Abstract
Background
Gene polymorphisms encoding the enzyme NADPH–cytochrome P450 oxidoreductase (POR) contribute to inter-individual differences in drug response.
Aim
To estimate polymorphic allele frequencies of the POR gene in a Czech Slavic population.
Materials & Methods
The gene POR was analyzed in 322 Czech Slavic individuals from a control cohort by sequencing and HRM analysis.
Results
Twenty-five SNP genetic variations were identified. Of these variants, 7 were new, unreported SNPs, including two SNPs in the 5´flanking region (g.4965 C>T and g.4994 G>T), one intronic variant (c.1899 −20C>T), one synonymous SNP (p.20Ala=) and three nonsynonymous SNPs (p.Thr29Ser, p.Pro384Leu and p.Thr529Met). The p.Pro384Leu variant exhibited reduced enzymatic activities compared to wild type.
Conclusion
New POR variant identification indicates that the number of uncommon variants might be specific for each subpopulation being investigated, particularly germane to the singular role that POR plays in providing reducing equivalents to all CYPs in the endoplasmic reticulum.
Keywords: POR, CYP, P450 oxidoreductase, allele frequencies, haplotype, Czech Slavic population, pharmacogenetics
Introduction
Cytochrome P450 oxidoreductase (POR, E.C. 1.6.2.4.) belongs to a small family of diflavin reductases that donate electrons to a wide spectrum of heme-containing enzymes. POR is located on the cytosolic side of the endoplasmic reticulum and is essential in several biochemical processes such as steroidogenesis, xenobiotic metabolism, catabolism of bile and fatty acids and heme and squalene oxygenation [1, 2]. As inferred from its name, the main redox partners of POR constitute a group of microsomal cytochrome P450 enzymes (CYPs) [3]. The human genome contains 50 genes coding for microsomal CYPs, among which are several steroidogenic and many drug-metabolizing proteins [1]. CYP enzymes from families 1 to 3 are responsible for the metabolism of ~80–90% of all phase I-dependent, clinically used drugs [4, 5]. Since POR functions as the unique electron donor to all microsomal CYPs, pharmacogenomics interest has focused on POR genetic variants in recent years.
Human POR is a ~79 kDa protein comprised of 680 amino acids. It contains two flavin cofactors, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). The atomic structure of the soluble human POR protein has been described recently [6]. The molecule consists of three domains, a FMN-binding domain, a connecting domain and a FAD/NADP(H)-binding domain [6]. The connecting domain performs an important function as a flexible hinge, which brings two cofactor-binding domains into close proximity and thus facilitates the electron flow within the POR molecule [7, 8]. Electrons are transferred from NADPH through FAD and FMN to the respective acceptors and electron flow is controlled by the redox properties of the flavin cofactors [9], which are dictated by the protein structure. Molecular changes in the POR gene cause disruption in POR catalytic activities [10–18]. In mice, complete loss of the Por gene is embryonically lethal [19, 20]. Liver-specific knockout of Por generates phenotypically normal mice with seriously affected hepatic drug metabolism [21]. Initial studies of POR variants focused on catalytic assays with steroid-metabolizing CYPs [10–13], but recent interest has been on drug-metabolizing enzymes [14–17] and several in vivo studies have been performed [22–25]. With a growing number of POR assays becoming available, it has been demonstrated that the catalytic ability of one POR mutant with a particular CYP cannot predict its catalytic ability with another CYP [15, 17], or with another redox partner such as heme oxygenase [26]. POR function may also vary with CYP isoform [27] and the specific substrate metabolized [15, 17]. Thus, every single mutant must be individually assayed with the specific CYP of interest combined with the unique substrate being investigated.
The POR gene was identified by Shephard et al. [28] and it was localized to human chromosome 7q11.2. The gene contains 15 coding exons and a single untranslated exon termed 1U [29]. Molecular changes in the gene coding for POR were for the first time reported in 2004 [10, 30, 31]. Genetic variations identified to date are summarized on the official web site of POR polymorphisms (http://www.cypalleles.ki.se/por.htm) and, so far, 48 POR alleles/haplotypes have been published there. Recessive mutations in the POR gene have been associated with Antley-Bixler syndrome (ABS), disordered steroidogenesis, ambiguous genitalia, congenital adrenal hyperplasia (CAH) and polycystic ovary syndrome [32, 33]. Patients usually present with a combination of these symptoms and the syndrome caused by defects in POR has been termed P450 oxidoreductase deficiency (PORD) [32]. To date, five population genetic studies addressing the distribution of POR genetic differences within various populations have been performed [12, 34–37]. Specific subgroups of the Caucasian population were represented in several of these reports [12, 34, 35]. This study was undertaken to further define POR allele frequencies in the, so far unreported, Caucasian subpopulation, specifically in the Czech Slavic population.
Methods
Ethics
The study was carried out in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Committee of Medical Ethics at the General University Hospital in Prague. Informed consent was obtained from all adult participants and from parents of underage individuals.
Samples
The study enrolled a total of 322 subjects, including 144 males and 178 females. 227 DNA samples of adults were acquired during the longitudinal collection of control samples of healthy individuals from Czech Slavic population in our laboratory. In addition, the DNA of 95 neonates was extracted from cord blood [38].
DNA analysis
Genomic DNA was extracted from peripheral blood samples anticoagulated with EDTA according to a standard protocol or from cord blood using previously described methods [38]. All 16 exons of the POR gene with surrounding exon/intron boundaries (more than 20 bp of their flanking regions) were amplified by PCR using specific primers [37]. DNA analysis (HRM analysis and DNA sequencing) were implemented according to previously described techniques [37] with minor changes. We extended the region of exon 1U (E1U) by using following primers: Fw 5´CGAAGGAGGAGGCTAGACCG-3´ and Rev 5´-AAGCTGTGGAAAAGTCGACCC-3´. The PCR products of E1U region were thus 651 bp long. The PCR reactions were carried out in a total volume of 12.5 µl, including 25 ng of genomic DNA, 0.1 µM of each primer, 8% DMSO and 1× Plain PP Master Mix (Top-Bio, Prague, Czech Republic). Initial denaturation was at 94°C for 2 min, followed by 33 cycles of 30 s denaturation at 94°C, 30 s annealing at 65 °C and 45 s elongation at 72°C, with a final extension at 72°C for 5 min.
