Abstract
Importance
After chemoradiation for head and neck cancer, over ninety percent of patients who achieved a complete response by imaging were regionally controlled without post-radiotherapy neck dissections (PRND). Since several groups have reported that lymph node involvement also predicted failure at both primary and distant sites, it remains unclear the extent to which PRND impacts non-regional sites of disease.
Objective
Here, we evaluated how PRND impacted local and distant control in patients who achieved a clinical complete response.
Design
We retrospectively reviewed patients treated for stage III/IV disease with definitive chemoradiation between 1990 to 2012.
Setting
University of Illinois at Chicago.
Participants
287 patients were treated with definitive CRT, of whom seventy-four underwent PRND. Median follow up was 25.4 months.
Interventions
Chemoradiation followed by lymph node dissection or observation.
Main Outcomes and Measures
Endpoints evaluated included local control (LC), regional control (RC), freedom from distant metastasis (FFDM), progression free survival (PFS) and overall survival (OS) using first-failure analysis.
Results
Patients with advanced nodal disease (≥N2b; n=176) had improved PFS (74.6% vs. 39.1%; P<.001) while patients with lesser nodal disease had similar PFS. For patients with advanced nodal disease, PRND improved 2-year LC (85.5 vs. 53.5%; p<.001), locoregional control with PRND (78.9% vs. 45.7%; P<.001), FFDM (79.5% vs. 67.5%%; P=.03) and OS (84.5% vs. 61.7%; P=.004) but not RC (96.9% vs. 90.1%; P=.21) The benefit in LC (87.4% vs. 66.2%; P=.02) and PFS (80.7% vs. 53.4%; P=.01) persisted for those with negative post-treatment imaging who underwent PRND. On univariate analysis, PRND, alcohol use, nodal stage and chemoradiation significantly impacted 2 year LC and/or PFS. On multivariate analysis, PRND remained strongly prognostic for 2 year LC (HR 0.22; P=.0007) and PFS (HR 0.42; P=.002).
Conclusions and Relevance
PRND improved control of non-regional sites of disease in patients with advanced nodal disease who achieved a complete response after chemoradiation. Thus, PRND may impact the control of non-nodal sites through possible mechanisms such as clearance of incompetent lymphatics and prevention of re-seeding of the primary and distant sites.
Trial Registration
None.
INTRODUCTION
In squamous cell cancer of the head and neck (HNSCC), lymph node positivity correlates with worse progression free survival (PFS) and overall survival (OS) 1, 2. Traditionally, patients with nodal disease at presentation or with residual palpable neck disease after definitive therapy have been considered for post-radiotherapy neck dissection (PRND) 3, 4. As attempts are made to reduce treatment-related morbidity, the continued necessity of PRND has been questioned. Recently, several reports have used computed tomography (CT) with or without positron emission tomography (PET-CT) to select patients who can avoid PRND 3, 5–15. However, these imaging studies assumed that PRND only impacted persistent disease in the nodal basins as measured by pathological findings or subsequent regional control (RC) rates. In detecting residual cancer cells in surgical specimens, CT and PET-CT had a negative predictive value (NPV) between 86–100% and 95–100%, respectively, 3, 6, 12, 13, 15–20. Other reports demonstrated that a complete response on PET-CT or CT (cCR) predicted for RC in 85–100% of patients 5, 7, 9, 13, 21. Thus, patients achieving a cCR by imaging may avoid PRND after definitive chemoradiation (CRT) with little detriment to regional control.
However, involved cervical lymph nodes have also predicted for worse local (LC) and distant control (DC). For example, in patients treated with definitive surgery, Leemans et al. demonstrated that 3 or more involved nodes increased local recurrence by 10% 22. Furthermore, for patients undergoing definitive radiotherapy, involved lymph nodes predicted for worse LC across multiple head and neck sites 23–25. Regarding DC, three or more involved nodes correlated with a 4.5-fold increase in distant metastases 2. Therefore, it remains unclear whether clearance of lymph node metastasis may also impact local or distant disease control.
Here, we examined the impact of lymph node dissection in patients with locally advanced HNSCC. For patients with N2b or greater nodal involvement, we find that PRND improved LC and PFS in patients achieving a cCR with CRT. Thus, these findings are hypothesis generating in that clearance of involved lymphatics may improve outcomes at non-regional disease sites.
METHODS
Eligible study population
Between 1990 and 2012, 457 patients with head and neck cancer and without distant metastatic disease were treated with definitive radiotherapy (RT) at the University of Illinois at Chicago Medical Center (UIMC), including 356 patients with locally advanced HNSCC. We excluded 11 patients who had persistent disease after radiotherapy, 13 patients with a very-high Charlson26 comorbidity index which may impact disease control and 45 patients who had follow-up times less than one year and were without evidence of disease. A total of 287 patients were used for analysis. Data was collected in accordance with The University of Illinois at Chicago Institutional Review Board guidelines (protocol 2011–1075). A single physician (MTS) collected all patient data from available physical and electronic medical records. Patients were staged according to the American Joint Committee on Cancer staging system at the time of diagnosis.
Workup, Treatment and Follow-up
In general, patients underwent history and physical examination, endoscopic evaluation of primary tumor, dental evaluations and CT of the head, neck, and chest. All patients received radiotherapy as a component of their care, and over 93.0% of patients received CRT. Follow-up data was acquired from visits within any UIMC department. Post-treatment imaging (PTI) consisted of CT imaging of the head and neck obtained at a median of 6 weeks post-radiotherapy. Patients were then followed every 2–4 months as per the clinic routine. PET-CT was not uniformly employed and was not analyzed in this report. Positive imaging findings on CT were defined as residual lymphadenopathy, extracapsular spread or residual disease at the primary site. Residual local disease was determined by imaging, physical exam and direct laryngoscopy. Work-up of potentially recurrent disease was ordered at the discretion of the treating physician. The incidence of late treatment-related morbidity was recorded at subsequent follow up visits.
