Synopsis
In this chapter, I will review the long-term outcomes and their precursors of type 1 diabetes (T1D) starting in youth. I will also contrast the changing incidence of these long-term complications as we have moved from the pre-DCCT to the post-DCCT standard of care and will review the emerging data related to complications in youth with type 2 diabetes (T2D). Finally, I will review the recent understanding related to the effects of diabetes on the brain and cognition.
Keywords: Diabetes mellitus, Retinopathy, Microalbuminuria, Diabetic neuropathy, CVD Risk Factors, Neurocognition, Neuroimaging
The Diabetes Control and Complications Trial (DCCT) and its ongoing longitudinal observational follow-up study, the Epidemiology of Diabetes Interventions and Complications (EDIC) study represent a major turning point in our understanding of the long-term outcomes of type 1 diabetes (T1D). The DCCT clearly demonstrated that intensive therapy of diabetes that lowered hemoglobin A1c (HbA1c) levels by about 2% (9.0% to 7.1%) reduced the incidence of onset and progression of diabetic retinopathy, diabetic nephropathy and diabetic neuropathy by 47–54%, 39% and 60%, respectively, in both young adults (18–39 years old) (1) and adolescents (13–18 years old) (2) with a diabetes duration of 1–15 years at the time of enrollment. During the EDIC follow-up study, the benefits on cardiovascular disease (CVD) outcomes also became apparent with a 42% reduction in CVD events after 17 years. (3) The ongoing EDIC Study subsequently showed that these benefits not only persisted, but indeed widened, at four (4,5) and ten (6,7) years after the end of the DCCT during a time of equivalent glycemic control between the original conventional and intensive groups in the DCCT; this has been called “metabolic memory”. The between group differences in complication rates in DCCT and EDIC and the “metabolic memory” phenomenon were almost entirely a result of the differences in HbA1c between the groups during the DCCT. (4–7) Other factors contributed little if any to these differences.
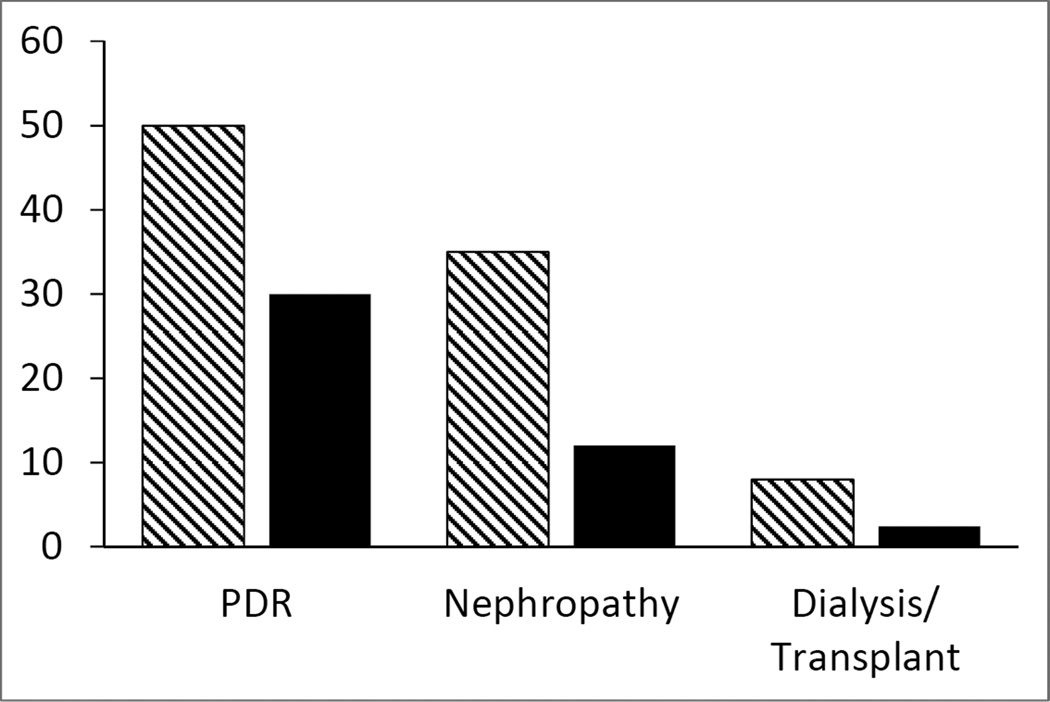
Intensive therapy, as implemented in the DCCT and along with many subsequent pharmacologic and technologic advances, has now become the standard of care for T1D. With this changing standard of care for T1D during the last two decades since the release of the DCCT results, the morbidity and mortality associated with the microvascular and macrovascular complication of T1D has been reduced or delayed, but not eliminated (8). Comparing complication rates from about 20 years earlier to those in the DCCT/EDIC cohort after 20 years of follow-up, the cumulative incidence of proliferative diabetic retinopathy (PDR) and nephropathy fell from 50% and 35%, respectively, to 30% and 12%, respectively; the rates of end-stage renal disease (ESRD) requiring dialysis or transplantation have also declined. (see Figure 1 below). The rates of other clinically severe complications also fell dramatically. There remains no cure or prevention of T1D and indeed the incidence of T1D and the overall impact of its complications appear to be increasing.
Figure 1.
Cumulative incidence of proliferative diabetic retinopathy (PDR), nephropathy (≥300 mg/day albumin, serum creatinine ≥2.0 mg/dL, or dialysis/transplantation) and end-stage renal disease requiring dialysis or renal transplantation during the pre-DCCT era (hatched bars) and the post-DCCT era (solid bars). Data adapted from data reported in reference 8.
Simultaneous with the changing climate surrounding T1D, and along with the increasing prevalence of childhood obesity not only in the United States, but also around much of the developed world, the incidence of type 2 diabetes (T2D) is rising. T2D now accounts for a substantial portion of new-onset diabetes in youth. Emerging evidence suggests that T2D starting during childhood or adolescence may have worse long-term outcomes than either T1D in youth or T2D presenting during the adult years (see below).
Here, I will review the outcomes of diabetes starting during childhood and adolescence with particular focus on the long-term complications of diabetes (retinopathy, nephropathy, neuropathy, macrovascular disease) and their precursors in T1D starting in youth, as well as the emerging, though still inadequate, data related to complications in youth with T2D. Also, I will review the recent understanding related to the effects of diabetes on the brain and cognition. This warrants important consideration in developing the best targets for managing diabetes in children and adolescents.
Overview of Diabetes-Related Complications
The outcomes of diabetes in youth include short-term and long-term complications (Table 1). Whereas the long-term complications rarely have clinically important manifestations during the years that youth are under the care of their pediatrician or pediatric endocrinologist, youth with T1D are at risk for the short-term complications every day.
Table 1.
Overview of Diabetes-Related Complications
| Short-term Complications |
| Diabetic ketoacidosis (DKA) |
| Hypoglycemia |
| Visual |
| Psychosocial |
| Long-term Complications |
| Microvascular |
| Retinopathy |
| Nephropathy |
| Neuropathy |
| Peripheral |
| Autonomic |
| Macrovascular |
| Coronary Artery Disease |
| Cerebrovascular Disease |
| Peripheral Vascular Disease |
Short-term Complications of T1DM
Diabetic ketoacidosis (DKA) is dealt with elsewhere in this volume and will not be addressed here.
Some hypoglycemia is unavoidable in most individuals who are insulin-treated. Hypoglycemia is best considered an adverse effect of insulin therapy (and potentially sulfonylurea therapy as well) instead of a complication of diabetes. Hypoglycemia can cause a myriad of symptoms and signs that are generally divided into neurogenic/autonomic and neuroglycopenic. Neurogenic symptoms are the result of low blood glucose triggering an autonomic response with adrenergic and cholinergic symptoms including shakiness or tremor, diaphoresis, tachycardia or palpitations, hunger or irritability. Neuroglycopenic symptoms are the result of reduced availability of glucose to the brain and include sleepiness or lethargy, confusion, loss of consciousness, seizure, coma and even death. Mild hypoglycemia is generally defined as hypoglycemia which the patient recognizes because of neurogenic/autonomic symptoms and self-treats with recovery before neuroglycopenic signs or symptoms. Mild hypoglycemia is largely unavoidable in well-managed insulin-treated patients with T1D using currently available treatment modalities. However, see the discussion on Brain and Cognitive Effects of Diabetes below.
Severe hypoglycemia, generally defined using the DCCT criteria as hypoglycemia resulting in neuroglycopenic symptoms or signs that render the patient unable to treat themself, represents a more significant concern. Severe hypoglycemia can result in injury (to self or others), seizure, coma or death. In addition, severe hypoglycemia, especially in young children, may contribute to subsequent neurocognitive deficits and altered regional brain anatomy. Severe hypoglycemia is a complication of diabetes management that should be avoided and goals of treatment and education should include prevention of severe hypoglycemia. (9,10)
Short-term visual effects of T1D are not uncommon. Blurred vision may be an acute symptom of hypoglycemia in some patients. More commonly, blurred vision is reported by those with high or rapidly fluctuating blood glucose. This is usually transient and resolves once the blood glucoses are stable for a while. This is thought to be due to changes in the osmotic characteristics of the lens. Refractive error may change acutely with wide fluctuation of blood glucose and many ophthalmologists and optometrists recommend postponing refraction for the purpose of prescribing glasses or contact lenses until the blood glucose has been stable. In rare cases, cataracts can develop at or soon after the diagnosis of T1D, even in children and teenagers. (11–14) If the visual disturbances do not clear within a couple of months after the onset of diabetes treatment, examination by an eye doctor should be strongly considered.
