 5099 (4593) | 4180 (3549) | 0.0003 | 4883 (4519; 5244) | 4636 (4451; 4896) | 247 (−162; 584) |
| MBD-related† | 2298 (2663) | 2004 (2422) | <0.0001 | 2343 (2117; 2585) | 2575 (2439, 2737) | −232 (−533, 52) |
| ADT | 449 (934) | 529 (989) | 0.8300 | |||
| LHRH agonists | 1440 (1817) | 1387 (1833) | 0.1172 | |||
| Bisphosphonates | 206 (767) | 56 (290) | <0.0001 | |||
| Opioids | 202 (625) | 33 (196) | <0.0001 | |||
| Total | ||||||
| All-cause | 37 004 (58 231) | 15 899 (27 067) | <0.0001 | 30 837 (26 717; 34 948) | 19 016 (17 291; 21 186) | 11 820 (7248; 16 058) |
| MBD-related† | 11 377 (26 196) | 5223 (12 634) | <0.0001 | 9271 (7685; 10 865) | 6180 (5463; 7104) | 3091 (1267; 4861) |
Adjusted for age, CDS in year prior to MBD index date, year of MBD index date (reference: 1998–1999), MBD status, and baseline resource utilization in year prior to MBD index date.
Resources considered to be indicative of MBD could also have been consumed by some control patients, e.g., for prevention or diagnosis.
Includes both inpatient stays and day visits. ADT: androgen deprivation therapy; CDS: chronic disease score; CI: confidence interval; LHRH: luteinizing hormone releasing hormone; MBD: metastatic bone disease; SD: standard deviation.
Skeletal-related events
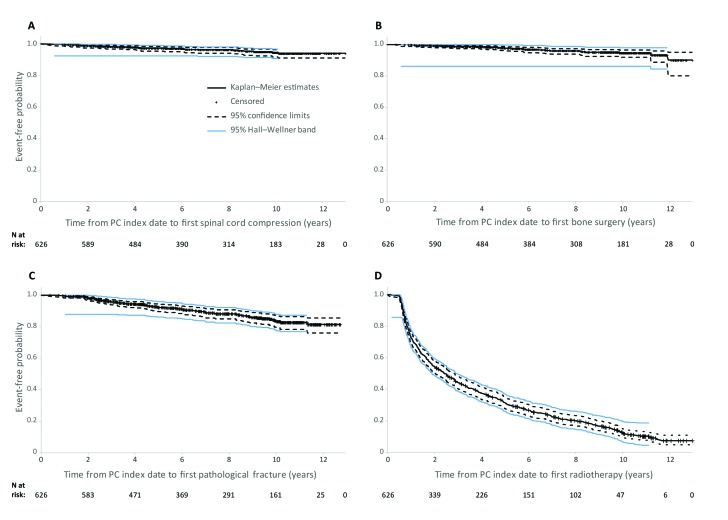
Radiotherapy was by far the most common SRE, received by 85% of MBD patients (Table 4). These results were confirmed by the Kaplan-Meier analysis (Fig. 2), which demonstrated that nearly 50% of MBD patients received radiotherapy within 2.5 years of their MBD diagnosis. Most patients in the MBD cohort exited the study without experiencing spinal cord compression, pathological fractures, or bone surgery.
Table 4.
Distribution of skeletal-related events in patients with metastatic bone disease (n = 618)
| Skeletal-related event | First event | All events | ||
|---|---|---|---|---|
|
| ||||
| n | Percent (%) | n | Percent (%) | |
| Bone surgery | 16 | 2.59 | 25 | 3.99 |
| Cord compression | 18 | 2.91 | 25 | 3.99 |
| Pathological fracture | 60 | 9.71 | 73 | 11.66 |
| Therapeutic radiotherapy | 524 | 84.79 | 534 | 85.30 |
Fig. 2.
Kaplan–Meier curves of time from PC index date to first skeletal-related event by type of event in patients with metastatic bone disease. PC: prostate cancer
Discussion
The present study is the first comprehensive assessment of both HCRU and associated costs among PC patients with MBD in Canada. Our results extend the finding of higher HCRU due to SREs reported by Habib and colleagues16 to other MBD-related resource use, and reveal the costs associated with this increased HCRU.
These Canadian results also support European and American studies, which found higher HCRU and costs for patients with MBD.8–10,13,15 A cost-of-illness study of MBD in the United States found that among PC patients, the presence of MBD nearly tripled mean per-patient direct medical costs (2004 US$56 281 vs. $19 781 for cases and controls, respectively).13 This larger difference than observed in our study, in which mean adjusted total healthcare costs were 62% higher in PC patients with MBD compared with controls, may be attributable to differences in healthcare prices and reimbursement structures between the United States and Canada.26
Instead of comparing MBD patients to controls without MBD, as done here, Hagiwara and colleagues compared costs in the period before and after development of bone metastases within a American cohort of PC patients with MBD.9 Adjusted total healthcare costs increased by 55% from the pre-MBD period (mean cost, 2008 US$23 047 per person-year) with development of bone metastases and no SREs ($35 827), and by 104% with development of bone metastases and SREs ($47 035). Thus, despite the different type of comparison, the cost impact was similar.
