Abstract
Background
Pesticide exposure has been found to cause renal damage and dysfunction in experimental studies, but epidemiological research on the renal effects of chronic low-level pesticide exposure is limited. We investigated the relationships between end-stage renal disease (ESRD) among wives of licensed pesticide applicators (N = 31,142) in the Agricultural Health Study (AHS) and (1) personal pesticide use, (2) exposure to the husband's pesticide use, and (3) other pesticide-associated farming and household activities.
Methods
AHS participants reported pesticide exposure via self-administered questionnaires at enrollment (1993–1997). ESRD cases were identified via linkage to the United States Renal Data System. Associations between ESRD and pesticide exposures were estimated with Cox proportional hazard regression models controlling for age at enrollment. Models of associations with farming and household factors were additionally adjusted for personal use of pesticides.
Results
We identified 98 ESRD cases diagnosed between enrollment and 31 December 2011. Although women who ever applied pesticides (56% of cohort) were less likely than those who did not apply to develop ESRD (Hazard Ratio (HR): 0.42; 95% CI: 0.28, 0.64), among women who did apply pesticides, the rate of ESRD was significantly elevated among those who reported the highest (vs. lowest) cumulative general pesticide use (HR: 4.22; 95% CI: 1.26, 14.20). Among wives who never applied pesticides, ESRD was associated with husbands' ever use of paraquat (HR = 1.99; 95% CI: 1.14, 3.47) and butylate (HR = 1.71; 95% CI: 1.00, 2.95), with a positive exposure–response pattern for husband’s cumulative use of these pesticides.
Conclusions
ESRD may be associated with direct and/or indirect exposure to pesticides among farm women. Future studies should evaluate indirect exposure risk among other rural populations.
Keywords: Pesticide exposure, End-stage renal disease, Farm women, Agricultural exposures
1. Introduction
Experimental animal studies and case studies of human poisonings suggest that pesticide exposure may cause permanent kidney damage (Chargui et al., 2012; Choudhary et al., 2003; Kackar et al., 1999; Kaur et al., 2012; Shah and Iqbal, 2010; Uyanikgil et al., 2009; Van Vleet and Schnellmann, 2003), but epidemiological research on the effects of prolonged low-level exposure is limited. In animal studies, renal damage and dysfunction have been observed with exposure to a range of pesticides, including organophosphate (Poovala et al., 1999; Shah and Iqbal, 2010), organochlorine (Choudhary et al., 2003; Sobel et al., 2005), carbamate (Kaur et al., 2012), and pyrethroid (Chargui et al., 2012) insecticides and triazine (Santa Maria et al., 1986) and chlorophenoxy (Uyanikgil et al., 2009) herbicides. We previously reported that long-term use of several specific pesticides was associated with end-stage renal disease (ESRD) among a large cohort of male pesticide applicators (Lebov et al., 2015). The wives of pesticide applicators are likely to have patterns of pesticide exposure that differ from those of their husbands, including less frequent use of pesticides and use of less toxic pesticides (Kirrane et al., 2004), and they may experience indirect exposures by virtue of living close to where pesticides are applied. Wives of pesticide applicators may also be exposed through take-home exposures, i.e. pesticide residues carried home on their husband's boots, clothing, and skin (Fenske et al., 2013) or by washing pesticide-contaminated clothing. Women who live on farms where pesticides are applied may experience exposure through spray drift and water contamination; proximity of household to pesticide application areas has been positively correlated with levels of pesticides found in household dust (Lu et al., 2000; Simcox et al., 1995), and several large drinking water surveys have found widespread contamination of community water systems and domestic wells by pesticides (Cohen et al., 1995; Fenske et al., 2002; U.S. Environmental Protection Agency, 1990; Wiles and Cook, 1994).
The Agricultural Health Study (AHS) provides a unique opportunity to study a variety of exposure pathways among a large population of wives of private pesticide applicators (i.e. farmers). Using AHS data, we examined rates of ESRD among wives in relation to their personal use of specific pesticides and pesticide use by their applicator husbands. We also evaluated the association between other non-application pesticide exposure opportunities and ESRD.
2. Methods
2.1. Study population and case definition
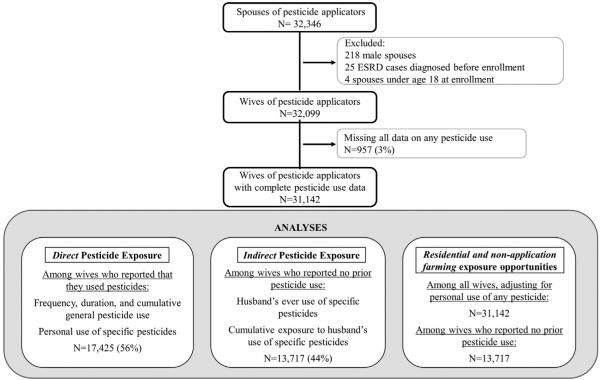
The AHS is a large, prospective study of Iowa and North Carolina pesticide applicators and their spouses (Alavanja et al., 1996). Approximately 84% (N = 52,394) of licensed private applicators in Iowa and North Carolina enrolled in the AHS between December 1993 and December 1997 by completing a questionnaire when they received or renewed their pesticide training certification. A total of 32,346 spouses of these applicators (75% of those eligible) enrolled in the study by completing a self-administered questionnaire (81%) or a telephone interview (19%). Enrollment questionnaires collected information on demographics, medical conditions, medication use, lifestyle factors, and pesticide use. Of the enrolled applicators, 44% also completed a take-home questionnaire, which collected additional information on specific pesticide use and pesticide application practices. Because the distribution of ESRD risk factors, including hypertension and diabetes, differ by gender (Arnetz et al., 2014; Zimmerman and Sullivan, 2013), and because <1% of all spouses were male, the current analysis includes only female spouses of pesticide applicators. We also excluded spouses under age 18 (N = 4), those who were diagnosed with end-stage renal disease prior to enrollment (N = 25; 20% of female spouses with diagnosed ESRD), and those who did not provide data on ever use of any pesticide (N = 957), leaving 31,142 wives for analysis (Fig. 1). Through a linkage with the United States Renal Data System (USRDS), we ascertained the first renal replacement therapy (i.e. dialysis initiation or renal transplantation) date for ESRD cases occurring between study enrollment and end of follow-up (December 31, 2011). Date of death from any cause was obtained by linking the cohort to state mortality files and the National Death Index. These mortality registry data were used to identify death dates for all participants; however, the USRDS also captures data on those who die of renal failure when a renal provider submits a required ESRD Death Notification (CMS-2746) form (U.S. Renal Data System, 2015). Therefore, we believe that few, if any, ESRD cases are missing from this analysis.
Fig. 1.
Study population and numbers used for sub-analyses.
2.2. Exposure assessment
Pesticide exposure information from the spouse enrollment questionnaire included: (1) ever/never use of 50 specific pesticides; (2) number of years (duration) and days per year (frequency) personally mixed or applied pesticides in general; (3) number of years lived or worked on a farm; (4) specific farm tasks performed; (5) performance of household tasks involving possible pesticide exposure; (6) distance from the participant's house to fields where pesticides were applied; (7) household practices that could increase pesticide exposure (e.g. storage of pesticides in the home); and (8) treatment of the home or lawn for pests. The applicator enrollment questionnaire elicited information on ever use of 50 pesticides and duration and frequency of use for 22 of those pesticides. On the applicator take-home questionnaire, applicators provided information on duration and frequency of use of the remaining 28 pesticides, as well as distance from private well to the nearest pesticide application area. The enrollment questionnaire was designed to capture the majority of pesticides commonly used at the time the study began or that were of interest because of their potential link to adverse health effects, including cancer, reproductive conditions, neurologic diseases, and other chronic diseases. (Questionnaires are available at: http://aghealth.nih.gov/collaboration/questionnaires.html.)
Direct exposure was defined as the wives' personal use of 50 specific pesticides, two functional and nine chemical classes of pesticides, and overall pesticide use. Indirect exposure was approximated by the husbands' ever and cumulative use of specific chemicals. Additionally, we evaluated ESRD in relation to several residential pesticide exposures, including contact with pesticide-contaminated materials and proximity of the home or well to pesticide mixing and application areas. Pesticide exposure may occur through contact with crops after a recent pesticide application and spending time outdoors during pesticide application (Deziel et al., 2015), and the opportunity for exposure is greater for those who have lived and/or worked on a farm for their whole lives compared to those who have spent less time on the farm. Therefore, we also considered the number of days spent working in the fields during the growing season prior to enrollment, the number of hours per day spent in the sun during the growing season, the number of years spent living or working on a farm over the lifetime, ever having a non-farm job, and specific farm work activities (other than pesticide application) as potential risk factors. This last cluster of potential exposures will be referred to as ‘non-application farming exposures’.
2.3. Statistical analyses
We used Cox proportional hazards models with age as the time scale to evaluate associations between ESRD and potential pesticide exposures. Participants accrued person-time from the date of enrollment to the earliest of ESRD therapy initiation, death (by any cause), or end of study follow-up.
Analyses of direct exposures, including duration, frequency, and cumulative use of pesticides in general, and ever use of specific pesticides, were limited to women who had ever personally mixed or applied pesticides (N = 17,425, 56%). Cumulative use of any pesticides (i.e. the product of duration and frequency of use) was categorized into quartiles, based on the distribution of use among the cases.
