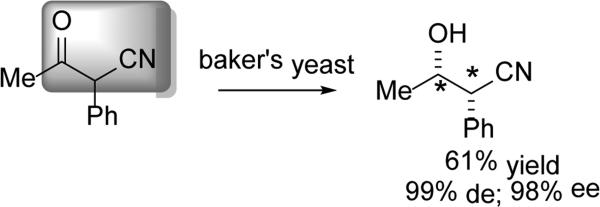Abstract
Over the past two decades, the domains of both frontline synthetic organic chemistry and process chemistry and have seen an increase in crosstalk between asymmetric organic/organometallic approaches and enzymatic approaches to stereocontrolled synthesis. This review highlights the particularly auspicious role for dehydrogenase enzymes in this endeavor, with a focus on dynamic reductive kinetic resolutions (DYRKR) to “deracemize” building blocks, often setting two stereocenters in so doing. The scope and limitations of such dehydrogenase-mediated processes are overviewed, as are future possibilities for the evolution of enzymatic DYRKR.
Keywords: Asymmetric synthesis, biocatalysis, dehydrogenase enzymes, deracemization, dynamic kinetic resolution
1 Introduction
The field of asymmetric synthesis is at an important crossroads currently. With both overall efficiency and control of stereochemistry being at a premium, organic chemists are active at the frontiers of methodology development, both on the more traditional organic/organometallic front, and now also on the biocatalytic front. In the former area, exciting developments include recent advances in organocatalysis[1] and base metal catalysis.[2] In the latter, the number and scope of enzymatic transformations continues to expand, particularly with advances in the directed evolution of protein catalysts in “the third wave of biocatalysis.”[3]
The time is now ripe for academic and process chemists to meld frontline synthetic organic methods with state-of-the-art biocatalytic methods. Indeed, over the past decade or so, in the domain of process chemistry particularly, one can see a clear evolution of thinking in this direction. Examples include the synthesis of pregabalin (Lyrica™), the first generation synthesis of which involved catalytic asymmetric hydrogenation, and the streamlined synthesis of which involves kinetic resolution with a lipase.[4] For the synthesis of atorvastatin (Lipitor™), hybrid synthetic organic/enzymatic approaches have been taken, wherein a dehydrogenase sets the stereochemistry of the side chain.[5] In streamlining process chemistry routes into a sister-statin; namely rosuvastatin (Crestor™), advantage has been taken of the power of aldolase technology.[6] Most recently, for the other major, natural product-derived statin category, simvastatin, can now be synthesized biocatalytically by means of an evolved “simvastatin synthase” enzyme, in pioneering work by Tang and co-workers.[7]
A particularly impressive case in process circles revolves around the synthesis of the dipeptidyl peptidase-4 inhibitor, sitagliptin (Januvia™), a useful anti-diabetic agent. The Merck process group won two Presidential Green Chemistry awards for distinct approaches into this target. Initially, the award was given for a route involving catalytic asymmetric hydrogenation.[8] The second generation route involved extensive remodeling a transaminase active site in a “directed evolution” endeavor jointly with Codexis, and provides a beautiful hybrid organic/enzymatic route into this complex heterocyclic target bearing a key core, β3-aromatic amino acid.[9]
This review will focus on a particularly auspicious area of biocatalysis wherein a racemic educt is effectively “deracemized” and two contiguous stereocenters are often set, in the same operation. Specifically, we will discuss Dynamic Reductive Kinetic Resolution (DYRKR) involving dehydrogenase enzymes. It is worth noting that other enzyme classes, namely transaminases[10] and benzaldehyde lyases,[11] have recently found application in similar strategies. The reader is also pointed to other reviews on dehydrogenase enzymes that may be of interest.[12] Our own interest in dehydrogenase enzymes stems from their utility as “reporting enzymes” in a method for catalyst discovery that we have developed known as In Situ Enzymatic Screening (ISES).[13] In this approach, an alcohol dehydrogenase (ADH) oxidizes the alcoholic product or byproduct of a reaction of interest. The concomitant increase in Abs340 associated with the formation of NAD(P)H is monitored spectroscopically. By utilizing two reporting ADH enzymes with complementary enantioselectivities, the experimentalist can glean information on relative rates and enantioselectivities of a series of catalysts or chiral ligands of interest, in a parallel screening format.[14]
In the course of evaluating dehydrogenase “reporting enzymes” for their substrate specificitiy and enantioselectivity, we have become very interested in also exploiting these enzymes for their potential in asymmetric synthesis.[15] Indeed, alcohol dehydrogenases provide a complementary and greener alternative to traditional Noyori-type asymmetric carbonyl hydrogenation, such as was exploited in the first generation Januvia™ synthesis.[8] Furthermore, the advancement of structural biology, computational chemistry, and molecular biology have allowed for the fine tuning of natural ADHs to tailor these to substrates of interest. These advantages have led to dehydrogenases being the preferred catalysts for ketone reductions in the Merck process group.[16] [17] In fact, it is estimated that 10% of current drug syntheses rely on a biocatalytic step.[18] This underscores the need for the synthetic chemist to work from a set of retrosynthetic transformations that complements the traditional Corey-esque set[19] with a new set of biocatalytic transform arrows. Turner and coworkers refer to this complementary view as “biocatalytic retrosynthesis.”[20]
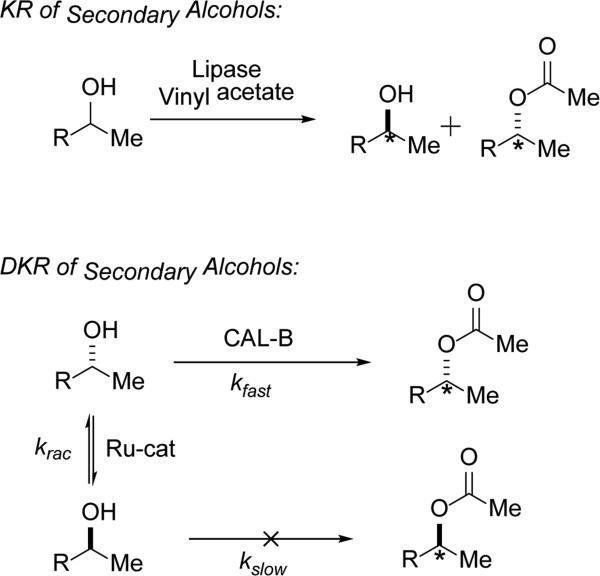
Here the focus is on synthetic exploitation of ADHs in dynamic kinetic resolutions. To be clear, for classical kinetic resolutions, in the ideal case, using a lipase, say, one can obtain enantiopure acylated product in 50% yield, while also recovering 50% of the antipodal unreacted alcohol (Scheme 1, top). While it can be advantageous to access both enantiomeric forms of a building block,[14a] this is not always desirable. To address this limitation, dynamic kinetic resolution (DKR) may be employed, where the stereocenter in question can be racemized. DKR has seen a large increase in application over the past couple of decades. In contrast to traditional KR, DKR can provide complete converstion to enantiomerically pure product from racemic starting material by combining the principles of classical KR with continuous racemization of the unreacted enantiomer (Scheme 1, bottom).
Scheme 1.
Representative examples of KR and DKR.
The racemization can be mediated by a chemical or biological catalyst,[21] or may even occur spontaneously under the reaction conditions.[21d] A successful DKR requires that the rate of racemization (krac) exceed the rate of processing the slow enantiomer (kslow) by the catalyst (Scheme 1, bottom). DKR has successfully been applied to a variety of compound classes including alcohols,[22] amines,[23] cyanohydrins,[24] and amino acids.[25]
DKR can also, of course, be extended to cases in which more than two antipodal products are possible as is the case for the lipase resolution shown above. In cases where a new stereogenic center is set, the process now has the ability of generating two stereocenters in one transformation, effectively dialing in one of four possible stereoisomeric products. A traditional chemo-catalytic method for this process is Noyori's Ru-catalyzed hydrogenation of α-substituted-β-ketoesters to afford α-substituted-β-hydroxyesters.[26] An enzymatic rival of this process can be achieved by a variety of alcohol dehydrogenases or microbes as demonstrated in Scheme 2. This process is termed dynamic reductive kinetic resolution (DYRKR). The first microbial DYRKR can be traced back to Deol in 1976[27] by employing baker's yeast in the reduction of the same compounds described in the Noyori system. The dynamic reductive processes that followed for the next two decades often employed whole cells due to availability. While these systems were able to achieve high enantiomeric excesses (ee) in some cases, the issue of several active enzymes often reduced overall success. Recent advances in molecular biology, genomic sequencing, and cofactor regeneration systems have allowed for the expression and isolation of individual alcohol dehydrogenases to overcome these earlier limitations.