We reported our results on an annual basis to convey the overall HCRU and cost impact of MBD among patients with PC. A more granular perspective was taken in a recent hospital claims database analysis, which reported an increased intensity of HCRU and higher costs for patients with MBD on a per-encounter rather than an annualized basis.15 In this American study, hospital length of stay was on average 2 days longer and the average total cost per hospital encounter was 31% higher for PC patients with MBD compared with control patients (2010 US$9728 vs. $7405, respectively). Comparable findings were reported by Oglesby and colleagues in a retrospective hospital database analysis of PC patients in the United Kingdom; mean costs of initial (index) hospitalizations were 37% higher among those with bone metastases and no SREs (UK£2557; year of costing not reported), and 93% higher among those with bone metastases and SREs (£3618), compared with patients with neither MBD nor SREs (£1871).10 In contrast, Pockett and colleagues found smaller changes in mean hospitalization costs for patients in Spain when comparing index admissions for PC (€3194; year of costing not reported) vs. subsequent admissions for MBD (€3180; −0.4%) or SREs (€3585; 12%),8 perhaps reflecting differences in hospital charges across countries.
Low utilization of bisphosphonates was observed in this cohort of PC patients in Québec. Awareness of the importance of bone health in PC among urologists in Québec was enhanced following regulatory approval of the bisphosphonate zoledronic acid in 2002. However, uptake of zoledronic acid in Québec was limited by a highly restrictive “Patient d’exception” reimbursement program before 2005, which required prescribers to submit requests for reimbursement on a per-patient basis to the Conseil du medicament. The reimbursement criteria applied were not transparent, and the success rate for obtaining reimbursement for zoledronic acid in PC patients was low. In 2005, the responsibility for reviewing the case-by-case reimbursement requests was reassigned to RAMQ, and the criteria were clarified (chronic disease, severe disease, and last-resort treatment), resulting in an increase in approvals for reimbursement of zoledronic acid. The study cohort would largely have been treated during the period of the greatest restrictions on coverage of zoledronic acid, since 74.4% of patients in the MBD cohort had their MBD index date prior to 2005, which may account for the observed low utilization of bisphosphonates.
The potential limitations of our study include the possibility of the ascertainment algorithms falsely identifying or failing to identify PC or MBD patients; the inability to definitively ascribe a diagnosis to the index date; and incomplete information with which to match MBD and control patients. In addition, while the RAMQ database is a comprehensive data source for outpatients, the only information it contains for inpatients is length of stay and reason for hospitalization. In particular, the only prescription drug dispensations recorded in the RAMQ database are those purchased at retail pharmacies, not those administered in hospitals. Finally, this analysis excludes the utilization and costs of chemotherapy. Since the use of chemotherapy in patients with PC is driven by the presence of symptomatic metastatic disease,27 it is likely that our study underestimates the total economic burden of MBD.
Conclusion
This study found a heavy burden of MBD. After adjusting for baseline variables, patients with MBD had $11 820 higher annual healthcare costs than controls, and most patients with MBD experienced their first SRE about 2.5 years after PC diagnosis. These results highlight the importance of effective therapy to reduce the clinical and economic impact of MBD among patients with PC.
Acknowledgments
This study was funded by Amgen Canada Inc., Mississauga, ON. W. Mark Roberts, PhD, Montreal, QC, provided medical writing and editorial assistance in the preparation of this manuscript, funded by Amgen Canada Inc.
Footnotes
Competing interests: Ms. Perrault is a consultant for Amgen Inc., SOBI, LEO Pharma, Pfizer, and Baxter. Dr. Fradet has participated in clinical trials in the past 2 years or is currently participating with the following companies: Aragon Pharmaceuticals, Argos/Scimega Research, Inc., Astellas, Bayer, Bristol-Myers Squibb, Cougar Biotechnology, Inc., Exelixis, GlaxoSmithKline, Imagistx, Janssen, Medivation Inc., Merck, Millenium, Roche, Sanofi, Sanofi Aventis Canada Inc., and Sanofi US Services Inc. He is also a member of the advisory boards for Sanofi, AstraZeneca, Janssen, Astellas, and Amgen. He has also received payment from SCF Pharma for RCT contribution. He has received research grants from Sanofi, Amgen, Jansen, and AstraZeneca. He also holds investments in Diagnocure. Ms. Lauzon is a consultant for Amgen Canada, Dymaxium Inc., SOBI, Leo Pharma, IMAC GMBH, and Pfizer Canada. Dr. LeLorier has received remuneration or is expecting it from the following companies: AstraZeneca, Bio-K+ International Inc., Campbell Alliance Group Inc., CUBIST Pharma Inc., GSK, Lundbeck Canada Inc., Merck Canada Inc., Novartis Canada Inc., Pfizer Canada Inc., Sanofi, and ZS Associates. Mr. Mitchell is a consultant for Amgen Canada, Pfizer Canada, Triton, Dymaxium, Pharmascience, JAMP, Leo Pharma, Athena Research, and Baxter. Dr. Habib has received payment from Amgen Canada as an employee and holds investments in the firm.