Definitions
We divided the decision to proceed with PRND into four categories: (1) “per protocol” where the patient had a cCR but underwent PRND due to advanced nodal disease at presentation; (2) “clinical suspicion despite cCR” where a cCR was documented in radiographic reports but a PRND was performed due to clinical suspicion; (3) “residual disease” in which the surgeon operated due to radiographic evidence of residual disease; and (4) “not stated”. We divided the decision not to proceed with PRND into 7 categories: (1) “surveillance” in which the patient achieved a cCR; (2) “non-compliance” in which the patient refused a recommended PRND; (3) “N0-N2a” nodal disease; (4) “comorbidity” that precluded surgery; (5) “myocardial infarction (MI) during/after RT” that precluded surgery; (6) “failure-to-thrive (FTT) after RT” that precluded surgery and (7) “not stated”. Patient comorbidity burden was approximated using a modified Charlson Comorbidity Index 26. Time to events for LC, RC, locoregional control (LRC), freedom from distant metastasis (FFDM), PFS and OS were determined from the last day of radiotherapy. Patterns of local, regional, or distant failure were documented as sites of first failure. RC was calculated for regional only failure. LC and FFDM were determined as failure with any component of local or distant failure, respectively. Of the 176 patients with N2b or greater nodal disease, 9 received radiotherapy alone, 18 received induction chemotherapy followed by radiotherapy and 149 received concurrent chemoradiation. For the patients receiving concurrent chemoradiation, 69 received taxol, hydroxyurea and 5-fluoruracil with radiation alternating in a week-on/week-off schedule, 58 received platinum based chemotherapy and 22 received other systemic agents.
Statistical analysis
Statistical analysis was performed using JMP version 9 (SAS Institute). All tests of statistical significance were two-sided, and significance was defined as p < 0.05. To compare differences between groups, chi-squared test was used for discreet variables and t-test for continuous variables. Survival estimates were obtained using the Kaplan-Meier method. All events were calculated using standard life table methods, and the differences were compared using Cox regression models. Positive predictive values, negative predictive values and accuracy of PTI were assessed in PRND patients regardless of length of follow-up (n=83). Survival analysis was performed for both Stage III–IVB patients and those with advanced nodal disease at presentation (defined as N2b or greater). Cox multivariable analysis (MVA) was performed to adjust for explanatory confounding prognostic factors including all variables with at least borderline significance on univariate analysis (UVA; p<.10) and those that are known to impact outcomes in HNSCC.
RESULTS
Patient, tumor and treatment characteristics
Median follow-up was 25.4 months for all patients. The median time to post-treatment imaging (PTI) was 5.0 weeks for those that underwent PRND and 6.9 weeks for those that did not (P=.0002). Baseline characteristics for those with advanced stage HNSCC are included in Table 1. In general, groups were balanced in terms of gender, KPS, comorbidities and alcohol or tobacco use. Patients who underwent PRND were younger (53.3 vs. 56.9 years, P=.04), had more advanced disease (stage IVA/B: 91.9% vs. 77.4%; P=.01) and had more oropharyngeal (46.0% vs. 29.1%; P=.007) and fewer laryngeal primaries (16.2% vs. 20.7%). Patients undergoing PRND also had more advanced nodal disease at presentation (P<.0001), but less locally advanced primary tumor stage (P=.05). CRT, either induction or concurrently, was employed more frequently in the PRND group (98.7% vs. 93.0%, P=.04). Finally, radiotherapy delays were more frequent in non-PRND patients.
TABLE 1.
Patient Characteristics (n = 287)
| Post-Radiotherapy Lymph Node Dissection (n = 74) | No Post-Radiotherapy Lymph Node Dissection (n = 213) | P value* | |
|---|---|---|---|
| Median age in years (IQR) | 53.3 (47.9 – 60.4) | 56.9 (48.9 – 64.4) | .04 |
|
| |||
| Median follow-up in months (IQR) | 35.6 (18.5 – 71.9) | 18.9 (10.1 – 52.1) | .0002 |
|
| |||
| Gender | .22 | ||
| Male | 60 (81.1%) | 150 (74.2%) | |
| Female | 14 (18.9%) | 55 (25.8%) | |
|
| |||
| KPS | .34 | ||
| ≥ 70 | 61 (82.4%) | 165 (77.5%) | |
| < 70 | 3 (17.7%) | 14 (6.6%) | |
| Not stated | 10 (13.5%) | 34 (16.0%) | |
|
| |||
| Comorbidity Score | .34 | ||
| Medium | 57 (77.0%) | 152 (71.4%) | |
| High | 17 (23.0%) | 61 (28.6%) | |
|
| |||
| Stage | .01 | ||
| III | 6 (8.1%) | 48 (22.5%) | |
| IVA | 50 (67.6%) | 127 (59.6%) | |
| IVB | 18 (24.3%) | 38 (17.8%) | |
