Abstract
Bone marrow lesions (BMLs) or using older terminology ‘Bone marrow edema' is characterised by excessive water signals in the marrow space on magnetic resonance imaging or ultrasound; BMLs constitute a central component of a wide variety of inflammatory and non-inflammatory rheumatologic conditions affecting the musculoskeletal system: BMLs are not only considered significant sources of pain but also linked to increased disease activity in many musculoskeletal conditions (for example, osteoarthritis, rheumatoid arthritis). The purpose of this review is to summarise current knowledge about the treatment of BMLs, with an emphasis on the clinical and histological features of this entity in inflammatory and non-inflammatory disease. We also try to pair this hypothesis with the apparent beneficial effects of various treatment regimens, mainly within the group of bone antiresorptive drugs (calcitonin, bisphosphonates) on symptoms associated with BMLs.
Introduction
After the introduction of magnetic resonance imaging (MRI) in clinical practice, bone marrow lesions (BMLs) have emerged as a central component of many different inflammatory and non-inflammatory diseases affecting the musculoskeletal system. Initial descriptions denoted such water signals on MRI bone marrow edema (BME). As later histological analyses of such lesions were unable to demonstrate oedematous changes at the tissue level in the vast majority of cases, the alternative term ‘bone marrow lesion' was introduced.1 Since then a wealth of publications have detailed the presence of BML in a wide variety of conditions (Table 1). The presence of BML has been shown to be associated with pain and progression of disease in numerous studies,2 and therefore various modalities have been tested as treatment options in the hope that they might reduce pain and progressions of disease. The purpose of this review is to summarise our current knowledge about the role of BMLs in inflammatory and non-inflammatory disease and the effects of current treatment regimens.
Table 1. Bone marrow lesion (BML) aetiology.
| (1) Trauma |
| Fracture (acute, osteoporotic and stress) |
| Local transient osteoporosis |
| Altered stress/biomechanics (plantar fasciitis, tendinitis/entesitis) |
| Bone bruise |
| Osteochondral injuries (osteochondritis dissecans) |
| (2) Degenerative lesions |
| Osteoarthritis (hip, knee, other) |
| MODIC lesions (spine) |
| (3) Inflammatory lesions |
| Inflammatory arthropathies and enthesitis (rheumatoid arthritis (RA), Ankylosing spondylitis, psoriasis) |
| Systemic chronic inflammation with fibrosis |
| (4) Ischaemic lesions |
| Avascular necrosis (AVN) |
| Complex regional pain syndrome (Sudeks atrophy of bone) |
| Sickle cell anaemia (SCA) |
| (5) Infectious lesions |
| Osteomyelitis |
| Diabetic foot, Charcot foot |
| Sepsis (bone incfarcts) |
| (6) Metabolic/endocrine lesions |
| Hydroxyapatite deposition disease (HADD) |
| Gout |
| (7) Iatrogenic lesions |
| Surgery |
| Radiotherapy |
| Immunosuppressants (glucocorticoids, cyclopsorin) |
| Cytostatics |
| (8) Neoplastic (and neoplastic-like) lesions |
Imaging
BMLs are not visible on plain X-ray or computed tomography images. Skeletal scintigraphy will show uptake at BMLs, but further differentiation requires more specific imaging technologies. Among those, MRI and Ultrasound (US) yield the most useful information.3
US has been of value in particular for imaging of enthesitis in relation to seronegative arthritis.4 It has even been claimed that US is superior to MRI when it comes to detecting early phases of enthesitis.5
BMLs give rise to a water signal on MRI, and it has been suggested that this water signal may be due to either capillary leakage caused by local change in the capillary wall (due to, for example, trauma, tumour) or by increased intravascular pressure either due to increased blood flow to the marrow or decreased venous clearance of the marrow space.1
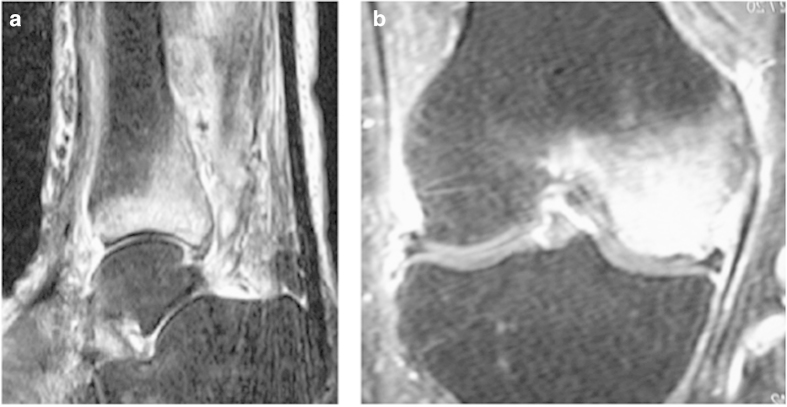
The MRI characteristics of BML include hypodense lesions on T1-weighted sequences and hyperdense lesions on T2-weighted sequences (Figure 1). Moreover, the lesions are characterised by homogeneity and the absence of sharp margins, and they cross anatomical boundaries. The best modality for detecting such lesions is achieved with water sensitive sequences such as fat suppressed T2-weighted, proton density-weighted, intermediate-weighted fast spin echo or short tau inversion recovery sequences.6,7
Figure 1.
