Abstract
Renal elimination and the resulting clearance of perfluorooctanoic acid (PFOA) from the serum exhibit pronounced sex differences in the adult rat. The literature suggests that this is largely due to hormonally regulated expression of organic anion transporters (OATs) on the apical and basolateral membranes of the proximal tubule cells that facilitate excretion and reabsorption of PFOA from the filtrate into the blood. Previously developed PBPK models of PFOA exposure in the rat have not been parameterized to specifically account for transporter-mediated renal elimination. We developed a PBPK model for PFOA in the male and female rat to explore the role of Oat1, Oat3, and Oatp1a1 in sex-specific renal reabsorption and excretion of PFOA. Descriptions of the kinetic behavior of these transporters were extrapolated from in vitro studies and the model was used to simulate time-course serum, liver, and urine data for intravenous (IV) and oral exposures in both sexes. Model predicted concentrations of PFOA in the liver, serum, and urine showed good agreement with experimental data for both the male and female rat indicating that in vitro derived physiological descriptions of transporter-mediated renal reabsorption can successfully predict sex-dependent excretion of PFOA in the rat. This study supports the hypothesis that sex-specific serum half-lives for PFOA are largely driven by expression of transporters in the kidney and contributes to the development of PBPK modeling as a tool for evaluating the role of transporters in renal clearance.
Keywords: PFOA, PBPK, Oatp1a1, Oat1, Oat3, IVIVE
Introduction
Perfluorinated compounds (PFCs) have been used since the 1950’s in a variety of industrial applications and consumer products. Perfluorooctanoic acid (PFOA) is among one of the most well studied members of this class of compounds (ATSDR, 2009) and is frequently found in oil, stain, grease, and water repellent coatings on carpets, textiles, leather, and paper (ATSDR, 2009). PFOA is particularly environmentally and biologically persistent due to its eight-carbon backbone, strong carbon-fluorine bonds, and metabolic stability (Calafat et al., 2007; Bartell et al., 2010). Despite recent reductions in manufacturing, PFOA has been identified as a ‘contaminant of emerging concern’ by the U.S. Environmental Protection Agency (EPA) due to its frequent detection in water systems across the United States (USEPA, 2008; USEPA, 2009; Bartell et al., 2010) as well as in the blood of the general U.S. population (Calafat et al., 2006; Calafat et al., 2007; Olsen et al., 2007; Olsen et al., 2012; USEPA, 2014a).
Epidemiological studies of occupational and community exposure to PFCs indicate associations between blood serum levels of PFOA and high cholesterol, other liver effects including increased liver enzymes and decreased bilirubin levels, chronic kidney disease, and early menopause (Olsen et al., 2000; Olsen et al., 2007; Sakr et al., 2007a; Sakr et al., 2007b; Vaughn et al., 2013; USEPA, 2014b). In rodent studies, PFOA has resulted in body-weight changes, developmental effects, liver effects, and decreased serum total cholesterol (Ikeda et al., 1985; Kawashima et al., 1995; Butenhoff et al., 2004; Guruge et al., 2006; Lau et al., 2006; Cui et al., 2009).
The pharmacokinetics of PFOA are well studied in rats. PFOA is known to be well absorbed in the gastrointestinal tract, highly bound in the serum albumin, not metabolized, and excreted unchanged primarily via the kidneys (Johnson et al., 1979; Vanden Heuvel et al., 1991). PFOA reaches steady-state in the serum very rapidly with daily dosing, but is eliminated slowly (Bartell et al., 2010). The serum half-life for PFOA is estimated to be between four and six days in the male rat and between two and four hours in the female rat (Kemper, 2003). In contrast, the serum half-life for PFOA in humans has been estimated to be between 2.3 and 3.8 years (Olsen et al., 2007; Bartell et al., 2010). This sex and species specificity in serum half-life is hypothesized to be due to hormonally-regulated, saturable renal reabsorption of PFOA via organic anion transporters (OATs) expressed on the apical and basolateral membranes of the proximal tubule cells (Kudo et al., 2002; Andersen et al., 2006; Nakagawa et al., 2007). The rat model described here uses in vitro to in vivo extrapolation (IVIVE) to incorporate physiological descriptions of these transporters to predict sex specific renal clearance of PFOA in the adult rat.