Psychosocial and behavioral issues are common among children with diabetes and their families. Discussion of these complications and outcomes is beyond the scope of this chapter, but it should be noted that regardless of whether the disorder or problem predated the onset or presented only after the onset of the diabetes, psychological, behavioral or emotional problems both interfere with successful management and contribute to worse outcomes associated with poor glycemic control. (15,16)
Long-term Complications of T1D
Overview
The long-term complications of diabetes are generally divided into microvascular and macrovascular. The microvascular complications include diabetic retinopathy (DR), diabetic nephropathy and diabetic neuropathy. The initial detectable lesions of diabetic DR are termed background diabetic retinopathy (BDR) and include microaneurysms, exudates and hemorrhages. BDR is generally benign and does not impact on vision. However, it does represent the first readily detectable ocular finding of diabetes in most patients. More sensitive and invasive tests such as 7-field stereo fundus photography, fluorescein angiography or vitreous fluorophotometry are generally not considered standard of care until retinal lesions are identified and treatment is being considered, but are often used as part of interventional or epidemiologic research studies. Swelling of the macula (clinically significant macular edema; CSME) represents an advanced form of retinopathy that will impact vision if not treated.
Proliferative diabetic retinopathy (PDR) represents more advanced disease with neovascularization, vitreous or preretinal hemorrhages, retinal detachment and other vision-impacting lesions. PDR and CSME warrant evaluation and close follow-up by an experienced ophthalmologist. Laser photocoagulation or other specialized forms of therapy may be necessary to preserve vision. DR is a leading cause of new-onset blindness in adults. However, clinically significant or vision-threatening retinopathy and end-stage renal disease are rarely detected during the years of pediatric follow-up. (17)
The earliest manifestation of renal involvement of T1D in children and adolescents, as well as adults, is hyperfiltration and an elevated renal plasma flow. Laborde et al (18) found in 45 diabetic children (age 12.5±4.0 years; duration 4.9±3.5 years) that both the glomerular filtration rate (GFR) (171±31 mL/min/1.73 m2 and 124±18 mL/min/1.73 m2, respectively) and renal plasma flow (RPF) ( 778±172 mL/min/1.73 m2 and 631±128 mL/min/1.73 m2 respectively) were higher in those with T1D than in control nondiabetic children. Studies report that hyperfiltration was associated with an increased risk of developing microalbuminuria (19, 20). Both nephromegaly (21) and higher ambulatory blood pressure (22) precede microalbuminuria in diabetic children. Nephropathy typically progresses from microalbuminuria (urinary albumin >30 mg/day or >30 mg/gram creatinine) to macroalbuminuria (urinary albumin >300 mg/day or >300 mg/gram creatinine) to falling glomerular filtration rate (GFR) and end-stage renal disease. Without intervention, diabetic nephropathy may progress to end-stage renal disease (ESRD) requiring dialysis or renal transplantation. Diabetic nephropathy is a leading cause of ESRD in adults. However, although microalbuminuria during adolescence is not uncommon, and it may be transient and/or intermittent, it is a predictor of possible future diabetic nephropathy. Macroalbuminuria, hematuria or renal insufficiency secondary to diabetes are rare during the pediatric years; (23) if present, strong consideration should be given to referral to a renal specialist.
Diabetic neuropathy can be manifest as peripheral neuropathy or autonomic neuropathy. Peripheral neuropathy most frequently presents with symptoms and findings in the feet, but can occur in any area of the body. Peripheral diabetic neuropathy is most often manifest with symptoms of numbness, tingling or burning and signs of reduced or absent reflexes and vibratory or temperature perception. Although the definitive diagnosis of diabetic neuropathy usually requires evaluation by a neurologist and/or a nerve conduction velocity study, screening using the Michigan Neuropathy Screening Instrument (MNSI) (24) has good sensitivity and specificity for the diagnosis of diabetic neuropathy and use of the 10-gram monofilament has good sensitivity from predicting the development of morbidity such as foot ulcer, infection or amputation. Peripheral neuropathy, along with poor circulation and wound healing, is a leading cause of non-traumatic amputation in adults.
Diabetic autonomic neuropathy has multiple manifestations including orthostatic hypotension, gastroparesis, pupillary dysfunction, bowel and bladder dysfunction, cardiac autonomic neuropathy with resting tachycardia and abnormal heart response to breathing and Valsalva, and erectile dysfunction.
Complications affecting the larger blood vessels, macrovascular complications, include coronary artery disease resulting in myocardial infarction, cerebrovascular disease resulting in stroke and peripheral vascular disease causing poor limb circulation resulting in claudication, infection or gangrene and amputation. Although these disorders are rarely if ever seen during the time of pediatric follow-up, the cardiovascular disease (CVD) risk factors (hypertension and dyslipidemia) and subclinical vascular abnormalities (intimal media thickening; stiffening of blood vessels; atherosclerotic plaque formation) certainly start during the adolescent years.
Screening for Diabetes Complications in Youth (Table 2)
Table 2.
Complication Screening Recommendations for Children and Adolescents with T1D
| ADA Recommendations (25) | ISPAD Recommendations (26) | |
|---|---|---|
| Retinopathy | Annual dilated funduscopic exam by an eye doctor at or after puberty or at age 10 and 3–5 years of diabetes | Annual fundus photography after age 11 and 2 years of diabetes, or at age 9 and 5 years of diabetes |
| Nephropathy | Annual urine albumin:creatinine ratio after 5 years of diabetes and after age 10 or puberty | Annual urine albumin:creatinine ratio or first morning albumin after 5 years of diabetes and after age 10 or puberty |
| Neuropathy | No specific guidelines in children | No specific guidelines in children |
| Macrovascular/CVD | Blood pressure annually Lipid profile at age 2 with a + family history, or age 10 or puberty without a + family history; if normal, repeat every 5 years |
Blood pressure annually Lipid profile every 5 years starting at age 12 |
ADA: American Diabetes Association; ISPAD: International Society for Pediatric and Adolescent Diabetes
Both the American Diabetes Association (ADA) and the International Society for Pediatric and Adolescent Diabetes (ISPAD) have guidelines for screening youth with both T1D and T2D for complications (summarized in Table 2) (25,26)
Comparison of Outcomes in the Pre- and Post-DCCT Eras
During the pre-DCCT era (before the publication of the results of the DCCT in 1993), the prevalence of retinopathy was reported to occur in 27%-89% of patients with T1DM for 19–30 years (27–31) (see Table 3 below) The prevalence of microalbuminuria (MA) was reported to be 19–28% and macroalbuminuria 16–20% after about 15 years duration (see Table 4 below) and of end-stage renal disease (ESRD) 2.2% and 7.8%, respectively, at 20 and 30 years duration. (32) Diabetic neuropathy, an outcome that is more difficult to document with certainty, was reported to occur in up to 60% of persons with the onset of T1D during childhood and adolescence. (see Table 5 below)
Table 3.
Prevalence of Diabetic Retinopathy in Youth-Onset T1D
| Reference | Author(s) | Year | Number of Subjects |
Age at Onset(yrs) Mean (Range) |
Duration (yrs) Mean(Range) |
Percent with Diabetic Retinopathy* |
|---|---|---|---|---|---|---|
| 36 | Malone et al | 1984 | 74 | “Youth” | 4.9±3.3 (1–13) |
BDR: 50 PDR: 14 |
| 37 | Verrotti et al | 1994 | 55 | “Children & Adolescent” | 6.9±3.1 (4.8–10) |
BDR: 16.4 |
| 52 | Kernell et al | 1997 | 557 | 14.6 | 8.0 | 14.5 |
| 39 | d’Annunzio et al | 1997 | 100 | 8.3±3.5 (1.2–16.4) |
10.4±1.9 (7.3–14.3) |
28 |
| 40 | Bognetti et al | 1997 | 317 | -- | -- | 22.7 |
| 41 | Holl et al | 1998 | 441 | “Children” | 7.6±6.3 | 16.3 |
| 27,28 | Olsen et al | 1999 | 339 | “Children & adolescent” | 13.2 | 60 |
| 29 | Skrivarhaug et al | 2006 | 294 | <15 | 24.3 (19.3–29.9) |
BDP: 89.1 PDR: 10.9 |
| 30 | Nordwall et al | 2006 | 80 | (7–21) | >13 | 27 |
| 42 | Majaliwa et al | 2007 | 99 | (5–18) | 4.76±3.58 | 22.7 |
| 46 | Nordwall et al | 2009 | 269 | 8.6±3.8 | 25.2±7.6 | BDR: 49.6 PDR: 26.1 |
| 43 | SEARCH | 2012 | 222 | <20 | 6.8±1.0 | 17 |
| 31 | Salardi et al | 2012 | 105 | (16–40) | 19.7 | 56.2 |
| 7 | DCCT/EDIC | 2010 | 156 | 13–18 | ~10 ~16 | Severe BDR: 16.1 PDR: 9.7 CSME: 1.7 Severe BDR: 19.5 PDR: 18.2 CSME:6.9 |
BDR: Background or nonproliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; CSME: clinically-significant macular edema
Table 4.