To evaluate direct and indirect exposure separately, husbands' ever and cumulative use of specific chemicals (i.e. indirect exposure) was evaluated among wives who reported no personal pesticide use (N = 13,717, 44%). Exposure–response analyses of husbands' cumulative use of specific chemicals accounted for the estimated amount of time that wives lived with their husbands prior to enrollment. We obtained information collected during AHS Phase 3 (2010–2012: N (applicators) = 24,171 and N (spouses) = 19,959) on the number of years that married participants reported living together prior to enrollment. For those who did not participate in Phase 3 or for whom this information was missing (38% of wives), we imputed values based on the age-specific mean number of years that Phase 3 wives reported living with their husbands before enrollment. This allowed us to estimate a date that couples began living together. The date of first use for each chemical was the midpoint of the husbands' reported decade of first use. On average, 6% of pesticide users did not report decade of first use. For pesticides banned from the market prior to enrollment (DDT in 1972 and toxaphene in 1990), the latest possible year pesticide exposure could have occurred was set to 5 years after the ban year. A buffer of 5 years was built in because we assume that farmers continued to use existing stocks of banned pesticides for a period of years after the ban. Based on the number of years the husband reported using the pesticide, and the number of years between the midpoint of the decade of first use and enrollment, an annual probability of use was calculated for each pesticide the husband reported using.
The wives' pesticide-specific indirect exposure duration was then defined as the number of years that wives could be exposed, based on the estimated start date for living together, multiplied by the annual probability of the husband's use. Thus, a husband's use of a specific pesticide prior to living with his wife would not count towards his wife's duration of exposure. For exposure–response analyses, we multiplied the wives’ indirect pesticide exposure duration by the husbands' frequency of use to obtain estimated lifetime-days of indirect exposure to specific chemicals, and then categorized lifetime-days into three levels: none, ≤ non-zero median lifetime-days, > median lifetime-days.
Initially, we evaluated associations with residential and non-application farming exposures among all wives. We also examined associations among farm wives who reported no personal pesticide use. Exposure–response trends for variables with more than two levels were assessed with linear trend tests, using the median value or the midpoint value of each category as the exposure value, as appropriate. We present hazard ratio estimates only for those exposures for which there were at least three cases in each exposure stratum.
State and education were identified as potential confounders through a literature review, but were not adjusted for in the present analyses because they were not strongly associated with ESRD in our study population. Though use of non-steroidal anti-inflammatory drugs (NSAIDs) was associated with ESRD, we did not adjust for this factor because there is no evidence to suggest that use of NSAIDs would affect pesticide use or exposure. Obesity and diabetes, risk factors for ESRD, may be on the causal pathway between personal pesticide use and ESRD (Lee et al., 2011; Montgomery et al., 2008; Starling et al., 2014), and there is no evidence to suggest that the wives' body mass index (BMI) and diabetes status would affect the husbands' use of pesticides; therefore, we did not adjust for these factors in models of the association between ESRD and personal use or husband’s use. For the few non-application exposures associated with diabetes and BMI, adjustment for these factors did not meaningfully change estimates (data not shown); therefore, diabetes and BMI were not included in final models.
Wives' ever use of pesticides in general was also evaluated as a potential confounder of associations with residential and non-application farming exposures, and was found to be significantly inversely associated with ESRD, associated with each exposure measure, and not on the causal pathway between exposure and ESRD. Therefore, models evaluating residential and non-application farming exposures among all wives were adjusted for wives' ever use of any pesticide, with very little loss of precision.
We used the AHS dataset release P1REL0310. All statistical analyses were done using SAS v9.3 (Cary, NC).
3. Results
Overall, a total of 98 cases (0.3% of wives) were diagnosed with ESRD during an average of 15.4 years of follow-up, resulting in an incidence rate of 20.4 ESRD cases per 100,000 person-years. After adjusting for age, ESRD incidence was significantly higher for obese participants, frequent NSAIDs users, and those who reported having doctor-diagnosed diabetes or hypertension (Table 1).
Table 1.
Demographic and medical characteristics of all wives in the Agricultural Health Study, 1993-1997 through end of follow up (December, 31, 2011).
| All wives |
Wives who reported no prior pesticide use |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics (reported at enrollment) |
Level | Non-cases N=31,044 (99.7%) |
ESRD cases N=98 (0.3%) |
HR | 95% CI | Non-cases N=13,653 |
ESRD cases N=64 |
HR | 95% CI | ||||
| N | % | N | % | N | % | N | % | ||||||
| Agea | 18–39 | 9585 | 31 | 8 | 8 | – | – | 4655 | 34 | 4 | 6 | – | – |
| 40–49 | 8850 | 29 | 20 | 20 | 2.74 | 1.21, 6.21 | 3507 | 26 | 17 | 27 | 5.69 | 1.91, 16.91 | |
| 50–59 | 7424 | 24 | 26 | 27 | 4.28 | 1.94, 9.46 | 2920 | 21 | 12 | 19 | 4.91 | 1.58, 15.22 | |
| 60–69 | 4170 | 13 | 32 | 33 | 9.83 | 4.53, 21.34 | 1986 | 15 | 23 | 36 | 14.51 | 5.02, 41.96 | |
| ≥70 | 1015 | 3 | 12 | 12 | 18.41 | 7.52, 45.06 | 585 | 4 | 8 | 12 | 21.08 | 6.34, 70.08 | |
| State | Iowa | 21,240 | 68 | 56 | 57 | – | 8461 | 62 | 33 | 52 | – | – | |
| North Carolina | 9804 | 32 | 42 | 43 | 1.32 | 0.88, 1.98 | 5192 | 38 | 31 | 48 | 1.22 | 0.75, 2.00 | |
| Education | <High school | 1512 | 5 | 10 | 11 | 1.19 | 0.6, 2.39 | 901 | 7 | 9 | 15 | 1.31 | 0.62, 2.77 |
| High school | 11,219 | 40 | 44 | 49 | – | 5215 | 42 | 31 | 51 | – | – | ||
| >High school | 15,051 | 54 | 35 | 39 | 0.82 | 0.52, 1.28 | 6426 | 51 | 21 | 34 | 0.77 | 0.44, 1.34 | |
| Pack years of smoking |
None | 21,825 | 73 | 64 | 67 | – | 9566 | 73 | 42 | 68 | – | – | |
| >0 to 9 | 4812 | 16 | 16 | 17 | 1.37 | 0.79, 2.38 | 2099 | 16 | 10 | 16 | 1.31 | 0.65, 2.62 | |
| >9 | 3337 | 11 | 15 | 16 | 1.55 | 0.88, 2.72 | 1470 | 11 | 10 | 16 | 1.46 | 0.73, 2.91 | |
| Body Mass Index (kg/m2) |
<25 | 13,552 | 49 | 29 | 35 | – | 6162 | 50 | 16 | 30 | – | – | |
| 25–29.99 | 8820 | 32 | 31 | 37 | 1.35 | 0.81, 2.24 | 3874 | 31 | 21 | 39 | 1.70 | 0.89, 3.27 | |
| ≥30 | 5116 | 19 | 24 | 29 | 1.86 | 1.08, 3.2 | 2326 | 19 | 17 | 32 | 2.33 | 1.17, 4.63 | |
| Number of years take NSAIDS nearly every |
Have not taken nearly every day day |
20,031 | 67 | 43 | 47 | – | – | 9066 | 70 | 28 | 47 | – | – |
| <1 year | 3786 | 13 | 10 | 11 | 1.06 | 0.53, 2.11 | 1443 | 11 | 5 | 8 | 0.96 | 0.37, 2.48 | |
| ≥1 years | 6051 | 20 | 38 | 42 | 2.01 | 1.29, 3.14 | 2522 | 19 | 27 | 45 | 2.44 | 1.42, 4.19 | |
| Diabetesb | No | 29,581 | 97 | 44 | 46 | – | – | 12,883 | 96 | 26 | 41 | – | – |
| Yes | 987 | 3 | 52 | 54 | 26.69 | 17.57, 40.53 | 515 | 4 | 38 | 59 | 28.16 | 16.7, 47.49 | |
| High blood pressureb |
No | 25,991 | 85 | 33 | 34 | – | 11,205 | 84 | 18 | 28 | – | – | |
| Yes | 4608 | 15 | 65 | 66 | 7.53 | 4.81, 11.81 | 2208 | 16 | 46 | 72 | 9.26 | 5.15, 16.64 | |
ESRD=End-stage renaldisease; HR=Hazard Ratio; CI=Confidence Interval.
For models evaluating age, time-on-study is the time scale.
Self-reported.
3.1. Wives' direct exposure to pesticides and indirect exposure through husbands' use
Among all wives, personal use of any pesticide was inversely associated with ESRD (Hazard Ratio (HR) = 0.42; 95% CI: 0.28, 0.64: data not shown). However, among women who personally mixed or applied pesticides, positive associations with ESRD were observed for the highest category of cumulative lifetime-days, frequency, and duration of use of any pesticides vs. the lowest category (Table 2).
Table 2.
Use of pesticides in general and specific pesticides by wives who reported personal pesticide use (direct exposure), adjusted for age, Agricultural Health Study, 1993–1997 through December 31, 2011.