Oat1 (Slc22a6) and Oat3 (Slc22a8) are expressed most highly in the proximal tubule cells of the rat kidneys and are localized to the basolateral membrane (Buist et al., 2002; Weaver et al., 2010). An extensive list of diverse substrates have been identified for Oat1, including PFOA. Oat3 has also been shown to be capable of PFOA transport (Nakagawa et al., 2007) . Together, these basolateral membrane transporters translocate PFOA from the blood into the proximal tubule cells and facilitate renal secretion. Oatp1a1 (Slco1a1) is expressed on the apical membrane of the proximal tubule cells in the rat and has been shown to transport PFOA from the urine back into the proximal tubule cells, thereby facilitating renal reabsorption (Weaver et al., 2010). The expression of these transporters is known to be sex-hormone regulated and have sex specific expression patterns in the adult rat (Buist et al., 2002). OATs are responsible for the movement of many pharmaceuticals and chemicals in the kidney. Sex-specific clearance and biological half-life has been demonstrated for several substrates of OATs including p-aminohippurate (PAH) (Reyes et al., 1998), zenarestat (Tanaka et al., 1991; Morris et al., 2003)), S-pentachlorophenyl-N-acetyl-L-cysteine (Smith and Francis, 1983), carnitine (Carter and Stratman, 1982), nilvadipine metabolite (M3) (Terashita et al., 1995) and 1-aminocyclohexanecarboxylic acid (Anton et al., 1986).
One other PBPK model for PFOA administration in the adult rat exists in the published literature (Loccisano et al., 2012). This model describes saturable reabsorption of PFOA from the filtrate compartment back into the kidney compartment via a single transporter with transporter maximum (Tm) and affinity constant (Kt) based on in vitro data describing Oatp1a1 uptake of PFOA. While this model was able to successfully describe PFOA kinetics in the adult rat, the information necessary to scale in vitro measurements of transporter activity to in vivo values for the transporters involved in both the excretion and renal reabsorption was not available at the time (Loccisano et al., 2012). The model described here applies recent in vitro data to expand upon the existing model by including physiological descriptions of both basolateral and apical membrane transporters in order to describe the sex specific kinetics of excretion and reabsorption in the kidneys. This evidence-based model confirms the findings of prior hypothesis-driven modeling efforts by showing that saturable reabsorption is necessary to achieve a consistent description of the experimental data. Further, it supports the hypothesis that sex-specific serum half-lives for PFOA are largely driven by expression and activity of transporters in the kidney and contributes to the development of PBPK modeling as a tool for evaluating the role of transporters in renal clearance.
Materials and Methods
Key Pharmacokinetic Studies in the Male and Female Rat
Pharmacokinetic data for PFOA in the adult rat were available for both oral gavage and intravenous (IV) dosing routes (Kemper, 2003; Kudo et al., 2007) for male and female Sprague-Dawley and Wistar rats. Datasets reporting PFOA concentrations in serum, urine, feces, and liver tissue following single IV and oral administration were used for development and evaluation of the model (Table 1).
Table 1.