Haplotyping studies
The linkage disequlibrium and haplotype block estimations analyses were performed as previously described [37]. In short, unphased genotype data of the whole cohort were entered into the Genotype Visualization and Algorithmic Tool (GEVALT) version 2 software [39]. Only polymorphisms passing the following thresholds were used for phasing and further steps: Hardy-Weinberg p > 0.001, minimum genotype % = 100%, minimum minor allele frequency = 0.001. Phasing, the linkage disequilibrium (LD) analysis and estimation of the haplotype block structure were performed utilizing the Genotype Resolution and Block Identification using the Likelihood (GERBIL) algorithm [40]. Comparative LD analysis was performed using the SNPs common to the current study and the previous report of POR polymorphisms in two Jewish cohorts [37].
Statistical method
Statistical analysis was performed by using software STATISTICA 10 (StatSoft, Czech Republic) and Pearson’s chi-squared test.
Results
In order to determine the frequency of POR genetic variations in the normal population, all 16 exons and exon/intron boundaries were examined in 322 Czech individuals. With the view of identifying potential regulatory variants, we extended the sequence of exon 1U in the region containing SP1 binding sites, which have been shown to play a critical role in POR transcription [41]. We identified 25 distinct POR genetic variations (Table 1). Four of them were found in the 5´-flanking region, 7 in intronic regions and 14 in the protein coding regions (exons). From the 14 variations found in exons, 7 were synonymous, 6 were missense single nucleotide polymorphisms (SNP) and one SNP was found in the first untranslated exon. Of the 25 variants identified, 7 were new SNPs, not described previously, including two SNPs in the 5´-flanking region (g.4965 C>T and g.4994 G>T), one intronic variant (c.1899 −20 C>T), one synonymous SNP (p.20Ala=) and three nonsynonymous SNPs (p.Thr29Ser, p.Pro384Leu and p.Thr529Met). All 7 novel SNPs were found as individual heterozygotes at allele frequencies 0.002 (0.2%). All SNPs found in the cohort are summarized in Table 1.
Table 1.
Genetic variations in the POR gene found in the Czech Slavic population. Newly found variants are shown in red, previously described ones in yellow.
| Exon | g. | c. | p. | SNP ID | CZ (644) | CZ | AJ | MJ | Hart | AA | CA | AS | ME | Gomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5´-Flanking | 4849 C>A | rs72553972 | 113 | 0,175 | - | - | - | 0,026 | 0,130 | 0,078 | 0,077 | |||
| 5´-Flanking | 4883 C>T | rs139824475 | 3 | 0,005 | - | - | - | - | - | - | - | |||
| 5´-Flanking | 4965 C>T | 1 | 0,002 | - | - | - | - | - | - | - | ||||
| 5´-Flanking | 4994 G>T | 1 | 0,002 | - | - | - | - | - | - | - | ||||
| Exon 1 | 5036 A>C | −47 A>C | rs3823884 | 159 | 0,247 | 0,224 | 0,221 | - | 0,812 | 0,266 | 0,311 | 0,377 | ||
| Intron 1 | 5099 C>T | −5 +21 C>T | rs72553978 | 5 | 0,008 | - | - | - | 0,000 | 0,009 | 0,000 | 0,003 | ||
| Exon 2 | 43951 C>T | 60 C>T | Ala20= | 1 | 0,002 | - | - | - | - | - | - | - | ||
| Exon 2 | 43976 A>T | 85 A>T | Thr29Ser | 1 | 0,002 | - | - | - | - | - | - | - | ||
| Exon 5 | 70258 A>G | 387 A>G | Pro129= | rs1135612 | 183 | 0,284 | 0,318 | 0,360 | 0,212 | 0,083 | 0,268 | 0,419 | 0,420 | 0,260 |
| Intron 5 | 70943 G>A | 517 −4 G>A | rs41299496 | 1 | 0,002 | - | - | - | - | - | - | - | ||
| Exon 10 | 73673 C>T | 984 C>T | Ala328= | rs72557941 | 7 | 0,011 | - | - | - | - | - | - | - | 0,007 |
| Intron 10 | 74663 C>G | 1067 −13C>G | rs4732516 | 634 | 0,984 | 0,991 | 0,938 | 0,953 | 0,609 | 0,959 | 0,870 | 0,869 | ||
| Exon 11 | 74721 G>A | 1112 G>A | Arg371His | 1 | 0,002 | - | - | - | - | - | - | - | ||
| Exon 11 | 74760 C>T | 1151 C>T | Pro384Leu | 1 | 0,002 | - | - | - | - | - | - | - | ||
| Exon 11 | 74800 G>A | 1191 G>A | Ser397= | rs72557928 | 1 | 0,002 | - | - | - | 0,000 | 0,000 | 0,003 | 0,000 | |
| Exon 11 | 74809 G>A | 1200 G>A | Ser400= | 1 | 0,002 | 0,000 | 0,004 | - | - | - | - | - | ||
| Intron 11 | 74869 C>T | 1248 +12C>T | rs2286822 | 222 | 0,345 | 0,385 | 0,393 | 0,359 | 0,208 | 0,319 | 0,360 | 0,407 | 0,293 | |
| Intron 11 | 74877 G>A | 1248 +20G>A | rs2286823 | 221 | 0,343 | 0,382 | 0,393 | 0,374 | 0,194 | 0,317 | 0,360 | 0,407 | 0,297 | |
| Exon 13 | 75534 T>C | 1455 T>C | Ala485= | rs2228104 | 632 | 0,981 | 0,991 | 0,952 | 0,923 | 0,675 | 0,967 | 0,881 | 0,868 | 0,990 |
| Exon 13 | 75587 C>T | 1508 C>T | Ala503Val | rs1057868 | 173 | 0,269 | 0,294 | 0,206 | 0,219 | 0,191 | 0,264 | 0,367 | 0,310 | 0,303 |
| Exon 13 | 75665 C>T | 1586 C>T | Thr529Met | 1 | 0,002 | - | - | - | - | - | - | - | ||
| Exon 14 | 75868 G>A | 1716 G>A | Ser572= | rs1057870 | 222 | 0,345 | 0,306 | 0,335 | 0,309 | 0,182 | 0,378 | 0,138 | 0,132 | 0,363 |
| Exon 15 | 76133 G>A | 1891 G>A | Val631Ile | rs145782750 | 1 | 0,002 | 0,000 | 0,004 | - | - | - | - | - | 0,007 |
| Intron 15 | 76153 G>T | 1898 +13 G>T | rs72557956 | 1 | 0,002 | - | - | - | 0,000 | 0,008 | 0,003 | 0,003 | ||
| Intron 15 | 76216 C>T | 1899 −20 C>T | 1 | 0,002 | - | - | - | - | - | - | - |
g. - Genomic position NG_008930.1
c. - Coding position NM_000941.2
p. - Amino acid change position NP_000932.3
The sign (+) represent nucleotides upstream the last base translated in the genomic sequence and the sign (−) represents nucleotides downstream the first base translated in the genomic sequence. MJ-Moroccan Jewish allele frequencies, AJ-Ashkenazi Jewish allele frequencies and AA-African American allele frequencies, CA-Caucasian American allele frequencies, AS-Chinese American allele frequencies, ME-Mexican American allele frequencies according to Huang et al. [12]. Frequency data were compiled from studies Hart et al. [34], Huang et al. [12], Gomes et al. [35], Saito et al. [36] and Tomkova et al. [37].