|
Non-casesa (N=17,391)
|
ESRD casesa (N=34)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Risk factor | Level | N | % | N | % | HR | 95% CI | P for trend |
| Cumulative lifetime-days of use of pesticides overallb | 1–24.5 | 5330 | 41.2 | 6 | 27.3 | – | – | – |
| 24.6–98 | 2988 | 23.1 | 5 | 22.7 | 1.16 | 0.35, 3.83 | ||
| 98.1–507.5 | 3982 | 30.8 | 6 | 27.3 | 0.96 | 0.30, 3.034 |
||
| >507.5 | 638 | 4.9 | 5 | 22.7 | 4.22 | 1.26, 14.2 | ||
| 0.024 | ||||||||
| Number of days per year personally mix or apply pesticides | 1–2.5 | 6455 | 49.6 | 9 | 40.9 | – | – | – |
| 2.6–7 | 2971 | 22.8 | 3 | 13.6 | 0.70 | 0.20, 2.54 | ||
| 7.1 –14.5 | 2249 | 17.3 | 4 | 18.2 | 1.20 | 0.37, 3.88 | ||
| >14.5 | 1339 | 10.3 | 6 | 27.3 | 2.80 | 0.99, 7.96 | 0.034 | |
| Number of years personally mixed or applied pesticides | 0.1–5 | 4750 | 36.5 | 6 | 27.3 | – | – | – |
| 5–20 | 5538 | 42.6 | 5 | 22.7 | 0.66 | 0.20, 2.16 | ||
| 21–30 | 1584 | 12.2 | 3 | 13.6 | 1.11 | 0.27, 4.47 | ||
| >30 | 1142 | 8.8 | 8 | 36.4 | 2.15 | 0.67, 6.86 | ||
| 0.155 | ||||||||
| Ever use of specific herbicides and herbicide chemical classes c | ||||||||
| Triazine herbicides | 1685 | 10.2 | 3 | 10.0 | 1.04 | 0.33, 3.22 | ||
| Atrazine | 1355 | 8.2 | 3 | 10.0 | 1.27 | 0.41, 3.96 | ||
| Chlorocetanilide herbicides | 1655 | 10.1 | 4 | 13.3 | 1.46 | 0.53, 4.02 | ||
| Alachlor | 1259 | 7.7 | 4 | 13.3 | 1.85 | 0.67, 5.12 | ||
| Phenoxy herbicides | 4438 | 26.6 | 9 | 30.0 | 1.10 | 0.50, 2.39 | ||
| 2,4-D | 4405 | 26.5 | 9 | 30.0 | 1.11 | 0.51, 2.41 | ||
| Other herbicides | ||||||||
| Chlorimuron-ethyl | 518 | 3.2 | 3 | 10.0 | 4.03 | 1.30, 12.51 |
||
| Imazethapyr | 902 | 5.5 | 3 | 10.0 | 2.37 | 0.76, 7.36 | ||
| Glyphosate | 10,281 | 60.6 | 16 | 51.6 | 0.83 | 0.41, 1.68 | ||
| Petroleum oil | 1060 | 6.5 | 3 | 10.0 | 1.69 | 0.55, 5.24 | ||
| Ever use of specific insecticides and insecticide chemical classes d | ||||||||
| Pyrethroid insecticides | 1423 | 8.9 | 3 | 10.3 | 1.57 | 0.50, 4.91 | ||
| Carbamate insecticides | 9590 | 57.2 | 23 | 71.9 | 1.50 | 0.69, 3.24 | ||
| Carbaryl | 9404 | 55.6 | 23 | 71.9 | 1.62 | 0.75, 3.50 | ||
| Organophosphate insecticides | 7834 | 45.5 | 13 | 41.9 | 0.78 | 0.38, 1.59 | ||
| Diazinon | 3068 | 18.6 | 5 | 16.1 | 0.88 | 0.35, 2.25 | ||
| Malathion | 5908 | 35.1 | 12 | 38.7 | 0.98 | 0.48, 2.03 | ||
ESRD=End-stage renal disease; HR=Hazard Ratio; CI=Confidence Interval.
The sum of the number of cases and non-cases from Table 2 and 3 will not add up to the total number of wives because 3% of study participants were missing data on ever personal use of any pesticide.
Among those who reported ever using any pesticide – 25% did not provide information on number of years mixed/applied or number of days per year mixed/ applied.
Herbicide chemical classes are: triazine herbicides (atrazine, cyanazine, and metribuzin); chlorocetanilide herbicides (alachlor and metolachlor); and phenoxy herbicides (2,4-D, 2,4,5-T, and 2,4,5-TP).
Insecticide chemical classes are: pyrethroid insecticides (permethrin or pyrethroid products); carbamate insecticides (aldicarb, carbaryl, and carbofuran); and organophosphate insecticides (chlorpyrifos, coumaphos, diazinon, dichlorvos, fonofos, malathion, methyl or ethyl parathion, phorate, terbufos, and trichlorfon).
Among the 17,425 women who applied pesticides, we identified 34 ESRD cases, and we had sufficient numbers to evaluate 10 specific chemicals and 6 chemical classes (Table 2). Among the 13,717 women who did not apply pesticides, we identified 64 ESRD cases, and there was sufficient use among their husbands to assess 43 specific chemicals for ever/never use and 27 for exposure–response (Table 3). ESRD was positively associated with ever use of alachlor for both direct (wives' personal use: HR = 1.85; 95% CI: 0.67, 5.12 – Table 2) and indirect exposures (husbands' use: HR = 1.63; 95% CI: 0.91, 2.91 – Table 3). The rate of ESRD was elevated in association with direct, but not indirect, exposure to the herbicides chlorimuron-ethyl (direct HR = 4.03; 95% CI: 1.30, 12.51; indirect HR = 1.04; 95% CI: 0.59, 1.85) and imazethapyr (direct HR = 2.37; 95% CI: 0.76, 7.36; indirect HR = 1.16; 95% CI: 0.66, 2.04), though only three cases reported using each of these pesticides.
Table 3.
Use of specific pesticides and pesticide chemical classes by husbands, among wives who did not apply pesticides (indirect exposure), adjusted for age, Agricultural Health Study, 1993–1997 through December 31, 2011.
|
Non-cases N=13,653 (43% of cohort)
|
ESRD cases N=64 (62% of cases)
|
|||||
|---|---|---|---|---|---|---|
| Exposed N | Exposed % | Exposed N | Exposed % | HR | 95% CI | |
| Ever use of fumigants and fungicides | ||||||
| Fungicides | ||||||
| Benomyl | 1394 | 11.4 | 3 | 5.5 | 0.46 | 0.15, 1.36 |
| Captan | 1276 | 10.5 | 4 | 7.5 | 0.84 | 0.32, 2.24 |
| Chlorothalonil | 1185 | 9.0 | 7 | 11.1 | 1.26 | 0.58, 2.71 |
| Metalaxyl | 2868 | 23.3 | 16 | 28.1 | 1.26 | 0.71, 2.24 |
| Dithiocarbamate fungicides | 1306 | 10.8 | 5 | 9.3 | 0.78 | 0.32, 1.90 |
| Maneb/Mancozeb | 1265 | 10.4 | 5 | 9.1 | 0.81 | 0.33, 1.97 |
| Fumigants | ||||||
| Methyl bromide | 2105 | 15.9 | 12 | 19.0 | 1.13 | 0.60, 2.11 |
| Carbon tetrachloride/carbon disulfide | 675 | 5.6 | 3 | 5.5 | 0.81 | 0.27, 2.43 |
| Ever use of specific herbicides and herbicide chemical classes | ||||||
| Triazine herbicides | 10,507 | 77.7 | 47 | 74.6 | 0.91 | 0.52, 1.62 |
| Atrazine | 9508 | 71.7 | 41 | 67.2 | 0.84 | 0.49, 1.44 |
| Cyanazine | 5052 | 40.9 | 23 | 46.9 | 1.31 | 0.75, 2.30 |
| Metribuzin | 5416 | 44.6 | 27 | 50.0 | 1.29 | 0.76, 2.21 |
| Chloroacetanilide herbicides | 8633 | 69.2 | 40 | 76.9 | 1.59 | 0.83, 3.04 |
| Alachlor | 6774 | 54.6 | 35 | 67.3 | 1.63 | 0.91, 2.91 |
| Metolachlor | 5750 | 46.5 | 25 | 49 | 1.23 | 0.71, 2.14 |
| Thiocarbamate herbicides | 4757 | 40.3 | 26 | 53.1 | 1.67 | 0.95, 2.93 |
| Butylate | 3797 | 31.4 | 24 | 45.3 | 1.71 | 1.00, 2.95 |
| EPTC | 2357 | 19.4 | 8 | 16.7 | 0.97 | 0.45, 2.07 |
| Dinitroaniline herbicides | 8487 | 69.5 | 35 | 68.6 | 1.01 | 0.56, 1.82 |
| Pendimethalin | 5432 | 44.5 | 24 | 44.4 | 1.11 | 0.65, 1.90 |
| Trifluralin | 6504 | 52.5 | 28 | 54.9 | 1.12 | 0.65, 1.95 |
| Phenoxy herbicides | 10,374 | 76.7 | 47 | 74.6 | 0.86 | 0.49, 1.51 |
| 2,4-D | 10,010 | 75.6 | 45 | 72.6 | 0.82 | 0.47, 1.43 |
| 2,4,5-T | 2667 | 22.1 | 12 | 22.6 | 0.69 | 0.36, 1.32 |
| 2,4,5-TP | 1092 | 9.1 | 5 | 9.6 | 0.88 | 0.36, 2.15 |
| Other herbicides | ||||||
| Chlorimuron-ethyl | 4541 | 37.5 | 18 | 34.0 | 1.04 | 0.59, 1.85 |
| Dicamba | 6072 | 49.4 | 28 | 54.9 | 1.39 | 0.8, 2.42 |
| Glyphosate | 9904 | 74.5 | 44 | 71.0 | 0.92 | 0.53, 1.60 |
| Imazethapyr | 5206 | 42.6 | 21 | 41.2 | 1.16 | 0.66, 2.04 |
| Paraquat | 2952 | 24.3 | 21 | 40.4 | 1.99 | 1.14, 3.47 |
| Petroleum Oil | 5682 | 47.1 | 20 | 39.2 | 0.75 | 0.43, 1.32 |
| Ever use of specific insecticides and insecticide chemical classes | ||||||
| Pyrethroid insecticides | 2798 | 23.1 | 7 | 13 | 0.68 | 0.31, 1.49 |
| Permethrin (crops) | 1608 | 13.2 | 2 | 3.8 | 0.40 | 0.11, 1.43 |
| Permethrin (animals) | 1466 | 11.9 | 5 | 9.3 | 1.13 | 0.46, 2.79 |
| Organochlorine insecticides | 6922 | 53.0 | 42 | 71.2 | 1.27 | 0.71, 2.28 |
| Aldrin | 2312 | 19.1 | 13 | 23.6 | 0.75 | 0.40, 1.42 |
| Chlordane | 3145 | 25.8 | 14 | 25 | 0.63 | 0.34, 1.16 |
| DDT | 3332 | 27.1 | 26 | 46.4 | 1.21 | 0.68, 2.14 |
| Heptachlor | 1873 | 15.6 | 10 | 18.9 | 0.75 | 0.37, 1.51 |
| Lindane | 2212 | 18.3 | 6 | 11.1 | 0.52 | 0.23, 1.20 |
| Toxaphene | 1749 | 14.5 | 10 | 18.5 | 0.90 | 0.45, 1.80 |
| Carbamate insecticides | 8315 | 64.7 | 35 | 61.4 | 0.70 | 0.41, 1.20 |
| Aldicarb | 1484 | 12.3 | 7 | 13.0 | 1.09 | 0.5, 2.38 |
| Carbaryl | 6878 | 55.1 | 30 | 53.6 | 0.78 | 0.46, 1.32 |
| Carbofuran | 3272 | 26.7 | 16 | 31.4 | 1.06 | 0.58, 1.91 |
| Organophosphate insecticides | 11,991 | 88.6 | 51 | 81.0 | 0.58 | 0.31, 1.08 |
| Chlorpyrifos | 5576 | 42.1 | 17 | 27.4 | 0.59 | 0.34, 1.04 |
| Diazinon | 3815 | 31.4 | 12 | 21.8 | 0.54 | 0.29, 1.03 |
| Dichlorvos | 1093 | 9.0 | 9 | 16.7 | 2.03 | 0.99, 4.15 |
| Fonofos | 2602 | 21.1 | 14 | 27.5 | 1.42 | 0.77, 2.63 |
| Malathion | 8793 | 70.2 | 36 | 64.3 | 0.71 | 0.41, 1.22 |
| Parathion | 1880 | 15.7 | 4 | 7.4 | 0.39 | 0.15, 1.04 |
| Phorate | 3963 | 32.7 | 19 | 34.5 | 1.00 | 0.57, 1.74 |
| Terbufos | 4809 | 38.8 | 20 | 38.5 | 1.07 | 0.61, 1.88 |
ESRD=End-stage renal disease; HR=Hazard Ratio; CI=Confidence Interval.
