Abstract
Background
Although blacks experience worse outcomes after treatment for squamous cell carcinoma of the head and neck (HNSCC), these conclusions were based on populations where blacks comprised a minority of patients. Here, we determined the impact of race on outcomes in HNSCC patients treated with radiotherapy at an institution where blacks comprise the majority of patients.
Methods
We performed a retrospective cohort study by reviewing 366 black and 236 white patients with non-metastatic HNSCC treated with radiotherapy between 1990 and 2012. The primary study outcome measurements were locoregional control, freedom from distant metastasis, progression-free survival, and overall survival.
Results
Median follow-up was 18.3 months for all patients. The 2-year locoregional control was 71.9% for blacks compared to 64.2% for whites (HR 0.72; P = .03). There was no difference between blacks and whites regarding 2-year freedom from distant metastasis, progression-free survival, and overall survival. For Stage III–IVB patients, blacks had similar outcomes as white patients. On multivariate analysis, race was not statistically significant for locoregional control, freedom from distant metastasis, progression free survival or overall survival. Despite these similar outcomes, black patients had worse socioeconomic factors as well as increased comorbidities but similar treatment compliance.
Conclusions
With more adverse prognostic factors, black patients experienced similar oncologic outcomes as white patients after radiotherapy for HNSCC. Our data suggests that centers treating large percentages of minority patients with radiotherapy for HNSCCs may overcome existing healthcare disparities through improved treatment compliance.
Keywords: Radiotherapy, Head and Neck Neoplasms, Minority Health, Outcomes Assessment, Minority Groups
Introduction
In the United States, racial disparities persist across multiple diseases and grow in importance as the country becomes increasingly diverse. This disparity also pervades oncology, where black race has correlated with worse 5-year overall survival (OS) rates, as compared to white patients, within almost every cancer subtype, including pediatric malignancies.1, 2 A recent Surveillance, Epidemiology, and End Results (SEER) report showed that black race predicted for increased HNSCC-specific and non-cancer mortality.3 Similarly, single institution series from the University of Florida, the University of Maryland and MD Anderson Cancer Center showed that OS rates of black patients with HNSCC approached half that of white patients.4–6 The difference also extended to decreased disease-free survival, cause-specific survival and freedom from distant metastases (FFDM). Thus, black race often predicted for worse oncologic outcomes in many cancer patients including those with HNSCCs.
However, the current literature is limited by the relative and absolute number of black patients analyzed. First, black patients comprised approximately 10–15% of the study sample in most series.3–5, 7–9 Second, the absolute number of black patients in individual institutional studies is low, often ranging from 50 to 100 patients.6, 10, 11 Third, for multi-institutional studies such as the SEER database, many patient and tumor variables were unknown and complicated analyses. Finally, in all studies, blacks had other confounding factors such as increased alcohol consumption, smoking and lower socioeconomic status. Thus, studying patient cohorts with greater black representation may help better characterize the impact of race on oncologic outcomes in HNSCC.
The University of Illinois at Chicago features a unique patient demographic where blacks comprise the majority of patients treated. To this end, we sought to determine the extent of racial disparities in the outcomes of HNSCC patients in which blacks comprise a large proportion of the population.
METHODS
Eligible study population
Between 1990 and 2012, we identified 694 patients with non-metastatic, HNSCC treated with radiotherapy at University of Illinois Medical Center (UIMC). We excluded 3 patients with inadequate treatment information, 20 Asian patients and 69 patients in whom race was not documented, resulting in 366 black and 236 white patients eligible for analysis. Data was collected in accordance with The University of Illinois at Chicago Institutional Review Board guidelines (protocol 2011–1075). A single attending physician (M.T.S.) collected all patient data from available physical and electronic medical records. Prior to treatment, patients were discussed at a multidisciplinary conference and underwent oncologic workup that included history and physical examination, endoscopic evaluation of the primary tumor and imaging. Gastrostomy and tracheostomy tubes were placed in patients at the discretion of the treating physician. All patients received radiotherapy (RT) as a component of their care. Patients were evaluated by a physician at least once weekly while receiving RT, during which acute toxicities were documented. Following treatment completion, patients were followed by providers within otolaryngology and/or medical and radiation oncology, and follow-up data was acquired from visits within any UIMC department. Patients underwent routine follow-up starting 1 month after radiotherapy and followed every 2 months for 2 years, every 4–6 months during years 3–5 and yearly thereafter. Work-up of potentially recurrent disease was ordered at the discretion of the treating physician.
Measures
Documentation of race was based on patients self-reporting on clinic or hospital intake sheets. Patient comorbidity burden was approximated using the Charlson Comorbidity Index.12 Performance status was assessed using the Karnofsky Performance Status (KPS).13 Patients were staged according to the American Joint Committee on Cancer staging system at the time of diagnosis. Median income data was captured by cross-referencing patient-reported zip codes with proprietary data, accessed from the US Census Bureau and Office of Management & Budget.14 Alcohol history was defined as ≥ or < 2 alcoholic drinks per day. Smoking was defined as ≥ or < 10 pack-years. Truncated RT course was defined as one shortened by more than 5 treatment fractions due to patient non-compliance. We defined RT delays as RT courses that were completed 5d or longer than the anticipated completion based on the initial start date. The expected timeframe of RT was based according to radiation dose prescribed and, therefore, was independent of definitive or post-operative RT course.