Nine of the 25 identified SNP variants had allele frequencies greater than 10% and are considered as common POR SNPs. No significant differences in the allele frequencies of these variants in the Czech population and the allele frequencies obtained in the previous studies were found. Likewise, no statistically significant difference in allele frequencies of the common SNPs was observed between male and female groups (Table 2) except for the uncommon SNP rs72557941 with 6 heterozygous men (out of 144) and only one heterozygous woman (out of 178) (p<0.05). The most common SNP resulting in an amino acid change, p.Ala503Val (POR*28), was present at the allele frequency of 0.269 (26.9%). All but one uncommon variant occurred with a frequency lower than 0.01 (1%). We reported the relatively high frequency of SNP rs72557941 (more than 0.01), but in previous studies, only Gomes et al. [35] reported this SNP and with lower frequency (0.007). We have not detected any minor (G) allele of rs10262966, which has been described in the Caucasian population in all previous studies, with frequencies between 0.007–0.045 (0.7–4.5%) [12, 34, 35].
Table 2.
Comparison of allele frequencies between male and female. Newly found variants are shown in red.
| Exon | c. | p. | SNP ID | F | M | P value |
|---|---|---|---|---|---|---|
| 5´-Flanking | 4849 C>A | rs72553972 | 0,197 | 0,149 | 0,1165 | |
| 5´-Flanking | 4883 C>T | rs139824475 | 0,008 | 0,000 | 0,1184 | |
| 5´-Flanking | 4965 C>T | 0,003 | 0,000 | 0,3680 | ||
| 5´-Flanking | 4994 G>T | 0,000 | 0,003 | 0,2659 | ||
| Exon 1U | 5036 A>C | −47 A>C | rs3823884 | 0,264 | 0,226 | 0,2618 |
| Intron 1U | 5099 C>T | −5 +21 C>T | rs72553978 | 0,003 | 0,014 | 0,1112 |
| Exon 1 | 60 C>T | Ala20= | 0,000 | 0,003 | 0,2659 | |
| Exon 1 | 85 A>T | Thr29Ser | 0,003 | 0 | 0,3680 | |
| Exon 4 | 387 A>G | Pro129= | rs1135612 | 0,284 | 0,285 | 0,9774 |
| Intron 4 | 517 −4 G>A | rs41299496 | 0,003 | 0 | 0,3680 | |
| Exon 9 | 984 C>T | Ala328= | rs72557941 | 0,003 | 0,021 | 0,0283 |
| Intron 9 | 1067 −13C>G | rs4732516 | 0,989 | 0,979 | 0,3274 | |
| Exon 10 | 1112 G/A | Arg371His | 0 | 0,003 | 0,2659 | |
| Exon 10 | 1151 C>T | Pro384Leu | 0,003 | 0 | 0,3680 | |
| Exon 10 | 1194 G>A | Ser397= | rs72557928 | 0,003 | 0 | 0,3680 |
| Exon 10 | 1200 G>A | Ser400= | 0,003 | 0 | 0,3680 | |
| Intron 10 | 1248 +12C>T | rs2286822 | 0,346 | 0,344 | 0,9628 | |
| Intron 10 | 1248 +20G>A | rs2286823 | 0,343 | 0,344 | 0,9777 | |
| Exon 12 | 1455 T>C | Ala485= | rs2228104 | 0,983 | 0,979 | 0,7104 |
| Exon 12 | 1508 C>T | Ala503Val | rs1057868 | 0,258 | 0,281 | 0,5159 |
| Exon 12 | 1586 C>T | Thr529Met | 0 | 0,003 | 0,2659 | |
| Exon 13 | 1716 G>A | Ser572= | rs1057870 | 0,362 | 0,323 | 0,2950 |
| Exon 14 | 1891 G>A | Val631Ile | rs145782750 | 0 | 0,003 | 0,2659 |
| Intron 14 | 1898 +13 G>T | rs72557956 | 0,003 | 0 | 0,3680 | |
| Intron 14 | 1899 −20 C>T | 0 | 0,003 | 0,2659 |
c. - Coding position NM_000941.2
p. - Amino acid change position NP_000932.3