No meaningful associations were found with the wives' use of the remaining herbicides with sufficient numbers for evaluation (atrazine, 2,4-D, glyphosate, petroleum oil); however, ESRD among non-applying wives increased with the husbands' use of thiocarbamate herbicides (butylate and EPTC). This association was driven primarily by a positive association observed between ESRD and husbands' ever use of butylate (HR = 1.71, 95% CI: 1.00, 2.95). We observed a corresponding positive trend with husbands' cumulative use of butylate, and an elevated ESRD rate for > median lifetime-days of EPTC (HR = 1.89; 95% CI: 0.70, 5.10 - Table 4). Ever use of the herbicide paraquat by the husbands was also significantly associated with ESRD among non-applying wives (HR = 1.99, 95% CI: 1.14, 3.47), with some evidence of a positive exposure–response trend. ESRD was positively associated with > median lifetime-days of husbands' use of pendimethalin (HR = 2.47; 95% CI: 0.87, 7.01) and petroleum oil (HR = 2.12; 95% CI: 0.75, 5.96).
Table 4.
Associations between the husbands’ cumulative lifetime use of specific chemicals and ESRD, among wives who reported no prior pesticide use, Agricultural Health Study, 1993–1997 through December 31, 2011.
|
Non-cases N=13,653
|
ESRD cases N=64
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pesticide | Lifetime-days of exposure | N | % | N | % | HR | 95% CI | p For trend |
| FUNGICIDES | ||||||||
| Chlorothalonil | 0.0 | 12,029 | 92.2 | 56 | 90.3 | – | – | |
| 1.0–45.2 | 574 | 4.4 | 3 | 4.8 | 1.21 | 0.41, 3.62 | ||
| >45.2 | 444 | 3.4 | 3 | 4.8 | 1.61 | 0.54, 4.79 | ||
| 0.3944 | ||||||||
| HERBICIDES | ||||||||
| Triazine herbicides | ||||||||
| Atrazine | 0.0 | 3853 | 29.8 | 20 | 34.5 | – | – | |
| 1.0–40.6 | 4465 | 34.5 | 19 | 32.8 | 0.91 | 0.49, 1.71 | ||
| >40.6–733.0 | 4626 | 35.7 | 19 | 32.8 | 0.76 | 0.41, 1.43 | ||
| 0.4091 | ||||||||
| Cyanazine | 0.0 | 7343 | 60.6 | 26 | 54.2 | – | – | |
| 1.0–37.2 | 2958 | 24.4 | 11 | 22.9 | 1.12 | 0.55, 2.27 | ||
| >37.2−262.9 | 1823 | 15.0 | 11 | 22.9 | 1.63 | 0.81, 3.31 | ||
| 0.1757 | ||||||||
| Metribuzina | 0.0 | 5034 | 67.6 | 21 | 65.6 | – | – | |
| 1.0–22.3 | 1656 | 22.3 | 6 | 18.8 | 1.00 | 0.41, 2.46 | ||
| 422.3–105.0 | 752 | 10.1 | 5 | 15.6 | 1.82 | 0.69, 4.76 | ||
| 0.217 | ||||||||
| Chloroacetanilide herbicides | ||||||||
| Alachlor | 0.0 | 5715 | 47.7 | 17 | 34.0 | – | – | |
| 1.0–37.5 | 3470 | 29.0 | 17 | 34.0 | 1.64 | 0.84, 3.21 | ||
| >37.5–491.4 | 2784 | 23.3 | 16 | 32.0 | 1.72 | 0.87, 3.40 | ||
| 0.2227 | ||||||||
| Metolachlor | 0.0 | 6690 | 55.7 | 26 | 54.2 | – | – | |
| 1.0–43.9 | 3118 | 26.0 | 11 | 22.9 | 1.05 | 0.52, 2.13 | ||
| >43.9–361.7 | 2194 | 18.3 | 11 | 22.9 | 1.36 | 0.67, 2.75 | ||
| 0.3934 | ||||||||
| Thiocarbamate herbicide | ||||||||
| Butylatea | 0.0 | 5678 | 76.3 | 17 | 54.8 | – | – | |
| 1.0–22.8 | 1025 | 13.8 | 7 | 22.6 | 2.70 | 1.12, 6.52 | ||
| >22.8–718.7 | 736 | 9.9 | 7 | 22.6 | 3.26 | 1.35, 7.87 | ||
| 0.0096 | ||||||||
| EPTC | 0.0 | 9835 | 81.7 | 40 | 83.3 | – | – | |
| 1.0–24.6 | 1561 | 13.0 | 4 | 8.3 | 0.83 | 0.31, 2.23 | ||
| >24.6–177.0 | 639 | 5.3 | 4 | 8.3 | 1.89 | 0.70, 5.10 | ||
| 0.2184 | ||||||||
| Dinitroaniline herbicides | ||||||||
| Pendimethalina | 0.0 | 4892 | 66.5 | 22 | 73.3 | – | – | |
| 1.0–51.5 | 2025 | 27.5 | 4 | 13.3 | 0.54 | 0.19, 1.54 | ||
| >51.5–718.7 | 438 | 6.0 | 4 | 13.3 | 2.49 | 0.88, 7.05 | ||
| 0.0622 | ||||||||
| Trifluralin | 0.0 | 5976 | 49.6 | 23 | 46.9 | – | – | |
| 1.0–36.9 | 2914 | 24.2 | 13 | 26.5 | 1.31 | 0.66, 2.59 | ||
| >36.9–357.5 | 3156 | 26.2 | 13 | 26.5 | 0.99 | 0.50, 1.95 | ||
| 0.8328 | ||||||||
| Phenoxy herbicide | ||||||||
| 2,4-D | 0.0 | 3349 | 26.1 | 17 | 28.3 | – | – | |
| 1.0–51.0 | 5063 | 39.4 | 22 | 36.7 | 0.94 | 0.50, 1.77 | ||
| >51.0–1032.5 | 4429 | 34.5 | 21 | 35.0 | 0.75 | 0.40, 1.42 | ||
| 0.3455 | ||||||||
| Other herbicides | ||||||||
| Chlorimuron ethyla | 0.0 | 5287 | 71.7 | 22 | 71.0 | – | – | |
| 1.0–7.5 | 922 | 12.5 | 5 | 16.1 | 1.76 | 0.67, 4.61 | ||
| >7.5–718.7 | 1162 | 15.8 | 4 | 12.9 | 1.02 | 0.36, 2.88 | ||
| 0.9876 | ||||||||
| Dicamba | 0.0 | 6277 | 52.7 | 23 | 47.9 | – | – | |
| 1.0–25.3 | 3175 | 26.6 | 13 | 27.1 | 1.30 | 0.66, 2.57 | ||
| >25.3–262.9 | 2463 | 20.7 | 12 | 25.0 | 1.42 | 0.7, 2.86 | ||
| 0.3802 | ||||||||
| Glyphosate | 0.0 | 3489 | 27.1 | 18 | 31.0 | – | – | |
| 1.0 to 20.3 | 4668 | 36.3 | 20 | 34.5 | 0.91 | 0.48, 1.71 | ||
| >20.3–1006.1 | 4712 | 36.6 | 20 | 34.5 | 0.89 | 0.47, 1.68 | ||
| 0.7925 | ||||||||
| Imazethapyr | 0.0 | 7067 | 59.6 | 30 | 61.2 | – | – | |
| 1.0–18.1 | 2955 | 24.9 | 10 | 20.4 | 0.97 | 0.47, 1.99 | ||
| >18.1–49.9 | 1831 | 15.4 | 9 | 18.4 | 1.47 | 0.69, 3.10 | ||
| 0.3237 | ||||||||
| Paraquata | 0.0 | 6319 | 84.8 | 24 | 80.0 | – | – | |
| 1.0–15.4 | 649 | 8.7 | 3 | 10.0 | 1.36 | 0.43, 4.30 | ||
| >15.4–102.8 | 486 | 6.5 | 3 | 10.0 | 1.78 | 0.56, 5.62 | ||
| 0.3201 | ||||||||
| Petroleum Oila | 0.0 | 6077 | 82.2 | 23 | 74.2 | – | – | |
| 1.0–30.0 | 772 | 10.4 | 4 | 12.9 | 1.75 | 0.62, 4.92 | ||
| >30.0–737.5 | 543 | 7.3 | 4 | 12.9 | 2.13 | 0.76, 5.98 | ||
| 0.1562 | ||||||||
| INSECTICIDES | ||||||||
| Organochlorine insecticides | ||||||||
| DDTa | 0.0 | 5925 | 79.8 | 19 | 67.9 | – | – | |
| 1.0–8.8 | 752 | 10.1 | 5 | 17.9 | 1.36 | 0.49, 3.80 | ||
| >8.8–236.0 | 745 | 10.0 | 4 | 14.3 | 1.11 | 0.37, 3.35 | ||
| 0.8801 | ||||||||
| Toxaphenea | 0.0 | 6635 | 88.8 | 25 | 80.6 | – | – | |
| 1.0–24.7 | 534 | 7.2 | 3 | 9.7 | 1.38 | 0.44, 4.36 | ||
| >24.7–48.2 | 299 | 4.0 | 3 | 9.7 | 2.10 | 0.66, 6.68 | ||
| 0.2042 | ||||||||
| Organophosphate insecticides | ||||||||
| Chlorpyrifos | 0.0 | 7735 | 59.5 | 45 | 72.6 | – | – | |
| 1.0–44.6 | 3621 | 27.9 | 9 | 14.5 | 0.49 | 0.24, 1.01 | ||
| >44.6–213.0 | 1643 | 12.6 | 8 | 12.9 | 0.91 | 0.43, 1.93 | ||
| 0.9139 | ||||||||
| Dichlorvos | 0.0 | 11,120 | 91.6 | 45 | 84.9 | – | – | |
| 1.0–33.5 | 540 | 4.4 | 4 | 7.5 | 2.08 | 0.78, 5.56 | ||
| >33.5–2585.5 | 476 | 3.9 | 4 | 7.5 | 2.18 | 0.81, 5.83 | ||
| 0.1254 | ||||||||
| Fonofos | 0.0 | 9776 | 80.0 | 37 | 77.1 | – | – | |
| 1.0–24.1 | 1474 | 12.1 | 6 | 12.5 | 1.18 | 0.5, 2.74 | ||
| >24.1–213.0 | 973 | 8.0 | 5 | 10.4 | 1.38 | 0.55, 3.42 | ||
| 0.4879 | ||||||||
| Malathiona | 0.0 | 3035 | 41.7 | 11 | 40.7 | – | – | |
| 1.0–13.5 | 1955 | 26.9 | 8 | 29.6 | 1.18 | 0.47, 2.93 | ||
| >13.5–217.0 | 2282 | 31.4 | 8 | 29.6 | 0.87 | 0.35, 2.16 | ||
| 0.6703 | ||||||||
| Phoratea | 0.0 | 5386 | 72.6 | 18 | 62.1 | – | – | |
| 1.0–18.1 | 1005 | 13.5 | 6 | 20.7 | 1.91 | 0.76, 4.77 | ||
| >18.1–360.1 | 1029 | 13.9 | 5 | 17.2 | 1.42 | 0.53, 3.77 | ||
| 0.4921 | ||||||||
| Terbufos | 0.0 | 7634 | 62.9 | 32 | 62.7 | – | – | |
| 1.0–37.5 | 2683 | 22.1 | 10 | 19.6 | 1.03 | 0.51, 2.10 | ||
| >37.5–357.5 | 1826 | 15.0 | 9 | 17.6 | 1.16 | 0.55, 2.43 | ||
| 0.6967 | ||||||||
| Carbamate insecticides | ||||||||
| Carbaryla | 0.0 | 4454 | 61.3 | 19 | 67.9 | – | – | |
| 1.0–19.1 | 1333 | 18.3 | 5 | 17.9 | 0.86 | 0.33, 2.28 | ||
| >19.1–1237.5 | 1482 | 20.4 | 4 | 14.3 | 0.53 | 0.18, 1.54 | ||
| 0.2547 | ||||||||
| Carbofuran | 0.0 | 9022 | 74.7 | 35 | 72.9 | – | – | |
| 1.0–53.1 | 2485 | 20.6 | 7 | 14.6 | 0.66 | 0.30, 1.46 | ||
| >53.1–236.0 | 578 | 4.8 | 6 | 12.5 | 2.22 | 0.95, 5.19 | ||
| 0.0536 | ||||||||
ESRD=End-stage renal disease; HR=Hazard Ratio; CI=Confidence Interval.