|
| |||
| Alcohol history | .24 | ||
| ≥ 2 drinks/day | 47 (63.5%) | 133 (62.4%) | |
| < 2 drinks/day | 18 (24.3%) | 34 (16.0%) | |
| Not stated | 9 (12.2%) | 46 (21.6%) | |
|
| |||
| Tobacco history | .44 | ||
| Yes (> 10 pack-years) | 59 (79.7%) | 161 (75.6%) | |
| No (≤ 10 pack-years) | 12 (16.2%) | 43 (20.2%) | |
| Not stated | 3 (4.1%) | 9 (4.2%) | |
|
| |||
| Primary site | .007 | ||
| Hypopharynx | 11 (14.9%) | 21 (9.9%) | |
| Larynx | 12 (16.2%) | 44 (20.7%) | |
| Nasopharynx | 1 (1.4%) | 22 (10.3%) | |
| Oral Cavity | 12 (17.5%) | 45 (21.1%) | |
| Oropharynx | 34 (46.0%) | 62 (29.1%) | |
| Other | 3 (4.1%) | 19 (8.9%) | |
|
| |||
| Tumor grade | .34 | ||
| Well Differentiated | 8 (10.8%) | 29 (13.6%) | |
| Mod. Differentiated | 42 (56.8%) | 104 (48.8%) | |
| Poorly Differentiated | 17 (23.0%) | 50 (23.5%) | |
| Not stated | 7 (9.5%) | 30 (14.1%) | |
|
| |||
| Tumor Stage | .05 | ||
| T1–2 | 24 (32.4%) | 56 (21.1%) | |
| T3–4 | 50 (67.6%) | 168 (78.9%) | |
|
| |||
| Nodal Stage | <.0001 | ||
| N0-2a | 10 (13.5%) | 101 (47.4%) | |
| N2b-3 | 64 (86.5%) | 112 (52.6%) | |
|
| |||
| Node levels IV/V/SCV involved | .05 | ||
| Yes | 27 (36.5%) | 52 (24.4%) | |
| No | 47 (63.5%) | 161 (75.6%) | |
| CRT | .04 | ||
| Yes | 73 (98.7%) | 198 (93.0%) | |
| No | 1 (1.4%) | 15 (7.0%) | |
| Induction | <.0001 | ||
| Yes | 53 (71.6%) | 97 (45.5%) | |
| No | 21 (28.4%) | 116 (54.5%) | |
| Concurrent | .008 | ||
| Yes | 69 (93.2%) | 173 (81.2%) | |
| No | 5 (6.8%) | 40 (18.8%) | |
|
| |||
| Intensity modulated radiotherapy | .53 | ||
| Yes | 42 (56.8%) | 112 (52.6%) | |
| No | 37 (43.2%) | 101 (47.4%) | |
|
| |||
| Radiotherapy delay >5 days | .006 | ||
| No | 59 (79.7%) | 128 (60.1%) | |
| Yes | 13 (17.6%) | 70 (32.9%) | |
| Truncated treatment course | 2 (2.7%) | 15 (7.0%) | |
|
| |||
| Extent of LND | N.A. | ||
| Radical LND | 3 (4.1%) | N.A. | |
| Modified radical LND | 35 (47.3%) | N.A. | |
| Selective LND | 31 (41.9%) | N.A. | |
| Lymph node biopsy | 1 (1.4%) | N.A. | |
| Not stated | 4 (5.4%) | N.A. | |
|
| |||
| Reasons for LND | N.A. | ||
| Per protocol | 33 (44.6%) | N.A. | |
| Clinical suspicion despite cCR | 20 (27.0%) | N.A. | |
| Residual disease on imaging | 10 (13.5%) | N.A. | |
| Not stated | 11 (14.9%) | N.A. | |
|
| |||
| Reasons for no LND | N.A. | ||
| Surveillance | N.A. | 40 (18.8%) | |
| Non-compliance | N.A. | 13 (6.1%) | |
| N0-N2a | N.A. | 101 (47.4%) | |
| Comorbidity | N.A. | 5 (2.3%) | |
| MI during/after RT | N.A. | 2 (0.9%) | |
| FTT after RT | N.A. | 1 (0.4%) | |
| Not stated | N.A. | 51 (23.9%) | |
For analysis of categorical variables, a two-sided chi-square test was used. For analysis of medians, a Wilcoxon/Kruskal-Wallis test was used.
IQR = Interquartile ratio.
KPS = Karnofsky Performance Status.
LND = Lymph node dissection
cCR = Complete clinical response
FTT = Failure to thrive.
MI = Myocardial infarction.
N.A. = Not applicable.
Post-radiotherapy imaging provides high NPV
Of the 74 patients undergoing PRND, 89.2% of patients underwent a modified radical neck dissection or a selective neck dissection. For patients undergoing a selective neck dissection, the most common nodal levels removed were levels II–III (n=8), followed levels I–III (n=6) followed by II–IV (n=4). The most common reason to proceed with PRND was per protocol (44.6%; n=33) where patients had a cCR by imaging and physical exam. The most common reason for patients with N2b or greater nodal disease to avoid PRND was for surveillance. A median of 16.5 (interquartile ratio of 9 to 24) nodes were resected. Twenty-three patients had residual disease, with a median of 1 involved node (range 1–8). Nine patients had multiple involved nodes and four had greater than 3 cm of residual disease. For all patients with radiographic evidence of disease on PTI, 21 and 11 patients had pathological positive and negative lymph nodes on PRND, respectively. For all patients without radiographic evidence of disease on PTI, 4 and 47 patients had pathological positive and negative lymph nodes on PRND, respectively. For all patients, the PTI positive predictive value was 63.6%, the negative predictive value was 92.2% and the accuracy was 81.9%. For N2b or greater patients with radiographic evidence of disease on PTI, 18 and 8 patients had pathological positive and negative lymph nodes on PRND, respectively. For N2b or greater patients without radiographic evidence of disease on PTI, 4 and 41 patients had pathological positive and negative lymph nodes on PRND, respectively. For patients with N2b or greater nodal disease, the PTI positive predictive value was 69.2%, the negative predictive value was 91.1% and the accuracy was 83.0%.
