Abstract
As in insects, frogs and birds, vocal activity in fishes tends to be more developed in males than in females, and sonic swimbladder muscles may be sexually dimorphic, i.e., either larger in males or present only in males. Male oyster toadfish Opsanus tau L produce a long duration, tonal boatwhistle advertisement call, and both sexes grunt, a short duration more pulsatile agonistic call. Sonic muscles are present in both sexes but larger in males. We tested the hypothesis that males would call more than females by inducing grunts in toadfish of various sizes held in a net and determined incidence of calling and developmental changes in grunt parameters. A small number of fish were recorded twice to examine call repeatability. Both sexes were equally likely to grunt, and grunt parameters (sound pressure level (SPL), individual range in SPL, number of grunts, and fundamental frequency) were similar in both sexes. SPL increased with fish size before leveling off in fish >200 g, and fundamental frequency and other parameters did not change with fish size. Number of grunts in a train, grunt duration and inter-grunt interval were highly variable in fish recorded twice suggesting that grunt parameters reflect internal motivation rather than different messages. Grunt production may explain the presence of well-developed sonic muscles in females and suggests that females have an active but unexplored vocal life.
Keywords: Behavioral ontogeny, Bioacoustics, Sexual dimorphism, Sound production, Sonic muscles, Agonistic behavior
Introduction
As in insects, frogs and birds (Ryan, 1985; Gerhardt & Huber, 2002; Catchpole & Slater, 2008), vocal activity in fishes is typically more developed in males than in females (Amorim, Vasconcelos & Fonseca, 2015). One of the major mechanisms of sound production in fishes utilizes sonic muscles that drive the swimbladder to vibrate, and these muscles are often sexually dimorphic (Ladich & Fine, 2006; Fine & Parmentier, 2015; Ladich, 2015a). Sexual dimorphism in various fishes includes two separate morphological states. In some species, i.e., batrachoidids, gadids, ophidiids, osphronemids males have larger sonic muscles (Ladich, 2015a), but in others muscles may be present exclusively in males as in most species in the family Sciaenidae (Chao, 1978; Ono & Poss, 1982; Hill, Fine & Musick, 1987; Borie et al., 2014). However, some sciaenids, including Japanese croaker (Ueng, Huang & Mok, 2007), Atlantic croaker Micropogonias chromis (Hill, Fine & Musick, 1987), whitemouth croaker M. funneri (Tellechea et al., 2010a) and black drum Pogonias chromis (Tellechea et al., 2010b), have sonic muscles in both sexes. As in toadfish, sonic muscles and swimbladders are larger in male Atlantic croaker (Hill, Fine & Musick, 1987). To our knowledge the question of what would select for these two divergent patterns (size differences or absence) has not been formally addressed.
The oyster toadfish Opsanus tau L (Fine & Thorson, 2008) and other members of the family Batrachoididae have been used as model species for various aspects of acoustic communication (Bass & McKibben, 2003; Modesto & Canario, 2003a; Modesto & Canário, 2003b; Rome, 2006; Amorim & Vasconcelos, 2008; Rice & Bass, 2009; Rice, Land & Bass, 2011; Jordao, Fonseca & Amorim, 2012; Mosharo & Lobel, 2012; Vasconcelos et al., 2012; Bass, Chagnaud & Feng, 2015). Male Opsanus tau and O. beta produce a long-duration tonal courtship boatwhistle call, which functions in male-male competition and female attraction (Fish, 1954; Tavolga, 1958; Fine, 1978b; Edds-Walton, Mangiamele & Rome, 2002; Thorson & Fine, 2002b). Males will enter and call from shelters (Gray & Winn, 1961), which allows experimental manipulation by playbacks that demonstrate call rate, frequency and duration affect male boatwhistle production (Winn, 1967; Fish, 1972; Winn, 1972). Calls are produced by intrinsic sonic muscles that line the lateral walls of the swimbladder (Fine, Burns & Harris, 1990; Fine, Bernard & Harris, 1993; Barimo & Fine, 1998). Extremely rapid contraction of these muscles determines the fundamental frequency of the boatwhistle (Skoglund, 1961; Fine et al., 2001; Elemans, Mensinger & Rome, 2014). The sonic muscles and swimbladder occur in both sexes and grow for life (Fine, Burns & Harris, 1990). However, both are larger in males than in females, and males have more but smaller muscle fibers (Fine, Burns & Harris, 1990). Toadfish of both sexes produce a less-studied shorter-duration more pulsatile grunt call in agonistic situations (Fish, 1954; Tavolga, 1958; Gray & Winn, 1961). Maruska & Mensinger (2009) have provided the most detailed study of grunts utilizing an unseen semi-natural population of males and females in a long shallow outdoor tank; their paper provides sonagrams and oscillograms demonstrating extensive variation in toadfish grunts. They quantify occurrence and differentiate sound parameters of large numbers of single, doublet and trains of grunts that occurred spontaneously and net grunts from netted individuals. Their fish also produced boatwhistles suggesting fish were exhibiting normal courtship behavior even under captive conditions. Grunt parameters have also been measured from several identified male toadfish calling in the York River (Barimo & Fine, 1998), and grunts often precede boatwhistles in Opsanus beta from the Florida keys (Thorson & Fine, 2002b). Finally, Elemans, Mensinger & Rome (2014) recorded net grunts from fish with implanted emg electrodes demonstrating that each grunt is caused by a quick burst of several muscle contractions.
There has been little behavioral work on grunts. Territorial males grunt, particularly when guarding eggs, if tethered toadfish (both males and females) and even blue crabs were brought close to the male’s nest (Gray & Winn, 1961). Male toadfish have been found to grunt on top of boatwhistles and grunts of nearby males, an acoustic tag, suggesting a dominance display (Thorson & Fine, 2002a; Mensinger, 2014). Finally grunts have been evoked by electrical brain stimulation in O. beta (Demski & Gerald, 1972; Demski & Gerald, 1974) and O. tau (Fine, 1979; Fine & Perini, 1994). Grunts in O. tau were divided into knock and burst grunts (shorter and longer respectively) and indicate that the fish can vary the number of muscle contractions in a grunt pulse as well as the number of pulses and the timing between pulses. We are left with the impression that males would be more likely to grunt than females although this hypothesis has not been tested (Ladich, 2015a).
In this study we examined the effects of size and sex on grunt incidence and parameters by recording individual male and female oyster toadfish held in air (net grunts) to describe ontogenetic changes and potential sex differences. Effects of recording in air will be dealt with in the discussion. Because fundamental frequency (muscle contraction rate) is determined by pattern generators in the brain (Bass, Chagnaud & Feng, 2015), we hypothesized that unlike in most fishes (Ladich, 2015b), grunt fundamental frequency would not decrease with fish size. Further, due to increases in sonic muscle and swimbladder size in larger fish (Fine, Burns & Harris, 1990), we predicted that sound pressure level would increase with fish size. We find call parameters and incidence of calling are equivalent in males and females suggesting that females are more vocal than previously demonstrated and accounting for the presence of a well-developed sonic system in females.
Materials and Methods
Oyster toadfish were collected in the York River, VA and kept in sea tables with running York-River water under ambient photoperiods at the Virginia Institute of Marine Science (VIMS). Other fish were transported to Virginia Commonwealth University (VCU) and kept in five 120 L tanks in half-strength artificial sea water (18‰) under a 14:10 LD cycle at 22 °C. Toadfish were weighed and then sexed using the presence of a cloaca (a second opening) between the anus and the urogenital papilla; the opening is present in females but not in males. A test of fish weighing between 113 and 786 g correctly determined the sex of 20 of 20 toadfish (10 males and 10 females) correctly as verified by gonadal inspection. Protocols were approved by the VCU Animal Care and Use Committee (IACUC no. AD20216).