Prevalence of Microalbuminuria in Youth-Onset T1D
| Reference | Author(s) | Year of Report |
Number of Subjects |
Age of Onset (yrs) Mean (Range) |
Duration (yrs) Mean (Range) |
Percent with MA/MacroA* |
|---|---|---|---|---|---|---|
| 54 | Joner G, et al | 1992 | 371 | -- | 10.5 (6.2–17.3) | 12.5 |
| 50 | Rudberg et al | 1993 | 156 | <20 | 6.9±4.5 | 24.2 |
| 91 | Janner et al | 1994 | 1640 | “Children & Adolescents” | -- | 19.5 |
| 40 | Bognetti et al | 1997 | 317 | 11 | ||
| 55 | Jones et al | 1998 | 233 | 7.7 (median) | 8.5 | 14.5 |
| 57 | Schultz, et al | 1999 | 514 | <16 | 5 | 12.8 |
| 48 | Holl et al | 1999 | 447 | “Children” | 10 13 |
5 10 |
| 27,28 | Olsen et al | 1999 | 339 | “Children & adolescent” | 13.2 | Micro: 9.0 Macro: 3.7 |
| 59 | Moore, et al | 2000 | 1,007 | <20 | 7.8 (median) (1.7–15.9) |
9.7 |
| 62 | Levy-Marchal et al | 2000 | 702 | “Children & Adolescents” | 7.6±3.1 | 5.1 |
| 57 | Dahlquist et al | 2001 | 60 | 5.7±3.0 | 29±3 | Micro: 28 Macro: 12 |
| 92 | Amin et al | 2005 | 308 | 9.8 (.3–15.9) | 10.9 (6.0–17.8) | 11.4 |
| 30 | Nordwall et al | 2006 | 80 | (7–21) | >13 | 5 |
| 60 | Skrivarhaug et al | 2006 | 299 | <15 | 24 (19.3–29.9) | 14.9 |
| 61 | Gallego, et al | 2006 | 950 | <16 | 7.6 | 13.4 |
| 93 | Amin, et al | 2009 | 527 | <16 | 10 | 20.9 |
| 49 | Raile et al | 2007 | 27,805 | 12.9 | 8.3 | Micro: 3 Macro: 0.2 |
| 53 | SEARCH | 2007 | 3,259 | <20 | (0–5) | 9.2 |
| 94 | Chiarelli et al | 2008 | 340 | <18 | 16 | 9.4 |
| 86 | Dart et al | 2012 | T1D: 1,011 T2D: 342 |
(1–18) | -- | 13.5 27.1 |
MA; Microalbuminuria; MacroA: Macroalbuminuria
Table 5.
Prevalence of Diabetic Neuropathy in Youth with T1D
| Reference | Author(s) | Year of Publication |
Number of Subjects |
Age at Onset(yrs) Mean (Range) |
Duration (yrs) Mean (Range) |
Percent with a Finding Compatible with Diabetic Neuropathy |
|---|---|---|---|---|---|---|
| 40 | Bognetti, et al | 1997 | 317 | -- | -- | 18.5 |
| 27,28 | Olsen et al | 1999 | 339 | <20 | 13.2 | 62.5 |
| 63 | Bao, et al | 1999 | 38 | <20 | 7.2 | 68.4 |
| 30 | Nordwall et al | 2006 | 80 | (7–21) | >13 | 59 |
| 66 | Jaiswal, et al, SEARCH | 2013 | 329 | <20 | 6.2±0.9 | 8.2 |
During the post-DCCT period, the prevalence of diabetes complications has fallen considerably compared to the pre-DCCT years. (8,32–34) Hovind et al (34) reported a reduction in the prevalence of diabetic nephropathy at an average age of 20 years with T1D diagnosed either during 1965–1969 and 1970–1974. Those diagnosed from 1965–1975 had a prevalence of about 40% whereas those diagnosed in later years (1975–1984) had a prevalence of 13.7–18.9%, a 56% reduction. Proliferative diabetic retinopathy (PDR) occurred in 30.3–32.1% at 20 years for those diagnosed in 1965–1974 and the prevalence was about 55% after 40 years. Similar to nephropathy, for those diagnosed during later years, the prevalence of PDR had fallen substantially. Likewise, Nordwall et al (35) reported similar reductions in the prevalence of both diabetic nephropathy and PDR at 25 and 30 years duration in those diagnosed in 1961–1965 compared to those diagnosed in 1970–1974. Figure 1, using data adapted from reference 8, compares the cumulative incidence of PDR, nephropathy and end-stage renal disease between the pre-DCCT and the post-DCCT era.
Retinopathy (Table 3)
Reports of the prevalence of diabetic retinopathy in patients diagnosed with T1D during childhood and adolescence are summarized is Table 3. In these studies, the prevalence of background diabetic retinopathy (BDR) ranges from 14.5–50% at 5–10 years duration and from 37–90% at or beyond 20 years duration of T1DM. The highest prevalence rates for BDR were from studies published in the 1980s and following patients during the pre-DCCT era. Malone et al (36) reported the prevalence of BDR of 50% at 5 years duration. However, Verrotti et al (37) and Palmberg et al (38) reported a prevalence of 16.4% and13% at 6.2 and 4–5 years duration, respectively, and Palmberg et al (38) reported a prevalence of 50% and 95% at 10–12 and 26–50 years duration, respectively. The prevalence of proliferative diabetic retinopathy (PDR) was 26% in one study at 26–50 years duration (38) and varied from 10.9–26.1% at and beyond 20 years duration. It should be noted that Palmberg’s study (38) reported on those diagnosed before age 30 and did not separate out those diagnoses during the pediatric years (defined herein as <20); for this reason, Palmberg’s data are not included in Table 3. Although, there appears to have been a reduction in the prevalence of BDR from reports during the post-DCCT era, BDR still appears in about 20% by 5–10 years and 40% by 20 years.
In all the studies listed in Table 3 in which it was examined (28,29,39–46), poorer glycemic control (higher HbA1c) was consistently associated with a greater risk of retinopathy. Other factors that were reported to be associated include microalbuminuria (28,37,44), puberty (31,39), blood pressure (44,45), female (29) or male (36) gender, BMI (44) and LDL cholesterol (43); however, these associations were not explored in all studies and not consistently associated when they were explored.
Retinopathy was the primary outcome of the DCCT and this cohort continues to be followed into EDIC. Of the “adolescent” participants (enrolled before the age of 18 years), 16.1%, 9.7% and 1.2%, and 19.5%, 19.5% and 6.9% of the conventional group had developed severe BDR, PDR and CSME, respectively, at about 10 and about 16 years of T1D, respectively. (7)
Diabetic retinopathy of any degree is rare in young children. Lueder et al (47) found no cases of retinopathy in 51 children diagnosed before the age of 2 who were followed and evaluated at a mean duration of 13.7 years. They pointed out that in other studies in the literature at that time, no child under 10 years old had been identified with diabetic retinopathy requiring treatment.
Microalbuminuria (Table 4)
There are many reports of the prevalence of micro- or macroalbuminuria in the literature. Table 4 summarizes those that include patients diagnosed with T1D during childhood and adolescence. Rigorous comparison across studies is difficult since the reported patient characteristics are not standardized and the urine collection techniques and definitions of micro-and macroalbuminuria vary somewhat between studies. In addition, many reports use only a single value for microalbumin whereas others reports use persistent (2 or 3 elevated values).
The prevalence of microalbuminuria (MA) in these studies of childhood-onset T1D varies from as low as 3–5% after a duration of 8–13 or more years (30,48,49) to 19–29% at 10–13 years (31,48,50). The highest reported prevalence among these studies is 24–29% at diabetes durations >15 years. (50,51)
We examined the prevalence of MA in our entire population of patients seen over a 1-year period (in 2012) in our Pediatric Diabetes Clinic. Of the 836 unique patients with T1D, 572 met the ISPAD criteria (26) for MA screening and of these 496 (87%) were screened during that year. These patients had a mean (±SD) age of 15.9±8.0 years (range: 3–25) and diabetes duration of 7.9±3.9 years (range: 0–21). Mean age at diabetes onset was 7.9±4.0 years and 52.2% were female. Eighty-eight (17.7%) had a positive urine microalbumin screen (albumin:creatinine ratio ≥30 mg/gram creatinine) and of these, 71 (80.7%) had a second confirmatory determination. Fourteen (19.7%) of these 71 and 2.9% of the entire cohort met criteria for persistent microalbuminuria based on two consecutive elevated albumin:creatinine ratios. These results are slightly higher though similar to the other reports noted in Table 4 though all these patients were diagnosed with diabetes during the post-DCCT era. The presence of MA was associated with higher HbA1c (9.5±1.4% vs. 9.1±1.8%; p=0.017 and persistent MA was associated with even a higher HbA1c (9.8±1.1% at the time of the test and 9.7±1.2% average over the past year). By univariant analysis, longer disease duration, higher systolic (p=0.017), but not diastolic (p=0.061), blood pressure, and lower height (p=0.02) were associated with the presence of a positive MA screen; in this initial analysis, blood pressure and height were not corrected for age or sex, however. When analyzed in a multivariant logistic regression model, older chronological age, higher systolic blood pressure and higher HbA1c were associated with the presence of MA.