F – POR allele frequencies in female group
M – POR allele frequencies in male group
Haplotyping Studies
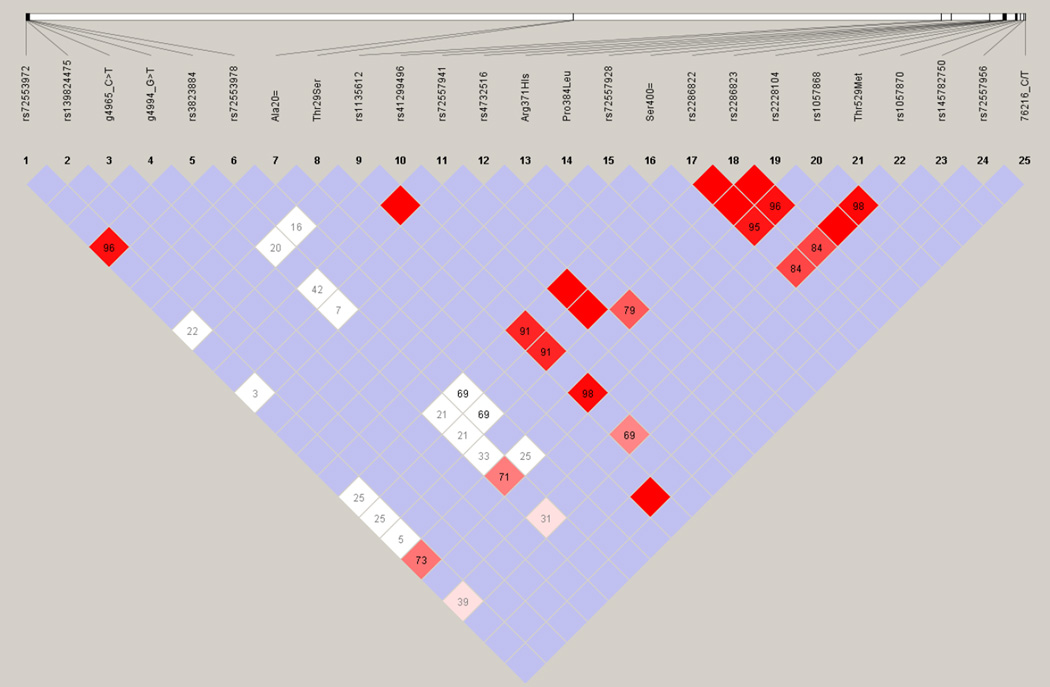
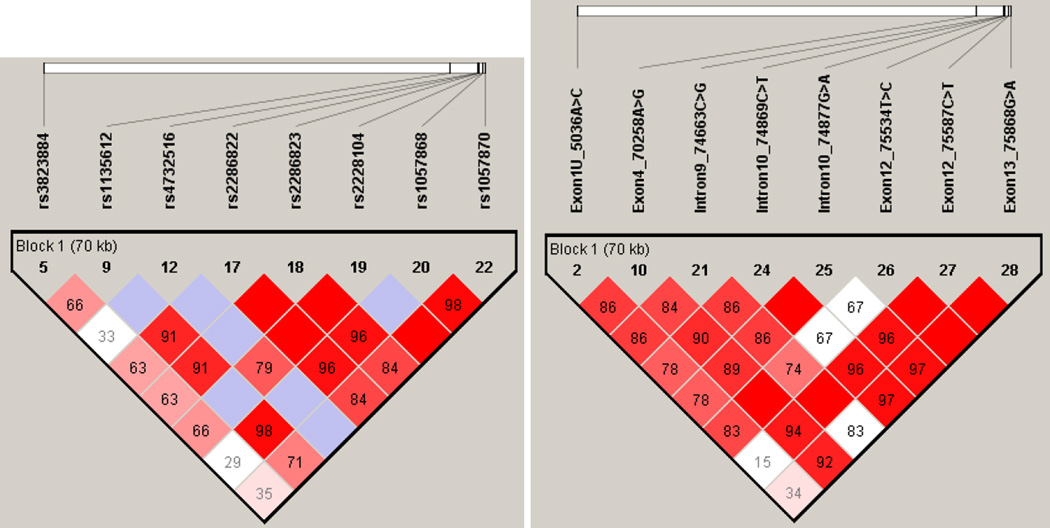
All of the 25 genotyped single nucleotide polymorphisms were used for LD assessment and haplotype block identification analyses as they passed the quality criteria (see Methods). Figure 1A depicts the relatively strong LD pattern across the POR gene, evident particularly from the markers with higher MAF. Four haplotype blocks with frequencies over 1% were inferred with indication of high values of multiallelic D´ between the blocks (Figure 1B). Almost half of the studied cohort were carriers of the POR*1 haplotype, i.e., either corresponding to the reference sequence or its variants without amino acid changes. POR*28 haplotypes were present in 150 individuals, the remaining haplotype block combinations were present in low frequencies.
Figure 1. Linkage equilibrium analysis of POR in the Czech population.
Linkage disequilibrium pattern of the analyzed polymorphisms across the POR gene in the Czech cohort (GEVALT v.2. software). The values of D’ are indicated in number and by color (for LOD >2: bright red: D=1, shades of red/pink: D<1; for LOD <2: blue: D=1, white D<1).
„Data not shown“: LD plot derived from SNPs common to AJ/MJ study and the current one.
LEFT: Czech cohort, RIGHT: combined sample of MJ and AJ. Similarly strong LD.
Functional Analysis of the p.Pro384Leu POR Missense Variation
Sequencing of the POR gene revealed a novel POR genetic variation p.Pro384Leu, which is a one-base substitution (c.1151C>T) on one allele in exon 10. To investigate this new POR variant, we expressed and purified the variant protein, determined flavin content, cytochrome c reduction and several CYP-mediated hydroxylation activities. Wild type (WT) POR and the p.Pro384Leu variant were bacterially expressed and purified as full length proteins (Supplementary Figure 1). For quantitative analysis of the proteins expressed in E. coli, SDS-polyacrylamide gel electrophoresis (PAGE) was performed and molecular mass of the proteins were determined to 77 kDa. Both fractions had greenish-brown color, which was expected due to spectral contribution of oxidized FAD (yellow) and air-stable FMN semiquinone (blue-gray). 11 mg and 7.25 mg of purified protein were produced per liter of culture for WT and the p.Pro384Leu variant, respectively.
HPLC-based analysis of flavin content was performed to assess the protein:FAD:FMN ratio. POR protein concentration was quantified by oxidized flavin absorbance (Δε = 21.4 mM−1cm−1) [42]) and compared to the microbicinchoninic acid (BCA) protein assay (Pierce) according to standard protocol. The purity of each protein preparation was determined by SDS-polyacrylamide gradient gel electrophoresis. These analyses showed that the p.Pro384Leu variant had the full complement of FAD and FMN and the protein: FAD: FMN ratio was the same as in the WT (1:1:1) (Supplementary Figure 2).
