Abstract
Urachal neoplasms are rare entities which may be classified as cystic or non-cystic. Literature surrounding patient outcomes remains limited to non-cystic, urachal adenocarcinomas. Literature focusing on mucinous cystic neoplasms of the urachus is sparse. These mucinous cystic lesions may be subclassified as benign mucinous cystadenomas, mucinous cystic tumours of low malignant potential, and mucinous cystadenocarcinomas. Mucinous subtypes have the potential to behave aggressively and may result in pseudomyxoma peritonei. We describe here the case of a 37-year-old male with a mucinous cystic tumour of low malignant potential after prior right orchiectomy and left hydrocelectomy. This case raises the interesting possibility of multiple genitourinary neoplasms arising in a similar time frame.
Introduction
The urachus is an embryological structure that rarely persists in adults, but may be a site of malignancy. Any primary neoplasm of the urachus may be grouped as mucinous or non-mucinous, and as cystic or non-cystic.1
Mucinous cystic lesions may be subclassified as benign mucinous cystadenomas, mucinous cystic tumours of low malignant potential, and mucinous cystadenocarcinomas. Mucinous subtypes have the potential to behave aggressively and may result in pseudomyxoma peritonei (PMP).2 However, natural progression of these lesions is difficult to interpret given the lack of reports. We attempt to further this understanding by describing the case of a 37-year-old male who presented with a mucinous cystic tumour of low malignant potential after prior right orchiectomy.
Case report
This 37-year-old male patient originally underwent right radical orchiectomy for a stage pT2, non-seminomatous germ cell tumour (NSGCT) (95% embryonal, 5% seminomatous) with lymphovascular invasion and elevated lactic acid dehydrogenase in early 2013. During workup, computed tomography (CT) demonstrated a 3.5-cm cystic, non-enhancing lesion with calcification contiguous with the right upper aspect of the bladder (Fig. 1). Cystoscopy demonstrated no bladder connection. This mass was determined to be unrelated to the testicular tumor given its appearance and lack of retroperitoneal lymphadenopathy. There were no pulmonary, hepatic, or skeletal metastases. Following orchiectomy, the patient underwent 2 cycles of adjuvant BEP (bleomycin, etoposide, cisplatin) chemotherapy with no recurrence of testicular tumour.
Fig. 1.
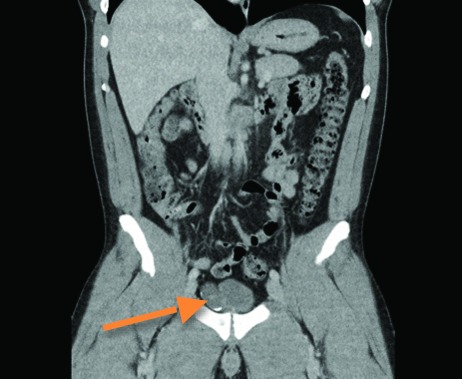
Computed tomography scan demonstrating an irregular cystic lesion with calcification contiguous with the right anterior upper aspect of the bladder.
Eighteen months later, the patient underwent left varicocelectomy for a varicocele, but developed a left hydrocele as sequelae. It was decided that the urachal mass be removed at the time the hydrocele was addressed. The patient then underwent concurrent left hydrocelectomy and partial cystectomy. At time of surgery, there was no evidence of PMP.
Pathology findings
Gross examination revealed a cystic structure containing cloudy, mucinous material that measured 4.0 cm in diameter with a wall thickness of 0.2 cm. Microscopic sections through the bladder resection revealed a thin-walled fibrous diverticulum (Fig. 2). The inner wall of the diverticulum contained granular calcified debris eliciting a multinucleated giant cell reaction. An area of intact mucosa was composed of columnar epithelium showing mucinous metaplasia with papillary architecture. Mucin was present in the lumen of the diverticula and had extravasated into the wall. The tumour was positive for cytokeratin 7 and cytoplasmic beta catenin. There was no invasive component. A nodule of smooth muscle contained cystic spaces lined by urothelium, a feature diagnostic of urachal remnant (Fig. 3). It was determined that the lesion was consistent with a diagnosis of mucinous cystic tumour of low malignant potential (MCTLMP).
Fig. 2.