Data only available for wives whose husbands returned the take-home questionnaire.
Results were mixed for insecticides. ESRD was elevated with husbands' use of the organophosphate insecticide dichlorvos. Non-significant inverse associations were found with husbands' use of several insecticides: chlorpyrifos, diazinon, and parathion (organophosphates), permethrin for crops (pyrethroids), and lindane (organochlorine). Among the carbamate insecticides, direct, but not indirect, exposure to carbaryl appeared to be associated with an elevated rate of ESRD, and > median lifetime-days of carbofuran use by the husbands was associated with ESRD (HR = 2.22; 95% CI: 0.95, 5.19). No clear associations or patterns were observed among the other pesticides with sufficient numbers of cases for analysis .
3.2. Residential and non-application farming exposures
We found little evidence of association between other measures of potential pesticide exposure and ESRD among all wives (Table 5). The rate of ESRD was elevated for > 10 h spent in the sun each day during the growing season (vs. < 1 h), and lower for those who spent less than 10 h in the sun (vs. < 1 h) during the growing season 10 years before enrollment. ESRD incidence was positively associated with never having a job off the farm.
Table 5.
Associations between selected measures of potential non-application farming and residential pesticide exposure and ESRD among wives who reported no prior pesticide use, Agricultural Health Study, 1993–1997 through December 31, 2011.
|
All wives
|
Wives who reported no personal use
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Time frame | Level | Non-cases N (%) N=31,044 |
ESRD cases N
(%) N=98 |
HR (95% CI) |
p For trend |
Non-cases N (%) N=13,653 |
ESRD cases N (%) N=64 |
HR (95% CI) |
p For trend |
| Potential exposure to pesticides through farming activities | ||||||||||
| Number of years lived or worked on farm over lifetime |
0–28 | 13,528 (44.1) | 31 (32.6) | – | 6689 (49.9) | 20 (32.8) | – | |||
| 29–49 | 11,102 (36.2) | 31 (32.6) | 0.81 (0.48, 1.35) |
4142 (30.9) | 18 (29.5) | 0.89 (0.46, 1.71) | ||||
| ≥50 | 6024 (19.7) | 33 (34.7) | 0.8 (0.46, 1.40) |
2577 (19.2) | 23 (37.7) | 1.09 (0.55, 2.15) | ||||
| 0.452 | 0.800 | |||||||||
| Number of days worked in field last season | None | 14,965 (48.8) | 59 (61.5) | – | 8208 (61.1) | 39 (61.9) | – | |||
| 1–30 | 11,415 (37.3) | 24 (25) | 0.71 (0.44, 1.15 ) |
4119 (30.6) | 15 (23.8) | 0.87 (0.48, 1.58) | ||||
| >30 | 4261 (13.9) | 13 (13.5) | 1.04 (0.56, 1.92) |
1113 (8.3) | 9 (14.3) | 1.71 (0.83, 3.54) | ||||
| 0.616 | 0.377 | |||||||||
| Number of hours per day generally spend in sun during growing season |
At enrollment | <1 | 6136 (27) | 27 (39.7) | − | 2089 (23.9) | 11 (27.5) | – | ||
| 1–2 | 7360 (32.4) | 18 (26.5) | 0.67 (0.37, 1.22) |
2398 (27.4) | 6 (15) | 52 (0.19, 1.41) | ||||
| 3–5 | 6455 (28.4) | 15 (22.1) | 0.67 (0.36, 1.28) |
2740 (31.4) | 10 (25) | 0.74 (0.31, 1.75) | ||||
| >5 | 2797 (12.3) | 8 (11.8) | 0.89 (0.40, 1.97) |
1191 (13.6) | 8 (20) | 1.29 (0.52, 3.22) | ||||
| 0.429 | 0.657 | |||||||||
| 10 years before enrollment |
<1 | 3785 (17.7) | 15 (22.4) | – | 2089 (23.9) | 11 (27.5) | – | |||
| 1–2 | 5519 (25.9) | 11 (16.4) | 0.58 (0.26, 1.26) |
2398 (27.4) | 6 (15) | 0.52 (0.19, 1.41) | ||||
| 3–5 | 7367 (34.5) | 20 (29.9) | 0.80 (0.40, 1. 57 ) |
2740 (31.4) | 10 (25) | 0.74 (0.31, 1.75) | ||||
| 6–10 | 3763 (17.6) | 14 (20.9) | 1.09 (0.52, 2.28) |
1191 (13.6) | 8 (20) | 1.29 (0.52, 3.22) | ||||
| >10 | 908 (4.3) | 7 (10.4) | 2.32 (0.95, 5.68) |
321 (3.7) | 5 (12.5) | 3.26 (1.14, 9.3) | ||||
| 0.127 | 0.092 | |||||||||
| Ever had a job off farm | Had a job off farm | 27,354 (89.2) | 75 (77.3) | – | 11,853 (88.2) | 48 (76.2) | – | |||
| Never had a job off farm | 3303 (10.8) | 22 (22.7) | 1.46 (0.9, 2.39) |
1586 (11.8) | 15 (23.8) | 1.53 (0.84, 2.78) | ||||
| Potential residential exposure to pesticides | ||||||||||
| Clothes worn during pesticide mixing or application are washed with family wash |
At enrollment (last 12 months) |
Noa | 26,998 (89.4) | 80 (87) | – | 11,869 (90.1) | 52 (85.2) | – | ||
| Yesa | 3208 (10.6) | 12 (13) | 1.12 (0.61, 2.06) |
1309 (9.9) | 9 (14.8) | 1.37 (0.67, 2.78) | ||||
| 10 years before enrollment |
No | 20,639 (84.7) | 62 (77.5) | – | 8495 (87.1) | 40 (76.9) | – | |||
| Yes | 3729 (15.3) | 18 (22.5) | 1.60 (0.94, 2.70) |
1254 (12.9) | 12 (23.1) | 1.88 (0.99, 3.59) | ||||
| Number of times per year personally wash clothes that were worn during pesticide application or mixing |
At enrollment (last 12 months) |
<5 | 10,829 (36.6) | 40 (44.4) | – | 3908 (41.2) | 20 (42.6) | – | ||
| 5–10 | 8220 (27.8) | 28 (31.1) | 1.12 (0.69, 1.82) |
2550 (26.9) | 8 (17) | 0.64 (0.28, 1.46) | ||||
| 11–15 | 4322 (14.6) | 5 (5.6) | 0.45 (0.18, 1.12 ) |
1257 (13.3) | 6 (12.8) | 1.05 (0.42, 2.63) | ||||
| 16–20 | 2467 (8.3) | 7 (7.8) | 1.07 (0.48, 2.37) |
718 (7.6) | 6 (12.8) | 1.77 (0.71, 4.43) | ||||
| >20 | 3770 (12.7) | 10 (11.1) | 1.09 (0.55, 2.19) |
1048 (11.1) | 7 (14.9) | 1.57 (0.66, 3.72) | ||||
| 0.752 | 0.654 | |||||||||
| 10 years before enrollment |
<5 | 8155 (34.2) | 27 (35.5) | – | 5493 (42.8) | 28 (47.5) | – | |||
| 5–10 | 6872 (28.8) | 20 (26.3) | 1.03 (0.58, 1.84) |
3331 (25.9) | 15 (25.4) | 0.96 (0.51, 1.8) | ||||
| 11 –15 | 3605 (15.1) | 11 (14.5) | 1.16 (0.58, 2.36) |
1622 (12.6) | 3 (5.1) | 0.43 (0.13, 1.41) | ||||
| 16–20 | 2118 (8.9) | 8 (10.5) | 1.43 (0.65, 3.17) |
923 (7.2) | 6 (10.2) | 1.48 (0.61, 3.59) | ||||
| >20 | 3091 (13) | 10 (13.2) | 1.36 (0.65, 2.82) |
1468 (11.4) | 7 (11.9) | 1.22 (0.53, 2.81) | ||||
| 0.279 | 0.135 | |||||||||
| Distance from well to nearest area where pesticides were applied as of enrollmentb (yards) |
Don't have private well/ no pesticides mixed on farm |
3522 (21.4) | 6 (13.6) | – | 1677 (22.9) | 2b (6.9) | – | |||
| >100 | 6073 (36.8) | 16 (36.4) | 1.58 (0.62, 4.00) |
2741 (37.5) | 9 (31) | 2.44 (0.58, 10.3) | ||||
| 51–100 | 4180 (25.4) | 14 (31.8) | 1.95 (0.75, 5.01) |
1765 (24.1) | 12 (41.4) | 4.71 (1.16, 19.18) | ||||
| ≤50 | 2714 (16.5) | 8 (18.2) | 1.77 (0.62, 5.04) |
1133 (15.5) | 6 (20.7) | 3.75 (0.83, 16.96) | ||||
| 0.225 | 0.270 | |||||||||
ESRD=End-stage renal disease; HR=Hazard Ratio; CI=Confidence Interval.
No=always use disposable clothing, clothes washed separately in family machine, washed in separate machine or sent out for cleaning; Yes=clothes washed with family wash or soaked separately and then washed with family wash.
Data only available for wives whose husbands returned the take-home questionnaire.
Among residential exposures, rates were elevated for participants who reported washing clothing worn during pesticide use with the family wash (compared to not washing such clothing with the family wash) 10 years prior to enrollment; however, associations with personally washing such clothing did not monotonically increase with increasing frequency of washing. Because the distance between private well and pesticide mixing activity was reported only by the 44% of applicator husbands who returned the take-home questionnaire, data for this variable were missing for nearly half of wives. Having a private well on a farm where pesticides are mixed (compared to no private well or no pesticide mixing on the farm) appeared to increase the rate of ESRD, but we did not see a clear trend with increasing distance between the well and the pesticide mixing area.