The Km for NAPDH was measured using cytochrome c as the electron acceptor (Supplementary Figure 3) for both WT and p.Pro384Leu. No difference was observed between WT and p.Pro384Leu (KmNADPH for WT = 1 µM and p.Pro384Leu = 0.9 µM), suggesting that the mutation has no significant effect on the apparent NADPH binding. However, the observed Vmax for p.Pro384Leu was ~60% of the WT, suggesting a possible impairment of electron flow in the mutant. As CYPs are the physiological electron acceptors for POR, various CYP activities were measured to assess the possible effect(s) of the mutation reflecting a physiological scenario (Supplementary Table 1). CYP2E1 activity in the presence of p.Pro384Leu showed a similar rate compared to the WT protein as measured by the conversion of p-nitrophenol to 4-nitrocatechol, whereas a slight decrease in the rate of arachidonic acid metabolism was observed with the p.Pro384Leu variant. It is interesting to note that the metabolism of 7-benzyloxy-4-trifluoro-methylcoumarin (BFC), which can be attributed due to both CYP3A11 and CYP1A2, showed a marked decrease compared to wild type. Such findings reiterate the fact that the characterization of POR mutations found in humans requires more rigorous testing, using various substrates of interest and the outcome should not be generalized.
Discussion
POR is an essential component of several enzyme systems, including systems containing drug-metabolizing CYPs. Several studies have raised the question of the importance of common POR variations in drug metabolism [12, 17, 34, 43]. Five studies have investigated the role of POR gene variants in relation to pharmacogenetics [12, 34–37]. We decided to look for POR SNP frequencies in an unstudied Czech Slavic population. The present study confirmed several of the already reported common POR SNPs (>10%) and their allelic frequencies were not significantly different from frequencies found in other ethnic groups (see Table 1). In contrast to our previous report [37], we also sequenced the proximal promoter region of the POR gene containing three SP1 binding sites shown to be important in the transcription of POR [41]. We have identified several heterozygous SNPs in this region (Table 1), but none of them lie within the crucial SP1 binding sites.
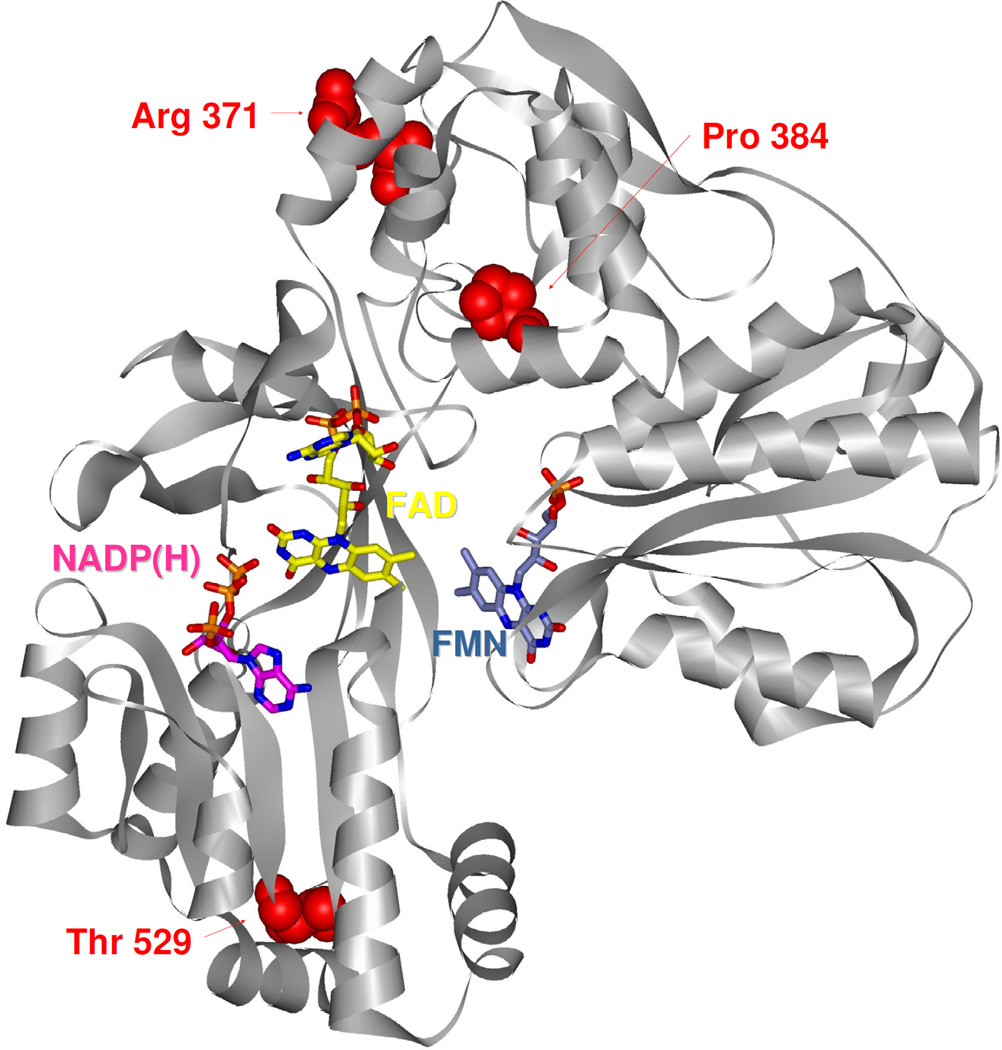
Our results revealed six amino acid changing variants (Table 1), among which four were not reported previously (p.The29Ser, p.Arg371His, p.Pro384Leu, p.Thr529Met) (Figure 3, variant p.The29Ser is not a part of the model, which is based on Δ66 truncation wild-type POR). During preparation of this manuscript, we found that the amino acid variant p.Arg371His has been described for the first time in the database of an Exome Variant Server (http://evs.gs.washington.edu/EVS/), but its impact on the POR function was not yet studied. Of the remaining known SNPs, p.Ala503Val is a common polymorphism with an allele frequency of 0.191 – 0.434 [12, 34–37]. According to recent studies, p.Ala503Val is a likely contributor to a pharmacogenetic variation in drug and xenobiotic metabolism [15, 17]. Amino acid variant p.Val631Ile was discussed in our previous work [37]. It is not a PORD-causing SNP [11], but it severely decreases the activity with some CYPs [14]. Therefore, we considered it as a potential biomarker for future POR pharmacogenetics screening [37].