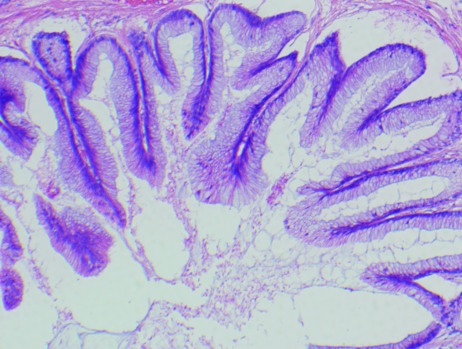
Pathology specimen through the bladder resection demonstrating a thin walled fibrous diverticulum with abundant inner wall granular calcified debris, a small area of intact mucosa showing mucinous metaplasia, and luminal mucin extravasating into the wall. No invasive components were noted.
Fig. 3.
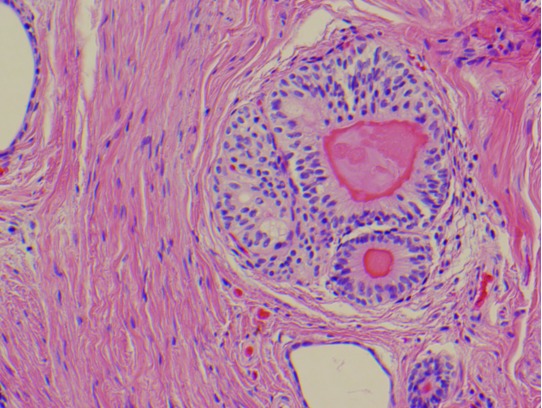
Pathology specimen containing a nodule of smooth muscle containing cystic spaces lined by urothelium; features diagnostic of urachal remnant.
Discussion
Glandular tumours of the urachus remain sparsely described due to rarity, degree of clinical correlation required to establish urachal origin, and lack of standardized nomenclature. A recent classification scheme was proposed by Amin and colleagues,1 assessing 55 cases at their institution. Of these, 22 were classified as MCTLMP. There are 5 other reports of lesions consistent with MCTLMPs.3–7 (Table 1).
Table 1.
Cases of MCTLMP or similar lesions thus far reported in case reports
| Reference | Age | Sex | Size (cm) | PMP | Diagnosis | Treatment | Symptoms | Patient history |
|---|---|---|---|---|---|---|---|---|
| Present case | 37 | M | 4 | Absent | MCTLMP | Partial cystectomy concurrently with left hydrocelectomy | Incidental finding | Prior right radical orchiectomy, prior left varicocelectomy |
| Carr and McLean3 | 72 | M | 4 | Absent | Mucinous tumour of uncertain malignant potential | Partial cystectomy | Hematuria (microscopic), nocturia | No other medical issues |
| Paul et al4 | 68 | M | 3 | Absent | Stage 0 mucinous adenocarcinoma in situ | Partial cystectomy | Hematuria, mucusuria | No other medical issues |
| Shinohara et al5 | 54 | M | 6 | Present | Mucinous cystic tumour with low malignant potential | Partial cystectomy | None | Mucus from ruptured urachal cyst found during left inguinal hernia repair, cyst excised at re-operation |
| Stenhouse et al6 | 54 | M | 11 | Present | Mucinous neoplasm of uncertain malignant potential | N/A | Abdominal pain, rectal bleeding | No other medical issues |
| Choi et al7 | 29 | F | 5.5 | Absent | Mucinous tumour of uncertain malignant potential | Partial cystectomy | Right flank pain | No other medical issues |
MCTLMP: mucinous cystic tumour of low malignant potential; PMP: pseudomyxoma peritonei; N/A: not available; M: male; F: female.