Scheme 2.
An example of the DYRKR process.
DYRKR strategies with reductive enzymes can be represented by systems as shown in Scheme 3. Adjacent to a carbonyl resides an epimerizable stereocenter, bearing an acidic proton. Under the reaction conditions, the two enantiomers are rapidly interconverted through an enol(ate) intermediate. This same principle applies to a number of related functionalities, including but not limited to, cylic and acyclic keto phosphonates, keto sulfones, α-cyano ketones, and even α-alkyl aldehydes. While the reduction of aldehydes is not typically associated with asymmetric synthesis, these systems serve as an entry point into enantiomerically enriched α-substituted primary alcohols.
Scheme 3.
Reduction of α-methyl-β-ketoesters
This review will focus on the current scope of DYRKR and its application in both academic laboratory and the industrial process group settings. An effort is made to provide a thorough overview of the application of this approach to various functional groups and scaffolds, including important synthetic and pharmaceuticalal targets as well as to offer a glimpse into future challenges for DYRKR.
A couple of notes on conventions employed in this review are in order here. On the one hand, a grey box is used to denote the functionality with the substrate that allows for stereochemical dynamism under the reaction conditions. On the other, color-coding is used to depict that absolute stereochemistry of the DYRKR product, with blue and red, indicating D- and L-stereochemistry, respectively. A typical product class would be a β-hydroxy ester, for example, and this convention specifically refers to the Fischer convention when considering the absolute stereochemsitry of the β-stereocenter with the carbonyl as reference point.
2 β-Keto Ester Reduction
The reduction of β-keto esters represents one of the most important DYRKR transformations. It is possible to see large differences in selectivities based on the nature of the ester, the α-alkyl group, or the R group flanking the ketone. One such example is reported by Häckh in the reduction of 2-Me-3-oxo esters (Scheme 3).[28] Replacement of an SNAC ester with an ethyl ester increased enantioselectivity from 43% ee to 92% ee. Interestingly, the same ester substitution for 2-Me-3-oxovalerate esters reversed the stereochemical course of the reduction from (2R,3R) to (2S,3S).
While a highly selective DYRKR may “surgically” deliver one of four possible stereoisomeric products, inevitably, less selective situations are often encountered, with an undesired isomer also being formed. To mitigate against this, it may be possible to employ individual enantiocomplementary or diastereocomplementary enzymes. Another approach is to use directed evolution, by generating a library of genetically engineered mutants and selecting for the stereochemistry of interest. As an example of the former strategy, Lüdeke and coworkers reported the identification of enzymes that generate three of the four possible stereoisomers of 5-hydroxy-4-methyl-3-oxohexanoate.[29] The three enzymes originate from distinct sources and display high enantio- and diastereoselectivities for complementary products (Scheme 4).
Scheme 4.
Tuning 3,5-diketo-ester reductions.
In an interesting new approach, a collaborative effort studied the application of six different ADHs to the reduction of α,α-dihaloacetophenones.[30] The initial screen of six dehydrogenases (ADH-A, R. ruber; RasADH, Ralstonia sp.; SyADH, Sphingobium yanoikuyae; LBADH, L. brevis; LKADH, L. kefir) across this family of substrates revealed that five demonstrated high enantioselectivity. The authors also investigated the ability of these enzymes to distinguish halogen atoms, for example, by reducing α-bromo-α-chloroacetophenone (Scheme 5). Although diastereoselectivity was limited (59% de, ADH-A), the enantioselectivity remained high (99% ee). The reduction of α-chloro-α-fluoroacetophenone proceeded with poorer diastereoselectivity, perhaps as a result of the enzyme being unable to distinguish the smaller fluorine atom when compared with the bulky bromine atom.
Scheme 5.
Reduction of α,α-dihalo-acetophenones.
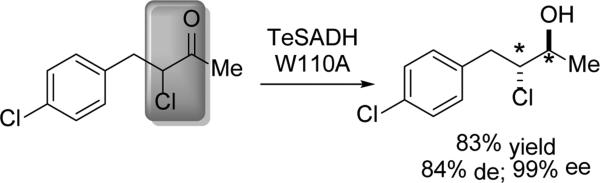
While many of the works highlighted in this review make use of whole cell or overexpressed dehydrogenases, Musa and Phillips demonstrated the power of rational design and site-directed mutagenesis to broaden the substrate scope of an enzyme. Thermoanaerobacter ethanolicus secondary ADH (TeSADH) had previously shown synthetic utility due to its ability to stereoselectively operate at elevated temperatures and in an organic solvent-rich milieu (30% v/v).[31] The W110A mutant not only reversed the enantiopreference of Thermoanerobacter ethanolicus ADH (TeSADH), but it also opened up the large pocket of the active site to accommodate bulkier aryl substitutents. Furthermore, this W110A mutant demonstrated the ability to catalyze the DYRKR of α-chloroketones with good diastereo- and excellent enantioselectivity as shown in Scheme 6. The chlorohydrin was transformed into the corresponding epoxide.
Scheme 6.
Exploitation of a useful TeSADH mutant.
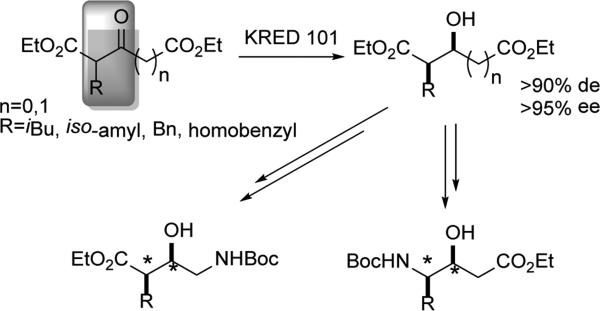
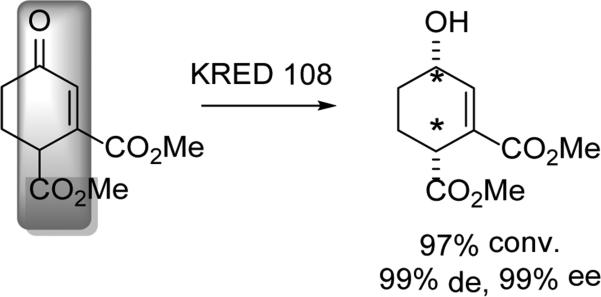
The diastereoselective DYRKR of various 3-ketoglutarates was reported by Kambourakis and Rozzell.[32] A range of R groups was successfully accommodated using a spectrum of KREDs (Scheme 7). For example, when R = iPr, KRED 101 provided the (3S,4R) product in 90% de and 99% ee. However, KRED 108 could select for the (3R,4R) product in 99% de and 99% ee. Regiodivergent modification of this common hydroxyl intermediate allowed for the synthesis of a number of statin analogues. Furthermore, the ability of KRED 108 to select for the anti reduction product granted access to individual diastereomers of these statines.
Scheme 7.
Access to γ−amino-β-hydroxy esters.
The Gotor group was able to access enantiopure 3,4-dihydroisocoumarins through a dynamic reductive kinetic resolution using ADH-A from Rhodococcus ruber.[33] Initial experiments proceeded more like of a classical kinetic resolutions. The enzymatic reaction only proceeded to ~38% conversion with high diastereo- (99% de) and enantioselectivity (99% ee). However, the remaining ketone was also found in 60% ee. This led the investigators to add Et3N (1% v/v) to facilitate racemization, ultimately resulting in a DYRKR process that yields the (2S,3S)-alcohol in good yield and excellent selectivity (Scheme 8). Following acid hydrolysis of the nitrile, lactonization ensues, providing for an elegant route into the desired dihydroisocomarin scaffold.
Scheme 8.
Stereocontrolled entry into 3,4-disubstituted dihydroisocoumarins
While baker's yeast served as the workhorse in early efforts of microbial reductions, new organisms are continuously being evaluated for similar biotransformations. The search for other microbial strains harboring ADHs capable of catalyzing similar biotransformations has led to a number of prospecting successes.[34] A schematic mapping of species vs. ideal β-keto ester (nitrile) substrate type is presented in Scheme 9. Note the high selectivities observed in these promising screens for DYRKR transformations of diverse β-keto functionalities. From these screens it is clear that valuable ADHs can be mined from Geotrichum candidum (α-alkyl-β-ketoesters),[35] Rhizopus arrhizus (α-cyano tetralone),[36] Mucor racemosus (cyclic β-ketoesters),[37] and Kloeckera magna (large cyclic β-ketoesters).[37] Other fungal sources have shown promise for the reduction of α-alkyl-β-ketoesters.[38]
Scheme 9.