Acute toxicity was scored according to the RTOG common toxicity criteria. Events for locoregional control (LRC), FFDM, progression-free survival (PFS), and OS were determined from the last day of radiotherapy. Patterns of local, regional or distant failure were documented as sites of first failure. LRC and FFDM was calculated as the time to locoregional or distant disease recurrence, respectively. PFS was calculated as the time to any failure or death from any cause. OS was calculated as the time to death from any cause.
Statistical analysis
All patients were included in analysis regardless of treatment compliance. Statistical analysis was performed using JMP version 9 (SAS Institute). All tests of statistical significance were two-sided, and significance was defined as a value of P < .05. The chi-square test was used to compare between discrete variables and t-test between continuous variables. Differences between medians was assessed using the Wilcoxon test. Survival analysis was performed for all patients, as well as stratified for Stage III–IVB patients alone to minimize treatment bias as this population is often more homogeneously treated than early stage HNSCC. Survival curves were plotted based on Kaplan Meier method and comparisons between categorical risk factors were conducted using the logrank test. Censoring is considered non-informative. For univariate analysis, we selected factors known to impact oncologic outcomes as well as patient and treatment characteristics that were different between black and white patients. We used Cox proportional hazards model to examine the effects of these different risk factors on event outcomes for LRC, FFDM, PFS and OS. Cox multivariate analysis was performed to adjust for explanatory confounding variables on univariate analysis. Nominal logistic regression was performed to adjust for explanatory confounding variables for truncated treatment courses and toxicity measures. Patient characteristics that were not recorded were not included during statistical analysis.
RESULTS
Population and Tumor Characteristics
Median follow-up for the entire group was 18.3 months overall, but was longer for black patients (21.5 vs. 15.4 months, P = .05; Table 1). Black and white patients presented with similar stages of disease, age, gender and KPS. Compared to white patients, black patients had a higher burden of high or very high medical comorbidities, lower median incomes and were more likely to be single. Black patients had more alcohol consumption, more than 10 pack-years of cigarette smoking and more illicit drug use. Black patients had more larynx (LX) primaries and white patients had more oral cavity (OC) primaries. When accounting for smoking, alcohol use and other socioeconomic factors, black race still accounted for a higher risk of laryngeal primaries (OR: 3.43; 95% CI 1.76–6.89; P = .0002). Black patients presented with more frequent lymphatic involvement of level IV, level V or the supraclavicular fossa (23.5% vs. 12.7%; P = .0008).
Table 1.
Patient Characteristics n = 602
| Black (n = 366) | White (n = 236) | P value | |
|---|---|---|---|
| Median age in years (IQR) | 57.7 (49.3 – 64.8) | 57.5 (48.9 – 63.2) | .83 |
| Median follow-up in months (IQR) | 21.5 (7.3 – 59.0) | 15.4 (6.3 – 46.3) | .05 |
| Gender | .20 | ||
| Male | 270 (73.8%) | 185 (78.4%) | |
| Female | 96 (26.2%) | 51 (21.6%) | |
| Karnofsky Performance Status | .18 | ||
| ≥ 70 | 305 (83.3%) | 184 (78.0%) | |
| < 70 | 25 (6.8%) | 9 (3.8%) | |
| Not stated | 36 (9.8) | 43 (18.2) | |
| Comorbidity index | .03 | ||
| Medium | 245 (66.9%) | 180 (76.3%) | |
| High | 105 (28.7%) | 51 (21.6%) | |
| Very high | 16 (4.4%) | 5 (2.1%) | |
| Stage | .08 | ||
| I | 33 (9.0%) | 12 (5.1%) | |
| II | 31 (8.5%) | 32 (13.6%) | |
| III | 64 (17.5%) | 50 (21.2%) | |
| IVA | 193 (52.7%) | 113 (47.9%) | |
| IVB | 45 (12.3%) | 29 (12.3%) | |
| Stage grouping | .72 | ||
| Early Stage (Stage I – II) | 64 (17.5%) | 44 (18.6%) | |
| Advanced Stage (Stage III – IVB) | 302 (82.5%) | 192 (81.4%) | |
| Median household income (IQR) | $28,203 ($25,143 – $36,334) | $42,774 ($36,670 – $55,301) | <.0001 |
| Relationship status | <.0001 | ||
| Divorced | 22 (6.0%) | 28 (11.8%) | |
| Married or re-married | 91 (24.8%) | 90 (38.1%) | |
| Single | 189 (51.6%) | 75 (31.8%) | |
| Widowed | 25 (6.8%) | 16 (6.8%) | |
| Not stated | 39 (10.7) | 27 (11.4%) | |
| Living situation | <.0001 | ||
| Lives alone | 150 (41.0%) | 81 (34.3%) | |
| Lives alone, with assistance | 78 (21.3%) | 29 (12.3%) | |
| Lives with others | 93 (25.4%) | 96 (40.7%) | |
| Not stated | 45 (12.3) | 30 (12.7%) | |
| Alcohol history | .02 | ||
| ≥ 2 drinks/day | 232 (63.4%) | 118 (50.0%) | |
| < 2 drinks/day | 73 (19.9%) | 60 (25.4%) | |
| Not stated | 62 (16.9%) | 58 (24.6%) | |
| Tobacco history | .01 | ||
| Yes | 306 (83.6%) | 180 (76.2%) | |
| No | 40 (10.9%) | 43 (18.2%) | |
| Not stated | 20 (5.5%) | 13 (5.5%) | |
| Illicit drug use | .01 | ||
| Yes | 56 (15.3%) | 20 (8.5%) | |
| No | 310 (84.7%) | 216 (91.5%) | |
| Primary site | <.0001 | ||
| Hypopharynx | 35 (9.8%) | 19 (8.3%) | |
| Larynx | 121 (34.0%) | 45 (19.7%) | |
| Nasal cavity | 1 (0.3%) | 5 (2.2%) | |
| Nasopharynx | 16 (4.5%) | 4 (1.8%) | |
| Oral cavity | 60 (16.9%) | 77 (33.6%) | |
| Oropharynx | 100 (28.1%) | 57 (24.9%) | |
| Other | 4 (1.1%) | 4 (1.8%) | |
| Paranasal sinus | 6 (1.7%) | 5 (2.2%) | |
| Major salivary gland | 2 (0.6%) | 4 (1.8%) | |
| Unknown primary | 11 (3.1%) | 9 (3.9%) | |
| Node levels IV/V/SCV involved | .0008 | ||
| Yes | 86 (23.5%) | 30 (12.7%) | |
| No | 280 (76.5%) | 206 (87.3%) |
SCV = supraclavicular fossa. IQR = interquartile ratio.