VIMS fish, collected for immunology projects unrelated to the current study, were auditioned for the presence or absence of sound production at approximately monthly intervals from May 23 to September 1, 1989. Different individuals were used in the various trials. Grunt sounds were evoked by holding the fish in a small net, i.e., the net grunts of Maruska & Mensinger (2009). Some fish called immediately when netted, some required gentle prodding to call, and others remained silent.
VCU fish were recorded in air 20 cm from a Uher microphone and 2002 Report L tape recorder (Assmann Electronics, Bad Homburg, Germany) at 3.75 ips. Four of the smallest fish produced lower amplitude sounds and were recorded 5 cm from the microphone. Assuming spherical spreading (Urick, 1975; Fine & Lenhardt, 1983; Mann, 2006), we converted the amplitude of these sounds to the level expected at 20 cm by subtracting 15.6 dB or 20 log (20/5). Pumps were turned off in the aquarium room during recording. Fish were netted and brought to the microphone, which was adjacent to the tanks. The recording commenced when the fish started to call. Fish that made long and rapid trains of grunts were recorded until the fish stopped calling or grunts became infrequent. Likewise fish producing few grunts were provided time to potentially add additional grunts. For these reasons fish were not recorded for a standardized time.
Sounds were analyzed on a Kay 505 Sonagraph (Kay Elemetrics, Murray Hill, New Jersey, USA). A calibration tone produced using a function generator connected to a speaker was recorded through the microphone allowing amplitude determination of grunt sounds in dB re: 20 µPa (dB SPL). We also measured the period in ms of the most intense cycle in the waveform and used its reciprocal to calculate fundamental frequency. Since grunt calls can be highly variable (Maruska & Mensinger, 2009) and individuals varied in their incidence of grunting, we attempted to record 25 fish twice to determine the degree of call fixity, i.e., do individual toadfish have a signature grunt. Seven of these fish called twice. For these fish we measured temporal parameters including number of grunts, grunt duration, and inter-grunt interval, the time between grunt pulses as measured from the call waveform.
For calling incidence, we set up contingency tables of calling and silent males and females and used Fisher’s exact probability test to determine sexual differences (GraphPad software, San Diego, California, USA). For call parameters, means for each fish were plotted against fish weight to determine developmental changes and sex differences. Data were fit with linear regressions except for SPL which was fit with a one phase association equation. Regressions for males and females were compared with analysis of covariance using fish weight as the covariate. For SPL comparisons, data were linearized with a log–log transform before comparison. If regression slopes were not significantly different from zero in both sexes, we used a t-test to compare parameters by sex. Male and female regressions were combined if sex differences were not significant. Temporal data for fish recorded twice were compared by regressing means from the first recording against means from the second.
Results
Grunt incidence in males and females
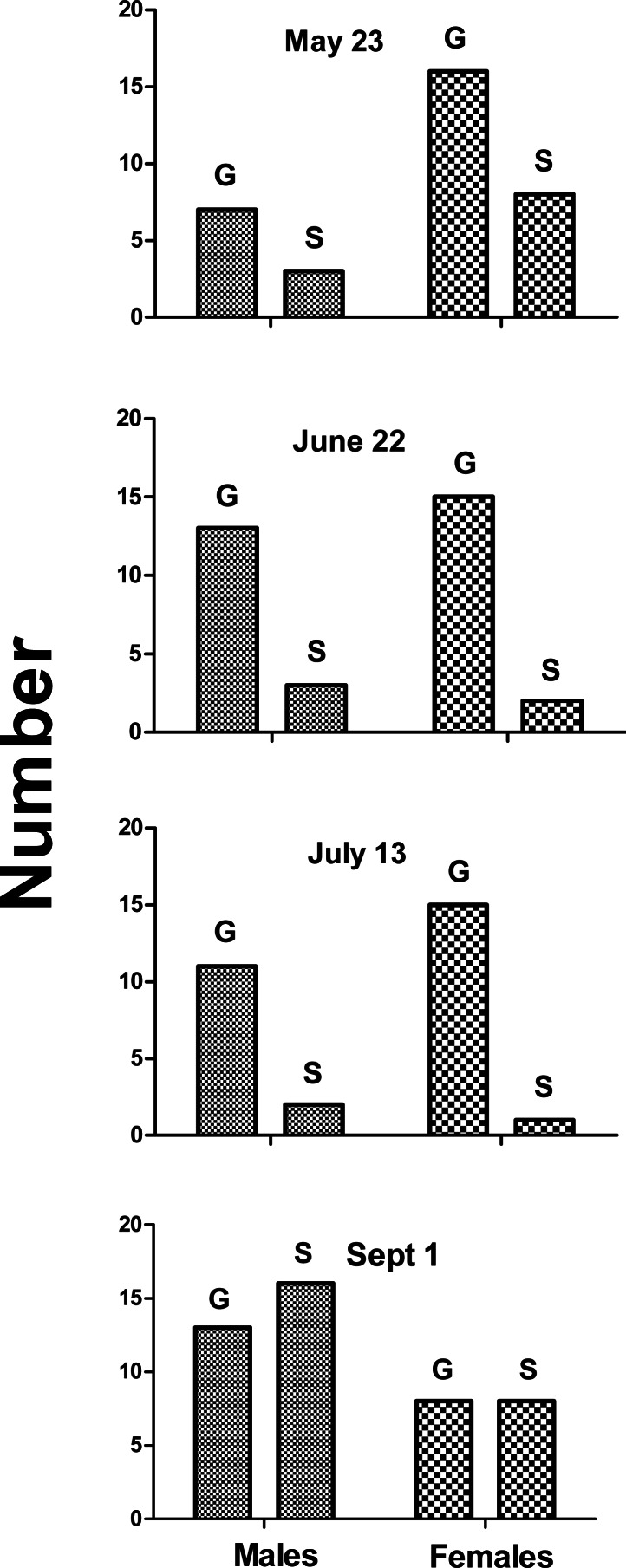
Over four recording sessions, we auditioned 128 different toadfish (59 males and 69 females) ranging in size from 15 to 835 g. Fisher’s Exact Probability test indicated no significant difference between the incidence of grunt production between males and females on any of the trial dates (Fig. 1). There was a decreased incidence in both sexes on Sept 1. Respectively 70% and 67% of males and females grunted on May 23 (P = 1.0), 82% and 88% on June 22 (P = 0.66), 81% and 94% on July 13 (P = 0.57), and 45% and 50% on Sept. 1 (P = 0.77). Thus males and females were equally likely to produce grunts when held.
Figure 1. Number of males and females that grunted (G) or were silent (S) on indicated dates.
Males and females were equally likely to grunt on all four dates (Fisher exact probability test), and incidence of grunting decreased in September.