Reducing select β-keto substrate motifs with various yeast strains.
The Stewart group has reported extensively on the use of baker's yeast, or the independently expressed ADH enzymes that comprise this sector of its genome, in the DYRKR of various substrates. Early efforts were made to develop a biocatalytic route into the phenyl isoserine side chain of the taxanes. One such example is the use of various yeast strains to reduce β-azido- α-ketoesters.[39] Although these reductions proceeded with excellent enantioselectivity (>98% ee) for three strains, limited diastereoselectivity (40% de, syn) was observed. In the same communication, the authors attempted a different route to the side chain via DYRKR of the Ojima lactam.[40] Unfortunately, this process appeared to suffer in selectivity when conversion exceeded 50%. It was thought that the low diastereoselectivities could perhaps be a result of multiple enzymes acting upon the substrate. This led to the systematic construction of a library of 19 GST-tagged reductases from the yeast genome.
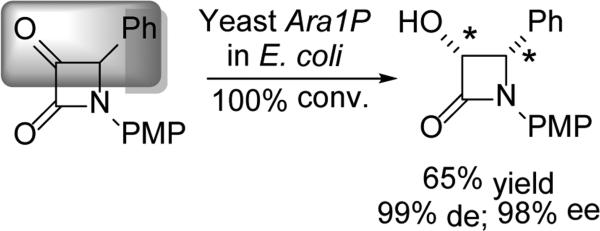
To address this shortcoming of the early attempts, Kayser went on to overexpress the yeast reductase, Ara1P, in E. coli.[41] What is key in this case is the importance of the experimental conditions. In a shake-flask, Ara1P yielded a 1.4:1 mixture of diastereomers (both in 99% ee). However, under fermenting conditions, only the (3S,4R) product was obtained (Scheme 10). Introduction of furyl or thiophenyl substituents, resulted in low diastereoselectivities, perhaps because the reduction rates of these substrates exceed the α-center racemization rates.
Scheme 10.
DYRKR entry into the taxane side chain.
Kalaitzakis and Smonou, in a joint effort with Biocatalytics, systematically explored the stereo- and chemoselectivity of a number of ADH enzymes and applied this to the reduction of β-ketoesters and 1,3-diones.[42] The next iteration employed a one pot, two enzyme approach to access 1,3-diols from 1,3-diketones.[43] As shown in Scheme 14, some of the examples are symmetrical diones. However, in other cases where no symmetry is present (Scheme 11), KRED 102 has remarkable regioselectivity in addition to diastereo- and enantioselectivity to produce the L-syn-α-alkyl-β-hydroxy ketones.
Scheme 14.
Reduction of vinylogous ketoesters.
Scheme 11.
Setting three contiguous centers with two consecutive ADH-mediated reductions
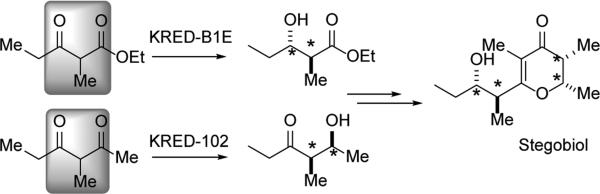
The authors do not observe any reduction to the diol with KRED 102 which demonstrates its ability to select exclusively for the dione. The second reduction to the diol with either KRED 101 or A1B is also noted to be very selective. Furthermore, KRED 101 and A1B display opposite facial selectivities, allowing for access to different 1,3-diol diastereomers. This represents an exciting development for the DYRKR-based synthesis of 1,3-diols of high optical purity and targeted stereochemistry. The authors were able to apply this DYRKR approach to the synthesis of a number of pheromones. In one case, the KRED-A1B-mediated reduction of methyl 2-methyl-3-oxopentanoate led to the stereoselective synthesis of sitophilate, a pheromone of the granary weevil.[44] Combining the chemoselectivity of KRED 102 in the reduction of 1,3-diketones with the stereoselectivity of KRED-B1E in the reduction of β–keto esters allowed for an impressive, enzymatically tuned entry into the beetle pheromones, stegobiol and steobinone (Scheme 12).[45]
Scheme 12.
Dual DYRKR entry into stegobiol.
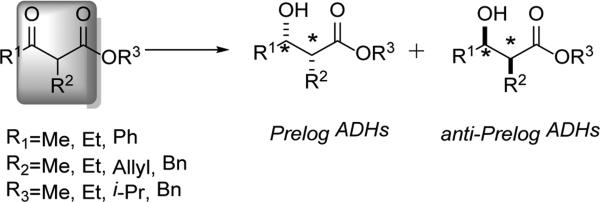
Collaborative work by Kroutil and Gotor examined a large series of dehydrogenases (ADH-A, CPADH, TeSADH, SyADH, RasADH, LBADH, LKADH) across various combinations of α-alkyl-β–ketoesters.[46] These enzymes were grouped according to the ability to handle certain steric requirement (termed ‘bulky-bulky’ substrates) or for the reduction to proceed in a Prelog or anti-Prelog fashion (Scheme 13).[47] While ADH-A, CPADH, and TeSADH tended to favor the formation of the (2R,3S)-product when R = small, RasADH [(2S,3S)-leading] and SyADH [(2R,3S)-leading] were able to accept bulkier substrates. The use of the anti-Prelog enzymes, LBADH and LKADH, provided access to the syn (2S,3R)-products.
Scheme 13.
Syn-Selectivity: Prelog vs. Anti-Prelog ADHs
The reduction of cylcohexenone derivatives presents its own unique problem as the regio- and chemoselectivity of 1,2- vs 1,4-reduction becomes a factor. Kosjek and coworkers screened a library of KREDS against a cyclohexenone with a γ–racemizable center (Scheme 14).[48] Under the reaction conditions, KRED 108 was able to reduce the vinylogous ketoester in high diastereoselectivity (99% de cis) and enantioselectivity (99% (S) with regard to the alcohol). This serves a representative example of enzymes being capable of distinguishing distal stereocenters in the enzymatic binding step.
Another example of such distal stereo-discrimination is seen in the YKER-I (from baker's yeast) reduction of sec-alkyl 2-methyl-3-oxobutyrates.[49] The active site of YKER-I not only is selective for the 2R-isomer but also for the 1’-R stereochemistry (Scheme 15). The result is that the reduction proceeds to give only one of eight possible stereoisomeric products. The recovered ketone is found to maintain a high enantiomeric excess at the 1’-position. By obtaining kinetic parameters for the individual 1’-enantiomers, the contributions of kcat and Km could be determined. Whereas the selectivity of lipases is often dominated by kcat,[50] HLADH appears to be controlled by Km.[51] The origin for this discrimination appears to be attributed to the differences in binding affinity of the 1’ stereoisomers to YKER-I. The Km for the 1’R substrate was found to be 10 times lower than that of the 1’S-isomer.
Scheme 15.
Discriminating distal stereocenters
From Stewart's collection of expressed yeast ADHs, an effort was made to characterize the enzyme for the ability to selectively catalyze the reduction of various β–keto-esters,[52] particularly, α–chloro-β–ketoesters.[53] Through extensive characterization, multiple enzymes were found to catalyze the reduction of at least two of the possible diastereomers with excellent selectivity (Scheme 16).[53] This led to the synthesis of both enantiomers of the taxol side chain by double inversion of the α–chloro-β–hydroxy ester, proceeding through an epoxide intermediate.
Scheme 16.
DYRKR of α-chloro-β-keto esters.
Recently, in our laboratory, a dehydrogenase from Clostridium acetobutylicum (CaADH) was expressed in E. coli and reported to be efficient on the same system. The CaADH reduction proceeded with excellent diastereo- (95% de, syn) and enantioselectivity (99% ee), even on a gram scale.[15b] Indeed, much more recent work indicates that this enzyme has enormous potential in asymmetric synthesis, displaying remarkable active site plasticity, while retaining stereochemical fidelity.[15a]
This represents a departure from the common α–alkyl-β–keto systems so far described. The presence of other α-heteroatomic functionality has been studied. In 1986, Sato reported the ability of Baker's yeast to catalyze the DYRKR of α–hydroxy-β–keto-esters.[54] Substrate scope was limited, but high enantioselectivities were observed in three instances. However, the common issue of diastereoselectivity persists, perhaps due to multiple active ADHs within the genome. This work was later expanded upon by Fadnavis and applied to the synthesis of chiral decalactones.[55] More recently, a panel of microbes was screened for the ability to selectively reduce 2-phenoxy-3-oxobutanoates.[56] This system maps onto that of clofibrate, a cholesterol-lowering compound. Adverse effects associated with clofibrate have driven the search for new analogues.