Treatment characteristics
White patients were more likely to receive surgery prior to RT (39.4% vs. 31.4%; P = .05; Table 2). When OC primaries, which are often treated with initial surgery, were excluded, there was no difference in post-operative RT between black and white patients (29.1% vs. 31.5%; P = .60). Similar proportions of black and white patients received chemotherapy in regards to both induction and concurrent chemotherapy. RT technique was similar between black and white patients, regarding usage of intensity modulated radiotherapy and frequency of RT delays. While black and white patients shared a similar frequency of RT delays, fewer black patients experienced a truncated RT course (3.3% vs. 8.1%, P = .01). When adjusting for comorbidities and postoperative radiotherapy, blacks still had fewer truncated RT courses (Odds Ratio 0.36; 95% CI 0.16–0.75; P = .007). Similar percentages of black and white patients were treated at distinct times during the study.
TABLE 2.
Treatment characteristics n = 602
| Black (n = 366) | White (n = 236) | P value | |
|---|---|---|---|
| RT timing | .05 | ||
| Postoperative | 115 (31.4%) | 93 (39.4%) | |
| Definitive | 251 (68.6%) | 143 (60.5%) | |
| Induction chemotherapy | .15 | ||
| Yes | 108 (29.5%) | 57 (24.2%) | |
| No | 258 (70.5%) | 179 (75.8%) | |
| Concurrent chemotherapy | .72 | ||
| Yes | 221 (60.3%) | 139 (58.9%) | |
| No | 139 (38.0%) | 97 (41.1%) | |
| Not stated | 6 (1.6%) | 0 (0%) | |
| Intensity modulated RT | .11 | ||
| Yes | 160 (43.7%) | 119 (50.4%) | |
| No | 206 (56.3%) | 117 (49.6%) | |
| RT delay | .70 | ||
| Yes | 97 (26.5%) | 56 (23.7%) | |
| No | 257 (70.2%) | 160 (67.8%) | |
| Not stated | 12 (3.3%) | 20 (8.5%) | |
| Truncated RT course | .01 | ||
| Yes | 12 (3.3%) | 19 (8.1%) | |
| No | 354 (96.7%) | 216 (91.9%) | |
| Era of RT | .63 | ||
| 1990–1997 | 66 (18.0%) | 49 (20.7%) | |
| 1998–2004 | 162 (44.3%) | 97 (41.1%) | |
| 2005–2012 | 38 (37.7%) | 90 (38.1%) |
RT = radiotherapy
Outcomes
Two-year LRC was 71.9% for black patients compared 64.2% for white patients and blacks had better LRC on univariate analysis (HR 0.72; 95% CI: 0.54 – 0.96; P = .03; Table 3). On multivariate analysis, race did not predict for LRC as only postoperative RT predicted for improved LRC (HR 0.73; 95% CI 0.52 – 1.05; P = .09). Despite having increased low neck node involvement, there was no difference in FFDM between black and white patients. Multivariate analysis showed that a history of consuming ≥ 2 alcoholic drinks daily, Stage III–IV disease, truncated RT course, era of RT and low neck node involvement independently predicted for worse FFDM.
Table 3.