(a) Bone marrow lesion (BML) in lower tibia in 64-year-old women with pain in her ankle and leg over a period of 6 months; (b) Lateral knee tendinitis and BML of lateral condyle in 35-year-old man training for marathon.
Histology
Our understanding of the pathology underlying BMLs is still fragmentary. Very few histological studies on BMLs have been performed. The lesion is not a typical edema by histologic criteria.1 Rather, it is characterised by fibrosis, lymphocytic infiltrates and increased vascularisation. It is probably the latter that is responsible for the water signal seen on MR.
One of the first studies on local transient osteoporosis reported diffuse or spotty areas of interstitial and intra-sinusoidal fluid in the marrow cavities, together with fat cell destruction or fibro vascular regeneration or both in areas exhibiting BML. Bone mineralisation was reduced on microradiographs, when compared with age-matched femoral heads without bone pathology. Several studies have reported signs of microfracture in areas containing BML.8,9 This finding together with the presence of active bone formation and live osteocytes in the area,10 however, points to increased repair capacity, which seems the key for the spontaneously reversible course of this syndrome. Increased marrow adiposity has also been reported in some case reports on BML.11,12
Analyses of needle aspirates from cancellous bone marrow have shown sign of high turnover in BML as reflected in significantly elevated levels of bone markers (Bone-specific alkaline phosphatase, osteocalcin, procollagen Type I N-terminal propeptide and C-terminal cross-linking telopeptide (ICTP)) with 4–16-fold higher values than measured in serum.13 Increased expression of angiogenesis factors (VEGF, CYR61 and CTGF) has also been demonstrated in BML, and it has been postulated that the elevated levels indicated a role of these proteins in the repair processes in osteonecrosis.14 However, the increased levels could also be a consequence of increased bone turnover.
In BMLs associated with inflammatory lesions of rheumatoid arthritis (RA), Schett et al. have provided ample evidence that the BML is caused by a combination of increased vascularisation, pannus formation and inflammatory lymphocytic infiltrates in the bone marrow, all invading the marrow space through cortical bone defects.15 Similar observations were reported in patients with ankylosing spondylitis.16
Impaired microcirculation causing increased intrasosseous pressure has also been invoked as a possible mechanism involved in the formation of BMLs, especially after renal transplantation.17
Thus, the histological and biochemical marker profiles summarised above suggest that BMLs constitute a local area of high bone turnover with increased expression of cytokines and angiogenic factors. Only few studies have reported tissue edema. Thus, all findings are in line with the notion that BMLs represent repair phenomena, elicited by inflammatory or non-inflammatory trauma to the bone.
The differentiation between osteonecrosis and BML is fluid. Several studies have considered BML of the knee and hip a preamble to later development of osteonecrosis, but the clinical data diverge. The MRI criteria for the diagnosis of osteonecrosis are not very precise18,19 and leave room for misclassification. It has been shown that BME correlates to necrotic volume at the femoral head,20 but another study looking at core specimens in patients ‘spontaneous osteonecrosis of the knee' (SONK) revealed no sign of necrotic tissue, as all trabeculae contained viable osteocytes and osteoblasts.10
Clinical features of BML syndromes
In inflammatory as well as non-inflammatory conditions, the presence of BML is usually associated with pain and progression of disease. The clinical features associated with the various BML aetiologies summarised in Table 1 will be summarised below.
Fractures
BML is the hallmark of recent vertebral fractures on MR and is used for the identification of vertebrae suitable for interventions like vertebroplasty and kyphoplasty. The MR signals may be present for a considerable time following the fracture with 28% of patients displaying continued signals 12 months after the first demonstration of the signal in the vertebrae.
