Abstract
Background
Although HPV vaccination has been recommended for use in girls and young women since 2007, HPV vaccine uptake is low in the US.
Methods
We conducted a retrospective cohort study using the 2008–2011 MarketScan data to examine HPV vaccine completion and dose adherence among commercially insured females aged 9–26 years. We performed multivariable logistic regression models to examine factors related to HPV vaccine completion and HPV vaccine dose adherence.
Results
Among 378,484 females aged 9–26 years who initiated HPV vaccination, only 29.4% completed HPV vaccination. Compared with females receiving vaccines from primary care providers, those receiving vaccines from OB/GYN providers were more likely to complete the vaccine series. Age at HPV vaccine initiation, health insurance plan, seasonal pattern, and flu vaccination were also significantly associated with vaccine completion. Among 111,286 females who completed HPV vaccination, 62.4% received all doses within 30 days of the recommended schedules. Similar factors relating to HPV vaccine completion were consistently associated with HPV vaccine dose adherence. However, younger age (<22 years) and receipt of flu vaccine were negatively related to HPV vaccine dose adherence.
Conclusions
Intervention programs to improve HPV vaccine reminding system and reduce logistic barriers for both physicians and patients are warranted.
Keywords: HPV vaccine completion, HPV vaccine dose adherence, Commercially insured females
Introduction
Human papillomavirus (HPV) infection is an established causal factor associated with about 5% of all human cancers, including cervical, anogenital and oropharyngeal cancers [1], [2]. In the United States (US), about 34,000 HPV-related cancers are diagnosed annually and medical expenses for preventing and treating HPV-related diseases are estimated to be $8 billion every year [3], [4]. HPV vaccination has been advocated as a safe and effective strategy for preventing HPV-associated cancers that are caused by vaccine-covered types [5], [6], [7], [8]. A significant decline in prevalence of HPV16, 18, 6, and 11 has been observed in adolescent girls aged 14–19 years and no serious safety concerns have been detected following HPV vaccine introduction in 2006 [8], [9], [10], [11]. Therefore, HPV vaccination is included in the U.S. Department of Health and Human Services Healthy People 2020 (HP2020) with a national objective of 80% 3-dose coverage rate for females by age 13–15 years [12].
Despite scientific evidence and strong recommendations, HPV vaccination in the US remains well below the HP2020 objective; the 3-dose coverage rate is only 39.7% among adolescent girls aged 13–17 years in 2014, and gaps exist in receiving all three doses within recommended schedules [13], [14], [15]. Because the overall effectiveness of HPV vaccination on reducing HPV-related diseases would be affected by a delayed or incomplete vaccination, immediate and multi-level actions are urged by the President Cancer Panel to increase HPV vaccine uptake as a national public health priority in the US [16].
In order to improve HPV vaccination, it is essential to understand current patterns of HPV vaccination. While previous research has primarily focused on HPV vaccine initiation and completion among adolescent girls, the adherence of recommended HPV dose schedules has not been well assessed [17], [18], [19], [20], [21], [22]. The objectives of this study were to: (1) examine HPV vaccine completion and dose adherence among commercially insured females who were aged 9 through 26 years old and had initiated HPV vaccination; (2) identify factors associated with HPV vaccine completion and dose adherence among these females. Our work will provide insights on compliance of HPV vaccine guidelines in the real-world context and shed lights on the potential areas where the general public, researchers, healthcare providers, health insurance companies and policy makers can contribute to enhance HPV vaccination in the US.
Materials and methods
Data source
We conducted a retrospective cohort study using the national health insurance claims data, the 2008–2011 MarketScan Commercial Claims and Encounters (CCE) database [23]. The MarketScan CCE database consists of reimbursed health care claims for employees, retirees, and their dependents of over 250 medium and large employers nationwide. Individuals included in the database are covered under commercial (private) insurance plans (Medicaid or Medicare data are not included). The database includes claims information from more than 130 payers, and describes the health care service use and expenditures for more than million covered individuals each year. We identified events of HPV vaccination from the in-patient and out-patient claims based on the Current Procedural Terminology 4th Edition [CPT-4] codes (90649 for Gardasil and 90650 for Cervarix) and linked multiple claims of HPV vaccination for each individual using a unique identifier (Enrollee ID). We also obtained information on individual’s age, gender, geographic location (US census region and state), type of health insurance plan, date(s) of HPV vaccination, receipt of seasonal flu vaccine, and type of provider who administered HPV vaccines.
Study population
To define a cohort of females who newly initiated HPV vaccination, we focused on females aged 9 through 26 years who had at least one claim of HPV vaccination during the period from 2009–2010 and had no claims of vaccination in 2008. We used the date of the first HPV vaccination claim in 2009–2010 as the index date and included females who had been continuously enrolled in an insurance plan from 12 months prior to the index date to 12 months post the index date to ascertain all HPV vaccination claims within 12 months. We chose the 12-month period for HPV vaccination because evidence indicated that 3 doses of HPV vaccine received within 12 months produced comparable immunogenicity compared to the standard 6-month HPV vaccination, but HPV vaccination with longer than a 12-month interval did not meet the non-inferiority criteria [24]. We excluded 1987 females with more than 3 HPV vaccination claims during this study period because we cannot verify if receipt of >3 doses is due to re-initiation of HPV vaccine series or a coding error. We also excluded 125,362 females who had HPV vaccination claims in 2008 because we did not have the 2006–2007 data to confirm the HPV vaccine initiate date.