Effects of size and sex on grunt parameters
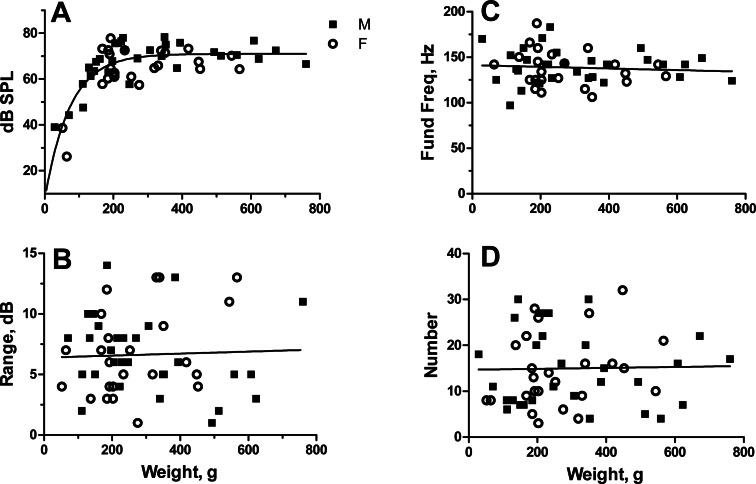
Male toadfish ranged between 29 and 760 g and females between 52 and 567 g. The largest four fish were males, which grow to larger sizes than females (Radtke, Fine & Bell, 1985). SPL increased nonlinearly from 39 to 78 dB SPL in males and 26 to 78 dB in females. Increase was rapid in both sexes to about 200 g and then leveled off (Fig. 2A). Analysis of covariance on regressions linearized with log–log transforms indicated no difference in SPL between males and females (Slopes: F1,52 = 2.59, P = 0.12; Intercepts: F1,53 = 1.65, P = 0.20). Data points for males and females co-scattered, and much of the difference between slopes came from one female with the lowest amplitude. Male and female data were combined and fit with a one-phase exponential equation ((y = 7.084 + 63.936 (1 − e−0.01378x)) with an r2 of 0.64 (Fig. 2A).
Figure 2. Relationship of sound pressure level in dB re: 20 µPa (A), range in dB (B), fundamental frequency (C) and number of grunts (D) to fish weight for male and female oyster toadfish.
Points represent the mean value for each individual except for number of grunts, which is the actual number. There were no sexual differences, and the regression line represents pooled data for males and females.
SPLs for individuals ranged from 1 to 14 dB with a mean of 6.6 ± 3.3 dB (SD) in males and 1–14 dB (mean 6.6 ± 3.6 dB) in females. Since range did not change with fish size (r2 = 0.002, P = 0.78) (Fig. 2B), mean range for males and females was compared with a t test indicating no sexual difference (t52 = 0.021, P = 0.98).
Fundamental frequency ranged from 97 to 183 Hz (mean 139.8 ± 18.2 Hz) in males and 106 to 187 Hz (mean 137.3 ± 19.6) in females and did not vary with fish size (r2 = 0.007, P = 0.56) (Fig. 2C). Fundamental frequency was similar between males and females (t52 = 0.49, P = 0.62).
Number of grunt pulses varied from 4 to 30 (mean 15.5 ± 8.5) in males and 3 to 32 (mean 14.4 ± 8.0) in females and did not change with fish size (r2 = 0.0004, P = 0.88) (Fig. 2D). Number of grunt pulses per fish was again similar between males and females (t52 = 0.49, P = 0.63).
Temporal properties and repeatability
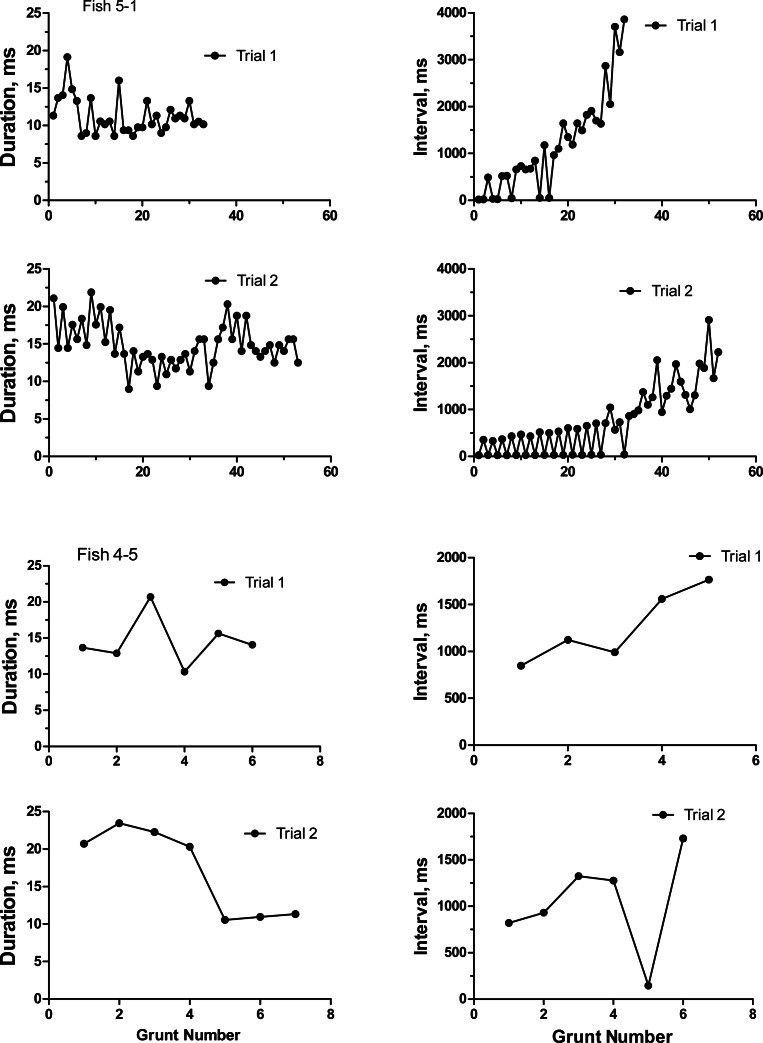
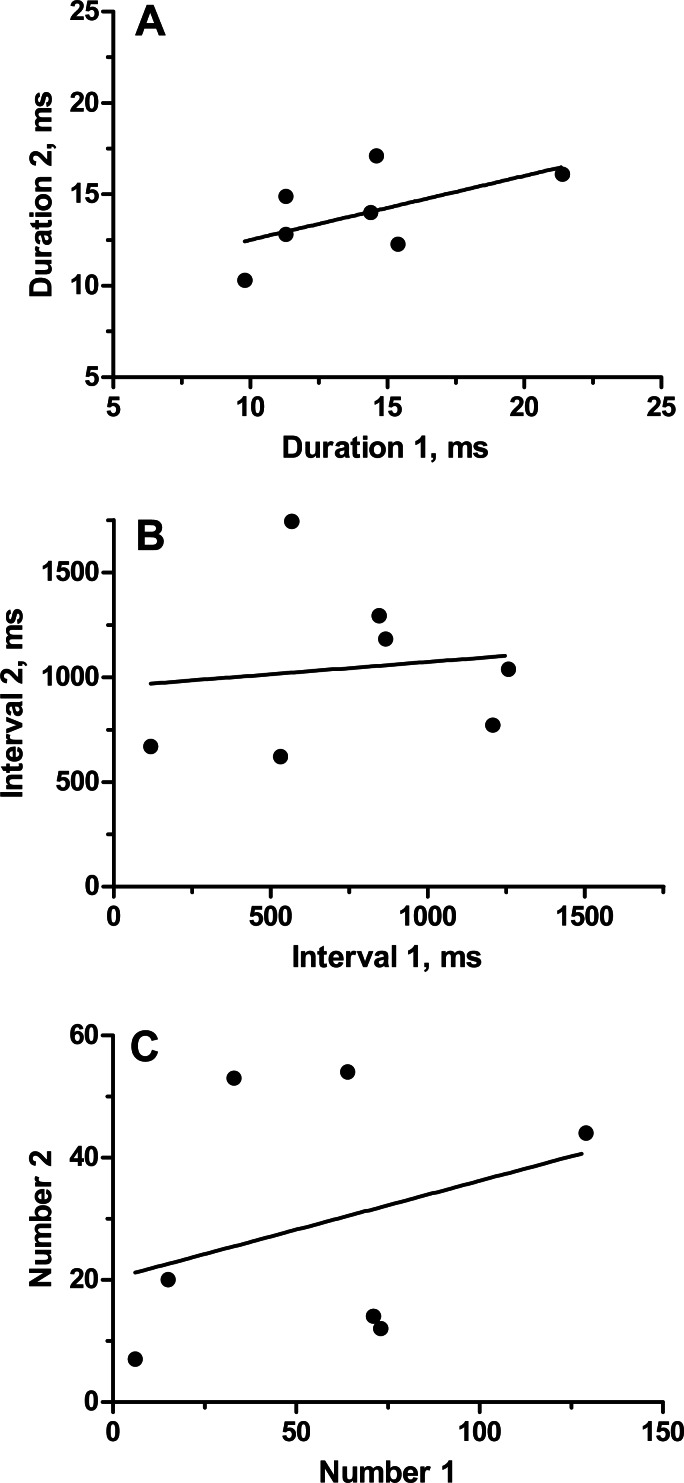
To categorize the variability and repeatability in grunts produced by individual toadfish, we recorded seven fish twice (August 19 and 24, 1989). Grunts were emitted in trains of pulses that varied extensively in number of grunts, inter-grunt interval and to a lesser extent in grunt duration (Table 1). Number of grunts across all fish varied from 6 to 129, and most grunts were rather short with a range in means from 10.3 to 21.3 ms. Inter-grunt intervals within trains were highly variable and tended to be bimodal with bursts of pulses wedged in between longer pauses coupled with a general trend for longer pauses toward the end of a grunt train (hence multimodal) (Fig. 3). Individual, pairs and burst grunts occurred. Means or medians do not adequately describe the intervals, which for fish 5-1 ranged between 18 ms to almost 4 s (Fig. 3). Two examples were chosen to illustrate the duration and inter-grunt interval of each grunt in a train recorded on both days (Fig. 3). These examples illustrate the range in variability among fish and suggest a degree of similarity between recording dates. Similarity was not evident in every individual (Table 1 and Fig. 4). Comparisons between pairs of recordings for individuals indicated a lack of correlation between sessions (Fig. 4): for duration (r2 = 0.33, P = 0.17), inter-grunt interval (r2 = 0.014, P = 0.80), number of grunts (r2 = 0.11, P = 0.47). Therefore even when there appear to be trends in qualitative pattern, numbers of grunts and quantitative measurements of their patterning are quite different. The number of grunts from individuals in the two recordings ranged from six and seven grunts (similar), 33 and 55 grunts (somewhat similar), to 73 to 12 (quite dissimilar). Although we suspect some toadfish may exhibit tendencies in the patterning of their grunts, overall the calls are extremely variable and did not demonstrate individual signatures.