Disease Control
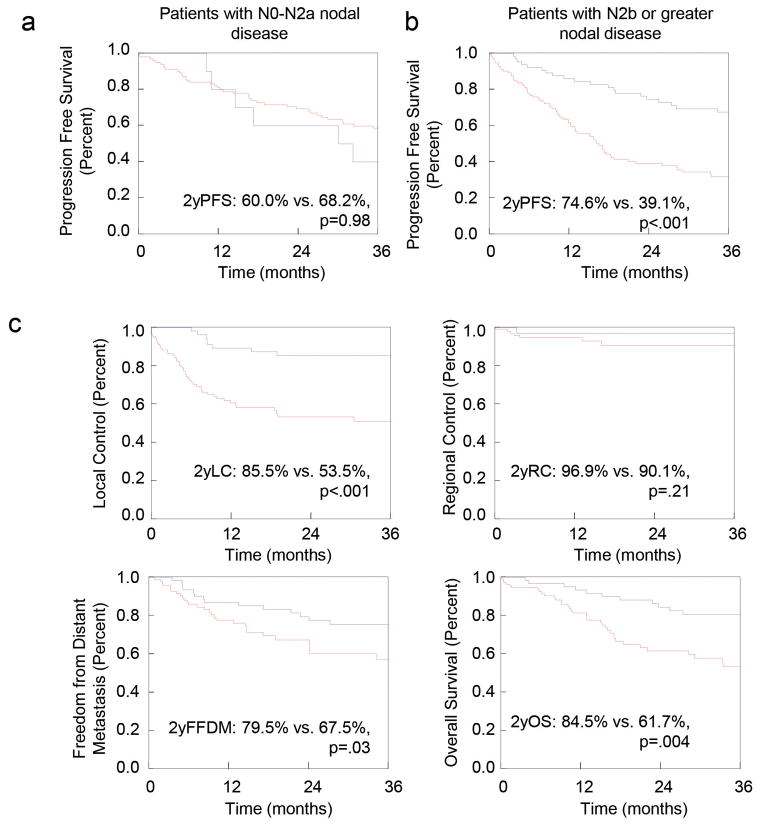
Patients with advanced nodal disease (N2b or higher; n=176) experienced improved PFS with PRND (74.6% vs. 39.1%; P<.0001), whereas those with N2a or less nodal disease did not (60.0% vs. 68.2%; P=.98; Figure 1). Patients with N2b or higher nodal disease undergoing PRND had superior LC (85.5% vs. 53.5%, P<.001), FFDM (79.5% vs. 67.5%; P=.03), and LRC (78.9% vs. 45.7%; P<.001) and OS (84.5% vs. 61.7%; P=.004) but similar RC. The benefit for PRND persisted for those with negative post-treatment scans (n=103) despite the high NPV in our dataset (Figure 2). In patients with a cCR by PTI, PRND continued to be associated with improved LC (87.4% vs. 66.2%; P=.02) and PFS (80.7% vs. 53.4%; P=.01). As shown in Figure 3, the impact of PRND on LC and/or PFS persisted across multiple disease sites including the hypopharynx (2y PFS: 63.6% vs. 18.5%; P=.02); larynx (2y PFS: 88.9% vs. 51.3%; P=.03), oral cavity (2y LC: 80.0% vs. 35.6%; P=.01) and oropharynx (2y OS: 88.5% vs. 35.6%; P=.01 and 2y PFS: 85.2% vs. 38.5%; P=.001).
Figure 1. Outcomes in Stage III–IV patients undergoing PRND.
Progression-free Survival for patients with (a) limited nodal disease (N0-N2a) and those with (b) advanced nodal disease (N2b-N3). (c) Local control, Regional Control, Distant Control and Overall Survival for those with N2b or greater nodal disease. The blue line represents the patients undergoing PRND; the red line represents patients not undergoing PRND.
Figure 2. PRND improves Local Control and Progression-Free Survival in N2b or greater patients with a clinical complete response on post-treatment imaging.
(a) Local Control, (b) Regional Control, (c) Progression-Free Survival and (d) Overall Survival. The blue line represents the patients undergoing PRND; the red line represents patients not undergoing PRND.
Figure 3. PRND improves Local Control and/or Progression-Free Survival in distinct head and neck sites.
(a) Local Control for N2b or greater patients stratified by the Hypopharynx, Larynx, Oral Cavity and Oropharynx sites. (b) Progression Free Survival for N2b or greater patients stratified by the Hypopharynx, Larynx, Oral Cavity and Oropharynx sites. The blue line represents the patients undergoing PRND; the red line represents patients not undergoing PRND.
On UVA, PRND, nodal stage, chemoradiation and alcohol history significantly impacted 2-year LC and/or PFS (Table 2). These factors and those known to impact outcomes in HNSCC were included on MVA. Table 3 details pertinent findings on MVA for PRND in terms of LC, FFDM, PFS and OS. Importantly, PRND remained strongly prognostic for 2-year LC (HR 0.22; P=.0007) and PFS (HR 0.42; P<.002). There were no statistically significant differences in outcomes between oropharyngeal and non-oropharyngeal sites.
Table 2.
Univariate analysis for patients with N2b or greater nodal disease. (n=176)
| 2-year Local Control | P value* | 2-year Progression Free Survival | P value* | |
|---|---|---|---|---|
| Post-Radiation Neck Dissection | ||||
| Yes | 85.5% | <.0001 | 66.0% | <.001 |
| No | 53.5% | 30.3% | ||
| KPS | ||||
| <70 | 51.4% | .15 | 35.6% | .09 |
| ≥70 | 69.1% | 51.6% | ||
| Primary Tumor Stage | ||||
| Early (T1–2) | 76.2% | .10 | 54.3% | .76 |
| Advanced (T3–4) | 62.2% | 51.5% | ||
| Nodal Stage | ||||
| N2b–c | 69.8% | .11 | 58.3% | .003 |
| N3 | 52.7% | 35.0% | ||
| Primary site | ||||
| Hypopharynx | 73.4% | .11 | 40.0% | .16 |
| Larynx | 78.8% | 63.2% | ||
| Nasopharynx | 73.0% | 51.9% | ||
| Oral cavity | 49.8% | 46.9% | ||
| Oropharynx | 68.6% | 59.3% | ||
| Other | 40.0 | 25.0% | ||
| Non-Oropharynx (vs. oropharynx) | 64.2% | .41 | 48.2% | .09 |
| Alcohol history | ||||
| ≥ 2 drinks/day | 63.7% | .17 | 43.2% | .003 |
| < 2 drinks/day | 79.6% | 77.6% | ||
| Tobacco history | ||||
| Yes (> 10 pack-years) | 65.2% | .29 | 52.3% | .28 |
| No (≤ 10 pack-years) | 73.7% | 55.0% | ||
| Node levels IV/V/SCV involved | ||||
| Yes | 72.5% | .17 | 55.2% | .47 |
| No | 61.6% | 50.1% | ||
| Chemotherapy | ||||
| Induction | ||||
| Yes | 70.6% | .04 | 56.6% | .09 |
| No | 59.2% | 46.1% | ||
| Concurrent | 66.1% | .38 | 53.1% | .14 |
| Yes | 64.7% | 48.2% | ||
| No | ||||
| Any | ||||
| Yes | 68.2% | .0004 | 53.9% | .02 |
| No | 16.2% | 22.0% | ||
| RT delay >5 days | ||||
| Yes | 65.2% | .41 | 50.6% | .58 |
| No | 69.5% | 60.0% | ||
All P-values are two sided. Univariate analysis was performed using log-rank test. 2y LC and PFS KPS = Karnofsky Performance Status
RT = Radiotherapy
TABLE 3.