Statistical analysis
We examined two dichotomous outcomes: (1) completion of HPV vaccination within 12 months among females who had initiated HPV vaccination in 2009–2010, which was defined as ‘Yes’ for those who received all 3 doses of HPV vaccines and ‘No’ for those who had 1 or 2 doses in a 12-month period; (2) adherence of recommended HPV vaccine dose schedules, which was determined by intervals between two consecutive HPV doses recommended by the Advisory Committee on Immunization Practices (ACIP) [8]. If an individual finished both the 2nd and 3rd doses of HPV vaccines no later than 30 days after the recommended interval, we considered the adherence as “Yes” (otherwise “No” if either dose was delayed). For Gardasil it is recommended that the 2nd and the 3rd doses to be completed at the end of the 2nd and 6th month; and for Cervarix, it is recommend that the 2nd and the 3rd doses be completed at the end of the 1st and 6th month. We defined the adherence intervals as within 90 days and 60 days after the date of the 1st dose for the 2nd dose of Gardasil and Cervarix, respectively, and within 210 days after the 1st dose for the 3rd dose of both Gardasil and Cervarix.
We calculated descriptive statistics, including the percentage, median and mean to depict the overall pattern of HPV vaccination. We examined HPV vaccine completion and dose adherence stratified by individuals’ characteristics (age at the 1st dose of HPV vaccination, US census region, urban or rural residence based on the Metropolitan Statistical Area [MSA] code, health insurance plan, type of provider who administered the 1st dose of HPV vaccine series, flu vaccination during the study period, and quarter of the year of HPV vaccine initiation). We evaluated the associations using the t test for continuous variables and the χ2 test or Fisher exact test for categorical variables. We then performed multivariable logistic regression models to identify factors that were significantly associated with the study outcomes (two-sided P-value <0.05) and reported adjusted odds ratios (aOR) and their 95% confidence intervals (CI). We conducted all statistical analyses using SAS version 9.3 (SAS Institute, Cary, NC). Given the very large sample size from claims data, there is tremendous statistical power to detect very small effect sizes. Therefore, the interpretations of statistically significant results need to take into account the practical implications in terms of odds ratios.
Results
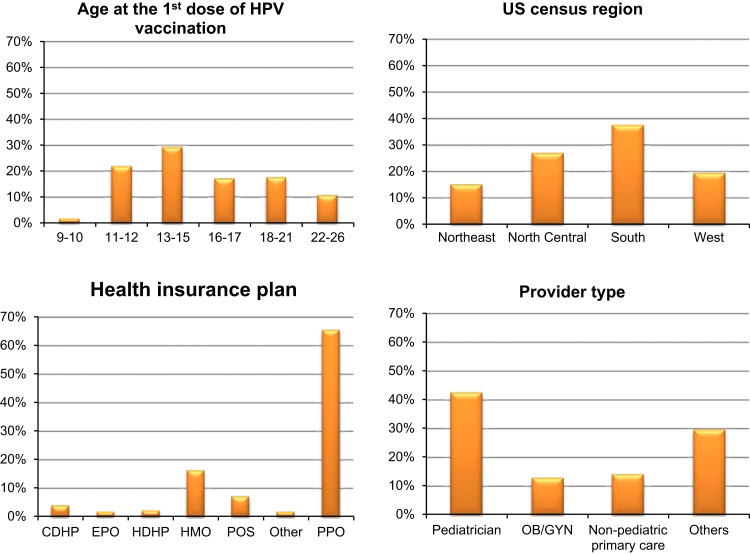
A total of 378,484 commercially insured females aged 9–26 years were included in this study, and the majority (99.2%) received the quadrivalent HPV vaccine (Gardasil, Merck & Co Inc, Whitehouse Station, New Jersey). Fig. 1 presented the cohort characteristics by age group at the 1st dose of HPV vaccination, geographic region, health insurance plan, and type of provider who administered the 1st dose of HPV vaccine series. Pre-adolescent girls aged 9–12 years accounted for 24.2% of the entire study population. The cohort represented four census regions with 37.8% from South, 27.2% from North Central, 19.7% from West and 15.3% from Northeast. Most people (88.7%) lived in the MSA (urban) and only 11.3% lived outside an MSA (rural). The majority had either preferred provider organization (PPO) health plan (65.8%) or health maintenance organization (HMO) plan (16.5%). Consistent with the age distribution, over 70% of females received vaccines from pediatricians (42.8%), non-pediatric primary care providers (14.3%), or obstetrics and gynecology (OB/GYN) providers (13.1%), while the rest (29.8%) received from other types of providers.
Fig. 1.
Characteristics of commercially insured females who were aged 9 through 26 years and had at least one claim of HPV vaccination during 2009–2010 (N=378,484). Footnotes: Health insurance plan that covers HPV vaccination: CDHP: consumer-driven health plan; EPO: Exclusive provider organizations; HDHP: high-deductible health plan; POS: point of service; PPO: Preferred provider organizations; HMO: Health maintenance organizations; Other: Comprehensive and POS capitation. Provider type represented providers who administered the 1st dose of HPV vaccine series. OB/GYN: obstetrics and gynecology.