Figure 3.
Missense variants found in the Czech Slavic population. The human POR structure [6] is depicted, gray ribbons indicate the peptide backbone. FAD (yellow) and FMN (blue–gray) cofactors and NADP(H) coenzyme (only 2´,5´-ADP of NADP+ was structurally resolved, pink) are shown in stick configuration. Amino acid residues corresponding to Czech Slavic variants are shown in space-filling configuration (see inset labels). FAD: Flavin adenine dinucleotide; FMN: Flavin mononucleotide.
POR variant p.Pro384Leu was identified within this population study. The substitution of a cyclic amino acid, proline, for a hydrophobic amino acid, leucine, and the important location of the Pro384 residue within the connecting domain and in a helical sequence, led us to express and purify this protein variant. The results of biochemical assays showed reduced activities of the p.Pro384Leu variant compared to WT. The Pro384 residue is located within the connecting domain of the POR molecule, a globular domain that was shown to be involved in the movement and interaction of the FAD- and FMN-binding domains [7, 44]. It is surprising, therefore, that POR activity was not more severely affected by the p.Pro384Leu substitution. It is not unexpected, however, that a minor loss of function from a single allele had no apparent effect on the health of the individual. There is a possibility that individuals exhibiting this variant may possess compromised capabilities to metabolize drugs as a consequence of challenging the system.
Subtle genetic variations and differences in patterns of LD, even within a single geoethnic group (e.g., Caucasian population) have been recognized as a potential source of complications in genome-wide association studies (GWAS). The biological and pharmacogenetic importance of rare variants arising from recently identified mutations emphasizes the importance of genetic characterization of relevant genes in distinct subpopulations [45], thus addressing personalized medicine. In agreement with the single allele frequency data, our results provide further evidence for the strong LD pattern across the POR gene region as well as the substantial representation of POR*1 and POR*28 haplotypes that we and others reported previously in several geoethnic groups [15, 25, 37].
Our study cohort consisted of 227 DNA samples obtained from adults and 95 samples obtained from cord blood of neonates. Clinical features of POR deficiency manifest already in newborn infants with symptoms including adrenal insufficiency, skeletal deformities and neonatal presentation with disordered sex development [46]. Therefore, we do not consider the age variations of the cohort to have impact on the POR allele frequency.
Several studies confirmed that some commonly occurring POR polymorphisms [17, 35] or polymorphisms affecting POR activity, but not associated with PORD disease [34], might have significant effects on drug-metabolizing CYP activities. Therefore, it is of further research interest to study polymorphisms found in the general population due to their potential role as possible additional challenges to the drug-metabolizing capacity of these individuals due to drug-drug interactions or induction of CYPs by environmental agents.
Conclusion
This first investigation of the Slavic population shows that allele frequencies of commonly found SNPs in the Czech Slavic population are similar to those from other studies investigating the POR gene in another Caucasian population [12, 34, 35]. We have not found any mutations associated with POR deficiency, suggesting that these are rare variants not commonly represented in the normal population. We described four new amino acid-changing variants, none of which occurred more than once suggesting that these are rare variants. Haplotype analyses did not reveal any statistically significant findings. Biochemical investigation of the new amino acid variant p.Pro384Leu showed reduction of its activity compared to WT, indicating that some of the uncommon variants can alter POR activity without causing POR deficiency. Observation of new variants in a subpopulation clearly indicates that the number of POR variants will be different depending on the cohort chosen for such studies. It is critical to continue these efforts in search of variants of such an important enzyme that plays a critical role in both endo- and xeno-biotic metabolism, including the metabolism of more than 90% of therapeutic drugs. This will be an important contribution to the future of personalized medicine and help to reduce possible negative outcomes due to adverse drug reactions.
Supplementary Material
Figure 2. Inferred Haplotypes in Czech Population.
The haplotype display of POR in the Czech cohort (GEVALT v.2. software, GERBIL algorithm) shows each haplotype in a block with its frequency in the respective population and connections from one block to the next (thin and thick lines refer to >10% and >50% chromosomes proceeding from the indicated haplotype on the left to the indicated haplotype on the right). In the crossing areas, a value of multiallelic D' (inversely related to the fraction of chromosomes that have experienced historical recombination) is shown. The numerical labels of the variants correspond to those shown in the Figure 1. Only haplotypes with frequency >1% in the Czech cohort are shown.
Future perspective.
Pharmacogenetics is a rapidly evolving field that will clearly lead to the development of pharmacogenetic tests. Since POR is an important drug metabolizing enzyme, in the future, the POR gene may become essential in pharmacogenetic screening.
Executive summary.
The role of NADPH–P450 oxidoreductase in pharmacogenetics
Polymorphisms in the gene encoding the enzyme NADPH–P450 oxidoreductase (POR) influence the activity of microsomal cytochromes P450 and thus modulate drug effects and contribute to inter-individual differences in drug response.
Population studies
To date, five population studies addressing the distribution of POR genetic differences within various populations have been performed. This study was undertaken to search for polymorphic variants of POR gene in the unstudied Czech Slavic population.
New POR variants in the Czech Slavic population
Analyzing of the POR gene in the Czech Slavic population showed a total of 25 different POR genetic variations, 7 of which were new, not described previously (two variants were found in flanking region: g.4965 C>T and g.4994 G>T, one intronic variant: c.1899 −20C>T and four exonic variants: p. 20Ala=, p.Thr29Ser, p.Pro384Leu and p.Thr529Met).
Biochemical characterization of the new p.Pro384Leu variant
Biochemical investigation of the new amino acid variant p.Pro384Leu showed reduction of its activity compared to WT, indicating that some of the uncommon variants can potentially alter POR activity without causing POR deficiency. This is particularly important due to the singular role that POR plays in providing reducing equivalents to all CYPs in the endoplasmic reticulum.