Multivariate Analysis in patients with N2b or greater nodal disease. (n = 176)
| Hazard Ratio (95% Confidence Interval)
|
||||
|---|---|---|---|---|
| Local Control | Freedom From Distant Metastasis | Progression Free Survival | Overall Survival | |
| PRND | 0.22 (0.07–0.54) | 0.53 (0.23–1.13) | 0.42 (0.23–0.74) | 0.58 (0.28–1.14) |
| P value | .0007 | .10 | .002 | .12 |
| Oropharynx (vs. non-oropharynx) | 1.44 (0.64–3.22) | 0.99 (0.42–2.17) | 0.92 (0.53–1.58) | 0.94 (0.47–1.85) |
| P value | .37 | .98 | .76 | .87 |
| Tumor Stage 3–4 | 1.72 (0.70–5.25) | 0.52 (0.24–1.19) | 0.84 (0.48–1.53) | 1.22 (0.63–3.10) |
| P value | .25 | .12 | .55 | .46 |
| Nodal Stage N3 (vs. N2b–c) | 1.94 (0.81–4.34) | 2.07 (0.86–4.78) | 1.82 (1.00–3.21) | 1.78 (0.82–3.66) |
| P value | .13 | .10 | .05 | .14 |
| Any chemotherapy | 0.10 (0.01–2.07) | 2.07x108 (0.14-∞) | 0.17 (0.04–1.10) | 7.69x107 (0.07-∞) |
| P value | .11 | .75 | .06 | .56 |
| Induction chemotherapy | 0.71 (0.32–1.59) | 0.72 (0.33–1.61) | 0.96 (0.56–1.68) | 1.02 (0.51–2.10) |
| P value | .41 | .42 | .96 | .96 |
| KPS < 70 | 1.48 (0.42–3.97) | 0.68 (0.11–2.45) | 1.18 (0.44–2.67) | 1.67 (0.22–2.10) |
| P value | .50 | .60 | .70 | .39 |
| High comorbidity | 1.26 (0.56–2.74) | 1.85 (0.80–4.14) | 1.49 (0.85–2.58) | 1.21 (0.58–2.39) |
| P value | .56 | .15 | .16 | .60 |
| Alcohol Abuse | 1.50 (0.56–5.23) | 3.57 (1.18–15.59) | 2.03 (1.02–4.50) | 3.19 (1.24–10.86) |
| P value | .44 | .02 | .04 | .01 |
| ≥ 10 Pack-Years Tobacco Use | 1.06 (0.35–4.61) | 0.61 (0.22–1.54) | 0.60 (0.28–1.38) | 0.41 (0.17–1.08) |
| P value | .92 | .36 | .21 | .07 |
| Radiotherapy Delay | 0.87 (0.38–1.91) | 0.68 (0.28–1.54) | 0.97 (0.54–1.70) | 1.00 (0.58–2.39) |
| P value | .73 | .36 | .92 | .60 |
All P-values are two sided. Multivariable Analysis was performed using Cox proportional hazard model.
Values in parenthesis indicate 95% Confidence Interval.
PRND = Post-Radiation Neck Dissection.
KPS = Karnofsky Performance Status.
Toxicity
The rates of late toxicity did not reach statistical significance for any differences between PRND and non-PRND groups including the need for permanent feeding tube (47.3% for PRND vs. 37.1% for no PRND; P=.13), tracheostomy tube (27.0% for PRND vs. 19.3% for no PRND; P=.19) and osteoradionecrosis (10.8%% for PRND vs. 9.4% for no PRND; P=.20).
DISCUSSION
Here, we show PRND improved control at non-nodal disease sites in patients with advanced nodal disease who achieved a cCR after CRT. Furthermore, advanced nodal stage correlated with decreased PFS in the absence of PRND. This increase in local control for the PRND group was not likely due to poor imaging technique because the NPV for CT-based cCR was 91.1% in this series and consistent with previous reports 4, 6, 15, 19, 20. Furthermore, improved local control occurred despite cCR at the primary site on initial post-therapy imaging and clinical exam. Therefore, it is unlikely that locally persistent disease would preferentially occur in the non-PRND group. By contrast, patients with less advanced nodal disease at presentation (N0-N2a) had similar rates of PFS whether or not they underwent PRND indicating that patients with limited nodal disease did not benefit from PRND. Thus, even with a cCR by imaging, PRND improved control at non-regional disease sites in HNSCC patients with advanced nodal disease.
Lymph nodes are often the first sites of metastatic spread and several groups have shown that increasing lymph node positivity correlates with distant spread 2. Additionally, several reports have correlated lymph node positivity with increased rates of local failure suggesting that lymph node metastases were a surrogate for locally aggressive disease 22–25. Several investigators found worse local control with advanced nodal disease in multiple head and neck sites and for both early and advanced T-stage tumors 22, 24, 25, 27, 28. In the absence of PRND, we found that patients with early nodal disease had better local control than patients with advanced nodal disease. Thus, increasing lymph node involvement adversely impacts local and distant control.