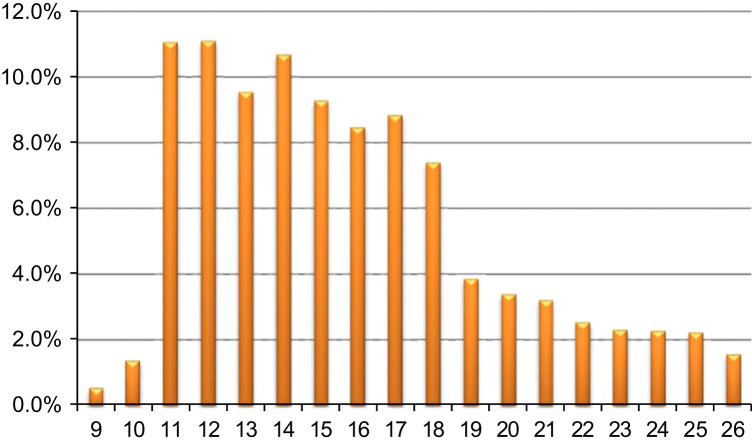
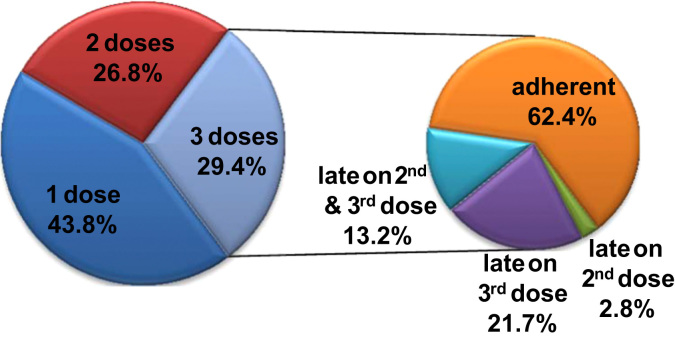
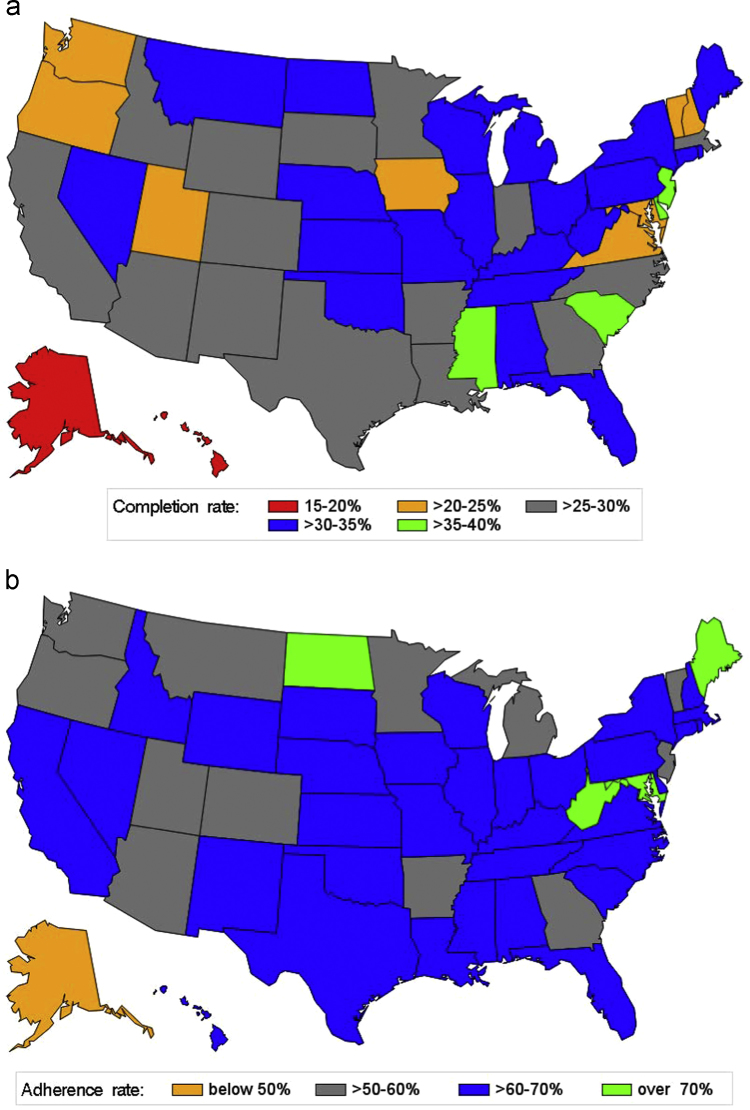
Fig. 2 showed the age distribution at the 1st dose of HPV vaccination. The mean age was 15.8 (standard deviation=4.0), a noticeable delay in initiating HPV vaccination according to the ACIP recommendation. Fig. 3 presented a snapshot of HPV vaccine completion and dose schedule adherence patterns in our study cohort. In particular, among females who had initiated HPV vaccination, 43.8% had only one dose, and 56.2% received ≥2 doses (29.4% received all 3 doses). Among 111,286 females who completed the 3-dose vaccine series, 62.4% (about 18% of the study population) had received both the 2nd and the 3rd doses within 30 days of the ACIP recommended intervals; 2.8% were late for the 2nd dose only; 21.7% were late for the 3rd dose only; and 13.2% were late for both doses. Fig. 4a and b indicated great variations in HPV vaccine completion and dose adherence across states. The state-level HPV vaccine completion rates ranged from 15.7% in the District of Columbia to 38.5% in Delaware, and the dose adherence rates were lower than 60% in 14 states.
Fig. 2.
Distribution of age at the 1st dose of HPV vaccination among 378,484 commercially insured females who were aged 9 through 26 years and had at least one claim of HPV vaccination during 2009–2010.
Fig. 3.
HPV vaccine completion among 378,484 commercially insured females who were aged 9 through 26 years and had at least one claim of HPV vaccination during 2009–2010 and HPV vaccine dose schedule adherence among 111,286 females who completed the 3-dose HPV vaccine series.
Fig. 4.
a. State-level HPV vaccine completion rate among 378,484 commercially insured females who were aged 9 through 26 years and had at least one claim of HPV vaccination during 2009–2010 Fig. 4b. State-level HPV vaccine dose schedule adherence rate among 111,286 females who completed the 3-dose HPV vaccine series.
Factors associated with HPV vaccine completion
We presented the vaccine completion rates stratified by different factors (Table 1). Univariate analysis results showed that vaccine completion was significantly associated with age at the 1st dose of HPV vaccination, geographic region, urban–rural residence, health insurance plan, type of provider who administered the 1st dose of HPV vaccine series, quarter of the year when the 1st dose of vaccine was administered, and whether the patient also had flu vaccination during the study period, with all p-values<0.0001, except the p-value=0.0408 for urban–rural residence.
Table 1.