Other farm-related and residential factors evaluated did not appear to be associated with ESRD [e.g. proximity of home to pesticide mixing and application areas, specific farm work activities (other than pesticide application), storage of pesticides in the home, and wearing boots during pesticide application in the home (data not shown)]. In general, patterns were similar but estimates were often greater in magnitude when we restricted analyses of these risk factors to spouses who reported no pesticide use, though statistical power was limited for these sub-analyses.
4. Discussion
Among wives who applied pesticides, ESRD was associated with the highest category of duration, frequency, and lifetime use of pesticides overall, as well as with ever use of several specific pesticides, suggesting that personal pesticide use may be a risk factor for ESRD. The rate of ESRD was elevated among wives whose husbands' reported ever using several specific chemicals, particularly paraquat and butylate. An apparent positive trend was observed for husbands' cumulative use of butylate, but no clear trends were observed for the 27 other chemicals for which we had sufficient numbers to conduct exposure–response analyses. Positive associations were observed with private well proximity to pesticide mixing areas, washing pesticide-exposed clothing with the family wash, and spending > 10 h in the sun during the growing season, though estimates were imprecise.
In an analysis including all spouses, we found that women who ever mixed and applied pesticides were significantly less likely to develop ESRD than women who reported no personal pesticide use. This may reflect a “healthy worker” effect whereby women with chronic diseases or other risk factors for ESRD were less likely to engage in pesticide application activities. Or, this finding may suggest other differences between AHS participants who work on the farm and those who do not. For example, we also observed a significant inverse association between ever use of pesticides and diabetes in the same dataset (Odds Ratio = 0.68, 95% CI: 0.60, 0.77: data not shown) and an inverse relationship between pesticide use and myocardial infarction among AHS spouses (Dayton et al., 2010). Those who mix and apply pesticides are significantly more physically active [(Odds Ratio = 1.45; 95% CI: 1.36, 1.55 for exercise vs. no exercise (referent)] and may otherwise have lifestyle characteristics that mitigate disease risk. On the other hand, in analyses limited to women who did mix and apply pesticides, an approach taken in other AHS studies of spouses (Starling et al., 2014) because of suspected differences between those who mix and apply and those who do not, we did find an increased risk among those in the highest quantile of cumulative pesticide use. This finding is consistent with the results of our previous study in male pesticide applicators, in which we found a significantly elevated rate of ESRD in the highest tertile of cumulative use for specific chemicals, compared to no use (Lebov et al., 2015). The only other study to evaluate the association between potential pesticide exposure and ESRD found a significant positive association with history of occupational exposure to “frequent or daily exposure to insect or plant spray” (Hsu et al., 2009).
Alachlor showed a consistently positive relationship with ESRD across analyses of direct and indirect exposures. Though these associations were not statistically significant, our finding of a moderately increased rate with alachlor use is similar to that observed in our previous analysis of applicators (Lebov et al., 2015). According to a report published by the California EPA (Office of Environmental Health Hazard Assessment, 1997), rats exposed to alachlor developed chronic nephritis and increased absolute kidney weights, but we were unable to find any additional studies to confirm these results. Though we saw an increased rate with personal use of chlorimuron-ethyl, the estimate was very imprecise, and this association was not replicated with exposure to the husbands' use. To our knowledge, the relationship between chlorimuron-ethyl exposure and renal function has not been investigated in laboratory or other epidemiological studies.
Several pesticides used by the applicator husbands were associated with increased rates of ESRD among wives who reported no personal pesticide use. Our finding of an increased rate with the husbands' use of butylate contrasts with results from our previous analysis, which did not indicate an association between butylate use and ESRD among pesticide applicators (Lebov et al., 2015). However, an experimental study in mice observed kidney lesions following administration of high doses of butylate (U.S. Environmental Protection Agency, 1984). To our knowledge, no other experimental studies have evaluated the renal effects of butylate, but, among AHS women, butylate use was significantly associated with gestational diabetes (Saldana et al., 2007), which is a risk factor for kidney disease (Mannisto et al., 2013).
Exposure to the husbands' carbofuran use appeared to be associated with ESRD at the > median lifetime use level, suggesting a possible threshold effect. Though we did not observe an association with carbofuran among male pesticide applicators (Lebov et al., 2015), nephrotoxic effects of carbofuran have been observed in rats (Kaur et al., 2012). Cumulative use of the herbicides pendimethalin and petroleum oil by the husbands was associated with an elevated rate of ESRD among the wives. In a prior study, we also observed an increased rate of ESRD with cumulative exposure to pendimethalin and petroleum oil among the applicator husbands (Lebov et al., 2015). Pendimethalin belongs to the class of dinitroaniline herbicides; a chemical used in the manufacture of dinitroaniline herbicides (4-Chlorobenzotrifluoride) was found to cause a dose-related toxic nephropathy in rats (Jameson and Yuan, 1992). However, we are unaware of any studies that have quantified any residual contamination of dinitroanilines by 4-chlorobenzotrifluoride. Additional research is needed to understand the mechanism by which pendimethalin exposure may lead to renal damage in humans.
Wives whose husbands' reported ever using paraquat had more than twice the rate of being diagnosed with ESRD compared to wives whose husbands did not use paraquat. Paraquat intoxication has been found to cause kidney damage in humans (Kim et al., 2009; Reigart et al., 1999; Wu et al., 2014); however, little is known about the effects of chronic low-level exposure to this chemical. In our previous study, we observed a significant positive trend in the rate of ESRD associated with increasing lifetime use of paraquat among pesticide applicators (Lebov et al., 2015). Exposure to paraquat produces reactive oxygen species (ROS) and related oxidative stress in renal cells (Wei et al., 2014), which can lead to renal cell apoptosis and necrosis (Kashihara et al., 2003); therefore, one possible biological mechanism for the association with paraquat, and other ROS-generating pesticides, is through repeated exposures over the lifetime, causing slow incremental renal cell damage and eventual renal dysfunction.