Table 1. Mean ± SD, minimum and maximum value for grunt duration, inter-grunt interval and number of grunts for seven individual oyster toadfish recorded on two separate days (a and b).
| Grunt duration, ms | Inter-grunt interval, ms | ||||||
|---|---|---|---|---|---|---|---|
| Fish | Minimum | Maximum | Minimum | Maximum | N | ||
| 3-1 a | 21.3 ± 6.4 | 12.1 | 54.0 | 840 ± 1,143 | 19.9 | 7,925 | 64 |
| b | 16.1 ± 4.0 | 11.7 | 26.6 | 1,182 ± 561 | 41.4 | 3,625 | 54 |
| 3-2 a | 9.8 ± 3.1 | 4.7 | 18.0 | 118 ± 384 | 7.8 | 2,294 | 129 |
| b | 10.3 ± 2.3 | 5.0 | 17.6 | 669 ± 2,935 | 12.5 | 18,105 | 44 |
| 4-4 a | 11.3 ± 3.7 | 4.7 | 16.0 | 845 ± 920 | 19.2 | 3,100 | 15 |
| b | 12.8 ± 4.0 | 7.8 | 21.9 | 1,293 ± 1,183 | 23.8 | 4,200 | 20 |
| 4-5 a | 14.6 ± 3.1 | 10.6 | 20.7 | 1,257 ± 349 | 847 | 1,766 | 6 |
| b | 17.1 ± 5.4 | 10.6 | 23.4 | 1,038 ± 496 | 145 | 1,731 | 7 |
| 5-1 a | 11.3 ± 2.4 | 8.6 | 19.1 | 1,207 ± 1,042 | 18.0 | 3,862 | 33 |
| b | 14.9 ± 2.9 | 9.0 | 21.9 | 771 ± 702 | 25 | 2,912 | 53 |
| 5-3 a | 15.4 ± 1.3 | 12.1 | 18.8 | 531 ± 1,190 | 17.6 | 9,455 | 73 |
| b | 12.3 ± 0.6 | 10.5 | 12.9 | 620 ± 430 | 24.6 | 1,600 | 12 |
| 5-5 a | 14.4 ± 3.4 | 7.8 | 23.8 | 567 ± 1,007 | 13.7 | 4,238 | 71 |
| b | 14.0 ± 5.9 | 7.0 | 28.1 | 1,743 ± 3,211 | 19.1 | 12,400 | 14 |
Figure 3. Duration and inter-grunt interval in milliseconds during the course of a grunt train for two oyster toadfish recorded on two occasions 6 days apart.
Figure 4. Relationship of parameters (average grunt duration, inter-grunt interval and number of grunts) for seven individual oyster toadfish recorded on two occasions.
Discussion
Toadfish live in murky water in Atlantic estuaries (Able & Fahay, 1998), and besides boatwhistles little is known about behavioral and acoustical interactions under natural conditions. We recorded individuals in air, which has the advantage of subjecting individuals to an equivalent stimulus under reasonable if abnormal acoustic conditions. Although underwater recordings would be preferable, tanks have complex acoustic fields that can alter signal frequency spectra due to resonance and decrease sound levels because of out-of-phase reflections from tank boundaries (Akamatsu et al., 2002; Parmentier et al., 2014). It would be difficult to record large numbers of toadfish underwater in close to free-field conditions. Three studies have examined calls from individuals of the same species recorded in air and underwater: two on pectoral stridulation sounds in catfishes (Knight & Ladich, 2014; Ghahramani, Mohajer & Fine, 2014) and one on swimbladder sounds in Atlantic croaker (Fine, Schrinel & Cameron, 2004). Croaker sounds therefore provide the most apt comparisons. Croakers were recorded in a shallow large boat harbor, which minimized but did not remove all acoustic complications from potential reflections. The peak frequency of croaker sounds in both media was identical because it is determined by timing of sonic muscle contractions as in the toadfish (Skoglund, 1961; Fine et al., 2001; Elemans, Mensinger & Rome, 2014) and not bladder resonance (Fine, 2012; Fine & Parmentier, 2015). Underwater croaker sounds damped more slowly (the waveform contained an extra cycle) and were more sharply tuned (higher Q) than in air, and it is likely therefore that grunts in this study would be several milliseconds longer if recorded underwater.