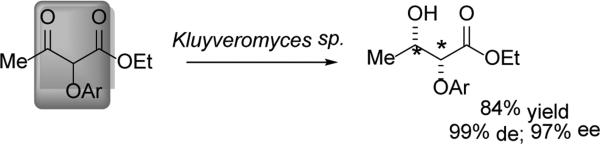
While baker's yeast was shown to reduce the butanoates with 92% de (syn) and 99% ee (2R,3S),[56] Kluyveromyces marxianus demonstrated better diastereoselectivity, providing the same product in 99% de and 97% ee (Scheme 17). The same group was able to isolate and purify the hypothesized ADH from K. marxianus and apply it to the reduction of α–(phthalamido)methyl-β–ketoesters.[57]
Scheme 17.
Useful DYRKR activity in K. marxianus
In 2013, the process group at Merck reported the selective reduction of α-amino-β-keto esters using in house-engineered dehydrogenases.[58] The syn amino alcohol was obtained in remarkable diastereo- (99% de) and enantioselectivity (99% ee) (Scheme 18). The amino alcohol was eventually transformed into cis-2,5-pyrrolidine, a core scaffold of β3-andrenergic receptor agonists. In this synthesis, the selective bio-reduction to generate two stereocenters allowed for a subsequent diastereoselective hydrogenation to set a third stereocenter.
Scheme 18.
Toward α-amino-β-hydroxy esters.
3 Other β-Keto-Systems
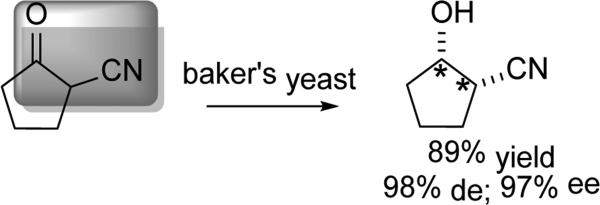
In work that expands the DYRKR domain beyond β-keto-esters, Delhi and Gotor explored the whole cell reduction of α-cyano-cyclopentanone.[59] Baker's yeast and Saccharomyces montanus provided the most efficient DYRKR with useful selectivity (98% de and 97% ee) (Scheme 19). Moving to the 6-membered ring provided similar selectivities. These findings allowed for the synthesis of cis-hydroxy nitriles, as opposed to the more common trans-diastereomers generated in the opening of epoxides or aziridines.
Scheme 19.
Reduction of β-keto nitriles
Acyclic systems of α-cyano-ketones have also been explored. The work of Itoh and coworkers demonstrated the ability of baker's yeast to reduce a number of 3-oxobutyronitriles (Scheme 20).[60] It is worth noting that the presence of the aryl group appears to be important in substrate recognition, as replacing the aryl group with other alkyl chains resulted in little diastereoselectivity (<10%). The absolute stereochemistry of this reduction is in good agreement with the work of Delhi.
Scheme 20.
Reduction of β-keto nitriles.
Phosphonates serve as non-hydrolyzable phosphate surrogates that have important utility in studying signaling pathways, inhibiting plant amino acid biosynthesis, or as reverse transcriptase inhibitors. As such, the development of asymmetric routes to phosphonate building blocks will continue to be important. One early example of this was the baker's yeast reduction of diethyl α-methyl- β-ketophosphonate.[61] Although the enantioselectivity was high (99% ee), the approach suffered from limited diastereoseletivity. Since then, efforts have been made to mine the yeast genome for suitable biocatalysts. Drawing from a similar approach used earlier by Stewart, Feske and coworkers screened 20 enzymes from this library against diethyl-α-chloro-α-ketophosphonate.[62] The group uncovered a number of enzymes that could reduce the ketophosphonate with ranging (12-95% de) diastereoselectivities for different products (Scheme 21). However, three enzymes were identified as catalysing the DYRKR with >90% de and >99% ee which allowed for a simple asymmetric, biocatalytic route into fosfomycin. Using whole cell baker's yeast, only 18% de was obtained, demonstrating again the utility of molecular biology and homology modeling in driving the frontiers of biocatalysis.
Scheme 21.
DYRKR of α-alkyl-β-keto-phosphonates.
Just as phosphonates are emerging as important targets in asymmetric synthesis, so too are sulfur-containing compounds, including sulfides, sulfoxides and sulfones. Such functionalities facilitate DYRKR processes by reducing the pKa of adjacent C-H bonds. These functionalities also provide useful binding handles for chemical biology and offer may be leveraged to facilitate subsequent carbon-carbon bond forming reactions.
As was the case for other substrate classes, early investigations into the reduction of sulfur-containing systems focused upon the study of native ADH activity in baker's yeast, as shown in Scheme 22.[63] While β-keto thioethers appear to be processed via simple (non-dynamic) kinetic resolution, β-keto sulfones under efficient DYRKR under these conditions. However, as the ring size increases to 7 for cyclic β-keto sulfones, a drastic decrease in yield is observed (8%, n=3).
Scheme 22.
DYRKR of β-keto sulfides and sulfones.
In a transposed version this system, the sulfone has been locked into the cyclic system in the form of 2-acetylsulfolane. The DYRKR still proceeds efficiently providing the β-hydroxy cyclic sulfone in an overall syn manner with high enantioselectivity.[63]
Interesting sulfur-containing functionalities are found in other ADH substrate classes.[64] Earlier, in Scheme 4, for example, we illustrated an SNAC thioester reduction catalyzed by a PKS KR. Other examples employing baker's yeast have attempted to reduce an α-alkyl-β-ketoxanthate. Surprisingly, this reduction results in increased diastereoselectivity (88% de) when compared to the ethyl ester analog (70% de), perhaps due to a more acidic α–proton.[65]
The common theme to this point is that ADH-mediated DYRKR can be applied to a broad range of keto-systems, with appropriately acidic α-protons (vide infra). The reduction of aldehydes is not normally thought of a stereoselective process (except, perhaps if carried out with deuteride equivalent). However, reductions of aldehydes with an adjacent stereocenter allow for a DYRKR. An early example of such a system was the baker's yeast-mediated reduction of a variety of 2-methyl-3-oxopropionates in which the ester chain was systematically lengthened.[66] Isobutyl 2-methyl-3-hydroxypropionate was obtained in 90% ee (R). This is in good agreement with the stereochemistry observed in the reduction of 2-methyl-3-oxobutanoates in which the (2R,3S) diastereomer predominates.
This α-alkyl aldehyde-variant of the DYRKR has been implemented in the stereoselective reduction of profenals in two laboratories recently.[67] Giacomini and coworkers, found that HLADH could effect the reduction of 2-phenylpropanal to (S)-2-phenylpropanol in 99% ee. Similar results were obtained for ibuprofenal. Since then, HLADH has also achieved high enantioselectivity in the reduction of other profenals.[68]
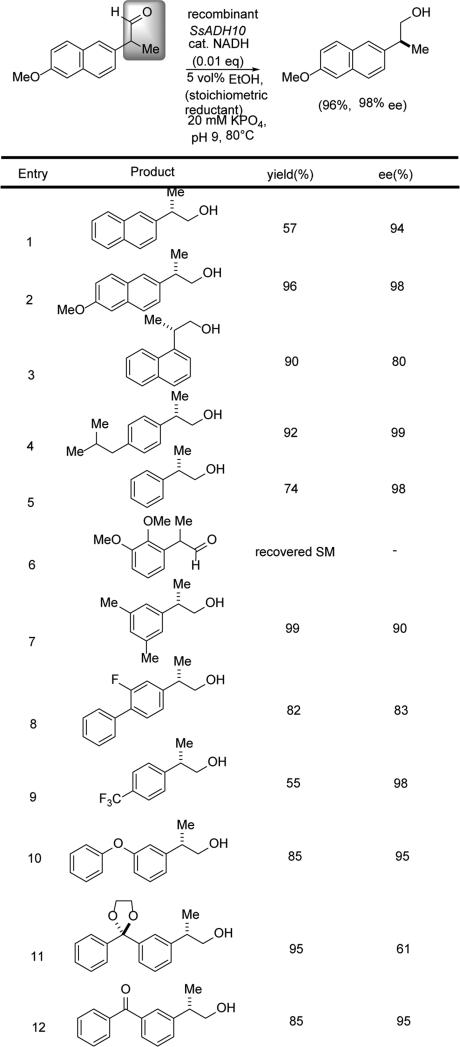
Parallel to those studies, a synergistic collaboration between the Berkowitz laboratory and that of Paul Blum, an archaeal microbiologist, led to the identification of a hyperthermophilic ADH with great potential for asymmetric catalysis. Namely, ADH isozyme 10 from Sulfolobus solfataricus (SsADH-10) was found to selectively mediate the DYRKR of a broad set of 2-(S)-profenaldehydes, generating the corresponding profenols in >94% ee (7 examples, Scheme 23).[15c]
Scheme 23.