HPV vaccine completion rate among 378,484 commercially insured females who were aged 9 through 26 years and had at least one claim of HPV vaccination during 2009–2010 (N=378,484)
| Characteristics | HPV vaccine completion N, % |
Multivariable logistic regression model |
|||
|---|---|---|---|---|---|
| Adjusted OR | 95% CI | P-value | |||
| Age group at the 1st dose of HPV vaccination | |||||
| 9–10 (N=7358) | 2,416, 32.8% | 1.34 | 1.26 | 1.41 | <.0001 |
| 11–12 (N=84,046) | 27,041, 32.2% | 1.36 | 1.32 | 1.40 | <.0001 |
| 13–15 (N=111,947) | 32,790, 29.3% | 1.19 | 1.15 | 1.22 | <.0001 |
| 16–17 (N=65,660) | 18,741, 28.5% | 1.12 | 1.09 | 1.16 | <.0001 |
| 18–21 (N=67,838) | 17,667, 26.0% | 0.90 | 0.88 | 0.93 | <.0001 |
| 22–26 (N=41,635) | 12,631, 30.0% | Ref. | |||
| US census region | |||||
| Northeast (N=58,035) | 19,227, 33.1% | 1.07 | 1.05 | 1.09 | <.0001 |
| South (N=143,070) | 40,307, 28.2% | 0.83 | 0.81 | 0.84 | <.0001 |
| West (N=74,409) | 19,570, 26.3% | 0.79 | 0.78 | 0.81 | <.0001 |
| North Central (N=102,970) | 32,182, 31.3% | Ref. | |||
| Residence | |||||
| Urban (MSA) (N=335,670) | 98,879, 29.5% | 1.03 | 1.01 | 1.05 | 0.0146 |
| Rural (non-MSA) (N=42,814) | 12,407, 29.0% | Ref. | |||
| Health insurance plan | |||||
| CDHP (N=15,605) | 5,291, 33.9% | 1.25 | 1.21 | 1.30 | <.0001 |
| Comprehensive (N=3703) | 1,053, 28.4% | 0.96 | 0.89 | 1.04 | 0.3144 |
| EPO (N=7207) | 2,183, 30.3% | 1.06 | 1.01 | 1.12 | 0.0288 |
| HDHP (N=9,002) | 2,545, 28.3% | 0.96 | 0.91 | 1.01 | 0.1166 |
| POS (27,501) | 8,258, 30.0% | 1.05 | 1.02 | 1.09 | 0.0022 |
| POS capitation (N=2717) | 815, 30.0% | 0.98 | 0.90 | 1.07 | 0.6862 |
| PPO (N=243,531) | 71,703, 29.4% | 1.04 | 1.02 | 1.06 | 0.0002 |
| HMO (N=61,079) | 17,110, 28.0% | Ref. | |||
| Type of provider who administered the 1st dose | |||||
| Pediatric (N=161,813) | 46,344, 28.6% | 0.85 | 0.83 | 0.87 | <.0001 |
| OB/GYN (N=49,633) | 18,006, 36.3% | 1.73 | 1.68 | 1.78 | <.0001 |
| Others (N=112,855) | 31,101, 27.6% | 0.99 | 0.96 | 1.01 | 0.3055 |
| Primary care (N=54,183) | 15,835, 29.2% | Ref. | |||
| Quarter of the year at the 1st dose | |||||
| Jan–Mar (N=93,848) | 25,894, 27.6% | 1.01 | 0.99 | 1.04 | 0.2593 |
| Apr–Jun (N=93,625) | 31,671, 33.8% | 1.39 | 1.35 | 1.42 | <.0001 |
| Jul–Sep (N=131,567) | 36,423, 27.7% | 0.99 | 0.98 | 1.02 | 0.9459 |
| Oct–Dec (N=59,444) | 17,298, 29.1% | Ref. | |||
| Flu vaccination | |||||
| Yes (N=135,117) | 46,847, 34.7% | 1.55 | 1.52 | 1.57 | <.0001 |
| No (N=243,367) | 64,439, 26.5% | Ref. | |||
Abbreviations: HPV: human papillomavirus; MSA: Metropolitan Statistical Area; CDHP: consumer-driven health plan; EPO: Exclusive provider organizations; HDHP: high-deductible health plan; POS: point of service; PPO: Preferred provider organizations; HMO: Health maintenance organizations; OB/GYN: obstetrics and gynecology; OR: odds ratio; CI: confidence interval.
Pre-adolescents had the highest vaccine completion, while the 18–21 age group had the lowest rate (26.0%). Geographic variation was also observed in HPV vaccine completion: the Northeast region had the highest completion rate (33.1%), followed by North central (31.3%); while the South and West regions had lower completion rates (28.2% and 26.3%, respectively).
HPV vaccine completion rates varied across types of health insurance plan as well as providers who administered the 1st dose of vaccines. In particular, among adult females aged 22–26 years, those with vaccine initiated by OB/GYN providers had a higher completion rate (34.4% versus 26.8% for patients with vaccine initiated by other providers in the 22–26 age group). Similarly at the entire cohort level, females initiated HPV vaccines by OB/GYN providers had higher rates of completing all 3 doses (36.3%) than did clients of pediatricians (28.6%), primary care providers (29.2%), and other specialists (27.6%).
We also observed a correlation between HPV vaccine completion and quarter of the year when the 1st dose of vaccine was administered. In particular, the April–June quarter has the best completion rate at 33.8%, while all other three quarters have rates below 30%. Our results also showed that 34.7% of whom received flu vaccination completed HPV vaccine series, compared to only 26.5% among those who did not get flu vaccination during the study period.
The multivariable Logistic regression results indicated that all factors found significant in the bivariate analysis (i.e. age, census region, health plan, provider type, flu vaccination, and quarter of the year) remained significantly associated with HPV vaccine completion. In particular, compared to the 22–26 age group, the 18–21 age group had the lowest odds of completion (aOR=0.90, 95% C.I.=0.88,0.93), while the 11–12 age group had the highest odds (aOR=1.36, 95% C.I.=1.32,1.40). Regarding geographic variation, the completion rate seemed higher in Northeast region, but was significantly lower in the South and West regions (South: aOR=0.83, 95% C.I.=0.81,0.84; West: aOR=0.79, 95% C.I.=0.78,0.81). Compared with HPV vaccination initiated by primary care providers, clients of pediatricians corresponded to the lowest odds of HPV vaccine completion (aOR=0.85, 95% C.I.=0.83,0.87), but vaccine initiation by OB/GYN providers was associated with higher odds of completion. Subgroup analyses stratified by age (9–10, 11–-12, 13–15, 16–17, 18–21 and 22–26) consistently showed the same patterns relating to providers across all the pertinent age groups.