Haplotyping studies
Our data provide evidence for the strong LD pattern across the POR gene region as well as the substantial representation of POR*1 and POR*28 haplotypes.
Conclusion
The observation of new POR genetic changes indicates that the number of uncommon POR variants might be specific for each subpopulation being investigated.
Reduction in the activity of p.Pro384Leu variant provides evidence that some uncommon POR variants might alter drug metabolism and thus POR variants might be important for the future of personalized medicine.
Footnotes
Disclaimer: The authors had full access to the data and take responsibility for its integrity. All authors have read and agree with the manuscript as written.
References
- 1.Guengerich FP, Wu ZL, Bartleson CJ. Function of human cytochrome P450s: characterization of the orphans. Biochem Biophys Res Commun. 2005;338(1):465–469. doi: 10.1016/j.bbrc.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 2.Masters BS, Marohnic CC. Cytochromes P450 - A family of proteins and scientists-understanding their relationships. Drug Metabolism Reviews. 2006;38(1–2):209–225. doi: 10.1080/03602530600570065. [DOI] [PubMed] [Google Scholar]
- 3.Lu AY, Coon MJ. Role of hemoprotein P-450 in fatty acid omega-hydroxylation in a soluble enzyme system from liver microsomes. J Biol Chem. 1968;243(6):1331–1332. [PubMed] [Google Scholar]
- 4.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32(3):210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286(5439):487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 6. Xia C, Panda SP, Marohnic CC, Martasek P, Masters BS, Kim JJ. Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency. Proc Natl Acad Sci U S A. 2011;108(33):13486–13491. doi: 10.1073/pnas.1106632108. The crystal structure of human soluble wild-type POR is presented, along with structures of two naturally occurring missense variants, V492E and R457H.
- 7.Ellis J, Gutierrez A, Barsukov IL, Huang WC, Grossmann JG, Roberts GC. Domain motion in cytochrome P450 reductase: conformational equilibria revealed by NMR and small-angle x-ray scattering. J Biol Chem. 2009;284(52):36628–36637. doi: 10.1074/jbc.M109.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent B, Morellet N, Fatemi F, et al. The closed and compact domain organization of the 70-kDa human cytochrome P450 reductase in its oxidized state as revealed by NMR. J Mol Biol. 2012;420(4–5):296–309. doi: 10.1016/j.jmb.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Munro AW, Noble MA, Robledo L, Daff SN, Chapman SK. Determination of the redox properties of human NADPH-cytochrome P450 reductase. Biochemistry. 2001;40(7):1956–1963. doi: 10.1021/bi001718u. [DOI] [PubMed] [Google Scholar]
- 10. Fluck CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36(3):228–230. doi: 10.1038/ng1300. The first report that mutations in gene POR cause the ABS phenotype with autosomal recessive inheritance and abnormalities in steroidogenesis.
- 11.Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76(5):729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang N, Agrawal V, Giacomini KM, Miller WL. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci U S A. 2008;105(5):1733–1738. doi: 10.1073/pnas.0711621105. Gene POR is sequenced in 824 healthy unrelated individuals in four ethnic groups: 218 African Americans, 260 Caucasian Americans, 179 Chinese Americans, and 185 Mexican Americans.
- 13.Fluck CE, Nicolo C, Pandey AV. Clinical, structural and functional implications of mutations and polymorphisms in human NADPH P450 oxidoreductase. Fundam Clin Pharmacol. 2007;21(4):399–410. doi: 10.1111/j.1472-8206.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics. 2008;18(7):569–576. doi: 10.1097/FPC.0b013e32830054ac. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal V, Choi JH, Giacomini KM, Miller WL. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet Genomics. 2010;20(10):611–618. doi: 10.1097/FPC.0b013e32833e0cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluck CE, Mullis PE, Pandey AV. Reduction in hepatic drug metabolizing CYP3A4 activities caused by P450 oxidoreductase mutations identified in patients with disordered steroid metabolism. Biochem Biophys Res Commun. 2010;401(1):149–153. doi: 10.1016/j.bbrc.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Sandee D, Morrissey K, Agrawal V, et al. Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet Genomics. 2010;20(11):677–686. doi: 10.1097/FPC.0b013e32833f4f9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey AV, Sproll P. Pharmacogenomics of human P450 oxidoreductase. Front Pharmacol. 2014;5:103. doi: 10.3389/fphar.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen AL, O'leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem. 2002;277(8):6536–6541. doi: 10.1074/jbc.M111408200. [DOI] [PubMed] [Google Scholar]
- 20.Otto DM, Henderson CJ, Carrie D, et al. Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic Acid homeostasis. Mol Cell Biol. 2003;23(17):6103–6116. doi: 10.1128/MCB.23.17.6103-6116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson CJ, Otto DM, Carrie D, et al. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem. 2003;278(15):13480–13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- 22.Oneda B, Crettol S, Sirot EJ, Bochud M, Ansermot N, Eap CB. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharmacogenet Genomics. 2009 doi: 10.1097/FPC.0b013e32833225e7. [DOI] [PubMed] [Google Scholar]
- 23.Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W. Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol. 2010;163(6):919–924. doi: 10.1530/EJE-10-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Li L, Ding X, Kaminsky LS. Identification of cytochrome P450 oxidoreductase gene variants that are significantly associated with the interindividual variations in warfarin maintenance dose. Drug Metab Dispos. 2011;39(8):1433–1439. doi: 10.1124/dmd.111.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jonge H, Metalidis C, Naesens M, Lambrechts D, Kuypers DR. The P450 oxidoreductase *28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5-expressing renal recipients. Pharmacogenomics. 2011;12(9):1281–1291. doi: 10.2217/pgs.11.77. [DOI] [PubMed] [Google Scholar]