Key Pharmacokinetic Studies in the Male and Female Rat
| Dose (mg/kg BW) | Route | Sex | Endpoint | Use in Model Development | |
|---|---|---|---|---|---|
| Kemper, Experiment 1 | 1.0 | Oral | M and F | cumulative dose in urine and feces | calibration |
| Kemper, Experiment 2 | 5.0 | Oral | M and F | cumulative dose in urine and feces | evaluation |
| Kemper, Experiment 3 | 25.0 | Oral | M and F | cumulative dose in urine and feces | evaluation |
| Kemper, Experiment 5 | 0.1 | Oral | M and F | serum concentration | evaluation |
| Kemper, Experiment 6a | 1.0 | Oral | M and F | serum concentration | calibration |
| Kemper, Experiment 6b | 1.0 | IV | M and F | serum concentration | calibration (M), evaluation (F) |
| Kemper, Experiment 7 | 5.0 | Oral | M and F | serum concentration | evaluation |
| Kemper, Experiment 8 | 25.0 | Oral | M and F | serum concentration | evaluation |
| Kudo, Experiment 1 | 0.041 | IV | M | serum and liver concentration | evaluation |
| Kudo, Experiment 2 | 16.56 | IV | M | serum and liver concentration | evaluation |
References: Kemper, R.A, 2003 and Kudo, N., et al., 2002.
For IV dosing, two data sets were available. In the first, four adult male and four adult female Sprague-Dawley rats were administered 1.0 mg/kg body weight [carbonyl-14C] ammonium perfluorooctanoate (14C-PFOA) via a surgically implanted jugular vein cannula (Kemper, 2003). In male rats, whole blood samples were collected from the cannula or from the tail vein pre-dose and at 0.25, 0.5, 1, 2, 4, 8, 12, 16, and 24 hours post-dose, at 24-hour intervals through 192 hours, and then at 48-hour intervals from 192 through 528 hours. In female rats, whole blood samples were collected from the cannula or the tail vein pre-dose and at 0.25, 0.5, 1, 2, 4, 8, 12, 16, 24, 36, 48, and 72 hours post-dose. Serum samples were analyzed for 14C-PFOA by liquid scintillation counting (LSC).
In the second IV dosing study, nine-week old male Wistar rats (four animals per dose group) were administered an IV dose of either 0.041 or 16.56 mg/kg body weight [1-14C] PFOA (Kudo et al., 2007). Whole blood samples were collected from the vena cava two hours after injection, after which animals were euthanized and tissue samples, including liver, kidney, intestine, testis, spleen, fat, heart, lung, brain, and stomach were collected. Serum and tissue samples were analyzed for 14C-PFOA by LSC.
One extensive dataset was available for oral dosing of PFOA and included measurement of PFOA in urine, feces and serum (Kemper, 2003). Four male and four female Sprague-Dawley rats per dose group were administered 14C-PFOA in a water vehicle via oral gavage at doses of 0.1, 1.0, 5.0 or 25.0 mg/kg body weight. For male rats, whole blood samples were collected from a surgically implanted jugular vein cannula or from the tail vein pre-dose and at 0.25, 0.5, 1, 2, 4, 8, 12, 16, and 24 hours post-dose, at 24-hour intervals through 192 hours, and then at 48-hour intervals from 192 through 528 hours. In female rats, whole blood samples were collected from the cannula or tail vein pre-dose at 0.25, 0.5, 1, 2, 4, 8, 12, 16, 24, 36, 48, 72, and 96 hours post-dose.
In a separate set of experiments (Kemper, 2003), four adult male and four adult female Sprague-Dawley rats per dose group were administered 14C-PFOA in a water vehicle via oral gavage at doses of 1.0, 5.0, or 25.0 mg/kg body weight. Following dosing, rats were housed individually in glass metabolism cages that allowed for collection of urine, feces, expired air, and volatile organics. For male rats, urine and feces were collected at 4, 8, 12, and 24 hours post-dose, and at 24-hour intervals through 336 hours. For female rats, urine and feces were collected at 4, 8, 12, and 24 hours post-dose, and at 24-hour intervals through 168 hours. Serum, urine, and feces samples were analyzed for 14C-PFOA by LSC.
Rat Model Development
A biologically-based compartmental model for PFOA in monkeys (Andersen et al., 2006) and a PBPK model for PFOA in rats (Loccisano et al., 2012) were used as a starting point to expand upon descriptions of transporter-mediated renal reabsorption. The model described here builds on this prior work using recently published data for in vitro to in vivo extrapolation in order to include physiologically-based descriptions of the basolateral and apical transporters associated with renal excretion and renal reabsorption.