Univariate analysis of outcomes in black and white patients n = 602
| Hazard Ratio (95% CI)
|
||||
|---|---|---|---|---|
| Locoregional Control | Freedom from Distant Metastasis | Progression Free Survival | Overall Survival | |
| Black | 0.72 (0.54 – 0.96) | 1.32 (0.87 – 2.03) | 0.89 (0.79 – 1.12) | 1.21 (0.87 – 1.69) |
| P value | .03 | .19 | .32 | .24 |
| KPS ≥ 70 | 0.75 (0.42 – 1.53) | 1.00 (0.42 – 3.28) | 0.62 (0.40 – 1.05) | 0.52 (0.29 – 1.03) |
| P value | .40 | .99 | .07 | .06 |
| High-very high comorbidity | 0.98 (0.89 – 1.43) | 0.79 (0.48 – 1.32) | 0.97 (0.74 – 1.24) | 1.0 (0.71 – 1.41) |
| P value | .89 | .31 | .79 | .97 |
| Advanced stage (III – IV) | 1.42 (0.96 – 2.18) | 5.11 × 109 (12.8 – ?) | 1.98 (1.40 – 2.90) | 2.78 (1.67 – 5.05) |
| P value | .08 | <.0001 | <.0001 | <.0001 |
| Income ≤ $35,000 | 0.99 (0.74 – 1.32) | 1.10 (0.75 – 1.65) | 1.04 (0.84 – 1.32) | 1.12 (0.83 – 1.53) |
| P value | .92 | .60 | .68 | .45 |
| Lives with others | 1.0 (0.72 – 1.39) | 1.14 (0.93 – 2.32) | 1.00 (0.78 – 1.30) | 1.09 (0.78 – 1.53) |
| P value | .99 | .11 | .96 | .63 |
| Primary site (Oropharynx referent) | ||||
| Hypopharynx | 0.88 (0.46 – 1.60) | 1.87 (0.96 – 3.56) | 1.22 (0.78 – 1.85) | 1.45 (0.84 – 2.45) |
| Larynx | 1.06 (0.70 – 1.61) | 0.95 (0.53 – 1.69) | 0.97 (0.70 – 1.34) | 0.86 (0.56 – 1.32) |
| Oral cavity | 1.43 (0.95 – 2.57) | 1.24 (0.70 – 2.21) | 1.30 (0.94 – 1.80) | 1.09 (0.70 – 1.69) |
| Other | 1.55 (0.94 – 2.52) | 1.56 (0.78 – 3.00) | 1.56 (1.05 – 2.27) | 1.51 (0.91 – 2.47) |
| P value | .15 | .19 | .07 | .15 |
| ≥ 2 alcoholic drinks/day | 1.49 (1.01 – 2.27) | 2.76 (1.53 – 5.49) | 1.44 (1.07 – 1.99) | 2.07 (1.33 – 3.40) |
| P value | .04 | .0004 | .02 | .0009 |
| > 10 pack-years smoking | 1.16 (0.48 – 1.83) | 0.95 (0.57 – 1.67) | 1.14 (0.82 – 1.62) | 1.77 (1.09 – 3.10) |
| P value | .48 | .84 | .43 | .02 |
| Illicit drug use | 1.21 (0.79 – 1.78) | 1.15 (0.64 – 1.93) | 1.17 (0.84 – 1.60) | 1.31 (0.84 – 1.95) |
| P value | .36 | .62 | .33 | .21 |
| Node IV/V/SCV involved | 1.31 (0.90 – 1.85) | 2.27 (1.47 – 3.44) | 1.58 (1.20 – 2.05) | 1.97 (1.38 – 2.76) |
| P value | .15 | .0003 | .001 | .0003 |
| Postoperative RT | 0.53 (0.37 – 0.73) | 0.76 (0.50 – 1.15) | 0.64 (0.50 – 0.82) | 0.68 (0.48 – 0.94) |
| P value | .0001 | .20 | .0003 | .02 |
| RT delay >5 days | 1.48 (1.06 – 2.03) | 0.96 (0.59 – 1.52) | 1.39 (1.07 – 1.78) | 1.49 (1.06 – 2.08) |
| P value | .02 | .89 | .01 | .02 |
| Truncated RT Course | 4.16 (2.39 – 6.75) | 3.71 (1.56 – 7.44) | 3.70 (2.34 – 5.55) | 4.52 (2.53 – 7.50) |
| P value | <.0001 | .005 | <.0001 | <.0001 |
| Intensity modulated RT | 1.24 (0.92–1.67) | 1.21 (0.81 – 1.79) | 1.42 (1.12 – 1.80) | 1.48 (1.07 – 2.03) |
| P value | .15 | .35 | .004 | .02 |
| Era of RT (1990–97 referent) | ||||
| 1998–2004 | 1.28 (0.86 – 1.93) | 2.35 (1.33 – 4.49) | 1.44 (1.06 – 2.00) | 1.46 (0.96 – 2.28) |
| P value | .23 | .003 | .02 | .07 |
| 2005–2012 | 1.54 (1.02 – 2.39) | 2.23 (1.18 – 4.45) | 2.07 (1.47 – 2.94) | 2.15 (1.36 – 3.47) |
| P value | .04 | .01 | < .0001 | .0009 |
KPS = Karnofsky performance status.
SCV = supraclavicular fossa.
RT = radiotherapy.