Fish sounds have been divided into fixed and variable interval calls (Winn, 1964), and the variability in toadfish grunts is striking. Grunts recorded at Woods Hole, Massachusetts were also variable and described as single, doublet and trains of grunts (Maruska & Mensinger, 2009). All three of these grunt types were emitted in this study, suggesting as in birds (Brown, 1975) different calls can occur in the same behavioral context. Long trains of variable-interval grunts with intervals increasing over time are reminiscent of following grunts evoked after the cessation of electrical brain stimulation (Fine, 1978a). Net grunts recorded by Maruska & Mensinger had an average duration of 178 ms, approximately ten fold longer than ones recorded in this study. Part of this difference could be due to geographical variation (Fine, 1978a; Fine, 1978b) between populations in Virginia and Woods Hole, Massachusetts, the northern limit of the toadfish’s geographical range. More likely, we suggest a difference in the fish’s central state since their outdoor tank included territorial males who boatwhistled during the mating season whereas fish in this study were held in smaller indoor tanks. The waveform of many of the Woods Hole grunts was somewhat boatwhistle-like and made by multiple muscle contractions in rapid succession whereas individual grunts in this study were caused by a single or small number of contractions (Fine et al., 2001; Elemans, Mensinger & Rome, 2014). Grunts recorded from identified males that were boatwhistling in the York River, VA fish varied from 48 to 147 ms in duration (Barimo & Fine, 1998), supporting the hypothesis that the grunts reflect different internal states of the fish under courtship and distress situations. Similar to the Woods Hole study (Maruska & Mensinger, 2009), the incidence of grunts decreased in September. The active part of the mating season ends in July in Chesapeake Bay estuaries, after which toadfish reduce calling (Fine, 1978b).
Behavior ontogeny
With the exception of SPL, grunt parameters were extremely variable but stable in fish from 29 to 760 g, equivalent to >100 to over 300 mm total length (Fine, 1975) and at least two to over 10 year-old individuals (Radtke, Fine & Bell, 1985). Although no juveniles were recorded in this study, we have heard grunts from fish <60 mm total length suggesting a lack of major changes in calls over most of the lifespan of the fish. Extreme variability even in fish recorded twice suggests that call variability may reflect the internal state at the moment rather than indicate different messages.
Although fish sounds typically increase in amplitude and decrease in frequency with fish size (Myrberg, Ha & Shamblott, 1993; Henglmüller & Ladich, 1999; Connaughton, Taylor & Fine, 2000; Wysocki & Ladich, 2001; Ladich, 2007; Lechner, Wysocki & Ladich, 2010; Tellechea et al., 2010a; Tellechea et al., 2010b), toadfish grunts differ in some ways from this pattern. SPL increased with size to 200 g and then unexpectedly stabilized. Sound amplitude is related to volume velocity (Bradbury & Vehrencamp, 1998), the product of speaker surface area and velocity of movement. Since swimbladder size increases continuously and dominant frequency does not change, sound amplitude should continue to increase as demonstrated with electrically-stimulated twitch sounds (Fine et al., 2001). However, that study increased stimulation voltage until maximal sound amplitude stabilized to ensure complete muscle fiber recruitment. In the current study dB ranges could be as much as 15 dB indicating that fish can control the amplitude of their sounds and potentially obscure a size effect on amplitude. It could also account for the lack of difference in SPL of male and female grunts. SPL increased continuously with fish size in the Portuguese toadfish Halobatrachus didactylus (Vasconcelos & Ladich, 2008) recorded in a shallow (12 cm) tub (30 × 45 cm). However, they plotted toadfish length data on a log scale suggesting that SPL in fact levels off in larger fish. Pulse period decreased with fish size suggesting longer sonic muscle twitch times in this species, which would at least partially explain a leveling off of SPL in amplitude in Halobatrachus. We are unsure why SPL levels off in the current study.
The absence of size effects on fundamental frequency was predicted because this variable is controlled by central pattern generators (Bass, Chagnaud & Feng, 2015) and twitch timing of the sonic muscles (Fine et al., 2001; Millot, Vandewalle & Parmentier, 2011) rather than resonant frequency of the swimbladder (Fine, 2012; Fine & Parmentier, 2015). Lower frequency sounds in larger fishes has historically been interpreted to result from lower resonant frequencies of larger swimbladders (Harris, 1964; Bergeijk, 1964). More recent work with swimbladder sounds suggests that apparently resonant sounds, ones that damp slowly, result from continuous vibration of a bone or tendon that continues to drive the swimbladder into vibration as in squirrelfish, glaucosomatids and carapids (Fine & Parmentier, 2015). Other cases in which swimbladders are driven directly by muscle twitches as in weakfish (Connaughton, Taylor & Fine, 2000) and Halobatrachus (Vasconcelos & Ladich, 2008) have more modest slopes when plotted against fish size because larger muscles take longer to complete a twitch and therefore produce longer pulses (Connaughton, Fine & Taylor, 2002; Parmentier & Fine, in press). We believe that the current study provides the first explicit demonstration that swimbladder size does not affect sound frequency although boatwhistle choruses recorded in the field (Fine, 1978b) contained fundamental frequencies that varied over as little as 10 Hz, therefore pointing to the same conclusion.
An ontogenetic study of hearing and sound production in Halobatrachus indicated that SPL increased, but sound duration, number of sounds and dominant frequency decreased with fish standard length (3–32 cm SL) (Vasconcelos & Ladich, 2008). Many of their smallest fish failed to grunt. The auditory brainstem response indicated that hearing was best below 300 Hz in fish of all sizes but that the smallest juveniles had lowest sensitivities (higher thresholds) at low (100 Hz) and high (800 and 1,000 Hz) frequencies. The authors suggest that acoustic communication might be absent in young juveniles. Auditory sensitivity has been determined in the oyster toadfish (Fish & Offutt, 1972; Yan et al., 2000) but only in adults, which also hear best below 300 Hz but appear to be less sensitive than Halobatrachus.
Male and female grunts
Incidence of grunting as well as number of grunts, grunt duration, inter-grunt interval and sound pressure level were similar in males and females. It is clear that both sexes possess a similar grunt repertoire under these conditions. Based on swimbladder and sonic muscle size and general assumptions, we would not have predicted these findings. Considering the possibilities of either smaller or no muscles in females of various species, this study suggests that female oyster toadfish participate in a vocal world and may account for their well-developed albeit smaller sonic system.
What would select for loss of sonic muscles in many female sciaenid species has not been formally considered. Assuming that both sexes in an ancestral sciaenid (Lo et al., 2015) had sonic muscles originally, the simplest hypothesis would be that females lost muscles in species in which sound function was restricted to male advertisement calls, and females had no role in sound production. However, male weakfish Cynoscion regalis and silver croaker Plagioscion squamisissimus produce advertisement calls (Connaughton & Taylor, 1996; Borie et al., 2014) but also disturbance calls when held (Connaughton, Fine & Taylor, 2002; Borie et al., 2014). Only males have sonic muscles in these species (Ono & Poss, 1982; Hill, Fine & Musick, 1987). Disturbance calls likely function in predator–prey interactions (Bosher, Newton & Fine, 2006) and should be equally useful for both sexes. Accordingly, the evolution of sexual dimorphism in fish sonic mechanisms and what would select for mute females requires further exploration.
Conclusions
Even though male toadfish produce a courtship boatwhistle and possess larger sonic swimbladder muscles than females, both sexes are equally likely to grunt under distress conditions. Grunts are extremely variable, and this variation is similar in both sexes. The lack of a size effect on fundamental frequency supports the notion that sounds are produced as a forced rather than a resonant response. We suggest that grunt variation reflects internal state of the caller rather than different messages and that calling in females explains the presence of sonic muscles, which are lost in some species of sonic fishes.