In these studies, the most consistent predictor of MA, aside from disease duration, was HbA1c. (48,49,52–61). Systolic, diastolic and/or mean blood pressure were also associated in some (49,53–56,62,) but not all (58) studies. Female sex is a frequent (48,53,57,61), but not consistent, (49,60) association. Other factors, such as shorter height, BMI, total or LDL cholesterol, triglycerides, retinopathy and smoking were not consistently associated with MA in studies in pediatric patients; of course it should be noted that retinopathy is infrequently found and smoking infrequently reported in this age group.
Neuropathy (Table 5)
Reliable and consistent data related to diabetic neuropathy in youth with T1D are limited. There are fewer studies than for retinopathy and microalbuminuria. In addition, many would consider the gold standard for peripheral diabetic neuropathy to include the performance of nerve conduction velocity studies. These are expensive, somewhat uncomfortable to painful and difficult to standardize, and therefore are infrequently done. The DCCT/EDIC study reported on rates of peripheral and autonomic neuropathy in T1DM, but this cohort is not entirely youth-onset and is, therefore, not discussed directly in this review. Bao et al (63) reported nerve conduction studies in a group of 38 youth-onset T1D and found a high prevalence of abnormalities but only 2 (5.3%) had symptomatic neuropathy and there was no control group.
Table 5 summarizes the studies that report the prevalence of findings compatible with peripheral neuropathy in cohorts of youth-onset T1D. The prevalence ranges from 8.2% at a mean duration of about 6 years to about 60% by a duration of 13 or more years. Although the DCCT clearly demonstrated that the rate of both peripheral and autonomic neuropathy is reduced by intensive therapy, the cross-sectional studies do not consistently show associations of neuropathy with glycemic control. (64–66) Some studies, but not all (64), report associations with HbA1c (63), male sex (28), blood pressure (28), elevated cholesterol and triglycerides (63) and the presence of microalbuminuria (28,63,66).
Verrotti et al (64) studied cardiovascular autonomic nerve function in 110 children with T1D. Forty-seven (43%) had one or more abnormalities. In this report, the was no association between the abnormal autonomic nervous system findings and glycemic control, sex, diabetes duration or the presence of retinopathy or microalbuminuria.
Cardiovascular Disease Risk Factors (Table 6)
Table 6.
Cardiovascular Disease Risk Factors in Youth with T1D
| Reference | Author(s) | Year of Publication | CVD Risk Factors* Affected |
|---|---|---|---|
| 77 | Krantz, et al | 2004 | ↑ cIMT |
| 69 | Rodriguez, et al., SEARCH | 2006 | “25% at age 10–19 had at least 2 CVD risk factors” |
| 73 | Kershnar, et al., SEARCH | 2006 | 48% had LDL >100 |
| 78 | Schwab et al | 2007 | ↑ cIMT; ↑ SBP |
| 72 | Petitti, et al., SEARCH | 2007 | ↑ TC, TG, non-HDL |
| 95 | Della Pozza, et al | 2007 | ↑ cIMT |
| 82 | Heilman, et al | 2009 | ↑ cIMT |
| 70 | Guy, et al., SEARCH | 2009 | ↑ TC, ↑ LDL, ↑non-HDL, ↑ apolipoprotein B, ↑ dense LDL particles |
| 83 | Urbin, et al., SEARCH | 2010 | ↑ arterial stiffness (PWV) |
| 80 | Babar, et al | 2011 | ↑ cIMT |
| 76 | Della Pozza, et al | 2011 | ↑ cIMT |
| 81 | Wadwa, et al., SEARCH | 2012 | ↓ FMD |
| 75 | Shah, et al., SEARCH | 2012 | ↑ arterial stiffness (PWV); ↑ adiponectin |
| 68 | Steigleder-Schweiger, et al | 2012 | 76.1% 1+ CVD risk factor 20.8% 2+ CVD risk factors 10.2% 3+ CVD risk factors 4.9% 4+ CVD risk factors |
| 84 | Dabelea, et al., SEARCH | 2013 | ↑ PWV; ↑ SBP |
| 79 | Urbina, et al., SEARCH | 2013 | ↑ cIMT |
| 71 | Maahs, et al., SEARCH | 2013 | ↑ TC,↑ LDL, ↑ TG, ↑ non-HDL |
| 74 | Kuryan, et al | 2014 | ↑ non-HDL |
| 67 | Alman, et al | 2014 | ↓ ICH; ↑ PWV |
CVD: Cardiovascular Disease; cIMT: Carotid intima-media thickness; TC: total cholesterol; LDL: LDL-cholesterol; HDL: HDL cholesterol; non-HDL: non-HDL cholesterol; TG: triglycerides; PWV: pulse wave velocity; SBP: systolic blood pressure; FMD: Flow-mediated dilation
Cardiovascular disease (CVD) risk factors are higher in persons with T1D than in controls and this is generally true in youth-onset T1D as well. Compared to non-diabetic control children and adolescents, who have been generally well matched for age, sex and race/ethnicity (but not always for BMI), patients with T1D generally tend to have more CVD risk factors (67–69), higher total cholesterol (TC) (70–72), LDL-cholesterol (LDL) (70,71,73), triglycerides (TG) (71,72), non-HDL cholesterol (non-HDL) (70–72,74), and Apolipoprotein B (70), more small LDL particles (70), lower HDL cholesterol (HDL) and higher adiponectin (75). However, considerable variability between studies reporting patients during the adolescent years exists. Table 6 represents a summary of studies reporting CVD risk factors in adolescents with T1D.
In addition to these easily monitored common risk factors for cardiovascular disease (CVD) such as glycemic control, hypertension and dyslipidemia, direct measures of vascular function have been performed in youth with both T1D and T2D. These measures include ultrasonographically obtained carotid artery intima-media thickness (cIMT), flow-mediated dilation (FMD) and pulse wave velocity (PWV). cIMT was increased (76–79), FMD decreased (76,80,81) and PWV increased (67,75,82–84), all indicators of vascular dysfunction, in T1D when compared to appropriately matched control subjects. Increased cIMT (85) and PWV (81) were also found in youth with T2D and these increases persisted after controlling for BMI.
Complications in Youth with Type 2 Diabetes Mellitus
Since type 2 diabetes (T2D) among youth has only become prevalent during the last 10–20 years, there are less data available about long-term complications in these subjects. However, the data available from three groups (86– 90) suggest that the complication risk for these patients may be higher than for patients with T1D diagnosed at a similar age and perhaps occur earlier in the course of the disease than in adults with T2D. Dart et al (86), from Manitoba Canada (an area where there is a high number of Oji-Cree native Canadians and the incidence of youth-onset T2D is very high) reported a higher burden of renal disease in youth-onset T2D than youth-onset T1D. They compared 1,011 subjects with youth-onset T1D to 342 with youth-onset T2D. Not unexpectedly, baseline difference included more females, higher BMI z-score and lower SES for the T2D group. Persistent MA was present in 26.9% vs. 12.7% and persistent macroalbuminuria in 4.7% vs. 1.6% (both p<0.001) in T2D vs. T1D, respectively. The age at onset of MA was similar, but the duration of diabetes was shorter (1.6± 1.5 [median 1.2]) in those with T1D than in those with T2D ( 6.3±3.9 [median 6.0] years) . In addition, T2D had an approximate 4-fold increased risk of renal disease (hazard ratio 4.03 [95% CI, 1.64–9.95]) and macroalbuminuria (hazard ratio 3.99 [95% CI, 1.50–10.0]), and a 3- to 5-fold increased risk of developing any renal complications, renal failure and end-stage renal disease for T2D vs. T1D at a similar age and diabetes duration. Survival analysis showed 100% renal survival out to 30 years duration in the T1D group compared to 100% renal survival at 10 years, 91.5% at 15 years and only 77.5% at 20 years in the T2D group.
Constantino et al (87) from Australia compared the outcomes of 354 patients with T2D to 470 patients with T1D, all diagnosed between 15 and 30 years of age, over a median 21.4 (interquartile range 14–30.7) year period. Mean age, duration of diabetes, year of diagnosis, HbA1c and smoking history were similar. As expected, BMI, systolic and diastolic blood pressure, total and LDL cholesterol were higher and HDL cholesterol lower in the T2D group. Survival was lower in the T2D group (hazard ratio 2.0 [95% CI 1.2–3.2]; p=0.003) with the cumulative mortality rate of 11% vs. 6.6%;, the rate of death due to cardiovascular disease was greater (50% vs. 30%, p<0.035; hazard ratio 3.5 [1.4–8.5]; p=0.004) in T2D vs. T1D,. Although retinopathy rates were similar (T2D: 37%; T1D: 41%), albuminuria, albumin:creatinine ratio and vibratory perception threshold were all higher (all p<0.0001) in those with T2D.