Fewer reports have addressed whether clearance of initially involved cervical lymphatics impacted local or distant disease. Demonstrating that nodal control impacted local control, Wall et al. reported that when the neck was controlled, LC was 65–80%; but when cancer recurred in the neck, LC decreased to 20–40% 25. Furthermore, Brizel et al. demonstrated that even with a cCR, patients with N2 nodal disease or greater had worse disease free survival if they were observed following definitive treatment (75% for PRND vs. 53% for observation) 23. Only 2 of the 16 patients who failed had only nodal recurrence. Compared to no failures in the PRND group (n=8), Soltys et al. demonstrated that 27% of patients that did not undergo PRND (n=48) had disease recurrence; 2 patients had regional-only failures while 11 patients had failures involving other sites 11. Similarly, we observed that PRND decreased local failures and trended to lower distant failures. Thus, our data and others suggest that clearance of involved lymphatics may impact disease control at non-regional sites.
By contrast, some groups have observed that cCR by imaging provides both high rates of regional as well as local control in the absence of PRND. Using both CT and PET-CT imaging, patients who achieved a cCR had local control rates of 85% or greater regardless of whether patients underwent PRND 3, 6, 14, 17. However in these series, 70% or more of the patients had oropharyngeal primaries which is associated with improved LC, PFS and OS that is often attributed to HPV positivity 28, 29. To this end, Shonka et al. showed that CRT cleared 82% of involved lymph nodes in p16-positive (and likely HPV-positive) cancers compared to only 50% of p16-negative cancers 30. We rely on other studies to address role of PRND for HPV-positive patients because, in our series, only 2 of the 60 cancers tested were positive for HPV. Therefore, due to our limited numbers of HPV-positive patients, our results suggesting a benefit of PRND on non-regional control likely applies to HPV-negative HNSCCs.
Studies in other disease sites also suggest that increasing lymph node positivity adversely impacted local control 31–33. For example, Stock et al. demonstrated that early stage cervical cancer had increased rates of local failure when regional nodes were involved 33. Similarly in non-small cell lung cancer, increasing numbers of involved lymph nodes portended worse local control with 50% of the failures occurring at the bronchial stump 32. In rectal cancer, addressing regional lymph nodes with pelvic RT and total mesorectal excision (TME) resulted in improved LC (3%) compared to TME surgery alone (11%) or non-TME surgery with RT (11%) 34, 35. Thus, in many types of cancer, LN involvement inversely correlated with local control and effectively clearing regional disease may improve outcomes at non-regional sites.
We can only postulate as to the mechanism by which removal of nodal disease impacted local control. This may include the biology of the tumor, immunological consequences of compromised lymphatics or other mechanisms. The beneficial impact of PRND may be more apparent in tumors that are less responsive to treatment and, therefore, inherently more likely to recur. Secondly, regional disease may act as a surrogate or even cause immune dysfunction that might be restored when the involved nodes are removed. In head and neck cancer patients, tumor infiltrating lymphocytes or lymph node lymphocytes were deficient in cytotoxic effector cells compared to the peripheral blood 36. Furthermore, CD8+ T cells in both hyperplastic and metastatic lymph nodes showed diminished responses to mitogenic stimuli 37. Similar immune dysfunction was observed in breast cancer lymph nodes and predicted for worse outcomes 38. In preclinical models, lymph node metastasis induce immunological tolerance due to inadequate costimulation 39. It is tempting to speculate that surgical clearance of these dysfunctional lymph nodes may improve systemic immune responses. Such a benefit would persist for those in cCR despite disease sterilization as demonstrated in this series. Indeed, Ma et al. demonstrated that immune dysfunction in patients with HNSCC recovered after lymph node dissection but not radiotherapy 40. Finally, others have proposed that reseeding of the primary from regional lymph node metastases might be important for LC 41. Therefore, PRND may have improved outcomes for tumors through clearance of involved lymphatics which may restore immune function or prevent reseeding of the primary.
PRND may increase morbidity and, thereby, negate any therapeutic benefit. While patients undergoing PRND trended to an increased risk of tracheostomy, they did not have significantly increased risks of feeding tubes or osteoradionecrosis in our experience. Caution is warranted as PRND was associated with increased morbidities in some series. For example, Narayan et al. observed that PRND was associated with significant complications in 17% of patients requiring either reoperation or long term medical treatment 16. Therefore, patients who might benefit from PRND need to be carefully selected in order to derive meaningful therapeutic benefit.
Limitations of the current analysis include the retrospective nature and potential bias in selecting patients for PRND. Although we used CT-based imaging, our NPV was 91.1% which is similar to the NPV of 86–100% for CT and PET-CT 6, 13, 15. Our regional control without PRND was similar to previous reports even though our study had a lower incidence of oropharyngeal primaries as compared to other series 3, 5, 6, 9, 11, 20. In our series, patients undergoing PRND had a greater proportion of oropharyngeal primaries and fewer T4 tumors, factors that impact LC. Addressing this issue, we observed no difference in LC when comparing oropharyngeal to non-oropharyngeal primaries (2y LC: 68.6% for oropharyngeal primary vs. 64.2% for non-oropharyngeal primary; p=.41). Furthermore, PRND still benefited patients with T3–T4 tumors with N2b or greater nodal disease and a cCR after CRT (2y LC: 87.5% for PRND vs. 73.2% for non-PRND; P=.04). Furthermore, PRND remained the only significant factor on MVA predicting for improved LC and PFS. In addition, given that induction chemotherapy was more common in patients undergoing PRND, it remains unclear the extent to which this neoadjuvant approach impacted disease control. Nevertheless, induction chemotherapy was not significant for LRC or PFS on multivariate analysis, which is consistent with other studies 42. Finally, our study was limited by the relatively short follow-up time and we await updated results with longer follow up times. Thus, the impact of PRND remains robust after accounting for several variables that may impact local control or distant control.