Supplemental Information
Acknowledgments
We thank Dr. Charles Rice for allowing us access to toadfish at the Virginia Institute of Marine Sciences and Uwe Kollenkirchen of Falcon Scientific Writing, D-14612 Falkensee, Karl-Liebknecht-Str. 87, Germany, who participated in the recording of VIMS fish and criticized the manuscript. Contribution number 60 from the Rice Center of Virginia Commonwealth University.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Michael L. Fine conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Tyler D. Waybright performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Virginia Commonwealth University IACUC AD20216.
References
- Able & Fahay (1998).Able KW, Fahay MP. The first year in the life of estuarine fishes in the Middle Atlantic Bight. New Brunswick, New Jersey: Rutgers University Press; 1998. [Google Scholar]
- Akamatsu et al. (2002).Akamatsu T, Okumura T, Novarini N, Yan HY. Empirical refinements applicable to the recording of fish sounds in small tanks. Journal of the Acoustical Society of America. 2002;112:3073–3082. doi: 10.1121/1.1515799. [DOI] [PubMed] [Google Scholar]
- Amorim & Vasconcelos (2008).Amorim MCP, Vasconcelos RO. Variability in the mating calls of the Lusitanian toadfish Halobatrachus didactylus: cues for potential individual recognition. Journal of Fish Biology. 2008;73:1267–1283. doi: 10.1111/j.1095-8649.2008.01974.x. [DOI] [Google Scholar]
- Amorim, Vasconcelos & Fonseca (2015).Amorim MCP, Vasconcelos RO, Fonseca PJ. Fish sounds and mate choice. In: Ladich F, editor. Sound communication in fishes. New York: Springer; 2015. pp. 1–33. [Google Scholar]
- Barimo & Fine (1998).Barimo JF, Fine ML. Relationship of swim-bladder shape to the directionality pattern of underwater sound in the oyster toadfish. Canadian Journal of Zoology. 1998;76:134–143. doi: 10.1139/z97-160. [DOI] [Google Scholar]
- Bass, Chagnaud & Feng (2015).Bass AH, Chagnaud BP, Feng NY. Comparative neurobiology of sound production in fishes. In: Ladich F, editor. Sound communication in fishes. New York: Springer; 2015. pp. 35–75. [Google Scholar]
- Bass & McKibben (2003).Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Progress in Neurobiology. 2003;69:1–26. doi: 10.1016/S0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bergeijk (1964).Bergeijk WAV. Directional and nondirectional hearing in fish. In: Tavolga WN, editor. Marine bio-acoustics. New York: Pergamon Press; 1964. pp. 281–299. [Google Scholar]
- Borie et al. (2014).Borie A, Mok HK, Chao NL, Fine ML. Spatiotemporal variability and sound characterization in Silver Croaker Plagioscion squamosissimus (Sciaenidae) in the Central Amazon. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0099326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher, Newton & Fine (2006).Bosher BT, Newton SH, Fine ML. The spines of the channel catfish, Ictalurus punctatus, as an anti-predator adaptation: an experimental study. Ethology. 2006;112:188–195. doi: 10.1111/j.1439-0310.2006.01146.x. [DOI] [Google Scholar]
- Bradbury & Vehrencamp (1998).Bradbury JW, Vehrencamp SL. Animal communication. Massachusetts: Sinauer; 1998. [Google Scholar]
- Brown (1975).Brown JL. The evolution of behavior. New York: WW Norton; 1975. [Google Scholar]
- Catchpole & Slater (2008).Catchpole CK, Slater PJB. Bird song: biological themes and variation. 2nd edition. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Chao (1978).Chao LN. A basis for classifying Western Atlantic Sciaenidae (Teleostei: Perciformes) vol. 415. National Oceanic and Atmospheric Administration; 1978. pp. 1–65. (Technical circular). [Google Scholar]
- Connaughton, Fine & Taylor (2002).Connaughton MA, Fine ML, Taylor MH. Weakfish sonic muscle: influence of size, temperature and season. Journal of Experimental Biology. 2002;205:2183–2188. doi: 10.1242/jeb.205.15.2183. [DOI] [PubMed] [Google Scholar]
- Connaughton & Taylor (1996).Connaughton MA, Taylor MH. Drumming, courtship and spawning behavior in captive weakfish, Cynoscion regalis. Copeia. 1996;1996:195–199. doi: 10.2307/1446957. [DOI] [Google Scholar]
- Connaughton, Taylor & Fine (2000).Connaughton MA, Taylor MH, Fine ML. Effects of fish size and temperature on weakfish disturbance calls: implications for the mechanism of sound generation. Journal of Experimental Biology. 2000;203:1503–1512. doi: 10.1242/jeb.203.9.1503. [DOI] [PubMed] [Google Scholar]
- Demski & Gerald (1972).Demski LS, Gerald JW. Sound production evoked by electric stimulation of the brain in toadfish (Opsanus beta) Animal Behaviour. 1972;20:507–513. doi: 10.1016/S0003-3472(72)80015-0. [DOI] [PubMed] [Google Scholar]
- Demski & Gerald (1974).Demski LS, Gerald JW. Sound production and other behavioral effects of midbrain stimulation in the free-swimming toadfish, Opsanus beta. Brain Behavior and Evolution. 1974;9:41–59. doi: 10.1159/000123654. [DOI] [PubMed] [Google Scholar]
- Edds-Walton, Mangiamele & Rome (2002).Edds-Walton PL, Mangiamele LA, Rome LC. Variations of pulse repetition rate in boatwistle sounds from oyster toadfish Opsanus tau around Waquoit Bay, Massachusetts. Bioacoustics. 2002;13:153–173. doi: 10.1080/09524622.2002.9753493. [DOI] [Google Scholar]
- Elemans, Mensinger & Rome (2014).Elemans CPH, Mensinger AF, Rome LC. Vocal production complexity correlates with neural instructions in the oyster toadfish (Opsanus tau) Journal of Experimental Biology. 2014;217:1887–1893. doi: 10.1242/jeb.097444. [DOI] [PubMed] [Google Scholar]
- Fine (1975).Fine ML. Sexual dimorphism of the growth rate of the swimbladder of the oyster toadfish Opsanus tau. Copeia. 1975;1975:483–490. doi: 10.2307/1443646. [DOI] [Google Scholar]
- Fine (1978a).Fine ML. Geographical variation in sound production evoked by brain stimulation in the oyster toadfish. Naturwissenschaften. 1978a;65:493. doi: 10.1007/BF00702846. [DOI] [Google Scholar]
- Fine (1978b).Fine ML. Seasonal and geographic variation of the mating call of the oyster toadfish Opsanus tau. Oecologia. 1978b;36:45–57. doi: 10.1007/BF00344570. [DOI] [PubMed] [Google Scholar]
- Fine (1979).Fine ML. Sounds evoked by brain stimulation in the oyster toadfish Opsanus tau. Experimental Brain Research. 1979;35:197–212. doi: 10.1007/BF00236611. [DOI] [PubMed] [Google Scholar]
- Fine (2012).Fine ML. Swimbladder sound production: the forced response versus the resonant bubble. Bioacoustics. 2012;21:5–7. doi: 10.1080/09524622.2011.647453. [DOI] [Google Scholar]
- Fine, Bernard & Harris (1993).Fine ML, Bernard B, Harris TM. Functional morphology of toadfish sonic muscle fibers: relationship to possible fiber division. Canadian Journal of Zoology. 1993;71:2262–2274. doi: 10.1139/z93-318. [DOI] [Google Scholar]