Transient regional osteoporosis, also denoted regional migratory osteoporosis or BME syndrome, is characterised by extensive BML starting in one skeletal region and often showing up in other skeletal regions later.21 It is often associated with active osteoporotic changes and low BMD,22,23 and therefore the phenomenon has been attributed to the presence of microfractures in the area.
Bone bruises or contusions may also result in BML, probably via the same mechanisms, although bleeding in the area has been implicated too.1 Bone bruises occur most often in the knee but also constitute important differentials in the hip, calcaneus and scaphoid.
Osteochondral trauma
Osteochondritis dissecans
Osteochondritis dissecans is an acquired, potentially reversible idiopathic disease of subchondral bone resulting in delamination and sequestration of cartilage, and the development of BML in subchondral bone is present in 50% of cases.24
Degenerative lesions
Osteoarthritis
The presence of BMLs in osteoarthritis (OA) has been related to mechanical loading and increased subchondral stress and has been shown to be associated with progression of disease, cartilage loss and subsequent risk of total joint replacement in most studies.25,26,27
MR studies have demonstrated lower bone mineral density and more severe disruption of subchondral bone architecture in patients with severe, progressive OA, which would be expected in a state of high turnover as reflected by the elevated bone marker levels in OA.28,29
Tendinitis
BML has been reported in relation to chronic, calcifying tendinitis in virtually every location of the axial and peripheral skeleton. One study reported that MRI showed positive marrow edema in 36% of cases, whereas isotope scans revealed uptake in 100% of cases.30
Inflammatory lesions
Seropositive inflammatory arthropathy (RA)
The BML pattern on MRI in RA is thought to reflect inflammatory infiltrates and increased vascularisation and may be seen even in the absence of erosive lesions.15,18 Similar to OA the presence of BML in RA also signals progressive disease, and diagnostically BML constitutes the most specific finding.31
Seronegative arthritis and enthesitis
Entheses are sites where tendons, ligaments, joint capsules or fascia attach to the bone. In psoriasis, enthesitis and joint involvement are frequent, despite the absence of clinical signs like pain and swelling.32
Ischaemic lesions
Avascular osteonecrosis
Osteonecrosis of the bone is invariably associated with BML, and the extent of BML correlates to persistence and intensity of pain in this condition.20
Complex regional pain syndrome
Complex regional pain syndrome (Reflex dystrophy, Sudecks atrophy of bone) is a severe and a debilitating condition after various traumatic events (fracture, inflammation, ulcerations and so on) affecting one or more extremities. Radiologically, the disease is characterised by bone loss leading to osteopenia and spotty osteolytic areas in the bone, and various areas may show the presence of BML.33
Infectious lesions
Osteomyelitis
Infectious lesions are associated with BML development. The emergence of a water signal is not surprising in light of the increased vascularisation and tissue edema accompanying inflammation. Granulomatous inflammations are frequently associated with imaging findings different from those seen with nonspecific bacterial infection.62 BMLs are also seen in association with osseous involvement during infections of the foot in diabetes, and MRI constitutes the best modality for evaluation of such lesions.34
MODIC changes associated with degenerative changes of the spine
BMLs in vertebrae in relation to degenerative changes in vertebrae (MODIC changes) have been shown to be associated with anaerobic infections, which are thought to spread from the disc to the surrounding bony tissues.35 This notion is further supported by the fact that antibiotic therapy has been shown to be effective in reducing back pain in these patients.36
Metabolic/endocrine lesions
Tissue Deposition of uric acid or hydroxyapatite crystals in the gout or connective tissue disorders are associated with BML.37,38
Latrogenic lesions
Damage to the bone marrow by surgery or radiotherapy also causes BML. Also, the use of corticosteroids, other immunosuppressive therapies and cytostatics have been implicated.17
Neoplastic (and neoplastic-like) lesions
The increased vascularisation especially in the periphery of skeletal metastases invariably leads to the formation of BML.
Treatment
Surgical
Core decompression
The earliest hypotheses pertaining to BML formation focused on reduced microcirculation, leading to ischaemia and increased intraosseous pressure.39 On the basis of this hypothesis, surgical drilling of holes in the area of BML formation to relieve pressure was introduced as one of the earliest interventions to reduce pain and increase function in OA.39,40 Later studies combined the core decompression procedure with insertion of bone graft material into the area through the canal provided.41 Even more recent studies have reported beneficial effects of combining core decompression and injection of hydroxyapatite cement in areas with BML and osteonecrosis with significant reduction in pain ensuing.42
Core decompressions followed by injection of autologous bone marrow stem cells have been introduced for the treatment of osteonecrosis of the hip in order to increase the number of committed osteogenic cells in areas of osteonecrosis,43 and also intravenous administration of autologous and allogenic stem cells has been tried.44 Complications to these extensive procedures have been reported as minimal, but the results, however, vary.45,46,47
Physical modalities
Extracorporal shock wave therapy
In extracorporal shock wave therapy, a mechanical shock of defined magnitude is delivered to the area with BML. The technique has mainly been used for the treatment of plantar fasciitis,48 but effects on BML at the hip have also been reported with significant pain relief and functional improvement of the hip and reduction in BME.49,50 Proponents of this method claim that the mechanical shocks improve blood flow in the area, thereby reducing or alleviating BMLs. The shocks could, however, also increase microdamage in the area, which—if BML signifies bone repair—is counterintuitive.