Factors associated with HPV vaccine dose adherence
Adherence of HPV vaccine dose schedule was significantly associated with geographic region, health plan, provider type, and the quarter of the year (1st dose) in a pattern similar to that for HPV vaccine completion (Table 2). However, in contrast to its association with HPV vaccine completion, pre-adolescents and adolescents (<18 years), urban residence, and having flu vaccination was related to significantly lower odds of HPV vaccine dose adherence.
Table 2.
HPV vaccine dose adherence rate among 111,286 commercially insured females who completed the 3-dose HPV vaccine series.
| Characteristics | HPV vaccine dose adherence N, % |
Multivariable logistic regression model |
|||
|---|---|---|---|---|---|
| Adjusted OR | 95% Confidence intervals | P-value | |||
| Age group at the 1st dose of HPV vaccination | |||||
| 9–10 (N=2,416) | 1,437, 59.5% | 0.75 | 0.68 | 0.83 | <.0001 |
| 11–12 (N=27,041) | 16,233, 60.0% | 0.79 | 0.75 | 0.84 | <.0001 |
| 13–15 (N=32,790) | 19,675, 60.0% | 0.74 | 0.70 | 0.78 | <.0001 |
| 16–17 (N=18,741) | 11,426,61.0% | 0.69 | 0.65 | 0.73 | <.0001 |
| 18–21 (N=17,667) | 11,164,63.2% | 0.64 | 0.61 | 0.68 | <.0001 |
| 22–26 (N=12,631) | 9,462, 74.9% | Ref. | |||
| US census region | |||||
| Northeast (N=19,227) | 12,257, 63.8% | 1.12 | 1.08 | 1.17 | <.0001 |
| South (N=40,307) | 25,406, 63.0% | 1.02 | 0.98 | 1.05 | 0.2933 |
| West (N=19,570) | 11,800, 60.3% | 0.94 | 0.91 | 0.98 | 0.0030 |
| North Central (N=32,182) | 19,934, 61.2% | Ref. | |||
| Residence | |||||
| Urban (MSA) (N=98,879) | 61,218, 61.9% | 0.87 | 0.84 | 0.91 | <.0001 |
| Rural (non-MSA) (N=12,407) | 4,228, 65.9% | Ref. | |||
| Health insurance plan | |||||
| CDHP (N=5,291) | 3,212, 60.7% | 1.01 | 0.95 | 1.08 | 0.7528 |
| Comprehensive (N=1,053) | 680, 64.6% | 1.14 | 0.99 | 1.30 | 0.0513 |
| EPO (N=2,183) | 1,326, 60.7% | 1.02 | 0.92 | 1.11 | 0.7423 |
| HDHP (N=2,545) | 1,581, 62.1% | 1.07 | 0.98 | 1.17 | 0.1263 |
| POS (N=8,258) | 5,114, 61.9% | 1.02 | 0.96 | 1.07 | 0.5530 |
| POS capitation (N=815) | 527, 64.7% | 1.09 | 0.94 | 1.27 | 0.2434 |
| PPO (N=71,703) | 45,030, 62.8% | 1.05 | 1.02 | 1.09 | 0.0040 |
| HMO (N=17,110) | 10,469, 61.2% | Ref. | |||
| Type of provider who administered the 1st dose | |||||
| Pediatric (N=46,344) | 26,243, 56.6% | 0.74 | 0.72 | 0.77 | <.0001 |
| OB/GYN (N=18,006) | 13,207, 73.4% | 1.41 | 1.34 | 1.48 | <.0001 |
| Others (N=31,101) | 15,755, 63.2% | 0.96 | 0.92 | 0.99 | 0.0402 |
| Primary care (N=15,835) | 10,203, 64.4% | Ref. | |||
| Quarter of the year at the 1st dose | |||||
| Jan–Mar (N=25,894) | 17,429, 67.3% | 1.30 | 1.25 | 1.36 | <.0001 |
| Apr–Jun (N=31,671) | 20,831, 65.8% | 1.26 | 1.21 | 1.31 | <.0001 |
| Jul–Sep (N=36,423) | 20,682, 56.8% | 0.89 | 0.86 | 0.92 | <.0001 |
| Oct–Dec (N=17,298) | 10,455, 60.4% | Ref. | |||
| Flu shot | |||||
| Yes (N=46,847) | 27,686, 59.1% | 0.92 | 0.90 | 0.95 | <.0001 |
| No (N=64,439) | 41,711, 64.7% | Ref. | |||
Abbreviations: HPV: human papillomavirus; MSA: Metropolitan Statistical Area; CDHP: consumer-driven health plan; EPO: Exclusive provider organizations; HDHP: high-deductible health plan; POS: point of service; PPO: Preferred provider organizations; HMO: Health maintenance organizations; OB/GYN: obstetrics and gynecology; OR: odds ratio; CI: confidence interval.