- Fine, Burns & Harris (1990).Fine ML, Burns NM, Harris TM. Ontogeny and sexual dimorphism of the sonic muscle in the oyster toadfish. Canadian Journal of Zoology. 1990;68:1374–1381. doi: 10.1139/z90-205. [DOI] [Google Scholar]
- Fine & Lenhardt (1983).Fine ML, Lenhardt ML. Shallow-water propagation of the toadfish mating call. Comparative Biochemistry and Physiology. 1983;76A:225–231. doi: 10.1016/0300-9629(83)90319-5. [DOI] [PubMed] [Google Scholar]
- Fine et al. (2001).Fine ML, Malloy KL, King CB, Mitchell SL, Cameron TM. Movement and sound generation by the toadfish swimbladder. Journal of Comparative Physiology. 2001;187A:371–379. doi: 10.1007/s003590100209. [DOI] [PubMed] [Google Scholar]
- Fine & Parmentier (2015).Fine ML, Parmentier E. Mechanisms of fish sound production. In: Ladich F, editor. Sound communication in fishes. Wien: Springer Verlag; 2015. pp. 77–126. [Google Scholar]
- Fine & Perini (1994).Fine M, Perini MA. Sound production evoked by electrical stimulation of the forebrain in the oyster toadfish. Journal of Comparative Physiology A. 1994;174:173–185. doi: 10.1007/BF00193784. [DOI] [PubMed] [Google Scholar]
- Fine, Schrinel & Cameron (2004).Fine ML, Schrinel J, Cameron TM. The effect of loading on disturbance sounds of the Atlantic croaker Micropogonius undulatus: air vs. water. Journal of the Acoustical Society of America. 2004;116:1271–1275. doi: 10.1121/1.1736271. [DOI] [PubMed] [Google Scholar]
- Fine & Thorson (2008).Fine ML, Thorson RF. Use of passive acoustics for assessing behavioral interactions in individual toadfish. Transactions of the American Fisheries Society. 2008;137:627–637. doi: 10.1577/T04-134.1. [DOI] [Google Scholar]
- Fish (1954).Fish MP. Character and significance of sound production among fishes of the Western North Atlantic. Bulletin of the Bingham Oceanographic Collection. 1954;14:1–109. [Google Scholar]
- Fish (1972).Fish JF. The effect of sound playback on the toadfish. In: Winn HE, Olla B, editors. Behavior of marine animals. vol. 2. New York: Plenum Press; 1972. pp. 386–434. [Google Scholar]
- Fish & Offutt (1972).Fish JF, Offutt GC. Hearing thresholds from toadfish, Opsanus tau, measured in the laboratory and field. Journal of the Acoustical Society of America. 1972;51:1318–1321. doi: 10.1121/1.1912977. [DOI] [PubMed] [Google Scholar]
- Gerhardt & Huber (2002).Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago: Chicago University Press; 2002. [Google Scholar]
- Ghahramani, Mohajer & Fine (2014).Ghahramani ZN, Mohajer YJ, Fine ML. Developmental variation in sound production in water and air in the blue catfish Ictalurus furcatus. Journal of Experimental Biology. 2014;217:4244–4251. doi: 10.1242/jeb.112946. [DOI] [PubMed] [Google Scholar]
- Gray & Winn (1961).Gray GA, Winn HE. Reproductive ecology and sound production of the toadfish Opsanus tau. Ecology. 1961;28:274–282. doi: 10.2307/1932079. [DOI] [Google Scholar]
- Harris (1964).Harris GG. Considerations on the physics of sound production by fishes. In: Tavolga WN, editor. Marine bio-acoustics. New York: Pergamon Press; 1964. pp. 233–247. [Google Scholar]
- Henglmüller & Ladich (1999).Henglmüller SM, Ladich F. Development of agonistic behaivour and vocalization in croaking gourami. Journal of Fish Biology. 1999;54:380–395. doi: 10.1111/j.1095-8649.1999.tb00837.x. [DOI] [Google Scholar]
- Hill, Fine & Musick (1987).Hill GL, Fine ML, Musick JA. Ontogeny of the sexually dimorphic sonic muscle in three sciaenid species. Copeia. 1987;1987:708–713. doi: 10.2307/1445663. [DOI] [Google Scholar]
- Jordao, Fonseca & Amorim (2012).Jordao JM, Fonseca PJ, Amorim MCP. Chorusing behaviour in the Lusitanian Toadfish: should I match my neighbours’ calling rate. Ethology. 2012;118:885–895. doi: 10.1111/j.1439-0310.2012.02078.x. [DOI] [Google Scholar]
- Knight & Ladich (2014).Knight L, Ladich F. Distress sounds of thorny catfishes emitted underwater and in air: characteristics and potential significance. Journal of Experimental Biology. 2014;217:4068–4078. doi: 10.1242/jeb.110957. [DOI] [PubMed] [Google Scholar]
- Ladich (2007).Ladich F. Females whisper briefly during sex: context- and sex-specific differencess in sounds made by croaking gouramis. Animal Behaviour. 2007;73:379–387. doi: 10.1016/j.anbehav.2006.04.014. [DOI] [Google Scholar]
- Ladich (2015a).Ladich F. Acoustic signalling in female fish. In: Ladich F, editor. Sound communication in fishes. New York: Springer; 2015a. pp. 149–173. [Google Scholar]
- Ladich (2015b).Ladich F. Ontogenetic development of sound communcation in fishes. In: Ladich F, editor. Sound communication in fishes. New York: Springer; 2015b. pp. 127–148. [Google Scholar]
- Ladich & Fine (2006).Ladich F, Fine ML. Sound-generating mechanisms in fishes: a unique diversity in vertebrates. In: Ladich F, et al., editors. Communication in fishes. vol. 1. Enfield, New Hampshire: Science Publishers; 2006. pp. 3–43. [Google Scholar]
- Lechner, Wysocki & Ladich (2010).Lechner W, Wysocki LE, Ladich F. Ontogentic development of auditory sensitivity and sound production in the squeaker catfish Synodontis schoutendeni. BMC Biology. 2010;8:10. doi: 10.1186/1741-7007-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo et al. (2015).Lo PC, Liu SH, Chao NL, Francis KE, Nunoo FK, Mok HK, Chen WJ. A multi-gene dataset reveals a tropical New World origin and early Miocene diversification of croakers (Perciformes: Sciaenidae) Molecular Phylogeneny and Evolution. 2015;88:132–143. doi: 10.1016/j.ympev.2015.03.025. [DOI] [PubMed] [Google Scholar]
- Mann (2006).Mann DA. Propagation of fish sounds. In: Ladich F, et al., editors. Communication in fishes. vol. 1. Enfield, New Hampshire: Science Publishers; 2006. pp. 107–120. [Google Scholar]
- Maruska & Mensinger (2009).Maruska KP, Mensinger AF. Acoustic characteristics and variations in grunt vocalizations in the oyster toadfish Opsanus tau. Environmental Biology of Fishes. 2009;84:325–337. doi: 10.1007/s10641-009-9446-y. [DOI] [Google Scholar]
- Mensinger (2014).Mensinger AF. Disruptive communication: stealth signaling in the toadfish. Journal of Experimental Biology. 2014;217:344–350. doi: 10.1242/jeb.090316. [DOI] [PubMed] [Google Scholar]
- Millot, Vandewalle & Parmentier (2011).Millot S, Vandewalle P, Parmentier E. Sound production in red-bellied piranhas (Pygocentrus natteri, Kner): an acoustical, behavioural and morphofunctional study. Journal of Experimental Biology. 2011;214:3613–3618. doi: 10.1242/jeb.061218. [DOI] [PubMed] [Google Scholar]