In conclusion, we observed a hypothesis generating observation where PRND may improve local control and likely distant control. The benefit of PRND on non-regional sites of disease persisted for patients with a cCR on imaging that had high predictive values. Currently, it remains unclear which patient, tumor and molecular characteristics may predict for improved local control with a PRND after a cCR. We propose that PRND should be reevaluated for patients with HPV-negative cancers and extensive nodal disease. Nevertheless, any potential benefit in PRND should be balanced with the increased morbidity following the procedure. While these observations contradict the current trends in the management of patients achieving a cCR, our results suggest that addressing metastatic lymph nodes may play a larger role than just the regional control of disease.
Acknowledgments
M.T.S. is supported by a grant from the Fanoni Anemia Research Foundation and the Burroughs Wellcome Career Award for Medical Scientists.
Footnotes
Meeting presentation: This work will be presented at the Annual Meeting for the American Society for Therapeutic Radiation Oncology (ASTRO), September 22–25, 2013, Atlanta, GA.
Conflict of Interest: None reported.
Author Contributions: M.T.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Spiotto. Acquisition of data: Spiotto and Abundo. Analysis and interpretation of data: Ranck and Spiotto. Drafting of manuscript: Ranck, Abundo and Spiotto. Critical revision of the manuscript important for intellectual content: Spiotto, Wiechselbaum, Kolokythias, Jefferson, Wenig. Statistical analysis: Ranck. Administrative, technical and material support: Ranck and Spiotto. Study supervision: Spiotto.
References
- 1.O’Brien CJ, Smith JW, Soong SJ, Urist MM, Maddox WA. Neck dissection with and without radiotherapy: prognostic factors, patterns of recurrence, and survival. Am J Surg. 1986 Oct;152(4):456–463. doi: 10.1016/0002-9610(86)90324-7. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993 Jan 15;71(2):452–456. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Argiris A, Stenson KM, Brockstein BE, et al. Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck. 2004 May;26(5):447–455. doi: 10.1002/hed.10394. [DOI] [PubMed] [Google Scholar]
- 4.Mendenhall WM, Villaret DB, Amdur RJ, Hinerman RW, Mancuso AA. Planned neck dissection after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2002 Nov;24(11):1012–1018. doi: 10.1002/hed.10187. [DOI] [PubMed] [Google Scholar]
- 5.Cho AH, Shah S, Ampil F, Bhartur S, Nathan CO. N2 disease in patients with head and neck squamous cell cancer treated with chemoradiotherapy: is there a role for posttreatment neck dissection? Arch Otolaryngol Head Neck Surg. 2009 Nov;135(11):1112–1118. doi: 10.1001/archoto.2009.148. [DOI] [PubMed] [Google Scholar]
- 6.Clavel S, Charron MP, Belair M, et al. The role of computed tomography in the management of the neck after chemoradiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. Feb 1;82(2):567–573. doi: 10.1016/j.ijrobp.2010.11.066. [DOI] [PubMed] [Google Scholar]
- 7.Corry J, Peters L, Fisher R, et al. N2–N3 neck nodal control without planned neck dissection for clinical/radiologic complete responders-results of Trans Tasman Radiation Oncology Group Study 98.02. Head Neck. 2008 Jun;30(6):737–742. doi: 10.1002/hed.20769. [DOI] [PubMed] [Google Scholar]
- 8.Lau H, Phan T, Mackinnon J, Matthews TW. Absence of planned neck dissection for the N2–N3 neck after chemoradiation for locally advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2008 Mar;134(3):257–261. doi: 10.1001/archoto.2007.49. [DOI] [PubMed] [Google Scholar]
- 9.Nayak JV, Walvekar RR, Andrade RS, et al. Deferring planned neck dissection following chemoradiation for stage IV head and neck cancer: the utility of PET-CT. Laryngoscope. 2007 Dec;117(12):2129–2134. doi: 10.1097/MLG.0b013e318149e6bc. [DOI] [PubMed] [Google Scholar]
- 10.Ong SC, Schoder H, Lee NY, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med. 2008 Apr;49(4):532–540. doi: 10.2967/jnumed.107.044792. [DOI] [PubMed] [Google Scholar]
- 11.Soltys SG, Choi CY, Fee WE, Pinto HA, Le QT. A planned neck dissection is not necessary in all patients with N2–3 head-and-neck cancer after sequential chemoradiotherapy. Int J Radiat Oncol Biol Phys. Jul 1;83(3):994–999. doi: 10.1016/j.ijrobp.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Vedrine PO, Thariat J, Hitier M, et al. Need for neck dissection after radiochemotherapy? A study of the French GETTEC Group. Laryngoscope. 2008 Oct;118(10):1775–1780. doi: 10.1097/MLG.0b013e31817f192a. [DOI] [PubMed] [Google Scholar]
- 13.Yao M, Graham MM, Hoffman HT, et al. The role of post-radiation therapy FDG PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004 Jul 15;59(4):1001–1010. doi: 10.1016/j.ijrobp.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Yao M, Hoffman HT, Chang K, et al. Is planned neck dissection necessary for head and neck cancer after intensity-modulated radiotherapy? Int J Radiat Oncol Biol Phys. 2007 Jul 1;68(3):707–713. doi: 10.1016/j.ijrobp.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 15.Yao M, Luo P, Hoffman HT, et al. Pathology and FDG PET correlation of residual lymph nodes in head and neck cancer after radiation treatment. Am J Clin Oncol. 2007 Jun;30(3):264–270. doi: 10.1097/01.coc.0000257611.65290.aa. [DOI] [PubMed] [Google Scholar]