In the SEARCH Study (43), 42% of those with T2D had retinopathy compared to 17% of those with T1D at a similar age and duration. SEARCH also reported a prevalence of peripheral neuropathy of 25.7% in 70 youth with T2D (age: 21.6±4.1 yrs.; duration: 7.8±1.8 yrs.) compared to a prevalence of 8.2% in a similar (slightly younger) group of 329 with T1D. (66)
The TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) Study can also provide present-day insight into the prevalence of complications in youth-onset T2D. The TODAY Study enrolled 699 subjects with youth-onset T2DM diagnosed at <18 years of age and with diabetes duration <2 years. At baseline enrollment, the mean age was 14.0±2.0 years and the duration was 7.8±5.9 months. Subjects in the TODAY Study were randomized to three treatment groups (metformin alone; metformin + rosiglitazone; metformin + and intensive lifestyle program) and followed for an average of 3.9 years (range: 2–8 years). During the last year of the study, 517 subjects had 7-field stereoscopic fundus photography with centralized reading performed. At an average age of 18.1±2.5 years and an average disease duration of 4.9±1.5 (range: 2.0–8.4) years, 13.7% had mild background retinopathy. None had severe nonproliferative or proliferative retinopathy or macular edema. (88) In the TODAY Study cohort, 11.6% had hypertension at baseline and 33.8% had developed hypertension by the end of the study. Hypertension was more common in males, but did not differ by race/ethnicity, treatment group, glycemic control or primary treatment outcome. 6.3% had MA (ACR ≥30 mg/gram creatinine) at baseline and by study end, 16.8% had MA. Fifty-seven (8.2%) developed macroalbuminuria (ACU ≥300 mg/gram creatinine) and <1% had renal insufficiency (GFR <70 mL/min). The incidence of MA was associated with higher HbA1c. (89) At baseline, 4.5% already had an elevated LDL-cholesterol and by 36 months of follow-up this had risen to 10.7%.
In the TODAY Study, LDL and non-HDL cholesterol and apolipoprotein B all rose during the first 12 months of follow-up; this rise was primarily related to HbA1c (p<0.0001). (90) Other studies have found similar CVD risk factor and lipid findings in youth with T2D to those seen in TODAY. The SEARCH study (100) found that 92% of youth with T2D, compared to 21% of youth with T1D, had two or more CVD risk factors. They also compared the lipid profile from 1,680 T1D to 283 T2D youth greater than 10 years old (73) and found that those with T2D had a higher percentage with elevated total cholesterol (TC) (>200 mg/dl), LDL-cholesterol (LDL) (>130 mg/dL) and triglycerides (TG) (>200 mg/dL) and a low HDL- cholesterol (HDL) (<40 mg/dL) than those with T1D. The SEARCH Study (72) also reported that although the rates of dyslipidemia (elevated levels of TC, LDL, TG or non-HDL, or lower HDL) were higher in T2D than T1D, the association of dyslipidemia with HbA1c was similar.
The rate of retinopathy in the TODAY cohort is at least as high or higher and the prevalence of microalbuminuria and cardiovascular risk factors (hypertension and dyslipidemia) seem higher than noted above for those with T1D of similar age and duration. Together with the data of Dart et al (86),Constantino et al (87) and the SEARCH Study (43,66), these data strongly suggest that youth-onset T2D is indeed a “more lethal phenotype of diabetes and is associated with a greater mortality, more diabetes complications, and unfavorable cardiovascular disease risk factors when compared with T1DM” [Constantino et al (87)] of similar age, duration and glycemic control.
The Brain and Cognitive Effects of Diabetes
Since the early reports in the 1980s by Ryan et al (96) and Rovet et al (97), the effect of diabetes on the brain and cognitive functioning has been an area of vigorous research and controversy. The long history of this subject has been recently reviewed by two groups active in this field (98–102). Reports from the DCCT (103–105) and from Wysocki et al (106) have suggested that there is little if any long-term risk to cognition associated with hypoglycemia occurring in older adolescents and young adults. In the DCCT, there was a slight decline of psychomotor efficiency associated with long-term metabolic control. (105)
There is, however, a robust body of evidence indicating that diabetes with its onset in early childhood is associated with both cognitive deficits and structural changes on MRI. Northam and her colleagues in Australia followed a cohort of children diagnosed with T1D from the time of diagnosis and for another 12 years. The initial mild psychological symptoms observed in children and their parents were largely resolved by one year. (107) At 2 years, there were deficits detected in memory and learning. (108) By 6 years, those with T1D had poorer performance on measures of intelligence, attention, processing speed and executive skills. Attention, processing speed and executive skills were associated with early onset (<4 years old) whereas intelligence was associated with a history of severe hypoglycemia. (109) At 12 years, these subjects were performing worse than controls on working memory, attention, new learning and mental efficiency. There was an association of verbal abilities, working memory and nonverbal processing speed with hypoglycemia and an association of working memory with hyperglycemia. (110) Also, after 12 years, the diabetic subjects had higher rates of mental health referrals and lower school completion. (111) Thus using this cohort followed since the onset of diabetes, Northam and her colleagues have shown the emergence of cognitive deficits in children with T1D over time.
The group at Washington University in St. Louis (Hershey, White, et al) have also provided a body of data supporting alteration of cognitive function and brain structure in youth with T1D, most notable those with very early (<5 years old) onset. Hershey et al (111) showed reduced performance on a spatial delayed memory task in early-onset children with T1DM and a history of severe hypoglycemia. Perantie et al, in both a retrospective (112) and prospective (113) analysis of T1D youth, showed effects of both hypoglycemia and hyperglycemia on regional brain volumes determined by voxel-based morphometry using MRI and Antenor-Dorsey et al (114) showed alterations in white matter structure using diffusion tensor imaging (DTI). As this work has progressed, it has become apparent that both hypoglycemia and hyperglycemia affect brain structure and function, at least in those that develop T1D at a very young age. These data suggest that hypoglycemia and hyperglycemia affect different cognitive domains and different regions of brain, indicating that the mechanisms underlying the effects of hypoglycemia and hyperglycemia may be different.
The DirecNet Study Group has also evaluated brain function and structure in a group of 144 children with T1D onset before age 8 years and 70 matched nondiabetic controls. Gray matter volumes (115), white matter structure (116) and cognitive function (117) were altered in the T1D group (compared to nondiabetic controls). Within the T1D group, these alterations were associated more strongly with hyperglycemia than with hypoglycemia. The mean HbA1c in this group of children was 7.9% and the associations with hyperglycemia in this group included detailed glycemic assessment using continuous glucose monitors (CGM).
Thus, the extant data strongly suggest that even at the current level on glycemic control, there is risk to the brain associated with both hyper- and hypoglycemia, especially in young children. This provides further support for the necessity of developing better approaches and technology to achieve blood glucose as close to normal as possible at all ages.
Key Points.
Clinically significant diabetes-related complications are uncommon in children and adolescents, but patients with youth-onset diabetes do develop life-altering complications during their young adult years.
Retinopathy, nephropathy (microalbuminuria) and neuropathy are associated with glycemic control; current levels of glycemic control appear inadequate to completely prevent these complications.
Cardiovascular disease associated with diabetes starts during adolescence and vigorous attention to CVD risk factors (dyslipidemia and hypertension) are important components of caring for children and adolescents with diabetes.
Type 2 diabetes with it onset in youth is likely associated with more and earlier diabetes-related micro- and macrovascular complications than type 1 diabetes.
Recent and emerging data show that hyperglycemia as well as hypoglycemia may have lasting effects on brain function and structure, especially in young children.