Discussion
HPV vaccine completion
Using a large national claims data we found that among commercially insured females who initiated HPV vaccination, only about 30% had actually completed the 3-dose vaccine series within 12 months during 2009-2011. Even among adolescent girls aged 13 through 15 years, the complete rate was only 29.3%. In other studies HPV vaccine completion rates widely ranged from 22% to 75% among HPV vaccine initiators because of different study populations, sampling frames and study periods [15], [25], [26], [27], [28], [29], [30], [31]. The completion rate in our study was comparable with the rate (22%) observed by Dunne et al. using the same data source [30], but was much lower than the rates reported from national surveys [15], [25], [29]. As we used more strict criteria for selecting the study cohort and for defining HPV vaccine completion, it is possible that the completion rate was underestimated in our study if some females received the 2nd and/or the 3rd dose(s) after 12 months of initiation. Consistent with previous research, we found that age was significantly associated with HPV vaccine completion; pre-adolescent girls aged 9–12 years had the highest vaccine dosage completion rate, while young adult women aged 18–21 had the lowest rate [27], [32]. The age distribution also showed a delay in initiating HPV vaccination, with only a quarter of vaccinated females received their first doses at age 9–12.
Variation in HPV vaccine completion across health insurance plans was possibly due to different reimbursement policies for HPV vaccines. Previous studies have reported that inadequate insurance reimbursement as a major barrier for providers to offer HPV vaccination, resulting in a lower vaccine completion rate in eligible female patients [33], [34], [35]. However, in our study we did not observe significant effects of medical expense (total and net costs) per HPV vaccination on the completion or adherence rate (data was not shown because of very small differences). We also observed a great geographic variation in HPV vaccination, particularly indicated by the lower vaccine completion rate in South and West regions. Along with the variation in HPV vaccine completion by health insurance plan, it was likely that different health insurance policies across the states may have partly contributed to this geographic variation in HPV vaccination. Interestingly the completion rate remains barely over 20% among commercially insured adolescent girls in Virginia (21.8%) and the District of Columbia (15.7%), in which HPV vaccination is mandatory for middle school students [36].
When examining the completion rate by provider type, we found that compared with females who initiated vaccination from primary care providers, those receiving their first doses from OB/GYN providers were more likely to complete the vaccine series; while those who received vaccines from pediatricians were significantly less likely to complete HPV vaccination [27]. As numerous studies have concluded that provider recommendations play an important role in promoting HPV vaccine uptake [34], [35], [37], [38], [39], [40], [41], [42], OB/GYN providers may have better knowledge and expertize in HPV-related diseases and HPV vaccination, therefore, they may be more likely to remind patients to complete their vaccine series. However, previous studies have found inconsistent results regarding the association between pediatric providers and HPV vaccine completion: some studies showed that patients of pediatric providers were more likely to complete the vaccine than those of general medicine providers, while some studies indicated lower HPV vaccine completion in patients seen by pediatricians [20], [26], [43].
We used seasonal flu vaccination as an indicator for healthcare-seeking behavior. As our results showed that females who received the flu vaccine were more likely to complete HPV vaccine series, it implies that individual’s behaviors toward preventive health services can influence HPV vaccination [44]. Additionally, providers may use flu vaccination as an opportunity to encourage patients to complete their HPV vaccine series. We also observed an interesting seasonal pattern in HPV vaccine completion: females who initiated the vaccination during the 2nd quarter of the year (April–June) were more likely to complete the vaccine series. This seasonal variation was not due to loss to follow up or changes in health insurance plans as all females were continuously enrolled for 12 months or longer. It was possible that flu vaccination during October–December may help physicians or patients to remember HPV vaccination, but more studies will need to be conducted to understand factors contributing to this seasonal pattern.
Adherence of recommended HPV vaccine dose schedules
Our study also provided new findings on the adherence of HPV vaccine dose schedules. Among females who have completed HPV vaccination, about 60% (18% in total vaccine-eligible females) have received the 2nd and 3rd doses no later than 30 days after the recommended intervals. A delay in the 3rd dose is more common, possibly because of the prolonged vaccine interval – 6 months after the first dose. Consistent with findings in HPV vaccine completion, females who were covered by consumer-driven health plan (CDHP) or PPO, initiated the vaccination from OB/GYN providers, or received the 1st dose in the first 2 quarters of the year were more likely to follow the vaccine schedules, while those receiving HPV vaccination from pediatricians were significantly less likely to complete the vaccine series within the 6-month interval. Interestingly, unlike in HPV vaccine completion, younger age (<22 years) and receipt of flu vaccine were negatively related to adherence of HPV vaccine schedules. These results could be due to some logistic barriers for younger females or their parents (e.g., school attendance, transportation, or inconvenient schedules for parents), or the possibility that people may wait to combine HPV vaccine with flu vaccine to reduce the number of visits.
The recommended HPV doses and schedules are based on immunogenicity and vaccine efficacy from clinical trials. New evidence suggests that the reduced doses (2-dose vaccines) or prolonged schedules (at 0 and 12 months) may be as efficient as the current 3-dose schedules, but would result in lower costs and better adherence [24], [45], [46]. While flexible dose schedules could be important for improving national HPV vaccination, in our study we detected that a delay for the 3rd dose was common. Thus, efforts will need to focus on establishing or improving the reminding system for HPV vaccination, increasing awareness of HPV vaccine schedules for patients (or parents of pre- and adolescent girls), and reducing logistic barriers for both patients and providers [47], [48], [49]. Special attention should also be given to females aged 18–21 as they have lower rates of completion and adherence of HPV vaccination.
Study limitations
Several caveats need to be discussed when interpreting our study results. First, although the MarketScan CCE database annually includes over 50 million people covered by commercial insurance, the study population is not representative of the US general population. We also excluded females without at least 12-month continuous enrollment, those with >3 HPV vaccine claims or those with HPV vaccine claim(s) in 2008. Therefore, our study findings may not be applicable to other populations, especially underinsured or underserved females. Second, as MarketScan CCE is a claims database, limited information was available to better evaluate possible barriers for HPV vaccination. Third, as we examined HPV vaccine completion within 12 months, we may miss some females who received HPV vaccine doses over the 12-month period. That we chose the 12-month period was based on non-inferiority immunogenicity between the original HPV vaccine schedule and the alternative vaccine schedule at 0, 6, and 12 months [24]. In addition, we focused on the delayed HPV vaccine dose schedules as a study outcome and did not assess early HPV vaccination (i.e., receiving the 2nd and 3rd doses earlier than the recommended schedules, accounting for about 2% of total eligible females). Despite of these limitations, our study still provides new and important results that can be utilized to improve HPV vaccination in the US.