Other mucinous cystic lesions of the urachus include mucinous cystadenoma and cystadenocarcinoma.8–10 Mucinous cystadenomas display no dysplastic change, MCTLMPs demonstrate dysplastic change without invasion, and mucinous cystadenocarcinomas demonstrate invasion. It is hypothesized that mucinous cystadenomas and MCTLMPs represent a continuum of lesion which may eventually degenerate into mucinous cystadenocarcinoma. MCTLMPs are managed surgically, although there is no evidence that definitively demonstrates the degeneration of MCTLMPs into mucinous cystadenocarcinoma. Adults suspected of having a urachal remnant of any sort may be at high risk of malignancy.11 Predictors of urachal malignancy include patients over 55 and patients with hematuria. Prophylactic excision of urachal anamolies in children is not recommended. Many patients likely undergo treatment to prevent a single case of urachal adenocarcinoma.12
MCTLMPs are most often incidental findings, although patients may present with an abdominal mass, pain, mucusuria, hematuria, urgency, or umbilical discharge. Patients may also demonstrate PMP originating from the urachal mass. Two prior cases of MCTLMP have demonstrated PMP,5,6 which is associated with complications such as cachexia and bowel obstruction if left untreated. Current treatment of PMP includes total urethrectomy, partial cystectomy, and peritonectomy followed by adjuvant chemotherapy.13
Our patient had a significant history of urologic issues, namely a right-sided, high-risk NSGCT and left varicocele. No other cases of MCTLMPs with prior urologic history have been documented. This patient’s NSGCT was primarily embryonal with positive lymphovascular invasion, stratifying it as high risk with up to a 50% chance of recurrence.14 Although there is debate regarding post-surgical management of high risk NSGCT, options include 1 to 2 cycles of adjuvant BEP or surveillance.15 If a tumour recurs during surveillance, 3 to 4 cycles of BEP may be required. Our patient was well-advised about these options and opted for adjuvant BEP.
Additionally, one-fifth to two-thirds of patients with tumours composed primarily of embryonal carcinoma have metastases at presentation, highlighting importance of ruling out this possibility.16 Lack of connection to the bladder, non-enhancement during imaging, and no evidence of retroperitoneal lymphadenopathy or other metastases suggested the lesion was unrelated to the testicular tumour. It was opted that surgical excision of this lesion wait until the patient had undergone complete treatment of his testicular cancer. This is a challenging clinical decision given the lack of data on possible malignant degeneration of urachal neoplasms.
We suspect our patient had underlying genetic predispositions due to his testicular and urachal neoplasms, and his young age at MCTLMP presentation. Monitoring this patient for recurrence or new lesions is warranted.
Conclusion
We have presented the case of a patient presenting with a urachal MCTLMP in the context of treatment for a right-sided testicular NSGCT. Clinical assessment of MCTLMP remains difficult and may present challenge in terms of timing of surgery. Lack of data on the natural history of these lesions and their possible malignant degeneration warrant that MCTMKP be treated with partial cystectomy.
Table 2.
A case series of MCTLMP or similar lesions
| Reference | Age | Sex | Size (cm) | Diagnosis | Treatment | Symptoms |
|---|---|---|---|---|---|---|
| Amin et al1 | 48 | F | 8 | MCTLMP with intraepithelial carcinoma | Excision of urinary bladder mass & umbilectomy | Suprapubic and umbilical mass |
| 26 | F | 2 | MCTLMP | Partial cystectomy | Suprapubic mass | |
| 74 | M | 6.5 | MCTLMP | Excision of tumour & sigmoid colectomy | Incidental finding | |
| 72 | M | 0.8 | MCTLMP | Partial cystectomy | Mucusuria | |
| 74 | M | 3 | MCTLMP | Partial cystectomy | Hematuria | |
| 50 | F | 2.1 | MCTLMP | Resection of urachus | Mass | |
| 45 | M | 3.5 | MCTLMP | Partial cystectomy | RLQ pain, Hematuria | |
| 58 | F | 1 | MCTLMP | Excision of lesion | Incidental finding | |
| 43 | F | 2.5 | MCTLMP | Partial cystectomy | Incidental finding | |
| 40 | F | 6 | MCTLMP | Partial cystectomy, urachectomy, and umbilectomy | Incidental finding | |