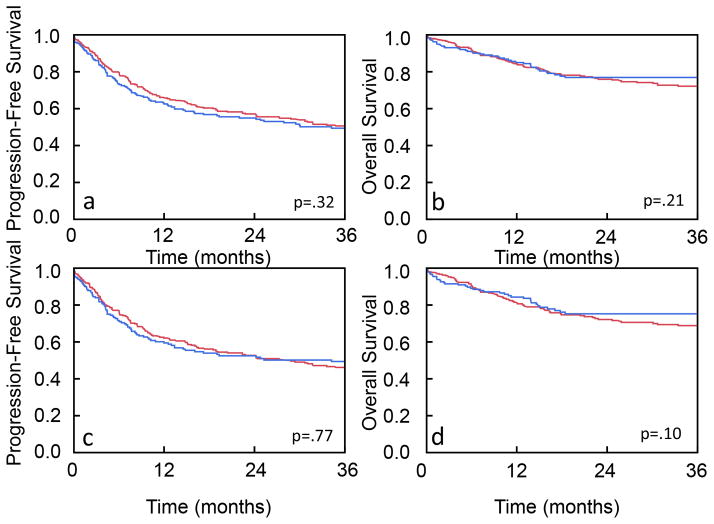
There was no difference in PFS or OS between blacks and whites (PFS: P = .32, OS: P = .21; Fig 1). Two-year PFS was 57.4% for black compared to 55.2% for white patients. Two-year OS was 76.2% for black compared to 77.7% for white patients. On univariate analysis, race did not impact PFS (HR 0.89; 95% CI 0.79 – 1.12; P = 0.32; Table 3) or OS (HR 1.21; 95% CI 0.87 – 1.69; P = 0.24). The log cumulative hazard function plots for OS and PFS were parallel and the global test failed to reject the proportional hazards assumptions with a P value of 0.41 and 0.23, respectively, indicating that the proportional hazard assumption holds for OS and PFS (data not shown). On multivariate analysis, definitive RT, Stage III–IV and increased alcohol history were associated with decreased PFS and OS (Table 4).
Figure 1. Kaplan-Meier analysis of outcomes in black and white patients.
(a) PFS and (b) OS for all HNSCC patients treated with radiotherapy. (c) PFS and (d) OS for Stage III–IV HNSCC patients treated with radiotherapy. Blue line represents white patients. Red line represents black patents. The logrank test was used to assess for differences in OS and PFS.
TABLE 4.
Multivariate analysis of outcomes in black and white patients (n = 602)
| Hazard Ratio (95% Confidence Interval)
|
||||
|---|---|---|---|---|
| Local Control | Freedom From Distant Metastasis | Progression Free Survival | Overall Survival | |
| Black | 0.73 (0.52–1.05), | 0.90 (0.66–1.83), | 0.90 (0.80–1.52), | 1.11 (0.71–1.77), |
| P value | .09 | .68 | .52 | .66 |
| Tumor stage III–IV | 1.59 (0.99–2.70) | 4.43 × 109 (7.54–7.79×1041), | 2.37 (1.48–4.03), | 2.87 (1.47–6.34), |
| P value | .05 | < .0001 | .0002 | .001 |
| ≥ 2 drinks per day | 1.30 (0.87–1.99), | 3.53 (1.77–8.12), | 1.46 (1.03–2.10), | 1.77 (1.08–3.06), |
| P value | .20 | .0001 | .03 | .02 |
| RT delay >5 days | 1.41 (0.96–2.02), | N.A. | 1.27 (0.92–1.73), | 1.31 (0.84–1.98), |
| P value | .08 | .15 | .23 | |
| Post-operative RT | 0.55 (0.36–0.81), | N.A. | 0.64 (0.46–0.89), | 0.62 (0.39–0.96), |
| P value | .003 | .007 | .03 | |
| Era of RT (1990–97 referent) | ||||
| 1998–2004 | 0.93 (0.59–1.51) | 2.19 (1.03–5.41) | 1.17 (0.76–1.84) | 1.53 (0.81–3.08) |
| P value | .76 | .04 | .48 | .20 |
| 2005–2012 | 1.23 (0.76–2.03) | 2.69 (1.19–6.91) | 1.68 (096–2.97) | 2.12 (0.96–4.87) |
| P value | .41 | .02 | .07 | .06 |
| Truncated RT course | N.A. | 4.08 (1.40–9.53), | N.D. | N.D. |
| P value | .01 | |||
| Node levels IV/V/SCV | N.A. | 1.79 (1.05–2.98), | 1.42 (0.98–2.03), | 1.31 (0.80–2.48), |
| P value | .03 | .06 | .27 | |
| KPS ≥ 70 | N.A. | 1.17 (0.48–3.89), | 0.90 (0.53–1.65), | 1.02 (0.49–2.48), |
| P value | .76 | .71 | .97 | |
| Intensity modulated RT | N.A. | N.A. | 1.06 (0.73–1.57) | 1.15 (0.69–1.90) |
| P value | .73 | 0.59 | ||
| > 10 pack-years | N.A. | N.A. | N.A. | 1.36 (0.60–3.93), |
| P value | .49 | |||
KPS = Karnofsky Performance Status.
N.A. = Not Applicable due to P value > .1 on univariate analysis.
N.D. = Not Done due to too few events for analysis.
Toxicity
Black patients experienced significantly less grade ≥ 3 acute mucositis (21.3% vs. 27.1%, P = .001) but similar rates of acute dermatitis, weight loss, and feeding-tube placement during RT as well as long terms dependence on feeding-tubes and tracheostomies. On multivariate analysis, black patients still experienced significantly less Grade 3 or greater mucositis during therapy (OR 0.50; 95% CI 0.29–0.84; P = .009; Table 5). Feeding tube placement during RT was associated with increased Stage, smoking history, concurrent chemotherapy and conformal radiotherapy usage.