In conclusion, HPV vaccination in the US remains well below the optimal goal. The reasons for non-completion or non-adherence of HPV vaccine series are multifactorial. In order to increase HPV vaccine uptake in the US, intervention programs to improve HPV vaccine reminding system and reduce logistic barriers for both physicians and patients are warranted.
Conflict of interest
None reported.
Author contribution
GL contributed toward the conception and design of the study, acquisition and analysis of data, drafting the manuscript and approving the final version. LK contributed toward the design of the study and analysis of data, drafting the manuscript and approving the final version. PD contributed toward the conception and design of the study, interpretation of data, drafting the manuscript, and final approval of the version to be submitted.
References
- 1.Satterwhite C.L., Torrone E., Meites E., Dunne E.F., Mahajan R., Ocfemia M.C. Sexually transmitted infections among us women and men: prevalence and incidence estimates, 2008. Sex Transm. Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.de Martel C., Ferlay J., Franceschi S., Vignat J., Bray F., Forman D. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Human papillomavirus-associated cancers-United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–261. [PubMed] [Google Scholar]
- 4.Chesson H.W., Ekwueme D.U., Saraiya M., Watson M., Lowy D.R., Markowitz L.E. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30:6016–6019. doi: 10.1016/j.vaccine.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr E., Sings H.L. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine. 2008;26:6244–6257. doi: 10.1016/j.vaccine.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 6.Kane M.A. Preventing cancer with vaccines: progress in the global control of cancer. Cancer Prev. Res. (Phila) 2012;5:24–29. doi: 10.1158/1940-6207.CAPR-11-0533. [DOI] [PubMed] [Google Scholar]
- 7.Van Kriekinge G., Castellsague X., Cibula D., Demarteau N. Estimation of the potential overall impact of human papillomavirus vaccination on cervical cancer cases and deaths. Vaccine. 2014;32:733–739. doi: 10.1016/j.vaccine.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz L.E., Dunne E.F., Saraiya M., Chesson H.W., Curtis C.R., Gee J. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 9.Markowitz L.E., Hariri S., Lin C., Dunne E.F., Steinau M., McQuillan G. Reduction in Human Papillomavirus (HPV) prevalence among young women following hpv vaccine introduction in the United States, national health and nutrition examination surveys, 2003–2010. J. Infect. Dis. 2013;208:385–393. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 10.Gee J., Naleway A., Shui I., Baggs J., Yin R., Li R. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the vaccine safety datalink. Vaccine. 2011;29:8279–8284. doi: 10.1016/j.vaccine.2011.08.106. [DOI] [PubMed] [Google Scholar]
- 11.Slade B.A., Leidel L., Vellozzi C., Woo E.J., Hua W., Sutherland A. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. Jama. 2009;302:750–757. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. Immunization and Infectious Diseases. IID–11.4 3 doses Human papillomavirus vaccine (HPV) for females by age 13 to 15 years. In. Washington, DC; 2012.
- 13.Stokley S., Jeyarajah J., Yankey D., Cano M., Gee J., Roark J., et al. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014--United States. MMWR Morb Mortal Wkly Rep. 2014,63:620-624. [PMC free article] [PubMed]
- 14.Williams W.W., Lu P.J., O׳Halloran A., Bridges C.B., Pilishvili T., Hales C.M. Noninfluenza vaccination coverage among adults-United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63:95–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Reagan-Steiner S., Yankey D., Jeyarajah J., Elam-Evans L.D., Singleton J.A., Curtis C.R. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years-United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The President’s Cancer Panel. Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer. A Report to the President of the United States from the President’s Cancer Panel. In. Bethesda, MD: National Cancer Institute; 2014
- 17.Wilson R.M., Brown D.R., Carmody D.P., Fogarty S. HPV vaccination completion and compliance with recommended dosing intervals among female and male adolescents in an inner-city community health center. J. Community Health. 2015;40:395–403. doi: 10.1007/s10900-014-9950-7. [DOI] [PubMed] [Google Scholar]
- 18.Dorell C.G., Stokley S., Yankey D., Markowitz L.E. Compliance with recommended dosing intervals for HPV vaccination among females, 13–17 years, National Immunization Survey-Teen, 2008–2009. Vaccine. 2012;30:503–505. doi: 10.1016/j.vaccine.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Harper D.M., Verdenius I., Harris G.D., Barnett A.L., Rosemergey B.E., Arey A.M. The influence of free quadrivalent human papillomavirus vaccine (HPV4) on the timely completion of the three dose series. Prev. Med. 2014;61:20–25. doi: 10.1016/j.ypmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Tan W., Viera A.J., Rowe-West B., Grimshaw A., Quinn B., Walter E.B. The HPV vaccine: are dosing recommendations being followed? Vaccine. 2011;29:2548–2554. doi: 10.1016/j.vaccine.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 21.Verdenius I., Harper D.M., Harris G.D., Griffith R.S., Wall J., Hempstead L.K. Predictors of three dose on-time compliance with HPV4 vaccination in a disadvantaged, underserved, safety net population in the US Midwest. PLoS One. 2013;8:e71295. doi: 10.1371/journal.pone.0071295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widdice L.E., Bernstein D.I., Leonard A.C., Marsolo K.A., Kahn J.A. Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics. 2011;127:77–84. doi: 10.1542/peds.2010-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomson Reuters (Healthcare) Inc. MarketScan Commercial Claims and Encounters Database and the Medicare Supplemental Database. In.