PBPK Model Structure for PFOA
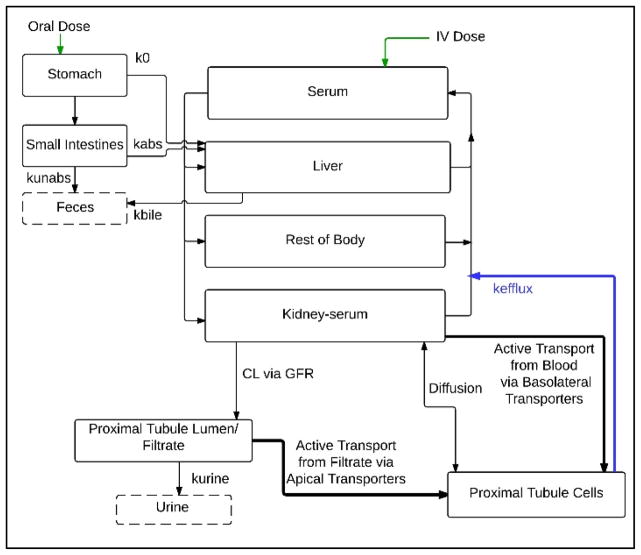
The model contains compartments for plasma, liver, stomach, small intestines, kidney serum, kidney proximal tubule cells, kidney filtrate, and a lumped compartment representing the rest of the body tissues (Figure 1). In contrast to previous models, a three-compartment kidney was used to describe renal excretion and reabsorption. PFOA is moved from the kidney blood into the filtrate in the lumen of the proximal tubule via glomerular filtration. PFOA in the filtrate is excreted in the urine via first-order rate constant kurine, or actively translocated into the proximal tubule cells via apical transporters. This non-linear process was described using Michaelis-Menten parameters, Vmax_apical and Km_apical. PFOA in the kidney blood is actively translocated into the proximal tubule cells by basolateral transporters, again described using Michaelis-Menten parameters, Vmax_baso and Km_baso, and diffusion into and out of the proximal tubule cells via a first-order passive diffusion rate constant, kdif. Studies of cellular uptake of PFOA by OATs and their role in mediating renal reabsorption have suggested that there may be an efflux pathway that pumps intracellular PFOA from the proximal tubule cells back into systemic circulation (Yang et al., 2010). The model described this movement via a first-order rate constant, kefflux.
Figure 1. Structure of PBPK model for PFOA in the adult rat.
Chemical is introduced via oral gavage into the stomach or intravenous injection into the plasma.
PFOA oral bolus gavage was described using a stomach compartment. Absorption of PFOA in the GI tract was described in the stomach and small intestines. PFOA absorbed in the GI tract is carried to the liver in the serum. PFOA administered intravenously enters directly into systemic circulation. PFOA is excreted from the system in the bile, urine, and feces.
Model code was written and simulations were performed using AcslX modeling software (AEgis Technologies, Huntsville, AL, version 3.0.2.1). Model code is available in the supplementary materials.
Model Parameterization
Physiological Parameters
Physiological parameters for the rat are shown in Table 2. Body weights reported in each study were used for simulations. For experiments with more than one animal per dose group, average body weight was used. Fractional tissue volumes, cardiac output, fractional plasma flow, and glomerular filtration rates were obtained from the literature and scaled to body weight (Table 2). PFOA does not partition into the red blood cells. This was accounted for in the model by adjusting blood flow rates to plasma flow rates by multiplying blood flow by 1 – hematocrit.
Table 2.