- Modesto & Canario (2003a).Modesto T, Canario A. Morphometric changes and sex steroid levels during the annual reproductive cycle of the Lusitanian toadfish, Halobatrachus didactylus. General and Comparative Endocrinology. 2003a;131:220–231. doi: 10.1016/S0016-6480(03)00027-3. [DOI] [PubMed] [Google Scholar]
- Modesto & Canário (2003b).Modesto T, Canário AVM. Hormonal control of swimbladder sonic muscle dimorphism in the Lusitanian toadfish Halobatrachus didactylus. Journal of Experimental Biology. 2003b;206:3467–3477. doi: 10.1242/jeb.00581. [DOI] [PubMed] [Google Scholar]
- Mosharo & Lobel (2012).Mosharo KM, Lobel PS. Acoustic signals of two toadfishes from Belize: Sanopus astrifer and Batrachoides gilberti (Batrachoididae) Envrionmental Biology of Fishes. 2012;94:623–638. doi: 10.1007/s10641-011-9969-x. [DOI] [Google Scholar]
- Myrberg, Ha & Shamblott (1993).Myrberg AA, Ha SJ, Shamblott HS. The sounds of bicolor damselfish (Pomacentris partitus): predictors of body size and a spectral basis for individual recognition and assessment. Journal of the Acoustical Society of America. 1993;94:3067–3070. doi: 10.1121/1.407267. [DOI] [Google Scholar]
- Ono & Poss (1982).Ono RD, Poss SG. Structure and innervation of the swimbladder musculature in the weakfish, Cynoscion regalis. Canadian Journal of Zoology. 1982;60:1955–1967. doi: 10.1139/z82-253. [DOI] [Google Scholar]
- Parmentier & Fine (in press).Parmentier E, Fine ML. Fish sound production: an exaptation? In: Suthers RA, Fitch T, editors. Vertebrate sound production and acoustic communication. vol. 53. New York: Springer; 2015. (Springer handbook of auditory research). In Press. [Google Scholar]
- Parmentier et al. (2014).Parmentier E, Tock J, Falguière JC, Beauchaud M. Sound production in Sciaenops ocellatus: preliminary study for the develpment of acoustic cues in aquaculture. Aquaculture. 2014;432:204–211. doi: 10.1016/j.aquaculture.2014.05.017. [DOI] [Google Scholar]
- Radtke, Fine & Bell (1985).Radtke RL, Fine ML, Bell J. Somatic and otolith growth in the oyster toadfish (Opsanus tau L.) Journal of Experimental Marine Biology and Ecology. 1985;90:259–275. doi: 10.1016/0022-0981(85)90171-6. [DOI] [Google Scholar]
- Rice & Bass (2009).Rice AN, Bass AH. Novel vocal repertoire and paired swimbladders of the three-spined toadfish, Batrachomoeus trispinus: insights into the diverstiy of the Batrachoididae. Journal of Experimental Biology. 2009;212:1377–1391. doi: 10.1242/jeb.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, Land & Bass (2011).Rice AN, Land BR, Bass AH. Nonlinear complexity in a fish ‘two-voice’ system. Proceedings of the Royal Society of London B. 2011;278:3762–3768. doi: 10.1098/rspb.2011.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome (2006).Rome LC. Design and function of superfast muscles: new insights into the physiology of skeletal muscle. Annual Review of Physiology. 2006;68:193–221. doi: 10.1146/annurev.physiol.68.040104.105418. [DOI] [PubMed] [Google Scholar]
- Ryan (1985).Ryan MJ. The tungara frog, a study in sexual selection and communication. Chicago: University of Chicago Press; 1985. [Google Scholar]
- Skoglund (1961).Skoglund CR. Functional analysis of swimbladder muscles engaged in sound productivity of the toadfish. Journal of Biophysics and Biochemical Cytology. 1961;10(Suppl):187–200. doi: 10.1083/jcb.10.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavolga (1958).Tavolga WN. Underwater sounds produced by two species of toadfish Opsanus tau and Opsanus beta. Bulletin of Marine Science. 1958;8:278–284. [Google Scholar]
- Tellechea et al. (2010a).Tellechea JS, Martinez C, Fine ML, Norbis W. Sound production in the whitemouth croaker and relationship between fish size and call characteristics. Environmental Biology of Fishes. 2010a;89:163–172. doi: 10.1007/s10641-010-9709-7. [DOI] [Google Scholar]
- Tellechea et al. (2010b).Tellechea JS, Norbis W, Olsson D, Fine ML. Calls of the black drum (Pogonius chromis: Sciaenidae): geographical differences in sound production between Northern and Southern Hemisphere populations. Journal of Experimental Zoology. 2010b;313A:1–8. doi: 10.1002/jez.551. [DOI] [PubMed] [Google Scholar]
- Thorson & Fine (2002a).Thorson RF, Fine ML. Acoustic competition in the gulf toadfish Opsanus beta: acoustic tagging. Journal of the Acoustical Society of America. 2002a;111:2302–2307. doi: 10.1121/1.1466865. [DOI] [PubMed] [Google Scholar]
- Thorson & Fine (2002b).Thorson RF, Fine ML. Crepuscular changes in emission rate and parameters of the boatwhistle advertisement call of the gulf toadfish, Opsanus beta. Environmental Biology of Fishes. 2002b;63:321–331. doi: 10.1023/A:1014334425821. [DOI] [Google Scholar]
- Ueng, Huang & Mok (2007).Ueng JP, Huang BQ, Mok HK. Sexual differences in spawning sounds of the Japanese croaker Argyrosomus japonicus (Sciaenidae) Zoological Studies. 2007;46:103–110. [Google Scholar]
- Urick (1975).Urick RJ. Principles of underwater acoustics. New York: McGraw-Hill; 1975. [Google Scholar]
- Vasconcelos et al. (2012).Vasconcelos RO, Carrico R, Ramos A, Modesto A, Fonseca PJ, Amorim MCP. Vocal behavior predicts reproductive success in a teleost fish. Behavioral Ecology. 2012;23:375–383. doi: 10.1093/beheco/arr199. [DOI] [Google Scholar]
- Vasconcelos & Ladich (2008).Vasconcelos RO, Ladich F. Development of vocalization, auditory sensitivity and acoustic communication in the Lusitanian toadfish Halobatrachus didactylus. Journal of Experimental Biology. 2008;211:502–509. doi: 10.1242/jeb.008474. [DOI] [PubMed] [Google Scholar]
- Winn (1964).Winn HE. The biological significance of fish sounds. In: Tavolga WN, editor. Marine bio-acoustics. New York: Pergamon Press; 1964. pp. 213–231. [Google Scholar]
- Winn (1967).Winn HE. Vocal facilitation and the biological significance of toadfish sounds. In: Tavolga WN, editor. Marine Bio-acoustics. vol. 1. New York: Pergamon Press; 1967. pp. 283–304. [Google Scholar]
- Winn (1972).Winn HE. Acoustic discrimination by the toadfish with comments on signal systems. In: Winn HE, Olla BL, editors. Behavior of marine animals: current perspectives in research Vol. 2. Vertebrates. New York: Plenum Press; 1972. pp. 361–385. [Google Scholar]
- Wysocki & Ladich (2001).Wysocki LE, Ladich F. The ontogenetic development of auditory sensitivity, vocalization and acoustic communication in the labyritnth fish Trichopsis vittata. Journal of Comparative Physiology A. 2001;187:177–187. doi: 10.1007/s003590100186. [DOI] [PubMed] [Google Scholar]
- Yan et al. (2000).Yan HY, Fine ML, Horn NS, Colon WE. Variability in the role of the gasbladder in fish audition. Journal of Comparative Physiology A. 2000;187:371–379. doi: 10.1007/s003590050443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.