DYRKR leading to profenols.
This case also serves to illustrate a potentially important feature of hyperthermophilic enzymes in organic synthesis. Namely, in this case, the DYRKR is conducted under essentially “organic solvent-free” conditions; that is, in aqueous buffer, containing 5% ethanol as terminal reductant. While the starting (±)-naproxenal substrate is insoluble at RT, heating to 70 °C both activates the SsADH-10 enzyme and solubilizes the substrate. Thus, heat substitutes for organic co-solvent. Upon reaction completion, cooling to room temperature results in precipitation of highly enantioenriched (S)-naproxenol, allowing for product isolation by simple filtration. The enzyme itself, SsADH-10 is recovered in the filtrate and remains efficient even when recycled five times (>94% ee). This example illustrates the great potential of thermophilic enzymes in stereocontrolled synthesis and/or process chemistry, and argues for much greater exploration of the enzymology of archaeal hyperthermophiles!
In summary, a variety of substrate classes have been explored as platforms for DYRKR. Early discussions focused on the importance of a racemizable stereocenter to insure that one has a ‘dynamic’ reductive kinetic resolution. Indeed, the racemization rate should be fast, relative to the enzymatic reduction rate, under the reaction conditions, to insure an effective DYRKR process. Looking at the successful substrates described as a whole, it becomes apparent that most such systems have α-C-H pKa values between 7.5 and 12.5 (Scheme 24).[59, 69] Note that for values not previously reported in H2O, predicted pKa values are given here, through application of the program Marvin (version 15.4.2).[70] This ‘acidity window’ is within 2-4 units of the pH regimes employed for the dehydrogenase-mediated DYRKR thereby facilitating the rapid racemization needed.
Scheme 24.
pKa values of various successful DYRKR classes
4 Future Challenges
a. More Complex Substrates
While much of this review has detailed the deployment of dehydrogenase enzymes across a battery of carbonyl compounds, most commonly bearing an additional acidifying functionality, there is certainly room to increase the complexity of such substrates, going forward. This is particularly true when one considers Turner's vision of incorporating biocatalytic transformations into standard retrosynthetic analysis. Indeed, given the power of directed evolution, along with continuing advances in structural/computational biology, there will undoubtedly be increasing opportunities to leverage enzymatic chemistry in later stages of both process chemistry and natural products synthesis. As alluded to earlier, one impressive example from pharma is the latest sitagliptin (Januvia™) process from a fruitful Merck/Codexis collaboration, in which a transaminase is employed in the late stages to efficiently reductively aminate a complex ketone substrate.[9] This stellar example of directed evolution and optimization of an enzyme toward an advanced synthetic intermediate was recognized with the 2010 Presidential Green Chemistry Challenge Award.
Indeed, examples of ADH-mediated DYRKR processes with increasingly complex substrates are beginning to appear. For example, Matsumae and coworkers evaluated a number of microorganisms for the ability to reduce a diltiazem precursor.[71] Diltiazen is a calcium channel blocker that finds application in the treatment of hypertension and arrhythmia. The native ADHs present in baker's yeast were found to efficiently perform DYRKR on this diltiazem precursor, reducing the ketone with outstanding diastereo- (96% de) and enantioselectivities (>99% ee) in an overall 94% yield (Scheme 25).
Scheme 25.
Reduction of β-substituted-α-ketoamides.
It is also incumbent upon the synthetic community to continue to dchallenge dehydrogenase enzymes with substrates presenting new organic functionalities, in order to expand the biocatalytic toolbox. One recent such example from the Berkowitz laboratory involves the reduction of the previously unexplored class of α-fluorinated β-ketophosphonates.[15a] Success was had with a Clostridial enzyme that had previously shown the ability to generate ω-hydroxy-esters from the corresponding ω-keto carboxylate esters, in high optical purity. Under DYRKR conditions, a precursor to the taxane side chain was produced with high diastereo- and enantioselectivity. In an effort to expand the substrate repertoire for CaADH, it was challenged with a variety of α,α–difluoro-β–ketophosphonates. Surprisingly, CaADH reduces a large array of ketophosphonates with excellent enantioselectivity. This represents the first example of dehydrogenase application to these systems. In this sense, CaADH demonstrates remarkable active site plasticity, yet retains a high degree of stereochemical fidelity. Interestingly, it was also observed that the facial selectivity for the reduction is reversed upon changing substrate scaffolds from the previously employed ω-keto carboxylate substrate (D-selectivity) to the more recent β-keto-α,α-difluorophosphonate ester substrates (L-selectivity).
In an attempt to understand this fascinating behavior, computational docking experiments were performed in the CaADH active site with a representative β–keto-ester, γ–keto-ester, and β–keto-phosphonate (Autodock 4).[72] The results of these experiments are displayed in Scheme 26. As shown, the keto group for the β– and γ–keto-esters are coordinated to S140, a typical binding motif for short-chain dehydrogenases.[73] The ester carbonyls for the β– and γ–keto ester are shown to be in hydrogen bonding distance to Y153 and K207, respectively. However, the keto-phosphonate docks with the keto group inverted, forming a hydrogen bond with K207. This orientation leads to the observed stereochemistry for this substrate class. Such a result could not have been predicted, but this example serves to demonstrate the potential of some ADH active sites to exhibit remarkable substrate plasticity, while retaining an impressive level of stereochemical fidelity.
Scheme 26.
Remarkable active site plasticity exhibited by CaADH.: a D-syn-selectivity with ethyl α-chloro benzoylacetate; b D-selectivity seen with γ-keto carboxylate esters; c L-selectivity seen with a diethyl α,α-difluoro-β-keto-phosphonate ester
b. Substrates with Higher pKa Values
Another challenge on the horizon for the expansion of ADH-mediated DYRKR in asymmetric synthesis revolves directly around that aforementioned C-H acidity question.. The acidity of this α-proton limits current DYRKR undertakings to a fairly specific set of substrate classes. As was noted above, most of the successful examples cited in this review have reported α-C-H pKa values between 7.5 and 12 (Scheme 24), within several pH units of typical pH regimes in which ADHs normally operate. One apparent example of this pKa limitation is in the aforementioned ADH-mediated reduction of β–keto sulfides and β–keto sulfones bearing a-stereocenters (Scheme 22 and accompanying discussion). Whereas the latter was found to provide an excellent DYRKR platform, the former appears to be limited to classical kinetic resolution. This result is perhaps explained through the large differences in acidity of the α–proton (Scheme 27).[69a, 69c] Predicted pKa values were again obtained using the Marvin program.
Scheme 27.
pKa values for some challenging DYRKR classes (the dashed box includes “solved” examples.
It is important to note that a high pKa value can be circumvented by a variety of approaches. In the case of Scheme 8, exogenous triethylamine was added to facilitate racemization. Another approach is the application of enzymes capable of operating in a more basic media, either through the use of extremophiles (Scheme 23) or through ADH engineering.
c. Substrates with a Third, Non-Dynamic Preexisting Stereocenter
Another challenge on the horizon will be to uncover biocatalysts that are capable of generating three contiguous stereocenters. Recall that, while examples have been illustrated for such systems (e.g. Scheme 15), only two stereocenters were actually set in the biotransformation. In other examples, two sequential ADHs were shown to control a total of three stereocenters. Other such examples exist where a third, non-dynamic and also ‘silent’ stereocenter is present prior to the DYRKR. In these cases, the dynamic reduction proceeds in a highly selective manner. However, the non-dynamic center results in two major diastereomeric products (Scheme 28).[63-64] Interestingly, in both cases here, two essentially enantiopure diastereomers are obtained, yet the reported diastereomeric ratio is not 1:1, nor is the overall yield quantitative, as it should be, in the ideal case of a ‘silent’ pre-existing stereocenter. It thus appears likely in these cases, that the pre-existing stereocenter is not ‘silent,’ but rather that the ADH (or ADHs) present act at a differential rate upon the two enantiomeric ketone substrates, sensing this center in this way. Even so, given sufficient time and enzyme, it should have been possible to push these reactions to completion to give a 1:1 ratio of the observed products. One is left to conclude that either, insufficient enzyme/reaction time was used, or these conditions do in fact lead to the formation of other products, comprising the remaining 30-35% of the material. Either way, given the power of directed evolution, it should be possible to fine tune ADHs in the future to be almost completely ‘silent’ to the remote stereocenter for such substrates, or highly selective for that center (see Scheme 15) depending on the desired outcome of the process.