Pharmaceutical options
Bisphosphonates
Bisphosphonates reduce pain, extension of BMLs and improve functional outcomes in benign condition like osteonecrosis,51,52 regional transient osteoporosis,53 enthesopathy in spondyloarthritis54 and regional pain syndromes.55 Most of the studies have been open label or interventional, but for osteonecrosis and regional pain syndrome significant effects have been demonstrated in randomised prospective trials. In several intervention studies, positive effects on pain have also been reported in local transient osteoporosis, stress fractures and various BML syndromes in athletes.53,56,57 One randomised placebo-controlled study on effects of the bisphosphonate Ibandronate on BMLs in the knee, did not, however, demonstrate significant effects.58
The way in which bisphosphonates exert these beneficial effects is still poorly elucidated, but several possible explanations have been invoked: (1) bisphosphonates possess anti-angiogenic properties59 and have been shown to reduce angiogenic factors in serum.60 Thus, they may inhibit hyper-vascularisation, in BMLs; (2) through their inhibition of osteoclast activity58,61 bisphosphonates may reduce hyper-remodelling in BMLs.57
Prostaglandin derivatives
Prostaglandins have an important role in inflammatory responses and cell differentiation. It is thought that prostaglandin I2 (or prostacyclin) and its analogues promote bone regeneration on a cellular or a systemic level and improve microcirculation in the area.62,63
One prostacyclin derivative, iloprost, has demonstrated beneficial effects on BML. The drug is given as intravenous infusions over 5 days at doses between 25 and 50 μg per day. It has been shown to be efficacious in the treatment of BML at various locations,64 avascular osteonecrosis, except for terminal cases,63 and regional transient osteoporosis.65,66
One study compared the effects of iloprost and the bisphosphonate ibandronate on BMLs in the knee and found similar results with around 47% reduction in BMLs and improvement of pain and functions scores.
Beckmann et al.67 compared the effects of iloprost vs core decompression and the two methods combined in relatively small groups of 12 patients each. All three groups exhibited symptom relief as assessed by various scores (Harris Hip Scores, WOMAC score, SF-36 scores and VAS) 3 months and 1 year after the intervention. The group subjected to a core decompression followed by iloprost infusion, however, showed the best results.
TNF-inhibitors
The reduction in disease activity and the reduction in erosions with tumour necrosis factor (TNF) inhibition in RA are associated with a reduction in BML,68 and TNF inhibition has also been effective in pain and BML reduction in Spondyloarthritis54,69 and psoriatic arthritis and enthesitis70
Conclusion
BMLs have been demonstrated in a wide variety of lesions in the bone, with the common denominator for these conditions being some kind of injury to the bone and bone marrow through mechanical stress, inflammation or ischaemia. It seems that most of these lesions show cortical or trabecular bone defects or microtrauma. Even in so-called ‘spontaneous' cases, similar to SONK or regional transient osteoporosis, underlying microtrauma has been demonstrated in many cases.
Whether the characteristics of BMLs elicited through mechanical and inflammatory injury are exactly the same remains to be determined. From the evidence available to date it seems that two different major mechanisms may be operating in the development of BMLs: (1) invasion of the marrow space from the outside in inflammatory lesions of RA, spondyloarthritis and enthesitis and (2) a localised increase in proinflammatory cytokines and vasoactive agents in the marrow space due to microtrauma or ischaemia in the area as seen typically in macrofractures, local transient osteoporosis, bone bruises and OA.