TABLE 5.
Multivariate analysis of toxicity (n = 602)
| Odds Ratio
|
||||||
|---|---|---|---|---|---|---|
| Acute toxicity | Late toxicity | |||||
|
| ||||||
| ≥ Grade 3 mucositis | ≥ Grade 3 dermatitis | Feeding tube during RT | ≥ 10% Weight loss | Feeding tube at failure or last follow-up | Tracheostomy at failure or last follow-up | |
| Black (95% CI) | 0.50 (0.29–0.84) | 0.90 (0.50–166) | 0.69 (0.42–1.12) | 0.63 (0.20–2.07) | 0.64 (0.39–1.06) | 1.34 (0.78–2.52) |
| P value | .0009 | .74 | .14 | .43 | .09 | .29 |
| Tumor stage III–IV (95% CI) | 1.67 (0.75–3.99) | 5.72 (2.00–19.58) | 26.82 (9.01–116.3) | 6.28×106 (0.75- ) | 42.30 (8.58–767.4) | 11.06 (3.11–70.84) |
| P value | .21 | .007 | <.001 | .06 | <.0001 | <.0001 |
| ≥ 2 drinks per day (95% CI) | 0.77 (0.44–1.37) | 2.07 (1.02–4.46) | 1.46 (0.85–2.52) | 1.58 (0.38–10.7) | 2.11 (1.17–3.91) | 1.90 (1.00–3.83) |
| P value | .38 | .04 | .17 | .56 | .01 | .05 |
| > 10 pack-years (95% CI) | 1.49 (0.56–4.40) | 7.30 (1.36–136.2) | 3.37 (1.40–8.79) | 1.00 (0.16–19.2) | 6.35 (2.03–28.21) | 4.97 (1.37–32.07) |
| P value | .44 | .02 | .006 | .99 | .0008 | .01 |
| High comorbidity (95% CI) | 0.71 (0.39–1.26) | 1.20 (0.64–2.25) | 1.24 (0.73–2.11) | 0.28 (0.01–1.56) | 1.34 (0.77–2.31) | 1.36 (0.76–2.41) |
| P value | .24 | .57 | .43 | .17 | .30 | .30 |
| Very high comorbidity (95% CI) | 3.37 (0.88–13.71) | 1.21 (0.16–6.28) | 0.66 (0.15–2.73) | 2.51×10−7 (0–15.7) | 0.83 (0.16–3.53) | 1.87 (0.36–7.79) |
| P value | .08 | .83 | .57 | .53 | .81 | 0.42 |
| KPS ≥ 70 (95% CI) | 1.03 (0.37–3.19) | 1.32 (0.44–4.56) | 1.04 (0.38–2.75) | 8.75×106 (0.32- ) | 1.27 (0.48–3.47) | 1.38 (0.49–4.56) |
| P value | 0.95 | .63 | .94 | .23 | 0.63 | 0.56 |
| Post-operative RT (95% CI) | 0.59 (0.32–1.06) | 0.34 (0.16–0.70) | 0.84 (0.49–1.43) | 0.94 (0.22–3.47) | 0.57 (0.32–0.99) | 1.40 (0.78–2.52) |
| P value | .08 | .003 | .52 | .93 | .05 | 0.26 |
| Concurrent chemotherapy (95% CI) | 1.89 (0.96–3.83) | 1.17 (0.55–2.51) | 2.80 (1.49–5.39) | 1.92 (0.43–10.8) | 3.02 (1.58–5.94) | 2.38 (1.22–2.52) |
| P value | .07 | .68 | .001 | .40 | .0007 | .01 |
| Intensity modulated RT (95% CI) | 0.76 (0.44–1.30) | 0.25 (0.13–0.45) | 0.26 (0.15–0.44) | 0.49 (0.13–1.74) | 0.33 (0.19–0.56) | 0.23 (0.13–0.41) |
| P value | .32 | <.001 | <.001 | 0.27 | <.0001 | <.0001 |
RT = radiotherapy.
KPS = Karnofsky Performance Status.
DISCUSSION
Here, we show similar outcomes between black and white patients treated with radiotherapy for HNSCC. The absence of racial disparity was not due to worse than expected outcomes in the white cohort. Namely, the 2-year OS and PFS were similar to other studies examining racial disparities.4, 6, 7 Conversely, the outcomes of black patients in our study were similar to those historically reported for white patients.6, 7 Given that the outcomes of blacks and whites are similar after radiotherapy, analyzing our results may enable us to determine how to overcome healthcare disparities within oncology and potentially other diseases.
Many reports have suggested that patient-specific socioeconomic factors drive outcome disparities in HNSCC.3, 7, 15 In our dataset, blacks had lower median incomes as well as higher rates of comorbidities, unmarried status and alcohol, tobacco and illicit drug use. Other studies also show that patient-specific socioeconomic factors offer an incomplete explanation.3, 4, 6, 7 When controlled for race, unmarried status, and socioeconomic status, a recent SEER analysis of 34,568 patients in which 12.4% of patients were black showed that black race remained independently predictive of increased HNSCC-specific mortality.3 Additionally, a study of 20,915 patients of which 8.4% were black showed that for all levels of poverty, the median OS for black patients remained less than that of white patients.7 By contrast, the black cohort in our study had similar oncologic outcomes as whites despite adverse factors. Taken together, our data suggest that traditional socioeconomic factors may inadequately explain racial disparities.