Physiological parameters for PBPK model for PFOA in adult rats.
| Parameter | Definition | Units | Value | Source |
|---|---|---|---|---|
| QCC | cardiac output | L/h/kg0.75 | 14.0 | Brown et al., 1997 |
| QLC | fraction blood flow to liver | Unitless | 0.183 | Brown et al., 1997 |
| QKC | fraction blood flow to kidney | Unitless | 0.141 | Brown et al., 1997 |
| Htc | Hematocrit | Unitless | 0.46 |
Davies and Morris, 1993 Brown et al., 1997 |
| VplasC | fraction volume of plasma | L/kg BW | 0.0312 |
Davies and Morris, 1993 Brown et al., 1997 |
| VLC | fraction volume of liver | L/kg BW | 0.035 | Brown et al., 1997 |
| VKC | fraction volume of kidney | L/kg BW | 0.0084 | Brown et al., 1997 |
| VfilC | fraction volume of filtrate | L/kg BW | 8.40 × 10−4 | 10% kidney volume |
| VPTCC | fraction volume of proximal tubule cells (PTC) | L/g kidney | 1.35 × 10−4 | calculated based on 60 million PTC/gram kidney (Hsu et al., 2014), 1 PTC = 2250 um3 (Milo et al., 2010) |
| Protein | amount of protein in proximal tubule cells | mg protein/proximal tubule cell | 2.0 ×10−6 | Addis et al., 1936 |
| GFRC | glomerular filtration rate (male) | L/hr/kg kidney | 62.1 | Corley et al., 2005 |
| glomerular filtration rate (female) | L/hr/kg kidney | 41.04 | Corley et al., 2005 |
Chemical Specific Parameters
Chemical specific parameters used in the model are shown in Table 3 and a few are explained in detail in the following sections.
Table 3.
Chemical specific parameters for adult rat PFOA PBPK model.
| Parameter | Definition | Units | Value | Source |
|---|---|---|---|---|
| Free | free fraction of PFOA in plasma | unitless | 0.09 | Fit to Kemper, Experiment 6b and 6b |
| Vmax_basoC | Vmax of basolateral transporters measured in in vitro studies (average of Oat1 and Oat3) | mg/h/kg BW0.75 | 0.04 | Calculated from Nakagawa et al., 2007 |
| Km_baso | Km of basolateral transporters (Oat1 and Oat3) | ug/mL | 27.20 | Nakagawa et al., 2007 |
| Vmax_apicalC | Vmax of apical transporters measured in in vitro studies (Oatp1a1) | mg/h/kg BW0.75 | 0.947 | Calculated from Nakagawa et al., 2007 |
| Km_apical | Km of apical transporters (Oatp1a1) | ug/mL | 52.3 | Weaver et al., 2010 |
| RAFapi | relative activity factor of apical transporters (male) | unitless | 35.0 | Fit to Kemper, Experiment 6b |
| relative activity factor of apical transporters (female) | unitless | 0.001356 | Fit to Kemper, Experiment 6a | |
| RAFbaso | relative activity factor of basolateral transporters (male) | unitless | 4.07 | Fit to Kemper, Experiment 6b |
| relative activity factor of basolateral transporters (female) | unitless | 0.01356 | Fit to Kemper, Experiment 6a | |
| PL | liver:blood partition coefficient | 2.2 | Kudo et al., 2007 | |
| PK | kidney:blood partition coefficient | 1.05 | Kudo et al., 2007 | |
| PR | Rest of body:blood partition coefficient | 0.11 | Kudo et al., 2007 | |
| k0c | rate of absorption of PFOA in stomach | /hr/kg0.25 | 1.0 | Fit to Kemper, Experiment 1 and Experiment 6a |
| kabsc | rate of absorption of PFOA in small intestines | /hr/kg0.25 | 2.12 | Fit to Kemper, Experiment 1 and Experiment 6a |
| kunabsc | rate of unabsorbed dose to appear in feces | /hr/kg0.25 | 7.06 × 10−5 | Fit to Kemper, Experiment 1 and Experiment 6a |
| keffluxc | rate of clearance of PFOA from proximal tubule cells into blood | /hr/kg0.25 | 2.49 | Fit to Kemper, Experiment 6a and 6b |
| kbilec | biliary elimination rate | /hr/kg0.25 | 0.004 | Fit to Kemper, Experiment 1 and Experiment 6a |
| kurinec | urinary elimination rate | /hr/kg0.25 | 1.6 | Fit to Kemper, Experiment 6a and 6b |
Free Fraction
More than 90% of PFOA is bound to the serum albumin in rat blood (Han et al., 2003). Only the free fraction of PFOA is available for distribution. This was accounted for in the model using a free fraction constant (Free) that was multiplied by the concentration of PFOA moving into and out of each compartment such that only the unbound PFOA was able to partition into the compartment, be moved from one compartment to another via transporters, or be excreted from the body in the urine and feces. This parameter was fit to experimental data (see Model Calibration). The available kinetic data for PFOA in rats all reported total PFOA concentrations in the serum. In order to compare model predicted serum concentrations to the serum concentrations measured in these studies, free PFOA was translated back into total PFOA to generate predicted serum concentrations curves.