The available histological data suggest that trauma universally elicits localised repair with high bone turnover and increased vascularisation. The increased vascularisation induced by angiogenic factors and capillary leakage induced by proinflammatory cytokines both contribute to the ‘water signal' seen on MRI. The presence of a localised high turnover state with increased ambient levels of proinflammatory cytokines and vasoactive agents may also explain the positive effects of antiresorptive drugs like bisphosphonates and TNF antagonists on BML extension and symptoms associated with the lesion. A reduction in turnover would eventually lead to lower levels of proinflammatory cytokines and vasoactive peptides in the lesion. Some bisphosphonates, in particular Zoledronic acid, even exert specific anti-angiogenic effects that might add to their efficacy. TNF antagonists would also reduce local cytokine concentrations by different mechanisms.
It seems that the proposed surgical techniques with or without subsequent injection of stem cells, osteogenic peptides or hydroxyapatite should be reserved to cases where pharmaceutical interventions with either bisphosphonates or iloprost have failed.
Musculoskeletal diseases with BMLs constitute a huge clinical problem, which is responsible for a large proportion of disability in society. Therefore, effective treatment options are highly needed, and it is conceivable that such treatments might reduce the need for—or at least postpone—implant surgery in cases affecting the hip and knee. It has to be acknowledged, however, that this area of research suffers from a large number of small-scale interventional studies and less large-scale randomised studies. It is also still worth noting that one of the best, randomised studies on the effects of Ibandronate on knee BMLs failed to demonstrate significant improvement over placebo.
Footnotes
I have received speaker fees and consultant fees from: Merck, Amgen, Eli Lilly & Co, Novartis and IDS.
References
- Thiryayi WA, Thiryayi SA, Freemont AJ. Histopathological perspective on bone marrow oedema, reactive bone change and haemorrhage. Eur J Radiol 2008; 67: 62–67. [DOI] [PubMed] [Google Scholar]
- Starr AM, Wessely MA, Albastaki U, Pierre-Jerome C, Kettner NW. Bone marrow edema: pathophysiology, differential diagnosis, and imaging. Acta radiol 2008; 49: 771–786. [DOI] [PubMed] [Google Scholar]
- Roemer FW, Frobell R, Hunter DJ, Crema MD, Fischer W, Bohndorf K et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage 2009; 17: 1115–1131. [DOI] [PubMed] [Google Scholar]
- Evangelisto A, Wakefield R, Emery P. Imaging in early arthritis. Best Pract Res Clin Rheumatol 2004; 18: 927–943. [DOI] [PubMed] [Google Scholar]
- Kamel M, Eid H, Mansour R. Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. J Rheumatol 2003; 30: 774–778. [PubMed] [Google Scholar]
- Roemer FW, Guermazi A, Lynch JA, Peterfy CG, Nevitt MC, Webb N et al. Short tau inversion recovery and proton density-weighted fat suppressed sequences for the evaluation of osteoarthritis of the knee with a 1.0 T dedicated extremity MRI: development of a time-efficient sequence protocol. Eur Radiol 2005; 15: 978–987. [DOI] [PubMed] [Google Scholar]
- Arndt WF 3rd, Truax AL, Barnett FM, Simmons GE, Brown DC. MR diagnosis of bone contusions of the knee: comparison of coronal T2-weighted fast spin-echo with fat saturation and fast spin-echo STIR images with conventional STIR images. AJR Am J Roentgenol 1996; 166: 119–124. [DOI] [PubMed] [Google Scholar]
- Martig S, Boisclair J, Konar M, Spreng D, Lang J. MRI characteristics and histology of bone marrow lesions in dogs with experimentally induced osteoarthritis. Vet Radiol Ultrasound 2007; 48: 105–112. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Schneider R, Bullough PG. Subchondral insufficiency fracture of the femoral head: histopathologic correlation with MRI. Skeletal Radiol 2001; 30: 247–254. [DOI] [PubMed] [Google Scholar]
- Berger CE, Kroner AH, Kristen KH, Grabmeier GF, Kluger R, Minai-Pour MB et al. Transient bone marrow edema syndrome of the knee: clinical and magnetic resonance imaging results at 5 years after core decompression. Arthroscopy 2006; 22: 866–871. [DOI] [PubMed] [Google Scholar]