- 16.Narayan K, Crane CH, Kleid S, Hughes PG, Peters LJ. Planned neck dissection as an adjunct to the management of patients with advanced neck disease treated with definitive radiotherapy: for some or for all? Head Neck. 1999 Oct;21(7):606–613. doi: 10.1002/(sici)1097-0347(199910)21:7<606::aid-hed4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Yao M, Smith RB, Graham MM, et al. The role of FDG PET in management of neck metastasis from head-and-neck cancer after definitive radiation treatment. Int J Radiat Oncol Biol Phys. 2005 Nov 15;63(4):991–999. doi: 10.1016/j.ijrobp.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 18.Ferlito A, Corry J, Silver CE, Shaha AR, Thomas Robbins K, Rinaldo A. Planned neck dissection for patients with complete response to chemoradiotherapy: a concept approaching obsolescence. Head Neck. Feb;32(2):253–261. doi: 10.1002/hed.21173. [DOI] [PubMed] [Google Scholar]
- 19.Langerman A, Plein C, Vokes EE, et al. Neck response to chemoradiotherapy: complete radiographic response correlates with pathologic complete response in locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009 Nov;135(11):1133–1136. doi: 10.1001/archoto.2009.154. [DOI] [PubMed] [Google Scholar]
- 20.Liauw SL, Mancuso AA, Amdur RJ, et al. Postradiotherapy neck dissection for lymph node-positive head and neck cancer: the use of computed tomography to manage the neck. J Clin Oncol. 2006 Mar 20;24(9):1421–1427. doi: 10.1200/JCO.2005.04.6052. [DOI] [PubMed] [Google Scholar]
- 21.Porceddu SV, Pryor DI, Burmeister E, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. Dec;33(12):1675–1682. doi: 10.1002/hed.21655. [DOI] [PubMed] [Google Scholar]
- 22.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer. 1994 Jan 1;73(1):187–190. doi: 10.1002/1097-0142(19940101)73:1<187::aid-cncr2820730132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Bernier J, Bataini JP. Regional outcome in oropharyngeal and pharyngolaryngeal cancer treated with high dose per fraction radiotherapy. Analysis of neck disease response in 1646 cases. Radiother Oncol. 1986 Jun;6(2):87–103. doi: 10.1016/s0167-8140(86)80015-9. [DOI] [PubMed] [Google Scholar]
- 24.Hahn SS, Spaulding CA, Kim JA, Constable WC. The prognostic significance of lymph node involvement in pyriform sinus and supraglottic cancers. Int J Radiat Oncol Biol Phys. 1987 Aug;13(8):1143–1147. doi: 10.1016/0360-3016(87)90186-6. [DOI] [PubMed] [Google Scholar]
- 25.Wall TJ, Peters LJ, Brown BW, Oswald MJ, Milas L. Relationship between lymph nodal status and primary tumor control probability in tumors of the supraglottic larynx. Int J Radiat Oncol Biol Phys. 1985 Nov;11(11):1895–1902. doi: 10.1016/0360-3016(85)90269-x. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Tankere F, Camproux A, Barry B, Guedon C, Depondt J, Gehanno P. Prognostic value of lymph node involvement in oral cancers: a study of 137 cases. Laryngoscope. 2000 Dec;110(12):2061–2065. doi: 10.1097/00005537-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Feng M, Jabbari S, Lin A, et al. Predictive factors of local-regional recurrences following parotid sparing intensity modulated or 3D conformal radiotherapy for head and neck cancer. Radiother Oncol. 2005 Oct;77(1):32–38. doi: 10.1016/j.radonc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul 1;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shonka DC, Jr, Shoushtari AN, Thomas CY, et al. Predicting residual neck disease in patients with oropharyngeal squamous cell carcinoma treated with radiation therapy: utility of p16 status. Arch Otolaryngol Head Neck Surg. 2009 Nov;135(11):1126–1132. doi: 10.1001/archoto.2009.153. [DOI] [PubMed] [Google Scholar]
- 31.Wharam MD, Meza J, Anderson J, et al. Failure pattern and factors predictive of local failure in rhabdomyosarcoma: a report of group III patients on the third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 2004 May 15;22(10):1902–1908. doi: 10.1200/JCO.2004.08.124. [DOI] [PubMed] [Google Scholar]
- 32.Higgins KA, Chino JP, Berry M, et al. Local failure in resected N1 lung cancer: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. Jun 1;83(2):727–733. doi: 10.1016/j.ijrobp.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Stock RG, Chen AS, Karasek K. Patterns of spread in node-positive cervical cancer: the relationship between local control and distant metastases. Cancer J Sci Am. 1996 Sep-Oct;2(5):256–262. [PubMed] [Google Scholar]
- 34.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001 Aug 30;345(9):638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 35.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997 Apr 3;336(14):980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 36.Snyderman CH, Heo DS, Johnson JT, D’Amico F, Barnes L, Whiteside TL. Functional and phenotypic analysis of lymphocytes in head and neck cancer. Arch Otolaryngol Head Neck Surg. 1991 Aug;117(8):899–905. doi: 10.1001/archotol.1991.01870200093016. [DOI] [PubMed] [Google Scholar]
- 37.Verastegui E, Morales R, Barrera JL, et al. Immunological approach in the evaluation of regional lymph nodes of patients with squamous cell carcinoma of the head and neck. Clin Immunol. 2002 Jan;102(1):37–47. doi: 10.1006/clim.2001.5130. [DOI] [PubMed] [Google Scholar]
- 38.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005 Sep;2(9):e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001 Jun 28;411(6841):1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 40.Ma XJ, Pan XL, Lv ZH, et al. Therapeutic influence on circulating and monocyte-derived dendritic cells in laryngeal squamous cell carcinoma patients. Acta Otolaryngol. 2009 Jan;129(1):84–91. doi: 10.1080/00016480802020459. [DOI] [PubMed] [Google Scholar]
- 41.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009 Dec 24;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009 Jul;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]