Scheme 28.
DYRKR with substrates containing a third, non-dynamic stereocenter.
d. Substrates with Two Dynamic Stereocenters
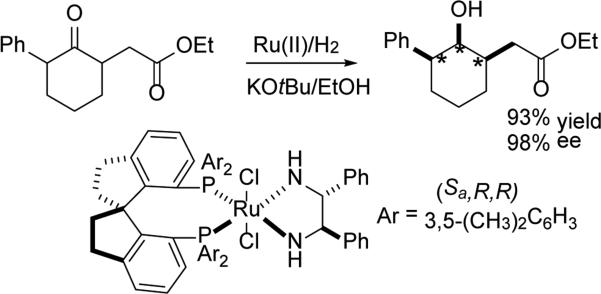
Finally, among the more powerful DYRKR processes to be developed in the future, would be those in which the dehydrogenase is able selectively to act upon a substrate with more than one dynamic center. Here, as is often the case in synthetic chemistry, inspiration to push the envelope in biocatalytic synthesis can be found in the organometallic chemistry literature. Indeed, in one nice such example, Zhou and co-workers have recently achieved such a feat utilizing Noyori-type asymmetric carbonyl reduction. Thus exposure α,α’-disubstituted cyclohexanones to Ru-catalyzed asymmetric ketone hydrogenation under basic, DYRKR conditions, leads to a remarkable result, in which three contiguous stereocenters are simultaneously set in one synthetic step.[74] Thus, in the presence of an appropriate chiral spirocyclic ligand, racemic α,α’ disubstituted cyclohexanones are transformed into essentially one of eight possible stereoisomeric α,α’ dialkylcyclohexanols. High cis,cis-selectivity (>99%) and high enantioselectivity are observed (Scheme 29). Given the remarkable ADH active site plasticity that has already been observed,[15a] and the power of directed evolution,[7c, 12b, 23a] a menu of such biocatalytic transformations may well be forthcoming in the not-too-distant future, in which ‘double, dynamic reductive kinetic resolutions’ are achieved, setting two different dynamic centers, while selectively reducing a central carbonyl functionality, all in a single operation.
Scheme 29.
Double DYRKR: Future challenges for ADH-mediated stereocontrolled synthesis?
Acknowledgements
The authors thank the NSF (DMR-1214019) for support. This research was facilitated by the IR/D (Individual Research and Development) program associated with DBB's appointment at the NSF. The authors thank the NIH (SIG-1-510-RR-06307) and NSF (CHE-0091975, MRI-0079750) for NMR instrumentation support and the NIH (RR016544) for facilities renovation.
Biography
 Gregory Applegate is pursuing his Ph.D. in Chemistry in the Berkowitz group at the U. Nebraska, with interests in synthetic methodology and chemical biology. He has studied the use of enzymes as both stereoselective catalysts and as ‘reporting enzymes’ in asymmetric synthesis, and also has interests in enzyme mechanism.
Gregory Applegate is pursuing his Ph.D. in Chemistry in the Berkowitz group at the U. Nebraska, with interests in synthetic methodology and chemical biology. He has studied the use of enzymes as both stereoselective catalysts and as ‘reporting enzymes’ in asymmetric synthesis, and also has interests in enzyme mechanism.
 David Berkowitz is Willa Cather Professor of Chemistry at the University of Nebraska and is currently serving as Director of the Chemistry Division at the NSF. His interests are at the interface of asymmetric synthesis and mechanistic enzymology. His honors include Alfred P. Sloan and JSPS Fellowships
David Berkowitz is Willa Cather Professor of Chemistry at the University of Nebraska and is currently serving as Director of the Chemistry Division at the NSF. His interests are at the interface of asymmetric synthesis and mechanistic enzymology. His honors include Alfred P. Sloan and JSPS Fellowships
References
- 1.a Martinez A, van Gemmeren M, List B. Synlett. 2014;25:961–964. [Google Scholar]; b Bisai V, Bisai A, Singh VK. Tetrahedron. 2012;68:4541–4580. [Google Scholar]; c Yang H, Carter RG. Org. Lett. 2010;12:3108–3111. doi: 10.1021/ol1011955. [DOI] [PubMed] [Google Scholar]; d Gustafson JL, Lim D, Miller SJ. Science. 2010;328:1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Jordan PA, Kayser-Bricker KJ, Miller SJ. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20620–20624. doi: 10.1073/pnas.1001111107. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Enders D, Narine AA. J. Org. Chem. 2008;73:7857–7870. doi: 10.1021/jo801374j. [DOI] [PubMed] [Google Scholar]; g Knudsen KR, Mitchell CET, Ley SV. Chem. Commun. 2006:66–68. doi: 10.1039/b514636d. [DOI] [PubMed] [Google Scholar]; h Steiner DD, Mase N, Barbas CF., III Angew. Chem., Int. Ed. 2005;44:3706–3710. doi: 10.1002/anie.200500571. [DOI] [PubMed] [Google Scholar]; i Agarkov A, Greenfield SJ, Ohishi T, Collibee SE, Gilbertson SR. J. Org. Chem. 2004;69:8077–8085. doi: 10.1021/jo049103g. [DOI] [PubMed] [Google Scholar]; j Brown SP, Brochu MP, Sinz CJ, MacMillan DWC. J. Am. Chem. Soc. 2003;125:10808–10809. doi: 10.1021/ja037096s. [DOI] [PubMed] [Google Scholar]
- 2.Friedfeld MR, Shevlin M, Hoyt JM, Krska SW, Tudge MT, Chirik PJ. Science. 2013;342:1076–1080. doi: 10.1126/science.1243550. [DOI] [PubMed] [Google Scholar]
- 3.Bornscheuer U, Huisman G, Kazlauskas R, Lutz S, Moore J, Robins K. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- 4.Burk MJ, De Koning PD, Grote TM, Hoekstra MS, Hoge G, Jennings RA, Kissel WS, Le TV, Lennon IC, Mulhern TA, Ramsden JA, Wade RA. J. Org. Chem. 2003;68:5731–5734. doi: 10.1021/jo034397b. [DOI] [PubMed] [Google Scholar]
- 5.Martinez CA, Hu S, Dumond Y, Tao J, Kelleher P, Tully L. Org. Process Res. Dev. 2008;12:392–398. [Google Scholar]
- 6.Oslaj M, Cluzeau J, Orkic D, Kopitar G, Mrak P, Casar Z. PLoS One. 2013;8:e62250. doi: 10.1371/journal.pone.0062250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a Tang Y. Abstracts of Papers, 249th ACS Natl Meeting; USA. March 22-26, 2015; Denver, CO; 2015. pp. CHED–1662. [Google Scholar]; b Tang Y, Gao X, Xie X. University of California, USA; 2011. p. 120. Application: 2010-US52038. [Google Scholar]; c Gao X, Xie X, Pashkov I, Sawaya MR, Laidman J, Zhang W, Cacho R, Yeates TO, Tang Y. Chem. Biol. 2009;16:1064–1074. doi: 10.1016/j.chembiol.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen KB, Hsiao Y, Xu F, Rivera N, Clausen A, Kubryk M, Krska S, Rosner T, Simmons B, Balsells J, Ikemoto N, Sun Y, Spindler F, Malan C, Grabowski EJJ, Armstrong Joseph JD., III J. Am. Chem. Soc. 2009;131:8798–8804. doi: 10.1021/ja902462q. [DOI] [PubMed] [Google Scholar]