The likelihood of developing an adverse health outcome related to pesticides depends on a variety of factors, including route of exposure. Much is known about the health effects of occupational dermal exposures (Roberts and Reigart, 2013), but research on the health effects of non-occupational dermal routes of pesticide exposure is limited. Though handling pesticide-contaminated clothing may result in dermal pesticide exposures, prior research has not shown an association between laundering practices for pesticide-contaminated clothes and pesticide biomarkers in urine (Deziel et al., 2015). In our study, rates were elevated for washing clothing worn during pesticide application 10 years prior to enrollment, though we did not see a clear trend with increasing frequency of washing contaminated clothing. However, low numbers of cases and broad exposure categories limited our ability to observe a trend if one exists.
Exposure monitoring studies have found detectable levels of pesticides in groundwater and drinking water sources in the U.S. (Ritter, 1990; U.S. Environmental Protection Agency, 1990). Investigators of chronic kidney disease of unknown origin (CKDu) in Sri Lanka suspect involvement of drinking water contaminated with heavy metals and/or pesticides in the etiology of the disease (Bandara et al., 2011; Jayasumana et al., 2014), but epidemiologic research is limited in this area. We found some evidence of an association with having a private well near a pesticide mixing area, compared to no private well or no mixing on site. It is unclear whether this relationship is indicative of groundwater contamination or simply a marker for increased pesticide use activities by the applicator and his/her family. Additional studies which track groundwater contamination over time are needed to support research on the association between pesticide-contaminated water and kidney disease.
Proximity to pesticide-treated farmland, as a surrogate for possible pesticide drift, is associated with higher detection rates and concentrations of common agricultural pesticides in household dust (Ward et al., 2006), but has not been consistently linked to higher levels of pesticides in urine or serum samples of women who live on farms (Deziel et al., 2015). We did not observe an association with closer proximity of the home to pesticide application areas in this study. However, there may not have been enough contrast between the exposed group (< 100 yds) and the referent group (> 300 yds) to observe a difference, and pesticide drift is highly dependent upon application method, pesticide formulation, and meteorology (Pimentel, 1995), which we were not able to evaluate in this study.
Wives who reported spending an average of 10 or more hours in the sun (compared to < 1 h) per day during the growing season 10 years before enrollment had an increased rate of ESRD diagnosis. The weaker association with this factor for the growing season immediately prior to enrollment may reflect a latency period for development of disease. In this study, those who spent more hours in the sun were more likely to engage in farming activities (data not shown). In the Farm Family Exposure Study, sample of families of licensed pesticide applicators in Minnesota and South Carolina (Baker et al., 2005), women who were “in the immediate vicinity of pesticide activities” had modestly higher concentrations of pesticide biomarkers compared to women who were not present during pesticide application (Alexander et al., 2006, 2007). Thus, extended periods of time spent in the sun each day during the growing season may be an indicator for increased potential pesticide exposure through farm work activities or through spray drift.
There were several limitations to this study. Despite the large sample size of the AHS cohort, evaluation of ESRD rates in relation to the wives' direct exposure was limited to 10 specific chemicals due to insufficient numbers of exposed cases, and we could not adjust for husbands' use when evaluating associations with wives' use of specific chemicals. As a result, we may have failed to identify important associations between ESRD and less commonly used pesticides. Accrual of additional cases over the next decade will permit evaluation of direct and indirect exposure to more pesticides. Additionally, low case numbers resulted in construction of exposure categories that may not have provided enough contrast for adequate evaluation of exposure–outcome relationships. Furthermore, spouse exposure to specific chemicals was based only on ever-use. Thus dose-related effects and timing of exposure could not be explored.
To evaluate indirect exposure to specific chemicals separately from direct exposure, we restricted analyses of husbands' use to women who reported that they never mixed or applied pesticides. Though this restriction resulted in limited power to evaluate exposure–response trends, we were still able to assess husbands' ever use of most reported chemicals in relation to wives' ESRD. Wives' pesticide exposure may have been misclassified due to the lack of available data on the specific year that husbands first used each pesticide or due to potentially inaccurate recall of pesticide use by the wives or husbands. However, because outcome data were ascertained prospectively, exposure misclassification is likely to be non-differential with respect to the outcome, thus biasing estimates toward the null. Recall of pesticide use by the husbands has been found to be reasonably reliable (Blair et al., 2002) and accurate (Hoppin et al., 2002). Though validity of the wives' responses regarding pesticide use has not been assessed, we have no reason to believe that recall would be different among wives.
Because we evaluated a large number of exposures, it is possible that some of our findings are due to chance. However, confidence in our results is increased because the magnitude and direction of the estimates for wives' personal use of individual pesticides are frequently similar to those of the estimates for personal use of the same chemicals by their applicator husbands (Lebov et al., 2015). Evaluation of residential and non-application farming exposures was limited to twelve activities or behaviors that were most likely to result in or be an indicator for long-term low-level exposure. It is noteworthy that we found similar and often stronger estimates for each of these factors when we restricted the cohort to women who had not personally applied pesticides, compared to the full cohort.
This study focuses on pesticide exposure prior to enrollment. Though additional pesticide exposure data were collected in subsequent phases of the AHS, we did not use data from later phases because 74% and 60% of spouses participated in Phase 2 and Phase 3, respectively, and with small numbers of cases we would not have had enough statistical power to evaluation associations. Moreover, because end-stage renal disease develops slowly over many years, more recent exposures may play a smaller role compared to pre-enrollment exposure. Yet, it is possible that unmeasured recent exposures may exacerbate existing renal dysfunction. Future studies, with additional accrued cases and person-time, will be conducted to evaluate the relative contribution of recent vs. long-term exposures to the development of chronic renal disease.
Although diabetes and obesity are related to risk for ESRD, we did not adjust for diabetes or obesity when exploring effects of personal pesticide use and ESRD risk because these conditions may be on the causal pathway. These variables were also not included in analyses focused on risk associated with husband's pesticide use because it is highly unlikely that these characteristics in the wife would be associated with the husband's pesticide use practices. We did initially adjust for diabetes and obesity in analyses of residential and non-pesticide farm exposures, but doing so did not meaningfully change the risk estimates and thus were not included in final models. Even so, it is also possible that diabetes may act as a modifier of associations between pesticide exposure and ESRD. Due to small case numbers, we were not able to evaluate diabetes or other conditions such as hereditary congenital nephropathy as an effect measure modifiers, but accrual of additional cases over time may allow us to study this in the future. Though it would be valuable to assess whether associations are stronger for certain sub-types of renal disease (e.g. glomerulonephritis, tubulointerstitial disease, etc.), our case numbers were too sparse to permit stratification by systemic vs. non-systemic forms of ESRD. Future studies could utilize renal biopsy data to evaluate associations with non-systemic vs. systemic ESRD.
This study also had several strengths. The large size of the cohort allowed for examination of an extensive range of potential occupational and para-occupational pesticide exposure pathways, none of which have been evaluated with respect to ESRD incidence among women. Our ability to consider the number of years that the couple lived together in estimating wives' cumulative exposure to their husbands' pesticide use was an improvement upon methods used in prior AHS studies which assessed health outcomes associated with husbands’ cumulative use. We were also able to evaluate use of the five most commonly used pesticides by AHS women (Kirrane et al., 2004), all of which were readily available at home and garden stores across the country through 2004 (United States Environmental Protection Agency, 2006a, 2006b). Most biomarkers of pesticide exposure are short lived, and exposures vary over time, thereby limiting inferences regarding accuracy of historical exposure classifications using contemporary biomarker data. To address this limitation, we have used questionnaires to collect specific pesticide use information from farmers and have documented that this information is reliable (Blair et al., 2002) and plausible (Hoppin et al., 2002). Lastly, we had essentially complete case ascertainment and reliable data on ESRD diagnosis date.
5. Conclusions
Though wives who ever used any pesticide had a lower rate of ESRD compared to wives who did not use pesticides, the rate of ESRD was elevated for personal use of several specific pesticides, and wives with the greatest cumulative lifetime-use of any pesticide had four times the rate of wives who seldom used pesticides. Additionally, potential indirect exposure to specific pesticides through the husbands’ use may be associated with an increased rate of ESRD, particularly for paraquat and butylate. Considering the widespread use of paraquat in developing countries (Wesseling et al., 2001), our findings in this and in our previous study of applicators may be relevant for farmers and farm families across the globe. Because this research is preliminary, and because many of our results are imprecise, additional studies are needed to confirm our findings.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZO1 ES 049030) and by an NIH, National Institute of Diabetes and Digestive and Kidney Diseases Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32 DK 007750; PI RJ Falk).
We thank the participants of the AHS for their contribution to this research. We also thank Aaron Blair, Freya Kamel, Honglei Chen, Cynthia J. Hines, and Laura Beane Freeman for reviewing this manuscript, and Stuart Long at Westat for his assistance with data management.
Footnotes
This research was supported in by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ZO1 ES 049030) and by an NIH, National Institute of Diabetes and Digestive and Kidney Diseases Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32 DK 007750; PI RJ Falk). The authors declare they have no actual or potential competing financial interests. This research has been approved by the Institutional Review Board at the University of North Carolina (Reference ID: 13-2276).