- Ryu KN, Jin W, Ko YT, Yoon Y, Oh JH, Park YK et al. Bone bruises: MR characteristics and histological correlation in the young pig. Clin Imaging 2000; 24: 371–380. [DOI] [PubMed] [Google Scholar]
- Kim SY, Koo KH, Suh KT, Kim YS, Cho YJ, Min BW et al. Fatty marrow conversion of the proximal femoral metaphysis in transient bone marrow edema syndrome. Arch Orthop Trauma Surg 2005; 125: 390–395. [DOI] [PubMed] [Google Scholar]
- Berger CE, Kroner AH, Minai-Pour MB, Ogris E, Engel A. Biochemical markers of bone metabolism in bone marrow edema syndrome of the hip. Bone 2003; 33: 346–351. [DOI] [PubMed] [Google Scholar]
- Radke S, Kenn W, Eulert J. Transient bone marrow edema syndrome progressing to avascular necrosis of the hip - a case report and review of the literature. Clin Rheumatol 2004; 23: 83–88. [DOI] [PubMed] [Google Scholar]
- Schett G. Bone marrow edema. Ann NY Acad Sci 2009; 1154: 35–40. [DOI] [PubMed] [Google Scholar]
- Appel H, Kuhne M, Spiekermann S, Kohler D, Zacher J, Stein H et al. Immunohistochemical analysis of hip arthritis in ankylosing spondylitis: evaluation of the bone-cartilage interface and subchondral bone marrow. Arthritis Rheum 2006; 54: 1805–1813. [DOI] [PubMed] [Google Scholar]
- Elder GJ. From marrow oedema to osteonecrosis: common paths in the development of post-transplant bone pain. Nephrology 2006; 11: 560–567. [DOI] [PubMed] [Google Scholar]
- Jergesen HE, Lang P, Moseley M, Genant HK. Histologic correlation in magnetic resonance imaging of femoral head osteonecrosis. Clin Orthop Relat Res 1990; 253: 150–163. [PubMed] [Google Scholar]
- Kubo T, Yamamoto T, Inoue S, Horii M, Ueshima K, Iwamoto Y et al. Histological findings of bone marrow edema pattern on MRI in osteonecrosis of the femoral head. J Orthop Sci 2000; 5: 520–523. [DOI] [PubMed] [Google Scholar]
- Ito H, Matsuno T, Minami A. Relationship between bone marrow edema and development of symptoms in patients with osteonecrosis of the femoral head. AJR Am J Roentgenol 2006; 186: 1761–1770. [DOI] [PubMed] [Google Scholar]
- Emad Y, Ragab Y, El-Shaarawy N, Rasker JJ. Transient osteoporosis of the hip, complete resolution after treatment with alendronate as observed by MRI description of eight cases and review of the literature. Clin Rheumatol 2012; 31: 1641–1647. [DOI] [PubMed] [Google Scholar]
- Guardiano SA, Katz J, Schwartz AM, Brindle K, Curiel R. Fracture complicating the bone marrow edema syndrome. J Clin Rheumatol 2004; 10: 269–274. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int 2006; 17: 1008–1012. [DOI] [PubMed] [Google Scholar]
- Choi YS, Cohen NA, Potter HG, Mintz DN. Magnetic resonance imaging in the evaluation of osteochondritis dissecans of the patella. Skeletal Radiol 2007; 36: 929–935. [DOI] [PubMed] [Google Scholar]
- Bennell KL, Creaby MW, Wrigley TV, Bowles KA, Hinman RS, Cicuttini F et al. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheum Dis 2010; 69: 1151–1154. [DOI] [PubMed] [Google Scholar]
- Roemer FW, Guermazi A, Javaid MK, Lynch JA, Niu J, Zhang Y et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis 2009; 68: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med 2003; 139: 330–336. [DOI] [PubMed] [Google Scholar]
- Dore D, Quinn S, Ding C, Winzenberg T, Jones G. Correlates of subchondral BMD: a cross-sectional study. J Bone Miner Res 2009; 24: 2007–2015. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Issever AS, Burghardt A, Lotz J, Arfelli F, Rigon L et al. Diffraction enhanced imaging of articular cartilage and comparison with micro-computed tomography of the underlying bone structure. Eur Radiol 2004; 14: 1440–1448. [DOI] [PubMed] [Google Scholar]
- Flemming DJ, Murphey MD, Shekitka KM, Temple HT, Jelinek JJ, Kransdorf MJ. Osseous involvement in calcific tendinitis: a retrospective review of 50 cases. AJR Am J Roentgenol 2003; 181: 965–972. [DOI] [PubMed] [Google Scholar]
- Conaghan PG, McQueen FM, Peterfy CG, Lassere MN, Ejbjerg B, Bird P et al. The evidence for magnetic resonance imaging as an outcome measure in proof-of-concept rheumatoid arthritis studies. J Rheumatol 2005; 32: 2465–2469. [PubMed] [Google Scholar]