- 9.Savile CK, Janey JM, Mundorff EC, Moore JC, Tam S, Jarvis WR, Colbeck JC, Krebber A, Fleitz FJ, Brands J. Science. 2010;329:305–309. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- 10.Cuetos A, Lavandera I, Gotor V. Chem. Commun. 2013;49:10688–10690. doi: 10.1039/c3cc46760k. [DOI] [PubMed] [Google Scholar]
- 11.Müller CR, Pérez-Sánchez M, de María PD. Org. Biomol.Chem. 2013;11:2000–2004. doi: 10.1039/c2ob27344f. [DOI] [PubMed] [Google Scholar]
- 12.a de Wildeman S, Sereinig N. Sci. Synth., Stereosel. Synth. 2011;2:133–208. [Google Scholar]; b Musa MM, Phillips RS. Catal. Sci. Technol. 2011;1:1311–1323. [Google Scholar]; c Hall M, Bommarius AS. Chem. Rev. 2011;111:4088–4110. doi: 10.1021/cr200013n. [DOI] [PubMed] [Google Scholar]; d Gamenara D, Seoane GA, Saenz - Méndez P, de María PD. Redox Biocatalysis: Fundamentals and Applications. 2012:101–179. [Google Scholar]; e Hollmann F, Arends IW, Holtmann D. Green Chem. 2011;13:2285–2314. [Google Scholar]; f Rowan AS, Moody TS, Howard RM, Underwood TJ, Miskelly IR, He Y, Wang B. Tetrahedron: Asymmetry. 2013;24:1369–1381. [Google Scholar]
- 13.a Berkowitz DB, Shen W, Maiti G. Tetrahedron Asymmetry. 2004;15:2845–2851. doi: 10.1016/j.tetasy.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Berkowitz DB, Maiti G. Org. Lett. 2004;6:2661–2664. doi: 10.1021/ol049159x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Berkowitz DB, Bose M, Choi S. Angew. Chem., Int. Ed. 2002;41:1603–1607. doi: 10.1002/1521-3773(20020503)41:9<1603::aid-anie1603>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a Ginotra SK, Friest JA, Berkowitz DB. Org. Lett. 2012;14:968–971. doi: 10.1021/ol203088g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dey S, Powell DR, Hu C, Berkowitz DB. Angew. Chem., Int. Ed. 2007;46:7010–7014. doi: 10.1002/anie.200701280. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Dey S, Karukurichi KR, Shen W, Berkowitz DB. J. Am. Chem. Soc. 2005;127:8610–8611. doi: 10.1021/ja052010b. [DOI] [PubMed] [Google Scholar]
- 15.a Panigrahi K, Applegate GA, Malik G, Berkowitz DB. J. Am. Chem. Soc. 2015;137:3600–3609. doi: 10.1021/jacs.5b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Applegate GA, Cheloha RW, Nelson DL, Berkowitz DB. Chem. Commun. 2011;47:2420–2422. doi: 10.1039/c0cc04585c. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Friest JA, Maezato Y, Broussy S, Blum P, Berkowitz DB. J. Am. Chem. Soc. 2010;132:5930–5931. doi: 10.1021/ja910778p. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Broussy S, Cheloha RW, Berkowitz DB. Org. Lett. 2009;11:305–308. doi: 10.1021/ol802464g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore JC, Pollard DJ, Kosjek B, Devine PN. Acc. Chem. Res. 2007;40:1412–1419. doi: 10.1021/ar700167a. [DOI] [PubMed] [Google Scholar]
- 17.a Patel JM. J. Mol. Catal. B: Enzym. 2009;61:123–128. doi: 10.1016/j.molcatb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wolberg M, Villela Filho M, Bode S, Geilenkirchen P, Feldmann R, Liese A, Hummel W, Mueller M. Bioprocess Biosyst. Eng. 2008;31:183–191. doi: 10.1007/s00449-008-0205-9. [DOI] [PubMed] [Google Scholar]; c Groeger H, Chamouleau F, Orologas N, Rollmann C, Drauz K, Hummel W, Weckbecker A, May O. Angew. Chem., Int. Ed. 2006;45:5677–5681. doi: 10.1002/anie.200503394. [DOI] [PubMed] [Google Scholar]; d Wolberg M, Hummel W, Wandrey C, Muller M. Angew. Chem., Int. Ed. 2000;39:4306–4308. doi: 10.1002/1521-3773(20001201)39:23<4306::AID-ANIE4306>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda T, Yamanaka R, Nakamura K. Tetrahedron Asymmetry. 2009;20:513–557. [Google Scholar]
- 19.Corey EJ, Long AK, Lotto GI, Rubenstein SD. Recl. Trav. Chim. Pays-Bas. 1992;111:304–310. [Google Scholar]
- 20.Turner NJ, O'Reilly E. Nat. Chem. Biol. 2013;9:285–288. doi: 10.1038/nchembio.1235. [DOI] [PubMed] [Google Scholar]
- 21.a Pellissier H. Adv. Synth. Catal. 2011;353:659–676. [Google Scholar]; b Pellissier H. Tetrahedron. 2011;67:3769–3802. [Google Scholar]; c Schnell B, Faber K, Kroutil W. Adv. Synth. Catal. 2003;345:653–666. [Google Scholar]; d Pamies O, Bäckvall J-E. Chem. Rev. 2003;103:3247–3262. doi: 10.1021/cr020029g. [DOI] [PubMed] [Google Scholar]
- 22.a Verho O, Bäckvall J-E. J. Am. Chem. Soc. 2015 doi: 10.1021/jacs.5b01031. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee JH, Han K, Kim MJ, Park J. Eur. J. Org. Chem. 2010;2010:999–1015. [Google Scholar]; c Hoyos P, Pace V, Alcantara AR. Adv. Synth. Catal. 2012;354:2585–2611. [Google Scholar]
- 23.a Carr R, Alexeeva M, Dawson MJ, Gotor - Fernández V, Humphrey CE, Turner NJ. ChemBioChem. 2005;6:637–639. doi: 10.1002/cbic.200400329. [DOI] [PubMed] [Google Scholar]; b Kim Y, Park J, Kim MJ. ChemCatChem. 2011;3:271–277. [Google Scholar]; c Alexeeva M, Enright A, Dawson MJ, Mahmoudian M, Turner NJ. Angew. Chem., Int. Ed. 2002;41:3177–3180. doi: 10.1002/1521-3773(20020902)41:17<3177::AID-ANIE3177>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.a Paizs C, Tähtinen P, Lundell K, Poppe L, Irimie F-D, Kanerva LT. Tetrahedron: Asymmetry. 2003;14:1895–1904. [Google Scholar]; b Inagaki M, Hiratake J, Nishioka T, Oda J. J. Org. Chem. 1992;57:5643–5649. [Google Scholar]
- 25.a Choi YK, Kim Y, Han K, Park J, Kim M-J. J. Org. Chem. 2009;74:9543–9545. doi: 10.1021/jo902034x. [DOI] [PubMed] [Google Scholar]; b Beard TM, Turner NJ. Chem. Commun. 2002:246–247. doi: 10.1039/b107580m. [DOI] [PubMed] [Google Scholar]
- 26.a Noyori R, Ikeda T, Ohkuma T, Widhalm M, Kitamura M, Takaya H, Akutagawa S, Sayo N, Saito T. J. Am. Chem. Soc. 1989;111:9134–9135. [Google Scholar]; b Noyori R, Ohkuma T, Kitamura M, Takaya H, Sayo N, Kumobayashi H, Akutagawa S. J. Am. Chem. Soc. 1987;109:5856–5858. [Google Scholar]
- 27.Deol BS, Ridley D, Simpson G. Aust. J. Chem. 1976;29:2459–2467. [Google Scholar]
- 28.Häckh M, Müller M, Lüdeke S. Chem.--Eur. J. 2013;19:8922–8928. doi: 10.1002/chem.201300554. [DOI] [PubMed] [Google Scholar]
- 29.Lüdeke S, Richter M, Müller M. Adv. Synth. Catal. 2009;351:253–259. [Google Scholar]
- 30.Kędziora K, Bisogno FR, Lavandera I, Gotor - Fernández V, Montejo - Bernardo J, García - Granda S, Kroutil W, Gotor V. ChemCatChem. 2014;6:1066–1072. [Google Scholar]