References
- Alavanja MC, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, et al. The agricultural health study. Environ. Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C, et al. Chlorpyrifos exposure in farm families: Results from the farm family exposure study. J. Expo. Sci. Environ. Epidemiol. 2006;16:447–456. doi: 10.1038/sj.jes.7500475. [DOI] [PubMed] [Google Scholar]
- Alexander BH, Mandel JS, Baker BA, Burns CJ, Bartels MJ, Acquavella JF, et al. Biomonitoring of 2,4-dichlorophenoxyacetic acid exposure and dose in farm families. Environ. Health Perspect. 2007;115:370–376. doi: 10.1289/ehp.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab. Syndr. Obes. 2014;7:409–420. doi: 10.2147/DMSO.S51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BA, Alexander BH, Mandel JS, Acquavella JF, Honeycutt R, Chapman P. Farm family exposure study: methods and recruitment practices for a biomonitoring study of pesticide exposure. J. Expo. Anal. Environ. Epidemiol. 2005;15:491–499. doi: 10.1038/sj.jea.7500427. [DOI] [PubMed] [Google Scholar]
- Bandara JM, Wijewardena HV, Bandara YM, Jayasooriya RG, Rajapaksha H. Pollution of river mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP, Sri Lanka. Environ. Geochem. Health. 2011;33:439–453. doi: 10.1007/s10653-010-9344-4. [DOI] [PubMed] [Google Scholar]
- Blair A, Tarone R, Sandler D, Lynch CF, Rowland A, Wintersteen W, et al. Reliability of reporting on life-style and agricultural factors by a sample of participants in the agricultural health study from Iowa. Epidemiology. 2002;13:94–99. doi: 10.1097/00001648-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Chargui I, Grissa I, Bensassi F, Hrira MY, Haouem S, Haouas Z, et al. Oxidative stress, biochemical and histopathological alterations in the liver and kidney of female rats exposed to low doses of deltamethrin (dm): a molecular assessment. Biomed. Environ. Sci. – BES. 2012;25:672–683. doi: 10.3967/0895-3988.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Choudhary N, Sharma M, Verma P, Joshi SC. Hepato and nephrotoxicity in rat exposed to endosulfan. J. Environ. Biol./Acad. Environ. Biol. India. 2003;24:305–308. [PubMed] [Google Scholar]
- Cohen B, Wiles R, Bondoc E. Weed Killers by the Glass: A Citizens' Tap Water Monitoring Project in 29 Cities. Environmental Working Group; Washington, DC, USA: 1995. [Google Scholar]
- Dayton SB, Sandler DP, Blair A, Alavanja M, Beane Freeman LE, Hoppin JA. Pesticide use and myocardial infarction incidence among farm women in the agricultural health study. J. Occup. Environ. Med./Am. Coll. Occup. Environ. Med. 2010;52:693–697. doi: 10.1097/JOM.0b013e3181e66d25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel NC, Friesen MC, Hoppin JA, Hines CJ, Thomas K, Freeman LE. A review of nonoccupational pathways for pesticide exposure in women living in agricultural areas. Environ. Health Perspect. 2015;123:515–524. doi: 10.1289/ehp.1408273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Barr D, Needham L. Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ. Health Perspect. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Negrete M, Galvin K. Breaking the take home pesticide exposure pathway for agricultural families: workplace predictors of residential contamination. Am. J. Ind. Med. 2013;56:1063–1071. doi: 10.1002/ajim.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Yucel F, Dosemeci M, Sandler DP. Accuracy of self-reported pesticide use duration information from licensed pesticide applicators in the agricultural health study. J. Expo. Anal. Environ. Epidemiol. 2002;12:313–318. doi: 10.1038/sj.jea.7500232. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch. Intern. Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson CW, Yuan J. Ntp technical report on the toxicity studies of parachloro-alpha,alpha,alpha trifluorotoluene (cas no: 98-56-6) administered in corn oil and alpha-cyclodextrin to f344/n rats and b6c3f1 mice in 14-day comparative gavage studies. Toxic. Rep. Ser. 1992;14:1–C2. [PubMed] [Google Scholar]
- Jayasumana C, Gunatilake S, Senanayake P. Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int. J. Environ. Res. Public Health. 2014;11:2125–2147. doi: 10.3390/ijerph110202125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kackar R, Srivastava MK, Raizada RB. Assessment of toxicological effects of mancozeb in male rats after chronic exposure. Indian J. Exp. Biol. 1999;37:553–559. [PubMed] [Google Scholar]
- Kashihara N, Sugiyama H, Makino H. Implication of apoptosis in progression of renal diseases. Contrib. Nephrol. 2003;139:156–172. doi: 10.1159/000071742. [DOI] [PubMed] [Google Scholar]
- Kaur B, Khera A, Sandhir R. Attenuation of cellular antioxidant defense mechanisms in kidney of rats intoxicated with carbofuran. J. Biochem. Mol. Toxicol. 2012;26:393–398. doi: 10.1002/jbt.21433. [DOI] [PubMed] [Google Scholar]
- Kim S.-j, Gil H-W, Yang J-O, Lee E-Y, Hong S-Y. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol. Dial. Transplant. 2009;24:1226–1232. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- Kirrane EF, Hoppin JA, Umbach DM, Samanic C, Sandler DP. Patterns of pesticide use and their determinants among wives of farmer pesticide applicators in the agricultural health study. J. Occup. Environ. Med./Am. Coll. Occup. Environ. Med. 2004;46:856–865. doi: 10.1097/01.jom.0000135521.15169.3e. [DOI] [PubMed] [Google Scholar]
- Lebov JF, Engel LS, Richardson D, Hogan SL, Hoppin JA, Sandler DP. Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the agricultural health study. Occup. Environ. Med. 2015 doi: 10.1136/oemed-2014-102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR., Jr. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PloS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ. Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP. Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003. Am. J. Epidemiol. 2008;167:1235–1246. doi: 10.1093/aje/kwn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Environmental Health Hazard Assessment . Public Health Goal for ALACHLOR in Drinking Water. California Environmental Protection Agency; California, USA: 1997. [Google Scholar]
- Pimentel D. Amounts of pesticides reaching target pests: environmental impacts and ethics. J. Agric. Environ. Ethics. 1995;8:17–29. [Google Scholar]
- Poovala VS, Huang H, Salahudeen AK. Role of reactive oxygen metabolites in organophosphate-bidrin-induced renal tubular cytotoxicity. J. Am. Soc. Nephrol. 1999;10:1746–1752. doi: 10.1681/ASN.V1081746. [DOI] [PubMed] [Google Scholar]
- Reigart JR, Roberts JR, Agency USEP. Recognition and Management of Pesticide Poisonings. US Environmental Protection Agency; Washington, DC: 1999. [Google Scholar]
- Ritter WF. Pesticide contamination of ground water in the united states – a review. J. Environ. Sci. Health Part B – Pestic. Food Contam. Agric. Wastes. 1990;25:1–29. doi: 10.1080/03601239009372674. [DOI] [PubMed] [Google Scholar]
- Roberts JR, Reigart JR. Recognition and Management of Pesticide Poisonings. U.S. Environmental Protection Agency; Washington, DC: 2013. [Google Scholar]
- Saldana TM, Basso O, Hoppin JA, Baird DD, Knott C, Blair A, et al. Pesticide exposure and self-reported gestational diabetes mellitus in the agricultural health study. Diabetes Care. 2007;30:529–534. doi: 10.2337/dc06-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Maria C, Vilas MG, Muriana FG, Relimpio A. Subacute atrazine treatment effects on rat renal functions. Bull. Environ. Contam. Toxicol. 1986;36:325–331. doi: 10.1007/BF01623515. [DOI] [PubMed] [Google Scholar]
- Shah MD, Iqbal M. Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem. Toxicol. – Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010;48:3345–3353. doi: 10.1016/j.fct.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ. Health Perspect. 1995;103:1126–1134. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J, Roberts SM. Acceleration of autoimmunity by organochlorine pesticides in (nzb x nzw)f1 mice. Environ. Health Perspect. 2005;113:323–328. doi: 10.1289/ehp.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA. Pesticide use and incident diabetes among wives of farmers in the agricultural health study. Occup. Environ. Med. 2014;71(9):629–635. doi: 10.1136/oemed-2013-101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Health Advisory: Butylate. U.S. Environmental Protection Agency; Washington, DC: 1984. [Google Scholar]
- U.S. Environmental Protection Agency National Pesticide Survey: Summary Results of EPA's National Survey of Pesticides in Drinking Water Wells. 1990 [Google Scholar]
- U.S. Renal Data System . 2014 Researcher's Guide to the USRDS Database. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. [Google Scholar]
- United States Environmental Protection Agency . Re-registration Eligibility Decision for Diazinon. Office of Pesticide Programs; 2006a. [Google Scholar]
- United States Environmental Protection Agency . Re-registration Eligibility Decision for Malathion. Office of Pesticide Programs; 2006b. [Google Scholar]
- Uyanikgil Y, Ates U, Baka M, Bicer S, Oztas E, Ergen G. Immunohistochemical and histopathological evaluation of 2,4-di-chlorophenoxyacetic acid-induced changes in rat kidney cortex. Bull. Environ. Contam. Toxicol. 2009;82:749–755. doi: 10.1007/s00128-009-9689-5. [DOI] [PubMed] [Google Scholar]
- Van Vleet TR, Schnellmann RG. Toxic nephropathy: environmental chemicals. Semin. Nephrol. 2003;23:500–508. doi: 10.1016/s0270-9295(03)00094-9. [DOI] [PubMed] [Google Scholar]
- Ward MH, Lubin J, Giglierano J, Colt JS, Wolter C, Bekiroglu N, et al. Proximity to crops and residential exposure to agricultural herbicides in Iowa. Environ. Health Perspect. 2006;114:893–897. doi: 10.1289/ehp.8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Tian W, Liu F, Xie G. Protective effects of exogenous beta-hydroxybutyrate on paraquat toxicity in rat kidney. Biochem. Biophys. Res. Commun. 2014;447:666–671. doi: 10.1016/j.bbrc.2014.04.074. [DOI] [PubMed] [Google Scholar]
- Wesseling C, van Wendel de Joode B, Ruepert C, Leon C, Monge P, Hermosillo H, et al. Paraquat in developing countries. Int. J. Occup. Environ. Health. 2001;7:275–286. doi: 10.1179/107735201800339209. [DOI] [PubMed] [Google Scholar]
- Wiles R, Cook KA. Tap Water Blues: Herbicides in Drinking Water. Environmental Working Group; Washington, DC, USA: 1994. [Google Scholar]
- Wu WP, Lai MN, Lin CH, Li YF, Lin CY, Wu MJ. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PloS One. 2014;9:e87568. doi: 10.1371/journal.pone.0087568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MA, Sullivan JC. Hypertension: what's sex got to do with it? Physiology. 2013;28:234–244. doi: 10.1152/physiol.00013.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]