- Erdem CZ, Tekin NS, Sarikaya S, Erdem LO, Gulec S. MR imaging features of foot involvement in patients with psoriasis. Eur J Radiol 2008; 67: 521–525. [DOI] [PubMed] [Google Scholar]
- Crozier F, Champsaur P, Pham T, Bartoli JM, Kasbarian M, Chagnaud C et al. Magnetic resonance imaging in reflex sympathetic dystrophy syndrome of the foot. Joint Bone Spine 2003; 70: 503–508. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Lipsky BA. Diagnosis and management of infection in the diabetic foot. Med Clin North Am 2013; 97: 911–946. [DOI] [PubMed] [Google Scholar]
- Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB et al. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 2013; 22: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert HB, Manniche C, Sorensen JS, Deleuran BW. Antibiotic treatment in patients with low-back pain associated with Modic changes Type 1 (bone oedema): a pilot study. Br J Sports Med 2008; 42: 969–973. [DOI] [PubMed] [Google Scholar]
- Carter JD, Kedar RP, Anderson SR, Osorio AH, Albritton NL, Gnanashanmugam S et al. An analysis of MRI and ultrasound imaging in patients with gout who have normal plain radiographs. Rheumatology 2009; 48: 1442–1446. [DOI] [PubMed] [Google Scholar]
- Bui-Mansfield LT, Moak M. Magnetic resonance appearance of bone marrow edema associated with hydroxyapatite deposition disease without cortical erosion. J Comput Assist Tomogr 2005; 29: 103–107. [DOI] [PubMed] [Google Scholar]
- Radke S, Rader C, Kenn W, Kirschner S, Walther M, Eulert J. Transient marrow edema syndrome of the hip: results after core decompression. A prospective MRI-controlled study in 22 patients. Arch Orthop Trauma Surg 2003; 123: 223–227. [DOI] [PubMed] [Google Scholar]
- Etemadifar M, Kooskzari M, Khalilollah N, Ali MK, Mahsa B. The results of core decompression treatment in patients with avascular necrosis of femoral head in patients at Isfahan City educational hospitals in 2010-2011. Adv Biomed Res 2014; 3: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CJ, Huang CC, Wang JW, Wong T, Yang YJ. Long-term results of extracorporeal shockwave therapy and core decompression in osteonecrosis of the femoral head with eight- to nine-year follow-up. Biomed J 2012; 35: 481–485. [DOI] [PubMed] [Google Scholar]
- Yang P, Bian C, Huang X, Shi A, Wang C, Wang K. Core decompression in combination with nano-hydroxyapatite/polyamide 66 rod for the treatment of osteonecrosis of the femoral head. Arch Orthop Trauma Surg 2014; 134: 103–112. [DOI] [PubMed] [Google Scholar]
- Hendrich C, Franz E, Waertel G, Krebs R, Jager M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev 2009; 1: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernigou P, Flouzat-Lachaniette CH, Delambre J, Poignard A, Allain J, Chevallier N et al. Osteonecrosis repair with bone marrow cell therapies: State of the clinical art. Bone 2015; 70C: 102–109. [DOI] [PubMed] [Google Scholar]
- Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. The Journal of bone and joint surgery American volume. 2004; 86-A: 1153–1160. [DOI] [PubMed]
- Chotivichit A, Korwutthikulrangsri E, Pornrattanamaneewong C, Achawakulthep C. Core decompression with bone marrow injection for the treatment of femoral head osteonecrosis. J Med Assoc Thai 2014; 97: S139–S143. [PubMed] [Google Scholar]
- Li X, Xu X, Wu W. Comparison of bone marrow mesenchymal stem cells and core decompression in treatment of osteonecrosis of the femoral head: a meta-analysis. Int J Clin Exp Pathol 2014; 7: 5024–5030. [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Johnson JE, Hirose CB, Bae KT. Chronic plantar fasciitis: acute changes in the heel after extracorporeal high-energy shock wave therapy–observations at MR imaging. Radiology 2005; 234: 206–210. [DOI] [PubMed] [Google Scholar]
- Lin PC, Wang CJ, Yang KD, Wang FS, Ko JY, Huang CC. Extracorporeal shockwave treatment of osteonecrosis of the femoral head in systemic lupus erythematosis. J Arthroplasty 2006; 21: 911–915. [DOI] [PubMed] [Google Scholar]
- d'Agostino C, Romeo P, Lavanga V, Pisani S, Sansone V. Effectiveness of extracorporeal shock wave therapy in bone marrow edema syndrome of the hip. Rheumatol Int 2014; 34: 1513–1518. [DOI] [PubMed] [Google Scholar]
- Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am 2005; 87: 2155–2159. [DOI] [PubMed] [Google Scholar]
- Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology 2005; 44: 352–359. [DOI] [PubMed] [Google Scholar]
- Ringe JD, Dorst A, Faber H. Effective and rapid treatment of painful localized transient osteoporosis (bone marrow edema) with intravenous ibandronate. Osteoporos Int 2005; 16: 2063–2068. [DOI] [PubMed] [Google Scholar]
- Maksymowych WP, Lambert R, Jhangri GS, Leclercq S, Chiu P, Wong B et al. Clinical and radiological amelioration of refractory peripheral spondyloarthritis by pulse intravenous pamidronate therapy. J Rheumatol 2001; 28: 144–155. [PubMed] [Google Scholar]
- Manicourt DH, Brasseur JP, Boutsen Y, Depreseux G, Devogelaer JP. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum 2004; 50: 3690–3697. [DOI] [PubMed] [Google Scholar]
- Simon MJ, Barvencik F, Luttke M, Amling M, Mueller-Wohlfahrt HW, Ueblacker P. Intravenous bisphosphonates and vitamin D in the treatment of bone marrow oedema in professional athletes. Injury 2014; 45: 981–987. [DOI] [PubMed] [Google Scholar]
- Ringe JD, Body JJ. A review of bone pain relief with ibandronate and other bisphosphonates in disorders of increased bone turnover. Clin Exp Rheumatol 2007; 25: 766–774. [PubMed] [Google Scholar]
- Meier C, Kraenzlin C, Friederich NF, Wischer T, Grize L, Meier CR et al. Effect of ibandronate on spontaneous osteonecrosis of the knee: a randomized, double-blind, placebo-controlled trial. Osteoporos Int 2014; 25: 359–366. [DOI] [PubMed] [Google Scholar]
- Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 2002; 62: 6538–6544. [PubMed] [Google Scholar]
- Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Effect of zoledronic acid on serum angiogenic factors in patients with bone metastases. Med Oncol 2008; 25: 346–349. [DOI] [PubMed] [Google Scholar]
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 2008; 19: 733–759. [DOI] [PubMed] [Google Scholar]
- Jager M, Tillmann FP, Thornhill TS, Mahmoudi M, Blondin D, Hetzel GR et al. Rationale for prostaglandin I2 in bone marrow oedema–from theory to application. Arthritis Res Ther 2008; 10: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager M, Zilkens C, Bittersohl B, Matheney T, Kozina G, Blondin D et al. Efficiency of iloprost treatment for osseous malperfusion. Int Orthop 2011; 35: 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meizer R, Radda C, Stolz G, Kotsaris S, Petje G, Krasny C et al. MRI-controlled analysis of 104 patients with painful bone marrow edema in different joint localizations treated with the prostacyclin analogue iloprost. Wien Klin Wochenschr 2005; 117: 278–286. [DOI] [PubMed] [Google Scholar]
- Aigner N, Petje G, Schneider W, Meizer R, Wlk M, Kotsaris S et al. Bone marrow edema syndrome of the femoral head: treatment with the prostacyclin analogue iloprost vs. core decompression: an MRI-controlled study. Wien Klin Wochenschr 2005; 117: 130–135. [DOI] [PubMed] [Google Scholar]
- Aigner N, Meizer R, Meraner D, Becker S, Meizer E, Landsiedl F. Bone marrow edema syndrome in postpartal women: treatment with iloprost. Orthop Clin North Am 2009; 40: 241–247. [DOI] [PubMed] [Google Scholar]
- Beckmann J, Schmidt T, Schaumburger J, Rath B, Luring C, Tingart M et al. Infusion, core decompression, or infusion following core decompression in the treatment of bone edema syndrome and early avascular osteonecrosis of the femoral head. Rheumatol Int 2013; 33: 1561–1565. [DOI] [PubMed] [Google Scholar]
- Hirose W, Nishikawa K, Hirose M, Nanki T, Sugimoto H. Response of early active rheumatoid arthritis to tumor necrosis factor inhibitors: evaluation by magnetic resonance imaging. Mod Rheumatol 2009; 19: 20–26. [DOI] [PubMed] [Google Scholar]
- Marzo-Ortega H, McGonagle D, Emery P. Etanercept treatment in resistant spondyloarthropathy: imaging, duration of effect and efficacy on reintroduction. Clin Exp Rheumatol 2002; 20: S175–S177. [PubMed] [Google Scholar]
- Marzo-Ortega H, McGonagle D, Rhodes LA, Tan AL, Conaghan PG, O'Connor P et al. Efficacy of infliximab on MRI-determined bone oedema in psoriatic arthritis. Ann Rheum Dis 2007; 66: 778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]