One possibility for the lack of racial disparities in our study may be due to a lower incidence of oropharyngeal primaries associated with human papillomavirus (HPV) in our study. In these cases, white patients have higher rates of favorable prognosis HPV-positive HNSCCs that approach half of all HNSCCs treated and, consequently, result in improved OS rates compared to black patients.16–18 Some studies have reported racial disparities in outcomes that were likely due to divergent outcomes in oropharyngeal primaries among races suggesting that the impact of HPV-positive cancers on racial disparities.6, 15 By contrast, in other series, black patients still had worse outcomes in subsites where HPV does not impact prognosis.8, 15 Since 2009, 9 of 105 patients with HNSCC in our series tested positive for the HPV biomarker p16 of which the majority were white patients with oropharyngeal primaries (15.4% white patients vs. 4.6% black patients; P = .06). While our series cannot account for HPV-positive cancers, our results indicate equivalent outcomes for black and white patients with non-HPV-positive HNSCCs that were treated with radiotherapy.
The large relative and absolute representation of blacks in our series may explain similar observations regarding the lack of healthcare disparity between black and white patients. Whereas most previous single institutional studies reported on blacks in only 10–15% of the total population 3–5, 7–9 and/or less than 100 patients in total,6, 10, 11 our observations were based on 366 black patients comprising 61% of the total population. In a patient population where 47% were black, Connell et al. showed that blacks and whites had statistically equivalent biochemical control after radiotherapy for prostate cancer.19 When blacks represented 55 to 60% of the patients, two separate studies on women with breast cancer demonstrated that race did not impact outcomes. 20, 21 Thus, we reason that black patients may experience less disparate outcomes when they comprise a larger proportion of the treated population.
We postulate several reasons may account for how the percent of blacks in the patient population impacts outcomes. One reason may be that the small sample sizes of blacks in previous studies are more prone to random variations and may complicate analyses. Another possibility may rely upon more effective communication between the healthcare provider and the patient. Black patients have described their healthcare visits as less informative, supportive, and partnering that may translate into inferior treatment compliance and outcomes.22 Since effective communication between healthcare providers and patients may improve treatment compliance, we observed that blacks and whites had similar rates of completing the intended radiotherapy course. Furthermore, effective communication resulting in improved treatment compliance may have improved outcomes because we observed that those who experienced truncations or delays in treatment had worse 2-year PFS and OS. In contrast to the racial variations in treatment delivery 23, white and black patients, in our series, received similar treatments with respect to surgery, chemotherapy, and radiotherapy. Thus, the similar types of treatment regimens and rates of compliance may be viewed as a surrogate for effective communication between healthcare providers and patients.
In addition, we also find that the incidence of OC and LX primaries differed between white and black patients while oropharynx primaries did not. While some groups have not seen racial differences in primary sites4, 7, other groups have shown increased percentages of oropharynx and oral cavity primaries in whites.6, 15 These racial differences in tumor incidences may be due to referral patterns of our institution as we have lower rates of HPV-positive cancers than would be expected. Nevertheless, it will require further study to determine whether factors unique to our institutional demographics or biological factors account for the differences in primary sites between races.
Our results are limited as with any retrospective review. First, our study relied on a relatively small number of patients over a 22 year time frame. Nevertheless, the number of black patients in this study approximates as many black patients as accrued in a multi-institutional study involving 100 centers.9, 24 Second, over 80% of our patients had locally advanced disease which may reflect both the lower socioeconomic status of our patients as well as referral patterns of our institution. Yet, our rate of advanced disease is similar to other reports documenting racial disparities in HNSCC.4, 5 Third, we restricted our analysis to patients who received radiotherapy and, therefore, cannot comment on the racial disparities in patients treated with only surgery or chemotherapy. While race may not impact outcomes after radiotherapy, our results are still applicable to the majority of patients as a recent SEER analysis demonstrated that 79.7% of HNSCC patients received radiation as a component of their care.25 Finally, the lack of disparity in our series may be due to a larger proportion of disadvantaged patients regardless of race. Still, white patients in our population had significantly better socioeconomic factors than black patients. Furthermore, the OS and LRC for black patients in our series was as good or better than white patients and on par with the outcomes of white patients reported in multiple other series.4–7, 15 Therefore, despite worse socioeconomic factors in black patients, we observed similar outcomes among black and white patients treated with radiotherapy.
Thus, we find that race does not predict outcomes in HNSCC when minorities comprise a large proportion of the patient population. It is likely that biologic and patient-specific socioeconomic factors cannot adequately explain racial differences in outcomes in HNSCCs. We propose that centers caring for a greater percentage of minority patients may have unique patient-healthcare provider relationships to overcome racial disparities in healthcare. These results may thus be applicable to other cancers and other non-malignant diseases.
Acknowledgments
M.T.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M.T.S. is supported by a grant from the Burroughs Wellcome Career Award for Medical Scientists.
Footnotes
Financial Disclosures: None.