Uptake and Elimination
A two-compartment GI tract was used to describe uptake of PFOA administered via oral gavage. Uptake in the stomach was described using a first-order rate constant, k0c. Uptake in the small intestine was described using a first-order rate constant, kabsc. Elimination was described via the urine, feces, and bile. A first-order urinary elimination rate, kurinec, was used to describe the excretion of PFOA from the filtrate compartment via the urine. The amount of unabsorbed dose to appear in the feces was described using a first-order rate constant, kunabsc. Experimental evidence suggests that PFOA is susceptible to biliary excretion (Kudo et al., 2007), however, both males and females are thought to excrete <1% of the administered dose via this route (Nakagawa et al., 2007). A first-order biliary excretion rate, kbilec, was used to account for PFOA excreted into the feces via the bile. First order rate constant, keffluxc, was used to account for the efflux pathway that pumps intracellular PFOA from the proximal tubule cells back into systemic circulation. Kbilec, k0c, kabsc, kunabsc, keffluxc, and kurinec were fit to experiment data (see Model Calibration) and scaled to body weight (BW0.25).
Tissue Partitioning
Tissue: plasma partition coefficients for PFOA in the kidney (PK), liver (PL), and rest of body (PR) were estimated from plasma and tissue data collected by Kudo et al. (2007). Male Wistar rats were administered a single IV dose of either 0.041 mg/kg body weight or 16.56 mg/kg body weight [1-14C] PFOA and serum and tissue samples were collected two hours post-dose. Concentrations measured in the serum, kidney, liver, and remaining body tissues following the low dose administration were used to calculate partition coefficients for the model. Partition coefficients used in this model are consistent with those used in previous modeling efforts (Loccisano et al., 2012).
Transport in the kidney compartment
In vitro to in vivo extrapolation was used to derive parameters describing transport of PFOA in the kidney compartment. Michaelis-Menten parameters describing Oat1 and Oat3 were calculated from data reported in in vitro studies that measured [14C] PFOA uptake by rat OATs expressed in human embryonic kidney (HEK293) cells (Nakagawa et al., 2007). Measurements of Vmax for Oat1 and Oat3 uptake of [14C] PFOA were averaged (Vmax_baso_invitro = 393.45 pmol/mg protein/min) and translated to in vivo values (Vmax_baso) by multiplying with a relative activity factor (RAFbaso) and an estimated mass of proximal tubule cells (protein) based on an estimated 60 million proximal tubule cells per gram kidney (Hsu et al., 2014). Relative activity factor information for the basolateral membrane transporters was not available in the literature, so this parameter (RAFbaso) was fit to experimental data (as described in the Model Calibration section of the text). The km value (Km_baso) was calculated by averaging the Km values reported for Oat1 and Oat3 uptake of [14C] PFOA (Yamada et al., 2007) and used directly in the model.