- 31.Musa MM, Ziegelmann-Fjeld KI, Vieille C, Zeikus JG, Phillips RS. J. Org. Chem. 2007;72:30–34. doi: 10.1021/jo0616097. [DOI] [PubMed] [Google Scholar]
- 32.a Kambourakis S, Rozzell JD. Tetrahedron. 2004;60:663–669. [Google Scholar]; b Kambourakis S, Rozzell JD. Adv. Synth. Catal. 2003;345:699–705. [Google Scholar]
- 33.Mangas-Sánchez J, Busto E, Gotor V, Gotor-Fernández V. Org. Lett. 2013;15:3872–3875. doi: 10.1021/ol401606x. [DOI] [PubMed] [Google Scholar]
- 34.Kamaruddin AH, Uzir MH, Aboul - Enein HY, Halim HNA. Chirality. 2009;21:449–467. doi: 10.1002/chir.20619. [DOI] [PubMed] [Google Scholar]
- 35.Buisson D, Azerad R, Sanner C, Larcheveque M. Biocatal. Biotransform. 1990;3:85–93. [Google Scholar]
- 36.Buisson D, Cecchi R, Laffitte J-A, Guzzi U, Azerad R. Tetrahedron Lett. 1994;35:3091–3094. [Google Scholar]
- 37.Danchet S, Bigot C, Buisson D, Azerad R. Tetrahedron: Asymmetry. 1997;8:1735–1739. [Google Scholar]
- 38.Ravía SP, Carrera I, Seoane GA, Vero S, Gamenara D. Tetrahedron: Asymmetry. 2009;20:1393–1397. [Google Scholar]
- 39.Kayser MM, Mihovilovic MD, Kearns J, Feicht A, Stewart JD. J. Org. Chem. 1999;64:6603–6608. doi: 10.1021/jo9900681. [DOI] [PubMed] [Google Scholar]
- 40.a Ojima I, Habus I, Zhao M, Georg GI, Jayasinghe LR. J. Org. Chem. 1991;56:1681–1683. [Google Scholar]; b Ojima I, Habus I, Zhao M, Zucco M, Park YH, Sun CM, Brigaud T. Tetrahedron. 1992;48:6985–7012. [Google Scholar]
- 41.Yang Y, Drolet M, Kayser MM. Tetrahedron: Asymmetry. 2005;16:2748–2753. [Google Scholar]
- 42.Kalaitzakis D, Rozzell JD, Kambourakis S, Smonou I. Org. Lett. 2005;7:4799–4801. doi: 10.1021/ol051166d. [DOI] [PubMed] [Google Scholar]
- 43.a Kalaitzakis D, Rozzell JD, Smonou I, Kambourakis S. Adv. Synth. Catal. 2006;348:1958–1969. [Google Scholar]; b Kalaitzakis D, Smonou I. J. Org. Chem. 2010;75:8658–8661. doi: 10.1021/jo101519t. [DOI] [PubMed] [Google Scholar]
- 44.Kalaitzakis D, Kambourakis S, Rozzell DJ, Smonou I. Tetrahedron: Asymmetry. 2007;18:2418–2426. [Google Scholar]
- 45.Kalaitzakis D, Smonou I. Eur. J. Org. Chem. 2012;2012:43–46. [Google Scholar]
- 46.Cuetos A, Rioz - Martínez A, Bisogno FR, Grischek B, Lavandera I, de Gonzalo G, Kroutil W, Gotor V. Adv. Synth. Catal. 2012;354:1743–1749. [Google Scholar]
- 47.Prelog V. Pure Appl. Chem. 1964;9:119–130. [Google Scholar]
- 48.Kosjek B, Tellers DM, Biba M, Farr R, Moore JC. Tetrahedron: Asymmetry. 2006;17:2798–2803. [Google Scholar]
- 49.Kawai Y, Hida K, Ohno A. Bioorg. Chem. 1999;27:3–19. [Google Scholar]
- 50.Okahata Y, Fujimoto Y, Ijiro K. J. Org. Chem. 1995;60:2244–2250. [Google Scholar]
- 51.Adolph HW, Maurer P, Schneider-Bernlöhr H, Sartorius C, Zeppezauer M. Eur. J. Biochem. 1991;201:615–625. doi: 10.1111/j.1432-1033.1991.tb16322.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaluzna IA, Matsuda T, Sewell AK, Stewart JD. J. Am. Chem. Soc. 2004;126:12827–12832. doi: 10.1021/ja0469479. [DOI] [PubMed] [Google Scholar]
- 53.Feske BD, Kaluzna IA, Stewart JD. J. Org. Chem. 2005;70:9654–9657. doi: 10.1021/jo0516077. [DOI] [PubMed] [Google Scholar]
- 54.Sato T, Tsurumaki M, Fujisawa T. Chem. Lett. 1986;15:1367–1370. [Google Scholar]
- 55.Fadnavis N, Vadivel SK, Sharfuddin M. Tetrahedron: Asymmetry. 1999;10:3675–3680. [Google Scholar]
- 56.Perrone MG, Santandrea E, Scilimati A, Tortorella V, Capitelli F, Bertolasi V. Tetrahedron: Asymmetry. 2004;15:3501–3510. [Google Scholar]
- 57.Perrone MG, Santandrea E, Scilimati A, Syldatk C. Adv. Synth. Catal. 2007;349:1111–1118. [Google Scholar]
- 58.Xu F, Chung JY, Moore JC, Liu Z, Yoshikawa N, Hoerrner RS, Lee J, Royzen M, Cleator E, Gibson AG. Org. Lett. 2013;15:1342–1345. doi: 10.1021/ol400252p. [DOI] [PubMed] [Google Scholar]
- 59.Dehli JR, Gotor V. J. Org. Chem. 2002;67:6816–6819. doi: 10.1021/jo0257288. [DOI] [PubMed] [Google Scholar]
- 60.Itoh T, Fukuda T, Fujisawa T. Bull. Chem. Soc. Jpn. 1989;62:3851–3855. [Google Scholar]
- 61.Żymańczyk-Duda E. Phosphorus, Sulfur Silicon. 2008;183:369–382. [Google Scholar]
- 62.Marocco CP, Davis EV, Finnell JE, Nguyen P-H, Mateer SC, Ghiviriga I, Padgett CW, Feske BD. Tetrahedron: Asymmetry. 2011;22:1784–1789. [Google Scholar]
- 63.Deasy RE, Maguire AR. Eur. J. Org. Chem. 2014;2014:3737–3756. [Google Scholar]
- 64.Deasy RE, O'Riordan N, Maguire AR. Catalysts. 2014;4:186–195. [Google Scholar]
- 65.Itoh T, Yonekawa Y, Sato T, Fujisawa T. Tetrahedron Lett. 1986;27:5405–5408. [Google Scholar]
- 66.Nakamura K, Miyai T, Ushio K, Oka S, Ohno A. Bull. Chem. Soc. Jpn. 1988;61:2089–2093. [Google Scholar]
- 67.Giacomini D, Galletti P, Quintavalla A, Gucciardo G, Paradisi F. Chem. Commun. 2007:4038–4040. doi: 10.1039/b712290j. [DOI] [PubMed] [Google Scholar]
- 68.Galletti P, Emer E, Gucciardo G, Quintavalla A, Pori M, Giacomini D. Org. Biomol. Chem. 2010;8:4117–4123. doi: 10.1039/c005098a. [DOI] [PubMed] [Google Scholar]
- 69.a Bordwell F, Van der Puy M, Vanier NR. J. Org. Chem. 1976;41:1885–1886. [Google Scholar]; b Bordwell F, Van der Puy M, Vanier NR. J. Org. Chem. 1976;41:1883–1885. [Google Scholar]; c Bordwell FG. Acc. Chem. Res. 1988;21:456–463. [Google Scholar]; d Bugg TD. Introduction to Enzyme and Coenzyme Chemistry. John Wiley & Sons; 2012. [Google Scholar]; e Olmstead WN, Bordwell FG. J. Org. Chem. 1980;45:3299–3305. [Google Scholar]
- 70.a Csizmadia F, Tsantili - Kakoulidou A, Panderi I, Darvas F. J. Pharm. Sci. 1997;86:865–871. doi: 10.1021/js960177k. [DOI] [PubMed] [Google Scholar]; b Szegezdi J, Csizmadia F. Abstracts of Papers, 227th ACS Natl Meeting; Anaheim, CA, USA. March 28-April 4, 2004.pp. COMP–233. [Google Scholar]; c Szegezdi J, Csizmadia F. Abstracts of Papers, 233rd ACS Natl Meeting; Chicago, IL, USA. March 25-29, 2007.pp. CINF–41. [Google Scholar]
- 71.Matsumae H, Douno H, Yamada S, Nishida T, Ozaki Y, Shibatani T, Tosa T. J. Ferment. Bioeng. 1995;79:28–32. [Google Scholar]
- 72.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. J. Comp. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B. Chemico-Biol. Interact. 2003;143:247–253. doi: 10.1016/s0009-2797(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 74.Liu C, Xie JH, Li YL, Chen JQ, Zhou QL. Angew. Chem. Int. Ed. 2013;52:593–596. doi: 10.1002/anie.201207561. [DOI] [PubMed] [Google Scholar]