Conflicts of Interest: None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Carroll WL. Race and outcome in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2061–3. doi: 10.1001/jama.290.15.2061. [DOI] [PubMed] [Google Scholar]
- 3.Rose BS, Jeong JH, Nath SK, Lu SM, Mell LK. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29(26):3503–9. doi: 10.1200/JCO.2011.35.7301. [DOI] [PubMed] [Google Scholar]
- 4.Al-Othman MO, Morris CG, Logan HL, Hinerman RW, Amdur RJ, Mendenhall WM. Impact of race on outcome after definitive radiotherapy for squamous cell carcinoma of the head and neck. Cancer. 2003;98(11):2467–72. doi: 10.1002/cncr.11822. [DOI] [PubMed] [Google Scholar]
- 5.Moore RJ, Doherty DA, Do KA, Chamberlain RM, Khuri FR. Racial disparity in survival of patients with squamous cell carcinoma of the oral cavity and pharynx. Ethn Health. 2001;6(3–4):165–77. doi: 10.1080/13557850120078099. [DOI] [PubMed] [Google Scholar]
- 6.Settle K, Taylor R, Wolf J, Kwok Y, Cullen K, Carter K, et al. Race impacts outcome in stage III/IV squamous cell carcinomas of the head and neck after concurrent chemoradiation therapy. Cancer. 2009;115(8):1744–52. doi: 10.1002/cncr.24168. [DOI] [PubMed] [Google Scholar]
- 7.Molina MA, Cheung MC, Perez EA, Byrne MM, Franceschi D, Moffat FL, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113(10):2797–806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 8.Nichols AC, Bhattacharyya N. Racial differences in stage and survival in head and neck squamous cell carcinoma. Laryngoscope. 2007;117(5):770–5. doi: 10.1097/MLG.0b013e318033c800. [DOI] [PubMed] [Google Scholar]
- 9.Wong SJ, Harari PM, Garden AS, Schwartz M, Bellm L, Chen A, et al. Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN): analysis of chemoradiation treatment approaches in the United States. Cancer. 2011;117(8):1679–86. doi: 10.1002/cncr.25721. [DOI] [PubMed] [Google Scholar]
- 10.Murdock JM, Gluckman JL. African-American and white head and neck carcinoma patients in a university medical center setting. Are treatments provided and are outcomes similar or disparate? Cancer. 2001;91(1 Suppl):279–83. doi: 10.1002/1097-0142(20010101)91:1+<279::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen LM, Li G, Reitzel LR, Pytynia KB, Zafereo ME, Wei Q, et al. Matched-pair analysis of race or ethnicity in outcomes of head and neck cancer patients receiving similar multidisciplinary care. Cancer Prev Res (Phila) 2009;2(9):782–91. doi: 10.1158/1940-6207.CAPR-09-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Karnofsky DA, Burchenal J. The Clinical Evaluation of Chemotherapeutic Agents in Cancer. Columbia Univ Press; 1949. [Google Scholar]
- 14.Datasheer, L.L.C. [Accessed April 8, 2012];2012 http://www.zip-codes.com/zip-code-database.asp. [Google Scholar]
- 15.Gourin CG, Podolsky RH. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116(7):1093–106. doi: 10.1097/01.mlg.0000224939.61503.83. [DOI] [PubMed] [Google Scholar]
- 16.Chernock RD, Zhang Q, El-Mofty SK, Thorstad WL, Lewis JS., Jr Human papillomavirus-related squamous cell carcinoma of the oropharynx: a comparative study in whites and African Americans. Arch Otolaryngol Head Neck Surg. 2011;137(2):163–9. doi: 10.1001/archoto.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settle K, Posner MR, Schumaker LM, Tan M, Suntharalingam M, Goloubeva O, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res (Phila) 2009;2(9):776–81. doi: 10.1158/1940-6207.CAPR-09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connell PP, Ignacio L, Haraf D, Awan AM, Halpern H, Abdalla I, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54–61. doi: 10.1200/JCO.2001.19.1.54. [DOI] [PubMed] [Google Scholar]
- 20.Chu QDSM, Williams M, Panu L, Johnson LW, Shi R, Li BDL, Glass J. Race/Ethnicity Has No Effect on Outcome for Breast Cancer Patients Treated at an Academic Center with a Public Hospital. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2157–61. doi: 10.1158/1055-9965.EPI-09-0232. [DOI] [PubMed] [Google Scholar]
- 21.Komenaka IK, Martinez ME, Pennington RE, Jr, Hsu CH, Clare SE, Thompson PA, et al. Race and ethnicity and breast cancer outcomes in an underinsured population. J Natl Cancer Inst. 2010;102(15):1178–87. doi: 10.1093/jnci/djq215. [DOI] [PubMed] [Google Scholar]
- 22.Gordon HS, Street RL, Jr, Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol. 2006;24(6):904–9. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 23.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 24.Ang KK, Chen A, Curran WJ, Jr, Garden AS, Harari PM, Murphy BA, et al. Head and neck carcinoma in the United States: First comprehensive report of the Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN) Cancer. 2012 doi: 10.1002/cncr.27609. [DOI] [PubMed] [Google Scholar]
- 25.Dansky Ullmann C, Harlan LC, Shavers VL, Stevens JL. A population-based study of therapy and survival for patients with head and neck cancer treated in the community. Cancer. 118(18):4452–61. doi: 10.1002/cncr.27419. [DOI] [PubMed] [Google Scholar]