- 24.Stanley M.A., Sudenga S.L., Giuliano A.R. Alternative dosage schedules with HPV virus-like particle vaccines. Expert Rev. Vaccines. 2014;13:1027–1038. doi: 10.1586/14760584.2014.935767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M., Laz T.H., McGrath C.J., Berenson A.B. Correlates of human papillomavirus vaccine series completion among young adult female initiators. Hum. Vaccin Immunother. 2014;10:2163–2167. doi: 10.4161/hv.29633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao C., Velicer C., Slezak J.M., Jacobsen S.J. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am. J. Epidemiol. 2010;171:357–367. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirth J.M., Tan A., Wilkinson G.S., Berenson A.B. Completion of the human papillomavirus vaccine series among insured females between 2006 and 2009. Cancer. 2012;118:5623–5629. doi: 10.1002/cncr.27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt M.A., Gold R., Kurosky S.K., Daley M.F., Irving S.A., Gee J. Uptake, coverage, and completion of quadrivalent human papillomavirus vaccine in the vaccine safety Datalink, July 2006–June 2011. J. Adolesc. Health. 2013;53:637–641. doi: 10.1016/j.jadohealth.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laz T.H., Rahman M., Berenson A.B. Human papillomavirus vaccine uptake among 18- to 26-year-old women in the United States: National Health Interview Survey, 2010. Cancer. 2013;119:1386–1392. doi: 10.1002/cncr.27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunne E.F., Stokley S., Chen W., Zhou F. Human papillomavirus vaccination of females in a large health claims database in the United States, 2006–2012. J. Adolesc. Health. 2015;56:408–413. doi: 10.1016/j.jadohealth.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du P., Camacho F., McCall-Hosenfeld J., Lengerich E., Meyers C.M., Christensen N.D. Human papillomavirus vaccination among adults and children in 5 US States. J. Public Health Manag. Pract. 2015;1:1. doi: 10.1097/PHH.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold R., Naleway A., Riedlinger K. Factors predicting completion of the human papillomavirus vaccine series. J. Adolesc. Health. 2013;52:427–432. doi: 10.1016/j.jadohealth.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Young J.L., Bernheim R.G., Korte J.E., Stoler M.H., Guterbock T.M., Rice L.W. Human papillomavirus vaccination recommendation may be linked to reimbursement: a survey of Virginia family practitioners and gynecologists. J. Pediatr. Adolesc. Gynecol. 2011;24:380–385. doi: 10.1016/j.jpag.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Holman D.M., Benard V., Roland K.B., Watson M., Liddon N., Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vadaparampil S.T., Malo T.L., Kahn J.A., Salmon D.A., Lee J.H., Quinn G.P. Physicians׳ human papillomavirus vaccine recommendations, 2009 and 2011. Am. J. Prev. Med. 2014;46:80–84. doi: 10.1016/j.amepre.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Conference of State Legislatures. HPV Vaccine: State Legislation and Statutes. In; 2012.
- 37.Dorell C.G., Yankey D., Santibanez T.A., Markowitz L.E. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics. 2011;128:830–839. doi: 10.1542/peds.2011-0950. [DOI] [PubMed] [Google Scholar]
- 38.Rosenthal S.L., Weiss T.W., Zimet G.D., Ma L., Good M.B., Vichnin M.D. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician׳s recommendation. Vaccine. 2011;29:890–895. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 39.Lau M., Lin H., Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children׳s Health. Vaccine. 2012;30:3112–3118. doi: 10.1016/j.vaccine.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Polonijo A.N., Carpiano R.M. Social inequalities in adolescent human papillomavirus (HPV) vaccination: a test of fundamental cause theory. Soc. Sci. Med. 2013;82:115–125. doi: 10.1016/j.socscimed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Ylitalo K.R., Lee H., Mehta N.K. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am. J. Public Health. 2013;103:164–169. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahman M., Laz T.H., McGrath C.J., Berenson A.B. Provider recommendation mediates the relationship between parental human papillomavirus (HPV) vaccine awareness and HPV vaccine initiation and completion among 13- to 17-year-old U.S. adolescent children. Clin. Pediatr. (Phila) 2015;54:371–375. doi: 10.1177/0009922814551135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen-Gunther J., Shank J.J., Ta V., Gardasil H.P.V. vaccination: surveillance of vaccine usage and adherence in a military population. Gynecol. Oncol. 2011;123:272–277. doi: 10.1016/j.ygyno.2011.07.094. [DOI] [PubMed] [Google Scholar]
- 44.Laz T.H., Rahman M., Berenson A.B. An update on human papillomavirus vaccine uptake among 11–17 year old girls in the United States: National Health Interview Survey, 2010. Vaccine. 2012;30:3534–3540. doi: 10.1016/j.vaccine.2012.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romanowski B., Schwarz T.F., Ferguson L.M., Ferguson M., Peters K., Dionne M. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum. Vaccin. Immunother. 2014;10:1155–1165. doi: 10.4161/hv.28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazcano-Ponce E., Stanley M., Munoz N., Torres L., Cruz-Valdez A., Salmeron J. Overcoming barriers to HPV vaccination: non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months. Vaccine. 2014;32:725–732. doi: 10.1016/j.vaccine.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 47.Patel A., Stern L., Unger Z., Debevec E., Roston A., Hanover R. Staying on track: a cluster randomized controlled trial of automated reminders aimed at increasing human papillomavirus vaccine completion. Vaccine. 2014;32:2428–2433. doi: 10.1016/j.vaccine.2014.02.095. [DOI] [PubMed] [Google Scholar]
- 48.Etter D.J., Zimet G.D., Rickert V.I. Human papillomavirus vaccine in adolescent women: a 2012 update. Curr. Opin. Obstet Gynecol. 2012;24:305–310. doi: 10.1097/GCO.0b013e3283567005. [DOI] [PubMed] [Google Scholar]
- 49.Cassidy B., Braxter B., Charron-Prochownik D., Schlenk E.A. A quality improvement initiative to increase HPV vaccine rates using an educational and reminder strategy with parents of preteen girls. J. Pediatr. Health Care. 2014;28:155–164. doi: 10.1016/j.pedhc.2013.01.002. [DOI] [PubMed] [Google Scholar]