- 26.Marohnic CC, Huber WJ, Iii, Patrick Connick J, et al. Mutations of human cytochrome P450 reductase differentially modulate heme oxygenase-1 activity and oligomerization. Arch Biochem Biophys. 2011;513(1):42–50. doi: 10.1016/j.abb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian M, Agrawal V, Sandee D, Tam HK, Miller WL, Tracy TS. Effect of P450 oxidoreductase variants on the metabolism of model substrates mediated by CYP2C9.1, CYP2C9.2, and CYP2C9.3. Pharmacogenet Genomics. 2012;22(8):590–597. doi: 10.1097/FPC.0b013e3283544062. [DOI] [PubMed] [Google Scholar]
- 28.Shephard EA, Phillips IR, Santisteban I, et al. Isolation of a human cytochrome P-450 reductase cDNA clone and localization of the corresponding gene to chromosome 7q11.2. Ann Hum Genet. 1989;53(Pt 4):291–301. doi: 10.1111/j.1469-1809.1989.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 29.Scott RR, Gomes LG, Huang N, Van Vliet G, Miller WL. Apparent manifesting heterozygosity in P450 oxidoreductase deficiency and its effect on coexisting 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(6):2318–2322. doi: 10.1210/jc.2006-2345. [DOI] [PubMed] [Google Scholar]
- 30.Adachi M, Tachibana K, Asakura Y, Yamamoto T, Hanaki K, Oka A. Compound heterozygous mutations of cytochrome P450 oxidoreductase gene (POR) in two patients with Antley-Bixler syndrome. Am J Med Genet A. 2004;128A(4):333–339. doi: 10.1002/ajmg.a.30169. [DOI] [PubMed] [Google Scholar]
- 31.Arlt W, Walker EA, Draper N, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363(9427):2128–2135. doi: 10.1016/S0140-6736(04)16503-3. [DOI] [PubMed] [Google Scholar]
- 32.Miller WL. P450 oxidoreductase deficiency: a new disorder of steroidogenesis with multiple clinical manifestations. Trends Endocrinol Metab. 2004;15(7):311–315. doi: 10.1016/j.tem.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Flück CE, Pandey AV. P450 Oxidoreductase Deficiency (PORD) In: New MI, Lekarev O, Parsa A, Yuen TT, O'malley B, Hammer GD, editors. Genetic steroid disorders. San Diego, USA: Academic Press; 2013. pp. 125–143. [Google Scholar]
- 34. Hart SN, Wang S, Nakamoto K, Wesselman C, Li Y, Zhong XB. Genetic polymorphisms in cytochrome P450 oxidoreductase influence microsomal P450-catalyzed drug metabolism. Pharmacogenet Genomics. 2008;18(1):11–24. doi: 10.1097/FPC.0b013e3282f2f121. The study shows that polymorphisms in the POR gene can affect POR and P450-catalyzed drug oxidation.
- 35. Gomes AM, Winter S, Klein K, et al. Pharmacogenomics of human liver cytochrome P450 oxidoreductase: multifactorial analysis and impact on microsomal drug oxidation. Pharmacogenomics. 2009;10(4):579–599. doi: 10.2217/pgs.09.7. Gene POR is sequenced in 150 individuals from Caucasian population and ten major POR drug-oxidation activities are measured in the liver microsomes.
- 36. Saito Y, Yamamoto N, Katori N, et al. Genetic polymorphisms and haplotypes of por, encoding cytochrome p450 oxidoreductase, in a Japanese population. Drug Metab Pharmacokinet. 2011;26(1):107–116. doi: 10.2133/dmpk.dmpk-10-sc-096. The study provides genetic variations and the haplotype structures of the POR gene in 235 Japanese subjects.
- 37. Tomkova M, Marohnic CC, Gurwitz D, Seda O, Masters BS, Martasek P. Identification of six novel P450 oxidoreductase missense variants in Ashkenazi and Moroccan Jewish populations. Pharmacogenomics. 2012;13(5):543–554. doi: 10.2217/pgs.12.21. POR gene is analyzed and molecular haplotypes are investigated in 301 Ashkenazi and Moroccan Jewish individuals.
- 38.Pejznochova M, Tesarova M, Honzik T, Hansikova H, Magner M, Zeman J. The developmental changes in mitochondrial DNA content per cell in human cord blood leukocytes during gestation. Physiol Res. 2008;57(6):947–955. doi: 10.33549/physiolres.931246. [DOI] [PubMed] [Google Scholar]
- 39.Davidovich O, Kimmel G, Shamir R. GEVALT: an integrated software tool for genotype analysis. BMC Bioinformatics. 2007;8:36. doi: 10.1186/1471-2105-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimmel G, Shamir R. GERBIL: Genotype resolution and block identification using likelihood. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):158–162. doi: 10.1073/pnas.0404730102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soneda S, Yazawa T, Fukami M, et al. Proximal promoter of the cytochrome P450 oxidoreductase gene: identification of microdeletions involving the untranslated exon 1 and critical function of the SP1 binding sites. J Clin Endocrinol Metab. 2011;96(11):E1881–E1887. doi: 10.1210/jc.2011-1337. The study revealed a pivotal role of the SP1 binding sites in the POR transcription.
- 42.Oprian DD, Coon MJ. Oxidation-reduction states of FMN and FAD in NADPH-cytochrome P-450 reductase during reduction by NADPH. J Biol Chem. 1982;257(15):8935–8944. [PubMed] [Google Scholar]
- 43.Oneda B, Crettol S, Jaquenoud Sirot E, Bochud M, Ansermot N, Eap CB. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharmacogenet Genomics. 2009;19(11):877–883. doi: 10.1097/FPC.0b013e32833225e7. [DOI] [PubMed] [Google Scholar]
- 44.Aigrain L, Pompon D, Morera S, Truan G. Structure of the open conformation of a functional chimeric NADPH cytochrome P450 reductase. EMBO Rep. 2009;10(7):742–747. doi: 10.1038/embor.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson MR, Wegmann D, Ehm MG, et al. An abundance of rare functional variants in 202 drug target genes sequenced in 14,002 people. Science. 2012;337(6090):100–104. doi: 10.1126/science.1217876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krone N, Reisch N, Idkowiak J, et al. Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab. 2012;97(2):E257–E267. doi: 10.1210/jc.2011-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marohnic CC, Panda SP, Martasek P, Masters BS. Diminished FAD binding in the Y459H and V492E Antley-Bixler syndrome mutants of human cytochrome P450 reductase. J Biol Chem. 2006;281(47):35975–35982. doi: 10.1074/jbc.M607095200. [DOI] [PubMed] [Google Scholar]
- 48.Massey V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.