Taken together, these considerations support the need for continuing research into new approaches and technology to improve the long-term overall glycemic control of those with diabetes of all ages, including young children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-term Complications in Insulin-dependent Diabetes Mellitus. N Eng J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.The D.C.C.T. Research Group. The Effect of Intensive Treatment on the Development and Progression of Long-Term Complications in Adolescents with Insulin-Dependent Diabetes Mellitus: The Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N Eng J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and Nephropathy in Patients with Type 1 Diabetes Four Years after a Trial of Intensive Therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Beneficial effects of intensive therapy of diabetes during adolescence: Outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT) J Pediatr. 2001;139:804–812. doi: 10.1067/mpd.2001.118887. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Prolonged effect of intensive therapy on the risk of retinopathy complications in patient with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch of Ophthal. 2008;126(12):1707–1715. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White NH, Sun W, Cleary PA, Tamborlane WV, Danis RP, Hainsworth DP, Davis MD for the DCCT-EDIC Research Group. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC : comparison of adults and adolescents. Diabetes. 2010;59(5):1244–1253. doi: 10.2337/db09-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration. Arch Intern Med. 2009;169(14):1306–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ly TT, Maahs DM, Rewers A, Dunger D, Oduwole A, Jones TW. ISPAD Guidelines 2014: Assessment and management of Hypoglycemia. Pediatric Diabetes. 2014;15(Suppl. 20):180–192. doi: 10.1111/pedi.12174. [DOI] [PubMed] [Google Scholar]
- 10.Elizabeth R Seaquist, John Anderson, Belinda Childs, Philip Cryer, Samuel Dagogo-Jack, Lisa Fish, Simon R Heller, Henry Rodriguez, James Rosenzweig, Robert Vigersky. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care. 2013;36:1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta V, Swift PGF, Woodruff GHA, Harris RP. Metabolic cataracts in newly diagnosed diabetes. Arch Dis Childhood. 1997;76:118–120. doi: 10.1136/adc.76.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehlich RM, Kirsch S, Daneman D. Cataracts in children with diabetes mellitus. Diabetes Care. 10(6):798–799. doi: 10.2337/diacare.10.6.798. [DOI] [PubMed] [Google Scholar]
- 13.Patel CM, Plummer-Smith L, Ugrasbul F. Bilateral metabolic cataracts in 10-yr-old boy with newly diagnosed type 1 diabetes mellitus. Pediatric Diabetes. 2009;10(3):227–9. doi: 10.1111/j.1399-5448.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato S, Oshika T, Numaga J, Kawashima H, Kitano S, Kaiya T. Influence of rapid glycemic-control on lens opacity in patients with diabetes mellitus. Ophthalmology. 2000;130(3):354–355. doi: 10.1016/s0002-9394(00)00546-8. [DOI] [PubMed] [Google Scholar]
- 15.Delamater AM, de Wit M, McDarby V, Malik J, Acerini CL. ISPAD Clinical Practice Consensus Guidelines 2014: Psychological care of children and adolescents with type 1 diabetes. Pediatric Diabetes. 2014;15(Suppl. 20):232–244. doi: 10.1111/pedi.12191. [DOI] [PubMed] [Google Scholar]
- 16.Anderson BJ, McKay SV. Barriers to glycemic control in youth with type 1 diabetes and type 2 diabetes. Pediatric Diabetes. 2011;12(3 Pt 1):197–205. doi: 10.1111/j.1399-5448.2010.00667.x. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Lee KE, Knudtson MD, Gangnon RE, Klein BEK. Changes in Visual Impairment Prevalence by Period of Diagnosis of Diabetes: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2009;116(10):1937–1942. doi: 10.1016/j.ophtha.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laborde K, Levy-Marchal C, Kindermans C, Dechaux M, Czernichow P, Sachs C. Glomerular filtration and microalbuminuria in children with insulin-dependent diabetes. Pediat Nephrol. 1990;4(1):39–43. doi: 10.1007/BF00858437. [DOI] [PubMed] [Google Scholar]
- 19.Chiarelli F, Verrotti A, Morgese G. Glomerular hyperfiltration increases the risk of developing microalbuminuria in diabetic children. Pediatr Nephrol. 1995;9(2):154–158. doi: 10.1007/BF00860729. [DOI] [PubMed] [Google Scholar]
- 20.Mauer M, Drummond K for the International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes. I. Study design and baseline characteristics of the study participants. Diabetes. 2002;51:1572–1579. doi: 10.2337/diabetes.51.5.1572. [DOI] [PubMed] [Google Scholar]
- 21.Lawson ML, Sochett EB, Chait PG, Balfe JW, Daneman D. Effect of puberty on markers of glomerular hypertrophy and hypertension in IDDM. Diabetes. 1996;45(1):51–55. doi: 10.2337/diab.45.1.51. [DOI] [PubMed] [Google Scholar]
- 22.Garg SK, Chase HP, Icaza G, Rothman RL, Osberg I, Carmain JA. 24-hour ambulatory blood pressure and renal disease in young subjects with type 1 diabetes. J Diabetes Complication. 1997;11(5):263–267. doi: 10.1016/s1056-8727(96)00067-0. [DOI] [PubMed] [Google Scholar]
- 23.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–83. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman EL, Stevens MJ, Thomas PK, et al. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of Medical Care in Diabetes—2014. Diabetes Care. 2014;37(Supplement 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 26.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K for ISPAD. Microvascular and macrovascular complications associated with diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Supplement):195–203. doi: 10.1111/j.1399-5448.2009.00576.x. [DOI] [PubMed] [Google Scholar]
- 27.Olsen BS, Johannesen J, Sjolie AK, Borch-Johnsen K, Hougarrdss P, Thorsreinsson B, et al. Metabolic control and prevalence of microvascular complication in young Danish patients with Type 1 diabetes mellitus. Danish Study Group of Diabetes in Childhood. Diabet Med. 1999;16(1):79–85. doi: 10.1046/j.1464-5491.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 28.Olsen BS, Sjolie A, Hougard P, Johannesen J, Borch-Johnsen K, Marinelli K, Thorsreinsson B, et al. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood. J Diabetes Complications. 2000;14(6):295–300. doi: 10.1016/s1056-8727(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 29.Skrivarhaug T, Fosmark DS, Stene LC, Bangstad HJ, Sandvik L, Hanssen KF, et al. Low cumulative incidence of proliferative retinopathy in childhood-onset type 1 diabetes: a 24-year follow- up study. Diabetologia. 2006;49(10):2281–2290. doi: 10.1007/s00125-006-0364-7. [DOI] [PubMed] [Google Scholar]
- 30.Nordwall M, Hyllienmark L, Ludvigsson J. Early diabetic complications in a population of young patients with type 1 diabetes mellitus despite intensive treatment. J Pediatr Endocrinol Metab. 2006;19(1):45–54. doi: 10.1515/jpem.2006.19.1.45. [DOI] [PubMed] [Google Scholar]
- 31.Salardi S, Porta M, Maltoni G, Rubbi F, Rovere S, Cerutti F, et al. Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology. Infant and toddler type 1 diabetes: complications after 20 years’ duration. Diabetes Care. 2012;35(4):829–833. doi: 10.2337/dc11-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finne P, Reunanen A, Stenman S, Groop P-H, Gronhagen-Riska C. Incidence of End-stage Renal Disease in Patients with Type 1 Diabetes. JAMA. 2005;294:1782–1787. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 33.Pambianco G, Costacou T, Ellis D, Becker D, Klein R, Orchard TJ. The 30-Year Natural History of Type 1 Diabetes Complications: The Pittsburgh Epidemiology of Diabetes Cpmplications Study Experience. Diabetes. 2006;55:1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 34.Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, et al. Decreasing Incidence of Severe Diabetic Microangiopathy in Type 1 Diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 35.Nordwall M, Bojestig M, Arnqvist HJ, Ludvigsson J. Declinng incidence of severe retinopathy and persisting decrease of nephropathy in an unselected populatioin of Type 1 diabetes--the Linkoping Diabetes Complications Study. Diabetologia. 2004;47:1266–1272. doi: 10.1007/s00125-004-1431-6. [DOI] [PubMed] [Google Scholar]
- 36.Malone JI, Grizzard S, Espinoza LR, Achenbach KE, Van Cader TC. Risk factors for diabetic retinopathy in youth. Pediatrics. 1984;73(6):756–761. [PubMed] [Google Scholar]
- 37.Vererotti A, Lobefala L, Chiarelli F, Mastropasqua L, Gallenga PE, Morgese G. Diabetic retinopathy. Relationship with nephropathy in pediatric age. Panminerva Med. 1994;36(4):179–183. [PubMed] [Google Scholar]
- 38.Palmberg P, Smith M, Waltman S, Krupin T, Singer P, Burgess D, et al. The natural history of retinopathy in insulin-dependent juvenile-onset diabetes. Ophthalmology. 1981;88(7):613–618. doi: 10.1016/s0161-6420(81)34975-6. [DOI] [PubMed] [Google Scholar]
- 39.d’Annunzio G, Malvezzi F, Vitali L, Barone C, Giacchero R, Klersy C, et al. A 3-19-year follow- up study on diabetic retinopathy in patients diagnosed in childhood and treated with conventional therapy. Diabet Med. 1997;14(11):951–958. doi: 10.1002/(SICI)1096-9136(199711)14:11<951::AID-DIA490>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Bognetti E, Calori G, Meschi F, Macellaro P, Bonfanti R, Chiumello G. Prevalence and correlations of early microvascular complications in young type I diabetic patients: role of puberty. J Pediatr Endocrinol Metab. 1997;10(6):587–592. doi: 10.1515/jpem.1997.10.6.587. [DOI] [PubMed] [Google Scholar]