| 80 | F | 2.5 | MCTLMP | Partial cystectomy and urachectomy | Mucusuria | |
| 37 | F | N/A | MCTLMP | N/A | Incidental finding | |
| 29 | F | N/A | MCTLMP | N/A | Bladder dome nodule | |
| N/A | N/A | N/A | MCTLMP | N/A | N/A | |
| 42 | F | 8 | MCTLMP | Excision of pelvic mass | Pelvic mass | |
| 42 | F | 6 | MCTLMP | N/A | Midline cystic mass | |
| 36 | F | N/A | MCTLMP | N/A | Incidental finding | |
| 39 | M | 6.5 | MCTLMP | Umbilectomy and urachectomy | Obstruction and umbilical discharge | |
| 57 | M | 2.8 | MCTLMP with intraepithelial carcinoma | Partial cystectomy | N/A | |
| 77 | F | 5.5 | MCTLMP | Partial cystectomy | N/A | |
| 43 | M | 7 | MCTLMP | Partial cystectomy, umbilectomy, and urachectomy | Incidental finding | |
| 26 | M | 8 | MCTLMP | Partial cystectomy | Urgency, abdominal pain |
MCTLMP: mucinous cystic tumour of low malignant potential; RLQ: right lower quadrant; N/A: not available; M: male; F: female.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Amin MB, Smith SC, Eble JN, et al. Glandular neoplasms of the urachus: A report of 55 cases emphasizing mucinous cystic tumors with proposed classification. Am J Surg Pathol. 2014;38:1033–45. doi: 10.1097/pas.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 2.Schell AJ, Nickel CJ, Isotalo PA. Complex mucinous cystadenoma of undetermined malignant potential of the urachus. Can Urol Assoc J. 2009;3:E39–41. doi: 10.5489/cuaj.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr NJ, McLean AD. A mucinous tumour of the urachus: Adenoma or low grade mucinous cystic tumour of uncertain malignant potential? Adv Clin Pathol. 2001;5:93–7. [PubMed] [Google Scholar]
- 4.Paul AB, Hunt CR, Harney JM, et al. Stage 0 mucinous adenocarcinoma in situ of the urachus. J Clin Pathol. 1998;51:483–4. doi: 10.1136/jcp.51.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinohara T, Misawa K, Sano H, et al. Pseudomyxoma peritonei due to mucinous cystadenocarcinoma in situ of the urachus presenting as an inguinal hernia. Int J Clin Oncol. 2006;11:416–9. doi: 10.1007/s10147-006-0594-1. [DOI] [PubMed] [Google Scholar]
- 6.Stenhouse G, McRae D, Pollock AM. Urachal adenocarcinoma in situ with pseudomyxoma peritonei: A case report. J Clin Pathol. 2003;56:152–3. doi: 10.1136/jcp.56.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J-W, Lee J-H, Kim Y-S. Urachal mucinous tumor of uncertain malignant potential: A case report. Korean J Pathol. 2012;46:83–6. doi: 10.4132/KoreanJPathol.2012.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prakash MR, Vijayalaxmi SV, Maitreyee R, et al. Complex mucinous cystadenoma of undetermined malignant potential of the urachus: A rare case with review of the literature. Malays J Pathol. 2014;36:145–8. [PubMed] [Google Scholar]
- 9.Fahed AC, Nonaka D, Kanofsky JA, et al. Cystic mucinous tumors of the urachus: Carcinoma in situ or adenoma of unknown malignant potential? Can J Urol. 2012;19:6310–3. [PubMed] [Google Scholar]
- 10.Agrawal AK, Bobiński P, Grzebieniak Z, et al. Pseudomyxoma peritonei originating from urachus—Case report and review of the literature. Curr Oncol. 2014;21:e155–65. doi: 10.3747/co.21.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley RA, Inman BA, Routh JC, et al. Urachal anomalies: A longitudinal study of urachal remnants in children and adults. J Urol. 2007;178:1615–8. doi: 10.1016/j.juro.2007.03.194. [DOI] [PubMed] [Google Scholar]
- 12.Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632–6. doi: 10.1016/j.juro.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Ishibashi H, Hirano M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei arising from urachus. Ann Surg Oncol. 2015:1–7. doi: 10.1245/s10434-014-4336-8. [DOI] [PubMed] [Google Scholar]
- 14.Isharwal S, Risk M. Management of clinical stage I nonseminomatous germ cell tumors. Expert Rev Anticancer Ther. 2014;14:1021–32. doi: 10.1586/14737140.2014.928593. [DOI] [PubMed] [Google Scholar]
- 15.Wit R, Bosl G. Optimal management of clinical stage I testis cancer: One size does not fit all. J Clin Oncol. 2013;31:3477–9. doi: 10.1200/JCO.2013.51.0479. [DOI] [PubMed] [Google Scholar]
- 16.Mills SE, Carter D, Greenson JK, et al. Sternberg’s Diagnostic Surgical Pathology. Lippincott Williams & Wilkins; 2012. p. 6247. [Google Scholar]