This paper has been peer-reviewed.
References
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014 [Google Scholar]
- 2.Saad F, Clarke N, Colombel M. Natural history and treatment of bone complications in prostate cancer. Eur Urol. 2006;49:429–40. doi: 10.1016/j.eururo.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 3.Luz MA, Aprikian AG. Preventing bone complications in advanced prostate cancer. Curr Oncol. 2010;17:S65–71. doi: 10.3747/co.v17i0.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 5.Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–83. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 6.Delea T, McKiernan J, Brandman J, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol. 2006;4:341–7. [PubMed] [Google Scholar]
- 7.Lage MJ, Barber BL, Harrison DJ, et al. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care. 2008;14:317–22. [PubMed] [Google Scholar]
- 8.Pockett RD, Castellano D, McEwan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755–60. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagiwara M, Oglesby A, Chung K, et al. The impact of bone metastases and skeletal-related events on healthcare costs in prostate cancer patients receiving hormonal therapy. Community Oncol. 2011;8:508–15. [Google Scholar]
- 10.Oglesby A, Pockett RD, McEwan P, et al. Hospital burden of disease associated with metastatic bone disease (MBD) and skeletal-related events (SREs) in patients with breast cancer (BC) and prostate cancer (PC) in the United Kingdom (UK) [Abstract presented at the 11th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Athens, Greece, 8–11 November 2008] Value Health. 2008;11:A476. [Google Scholar]
- 11.Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm. 2010;16:693–702. doi: 10.18553/jmcp.2010.16.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmood A, Ghazal H, Fink MG, et al. Health-resource utilization attributable to skeletal-related events in patients with advanced cancers associated with bone metastases: Results of the US cohort from a multicenter observational study. Community Oncol. 2012;9:148–57. [Google Scholar]
- 13.Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–42. doi: 10.1002/cncr.22678. [DOI] [PubMed] [Google Scholar]
- 14.Groot MT, Boeken Kruger CG, Pelger RC, et al. Costs of prostate cancer, metastatic to the bone, in the Netherlands. Eur Urol. 2003;43:226–32. doi: 10.1016/S0302-2838(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 15.Seal B, Sullivan SD, Ramsey SD, et al. Comparing hospital-based resource utilization and costs for prostate cancer patients with and without bone metastases. Appl Health Econ Health Policy. 2014;12:547–57. doi: 10.1007/s40258-014-0101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habib MJ, Merali T, Mills A, et al. Canadian health care institution resource utilization resulting from skeletal-related events. Hosp Pract (1995) 2014;42:15–22. doi: 10.3810/hp.2014.02.1087. [DOI] [PubMed] [Google Scholar]
- 17.Dragomir A, Dinea D, Vanhuyse M, et al. Drug costs in the management of metastatic castration-resistant prostate cancer in Canada. BMC Health Serv Res. 2014;14:252. doi: 10.1186/1472-6963-14-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamblyn R, Lavoie G, Petrella L, et al. The use of prescription claims databases in pharmacoepidemiological research: The accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 19.Levy AR, Tamblyn RM, Fitchett D, et al. Coding accuracy of hospital discharge data for elderly survivors of myocardial infarction. Can J Cardiol. 1999;15:1277–82. [PubMed] [Google Scholar]
- 20.Tamblyn R, Reid T, Mayo N, et al. Using medical services claims to assess injuries in the elderly: Sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–94. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 21.Monfared AA, Lelorier J. Accuracy and validity of using medical claims data to identify episodes of hospitalizations in patients with COPD. Pharmacoepidemiol Drug Saf. 2006;15:19–29. doi: 10.1002/pds.1131. [DOI] [PubMed] [Google Scholar]
- 22.Birnbaum HG, Cremieux PY, Greenberg PE, et al. Using healthcare claims data for outcomes research and pharmacoeconomic analyses. Pharmacoeconomics. 1999;16:1–8. doi: 10.2165/00019053-199916010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Institut national de santé publique Utilisation du fichier des paiements a l’acte de la RAMQ pour identifier les cas de cancers non déclarés au Fichier des tumeurs du Québec, Etude de faisabilité – Volet : Cancer de la prostate, Institue National de santé public du Québec, Février 2006. [updated 2006 Feb; cited 2013 Aug 5]. http://www.inspq.qc.ca/pdf/publications/488-CancersNonDeclareFichiersTumeurs.pdf. Accessed January 16, 2015.
- 24.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-G. [DOI] [PubMed] [Google Scholar]
- 25.Statistics Canada CANSIM Table 326-0020: Consumer Price Index (CPI), 2011 basket.
- 26.Pozen A, Cutler DM. Medical spending differences in the United States and Canada: The role of prices, procedures, and administrative expenses. Inquiry. 2010;47:124–34. doi: 10.5034/inquiryjrnl_47.02.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190:429–38. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]