- 41.Holl RW, Lang GE, Grabert M, Heinze E, Lang GK, Debatin KM. Diabetic retinopathy in pediatric patients with type-1 diabetes: effect of diabetes duration, preubertal and pubertal onset of diabetes, and metabolic control. J Pediatr. 1998;132(5):790–794. doi: 10.1016/s0022-3476(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 42.Majaliwa ES, Munubhi E, Ramaiya K, Mpembeni R, Sanyiwa A, Mohn A, Chiarelli F. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Dar es Salaam, Tanzania. Diabetes Care. 2007;30(9):2187–2192. doi: 10.2337/dc07-0594. [DOI] [PubMed] [Google Scholar]
- 43.Mayer-Davis EJ, Davis C, Saadine J, D’Agostino RB, Dabelea D, Dolan L, et al. SEARCH for Diabetesin Youth Study Group. Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med. 2012;29(9):1148–1152. doi: 10.1111/j.1464-5491.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein R, Knudson MD, Lee KE, Gangnon R, Klein BEK. The Wisconsin Epidemiologic Study of Diabetic retinopathy XXII. The twenty-five year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–1868. doi: 10.1016/j.ophtha.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yau J, Rogers S, Kawasaki R, Lamoureux E, Kowalski J, Bek T, et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care. 2012;35 doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Microalbuminuria Collaborative Study Group. Predictors of the development of microalbuminuria in patients with Type 1 diabetes mellitus: a seven-year prospective study. Diabet Med. 1999;16:918–925. [PubMed] [Google Scholar]
- 47.Nordwall M, Arnqvist HJ, Bojestig M, Ludvigsson J. Good glycemic control remains cruciao in prevention of late diabetic complications--the Linkoping Diabetes Complications Study. Pediatr Diabetes. 2009;10(3):168–176. doi: 10.1111/j.1399-5448.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 48.Lueder GT, Pradhan S, White NH. Risk of retinopathy in children with type 1 diabetes mellitus before 2 years of age. Am J Ophthalmology. 2005;140(4):930–931. doi: 10.1016/j.ajo.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Holl RW, Grabert M, Thon A, Heinze E. Urinary excretion of albumin in adolescents with type 1 diabetes: persistent versus intermittent microalbuminuria and relationship to duration of diabetes, sex and metabolic control. Diabetes Care. 1999;22(9):1555–1560. doi: 10.2337/diacare.22.9.1555. [DOI] [PubMed] [Google Scholar]
- 50.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW. Diabetic Nephropathy in 27,805 Children, Adolescents, and Adults With Type 1 Diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30:2523–2528. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 51.Rudberg S, Ullman E, Dahlquist G. Relationship between early metabolic control and the development of microalbuminuria--a lomgitudinal study in children with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36(12):1309–14. doi: 10.1007/BF00400811. [DOI] [PubMed] [Google Scholar]
- 52.Kernall A, Dedorsson I, Joansson B, Wickstrom CP, Ludvigsson J, Tuvemo T, et al. Prevalence of diabetic retinopathy in children and adolescents with IDDM: A population-based multicentre study. Diabetologia. 1997;40(3):307–310. doi: 10.1007/s001250050679. [DOI] [PubMed] [Google Scholar]
- 53.Maahs DM, Snively BM, Bell RA, Dolan L, Hirsch I, Imperator G, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. DiabetesCare. 2007;30(10):2593–2598. doi: 10.2337/dc07-0450. [DOI] [PubMed] [Google Scholar]
- 54.Joner G, Brinchmann-Hansen O, Torres CG, Hanssen KF. A nationwide cross-sectional study of retinopathy and microallbuminuria in young Norwegian type 1 (insulin-dependent) diabetic patients. Diabetologia. 1992;35(11):1049–1054. doi: 10.1007/BF02221680. [DOI] [PubMed] [Google Scholar]
- 55.Jones CA, Leese GP, Kerr S, Besstwick K, Isherwood DI, Vora JP, et al. Development and progression of microalbuminuria in a clinic sample of patients with insulin dependent diabetes mellitus. Arch Dis Childhood. 1998;78:518–523. doi: 10.1136/adc.78.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Tranplant. 2001;16(7):1382–1386. doi: 10.1093/ndt/16.7.1382. [DOI] [PubMed] [Google Scholar]
- 57.Schultz CJ, Konopelska-Bahu T, Dalton RN, Stratton I, Gale EAM, Neil A, et al. for the Oxford Regionial Prospecitve Study Group. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Diabetes Care. 1999;22(3):495–502. doi: 10.2337/diacare.22.3.495. [DOI] [PubMed] [Google Scholar]
- 58.Gorman D, Sochett E, Daneman D. The natural history of microalbuminuria in adolescents with type 1 diabetes. J Pediatr. 1999;134(3):333–337. doi: 10.1016/s0022-3476(99)70459-2. [DOI] [PubMed] [Google Scholar]
- 59.Moore THM, Shield JPH on behalf of the Microalbuminuria in Diabetic Adolescents and Children (MIDAC) research group. Prevalence of abnormal urinary albumin excretion in adolescents and children with insulin dependent diabetes: the MIDAC study. Arch Dis Child. 2000;83:239–243. doi: 10.1136/adc.83.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skrivarhaug T, Bangstad H-J, Stene LC, Sandvik L, Hanssen KF, Joner G. Low risk of overt nephropathy after 24 yr of childhood-onset type 1 diabetes mellitus (T1DM) in Norway. Pediatric Diabetes. 2006;7:239–246. doi: 10.1111/j.1399-5448.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 61.Gallego PH, Bulsara MK, Frazer F, Lafferty AR, Davis EA, Jones TW. Prevalence and risk factors for microalbuminuria in a population-based sample of children and adolescents with T1DM in Western Australia. Pediatric Diabetes. 2006;7:165–172. doi: 10.1111/j.1399-543X.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 62.Levy-Marchal C, Sahler C, Cahane M, Czernichow P GECER Study Group. Risk factors for microalbuminuria in children and adolescents with type 1 diabetes. J Ped Endocrinol Metab. 2000;13(6):613–620. doi: 10.1515/jpem.2000.13.6.613. [DOI] [PubMed] [Google Scholar]
- 63.Bao X-H, Wong V, Wang Q, Low LCK. Prevalence of peripheral neuropathy with insulin- dependent diabetes mellitus. Pediatric Neurology. 1999;20(3):204–209. doi: 10.1016/s0887-8994(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 64.Verrotti A, Chiarelli F, Blasetti A, Morgese G. Autonomic neuropathy in diabetic children. J Paediatr Child Health. 1995;31(6):545–548. doi: 10.1111/j.1440-1754.1995.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 65.dos Santos LH, Bruck I, Antoniuk SA, Sandrini R. Evaluation of sensorimotor polyneuropathy in children and adolescent with type 1 diabetes: associations with microalbuminuria and retinopathy. Pediatric Diabetes. 2002;3(2):101–108. doi: 10.1034/j.1399-5448.2002.30207.x. [DOI] [PubMed] [Google Scholar]
- 66.Jaiswal M, Lauer A, Martin CL, Bell RA, Divers J, Dabelea D, et al. SEARCH. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care. 2013;36(12):3903–3908. doi: 10.2337/dc13-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alman AC, Talton JW, Wadwa RP, Urbina EM, Dolan LM, Daniels SR, et al. SEARCH. Cardiovascular health in adolescents with type 1 daibetes: the SEARCH CVD Study. Pediatr Diabetes. 2014 Jan 22; doi: 10.1111/pedi.12120. [pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steigleder-Schweiger C, Rami-Merhar B, Waldhor T, Frohlich-Reiterer E, Schwarz I, Fritsch M, et al. Prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes in Austria. Eur J Pediatr. 2012;171(8):1193–1202. doi: 10.1007/s00431-012-1704-x. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, Imperatore G, Williams DE, Cell RA, et al. SEARCH. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2006;29(8):1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 70.Guy J, Ogden L, Wadwa RP, Hamman RF, Mayer-Davis EJ, Liese AD, et al. SEARCH. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes Care. 2009;32(3):416–420. doi: 10.2337/dc08-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maahs DM, Dabelea D, D’Agostino RB, Andrews JS, Shah AS, Crimmins N, et al. SEARCH. Glucose control predicts 2-year change in lipid profile in youth with type 1 diabetes. J Pediatr. 2013;162(1):101–107. doi: 10.1016/j.jpeds.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peitii DB, Imperaotore G, Palla SL, Daniels SR, Dolan LM, Kershnar AK, et al. SEARCH. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med. 2007;161(2):159–165. doi: 10.1001/archpedi.161.2.159. [DOI] [PubMed] [Google Scholar]
- 73.Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Marcovina S, et al. SEARCH. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2006;149(302):314–319. doi: 10.1016/j.jpeds.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 74.Kuryan RE, Jacobson MS, Frank GR. Non-HDL-cholesterol in an adolescent diabetes population. J Clin Lipidol. 2014;8(2):194–198. doi: 10.1016/j.jacl.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Shah AS, Dolan LM, Lauer A, Davis C, Dabelea D, Daniels SR, et al. SEARCH. Adiponectin and arterial stiffness in youth with type 1 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr Endocrinol Metab. 2012;25(7–8):717–721. doi: 10.1515/jpem-2012-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Della Pozza R, Beyerlein A, Thilmany C, Weissenbacher C, Netz H, Scmidt H, Bechtold S. The effect of cardiovascular risk factors on the longitudinal evolution of the carotid intima medial thickness in children with type 1 diabetes mellitus. Cardiovasc Diabetol. 2011;10:53. doi: 10.1186/1475-2840-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krantz JS, Mack WJ, Hodis HN, Liu CR, Liu CH, Kaufman FR. Early onset of subclinical atherosclerosis in young persons with type 1 diabetes. J Pediatr. 2004;145(4):452–457. doi: 10.1016/j.jpeds.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 78.Schwab KO, Doefer J, Krebs A, Krebs K, Schorb E, Hallelrmann K, et al. Early atherosclerosis in childhood type 1 daibetes: role of raised blood pressure in the absence of dyslipidemia. Eur J Pediatr. 2007;166(6):541–548. doi: 10.1007/s00431-007-0440-0. [DOI] [PubMed] [Google Scholar]
- 79.Urbina EM, Dabelea D, D’Agostino RB, Shah AS, Dolan LN, Hamman RF, et al. SEARCH. Effect of type 1 diabetes on carotid structure and function in adolescents and young adults: the SEARCH for Diabetes in Youth Study. the SEARCH CVD study. Diabetes Care. 2013;36(9):2597–2599. doi: 10.2337/dc12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Babar GS, Zidan H, Widlansky ME, Das E, Hoffmann RG, Daoud M, Alemzadeh R. Impaired endothelial function in preadolescent children with type 1 diabetes. Diabetes Care. 2011;34(3):681–685. doi: 10.2337/dc10-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodrigues BL, et al. SEARCH Study Group. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2012;33(4):881–886. doi: 10.2337/dc09-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heilman K, Zilman M, Zilman K, Lintrop M, Kampus P, Kals J, Tillmann V. Arterial stiffness, carotid artery intima-media thickness and plasma myeloperoxidase level in children with type 1 diabetes. Diabetes Res Clin Pract. 2009;84(2):168–173. doi: 10.1016/j.diabres.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, et al. SEARCH. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement stie and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156(5):731–737. doi: 10.1016/j.jpeds.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Dabelea D, Talton JW, D’Agostino R, Wadwa RP, Urbina EM, Dolan LM, et al. SEARCH. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type diabetes: the SEARCH CVD study. Diabetes Care. 2013;36(12):3838–3943. doi: 10.2337/dc13-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah AS, Dolan LM, Kimball TR, Gao Z, Khoury PR, Danials SR, Urbina EM. Influence of duration of diabetes, glycemic control, and traditional cardiovascular risk factors on early atherosclerotic vascular changes in adolescents and yount adults with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(10):3740–3745. doi: 10.1210/jc.2008-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265–1271. doi: 10.2337/dc11-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Constantino MI, Molyneax L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, et al. Long-terrm complications and mortality in young-onset diabetes. Type 2 diabetes is more hazaardous and lethal than type 1diabetes. Diabetes Care. 2013;36:3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.TODAY Study Group. Retinopathy in Youth With Type 2 Diabetes Participating in the TODAY Clinical Trial. Diabetes Care. 2013;36:1772–1774. doi: 10.2337/dc12-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.TODAY Study Group. Rapid rise in hypertension and nepropathy in yout with type 2 diabetes: The TODAY clinical trial. Diabetes Care. 2013;36:1735–1741. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.TODAY Study Group. Lipid and Inflammatory Cardiovascular Risk Worsens Over 3 Years in Youth With Type 2 Diabetes: The TODAY clinical trial. Diabetes Care. 2013;36:1758–1764. doi: 10.2337/dc12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janner M, Knill SE, Diem P, Zuppinger KA, Mullis PE. Eur J Pediatr. 1994;153(6):403–408. doi: 10.1007/BF01983401. [DOI] [PubMed] [Google Scholar]
- 92.Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, et al. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional Prospective Study. Kidney Int. 2005;68(4):1740–1749. doi: 10.1111/j.1523-1755.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 93.Amin R, Widmer B, Dalton RN, Dunger DB. Unchanged incidence of nicroalbuminuria in children with type 1 diabetes since 1986: a UK based inception cohort. Arch Dis Child. 2009;94:258–262. doi: 10.1136/adc.2008.144337. [DOI] [PubMed] [Google Scholar]
- 94.Chiarelli F, Giannini C, Verotti A, Mezzetti A, Mohn A. Increased concentrations of soluble CD40 ligand may help to identify type 1 diabetic adolescents and young adults at risk for developing persistent microalbuminuria. Diabetes Metab Res Rev. 2008;24(7):570–576. doi: 10.1002/dmrr.891. [DOI] [PubMed] [Google Scholar]
- 95.Della Pozza R, Bechtold S, Bonfig W, Putzker S, Kozlik-Feldmann R, Netz H, Schwarz HP. Age of onset of type 1 diabetes in chidren and carotid intima medial thickness. J Clin Endocrinol Metab. 2007;92(6):2053–2057. doi: 10.1210/jc.2006-2868. [DOI] [PubMed] [Google Scholar]
- 96.Ryan C, Vega A, Drash A. Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics. 1985;75:921–927. [PubMed] [Google Scholar]
- 97.Rovet JF, Ehrlich RM, Hoppe MG. Intellectual deficits associated with the early onset of insulin- dependent diabetes mellitus in children. Diabetes Care. 1987;10:510–515. doi: 10.2337/diacare.10.4.510. [DOI] [PubMed] [Google Scholar]
- 98.Arbelaez AM, Semenkovich, Hershey T. Glycemic extremes in youth with T1DM: The structural and functional integrity of the developing brain. Pediatr Diabetes. 2013;14:541–553. doi: 10.1111/pedi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryan C. Does moderately severe hypoglycemia cause cognitive dysfunction in children? Pediatr Diabetes. 2004;5:59–62. doi: 10.1111/j.1399-543X.2004.00044.x. [DOI] [PubMed] [Google Scholar]
- 100.Ryan C. Why is cognitive dysfunction associated with the development of diabetes in early life? The diathesis hypothesis. Pediatr Diabetes. 2006;7:289–297. doi: 10.1111/j.1399-5448.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 101.Biessels GJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 102.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 103.Jacobson, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Long-term effect of diabetes and its treatment of cognitive function. N Engl J Med. 2009;361(19):1914. [Google Scholar]
- 104.Musen G, Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, et al. DCCT/EDIC. Impactr of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care. 2008;10:1933–1938. doi: 10.2337/dc08-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacobson AM, Ryan CM, Cleary PA, Waberdki BH, Weinger K, Musen G, et al. DCCT/EDIC. Biomedical risk factors for dereased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2011;(54)(2):245–255. doi: 10.1007/s00125-010-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, White NH. Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care. 2003;6(4):1100–1105. doi: 10.2337/diacare.26.4.1100. [DOI] [PubMed] [Google Scholar]
- 107.Northam E, Anderson P, Adler R, Werther G, Warne G. Pyschosocial and family functioning in children with insulin-dependent diabetes at diagnosis. J Pediartr Psychol. 1996;21(5):699–717. doi: 10.1093/jpepsy/21.5.699. [DOI] [PubMed] [Google Scholar]
- 108.Northam EA, Anderson PJ, Werther GA, Warne GL, Andrewes D. Predictors of change in the neurophsychological profiles of children with type 1 diabetes 2 years after disease onset. Diabetes Care. 1999;22:1438–1444. doi: 10.2337/diacare.22.9.1438. [DOI] [PubMed] [Google Scholar]
- 109.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsycholopgical profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 110.Lin A, Northam EA, Rankins D, Werther GA, Comeron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes. 2012;11(4):235–243. doi: 10.1111/j.1399-5448.2009.00588.x. [DOI] [PubMed] [Google Scholar]
- 111.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes mellitus. Diabetes Care. 2005;28:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- 112.Perantie DC, Wu J, Koller JM, Lim A, Warren S, Black KJ, Sadler M, White NH, Hershey T. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care. 2007;30(9):2331–2337. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- 113.Perantie DC, Koller JM, Weaver PM, Lugar HM, Black KJ, White NH, Hershey T. Prospectively-determined impact of type 1 diabetes on brain volume during development. Diabetes. 2011;60(11):3006–3014. doi: 10.2337/db11-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antenor-Dorsey JA, Meyer E, Rutlin J, Perantie DC, White NH, Arbelaez AM, Shimony J, Hershey T. White matter microstructural integrity in youth with type 1 diabetes. Diabetes. 2013;62(2):581–589. doi: 10.2337/db12-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marzelli MJ, Mazaika PK, Barnea-Goraly N, Hershey T, Tsalikian E, Tamborlane W, Mauras N, et al. AL for the Diabetes Research in Children Network (DirecNet) Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes mellitus. Diabetes. 2014;63(1):343–353. doi: 10.2337/db13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. for the Diabetes Research in Children Network (DirecNet) Alterations in white matter structure in young children with type 1 diabetes mellitus. Diabetes Care. 2014;37(2):332–340. doi: 10.2337/dc13-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cato MA, Mauras N, Ambrosino J, Bondurant A, Conrad AL, Kollman C, et al. White NH, Hershey T for the Diabetes Research in Children Network (DirecNet) Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soc. 2014;20:238–247. doi: 10.1017/S1355617713001434. [DOI] [PMC free article] [PubMed] [Google Scholar]