Michaelis-Menten parameters for Oatp1a1 were calculated from values measured in in vitro studies (Weaver et al., 2010) that explored the role of rat OATs in transporting perfluorinated carboxylates of different chain lengths. Measurement of Vmax for Oatp1a1 uptake (Vmax_apical_invitro = 9,300 pmol/mg protein/min) of PFOA was translated to in vivo values (Vmax_apical) using a relative activity factor (RAFapi) and an estimated mass of proximal tubule cells (protein) based on an estimated 60 million proximal tubule cells per gram kidney (Hsu et al., 2014). The relative activity factor for the apical membrane transporter was not available in the literature, so this parameter (RAFapi) was fit to experimental data (as described in the Model Calibration section of the text). Km values reported for Oatp1a1 uptake of PFOA (Weaver et al., 2010) were used directly in the model (Km_apical).
Model Calibration
Time course PFOA serum and urine data resulting from experiments (Kemper Experiment 1 and Kemper Experiment 6a) in which adult male and female rats were administered a single oral gavage dose were used for calibration of the model and to fit parameter values for which data was not available (Table 1). Experiments (Kemper Experiment 6b) in which adult male rats were administered a single bolus IV dose of 1.0 mg/kg body weight (Kemper, 2003) were also used for calibration of the model and to fit parameter values for which data was not available (Table 1).
Parameter values that describe renal excretion and reabsorption (keffluxc, kurinec, RAFapi, and RAFbaso) and the extent of PFOA binding in the serum (Free) were optimized first using experimental data from the 14C-PFOA administration studies (Kemper, 2003; Kudo et al., 2007). To determine initial conditions, kurinec and Free were set to the values reported for the male rat in the Loccisano model (Loccisano et al., 2012), RAFapi and RAFbaso were set to the relative activity factor reported in the literature for human orthologues OAT1 and OAT3 (Yamada et al., 2007), and keffluxc was estimated based on the expectation that PFOA would be moved readily from the proximal tubule cells into systemic circulation. Parameter values were refined simultaneously to achieve a consistent description of the serum concentrations reported in Experiments 6a and 6b of the Kemper study. Initial and final calibrated values are shown in table 4.
Table 4.
Initial and Calibrated Parameter Values
| Parameter | Units | Initial Value | Source of Initial Value | Calibrated Value |
|---|---|---|---|---|
| Free | unitless | 0.006 | Loccisano, 2012 | 0.09 |
| RAFapi, male | unitless | 0.01356 | Yamada, 2007 | 35 |
| RAFapi, female | unitless | 0.01356 | Yamada, 2007 | 0.001356 |
| RAFbaso, male | unitless | 0.01356 | Yamada, 2007 | 4.07 |
| RAFbaso, female | unitless | 0.01356 | Yamada, 2007 | 0.01356 |
| k0c | /hr/kg0.25 | 1.0 | - | 1.0 |
| Kabsc | /hr/kg0.25 | 31.3 | Loccisano, 2012 | 2.12 |
| kunabsc | /hr/kg0.25 | 0.001 | Loccisano, 2012 | 7.06 × 10−5 |
| keffluxc | /hr/kg0.25 | 0 | - | 2.49 |
| Kbilec | /hr/kg0.25 | 0.35 | Loccisano, 2012 | 0.004 |
| Kurinec | /hr/kg0.25 | 0.1 | Loccisano, 2012 | 1.6 |
Free, Keffluxc and kurinec were treated as non sex-specific and a single value was calibrated for the male and female rat models. RAFapi and RAFbaso were calibrated separately for the male and female rat models based on reports that these transporters are expressed at significantly higher levels in the male rat than in the female rat (Yang et al., 2009; Weaver et al., 2010).
Subsequently, parameter values that govern absorption of PFOA in the gastrointestinal tract (k0c, kabsc, kunabsc, and kbilec) were simultaneously optimized using experimental serum and urine data from oral administration studies (Kemper, 2003). Kabsc, kunabsc, and kbilec were initially set to those values reported in the Loccisano model (Loccisano et al., 2012). The initial value for k0c was estimated based on the expectation that more PFOA is absorbed in the small intestine than in the stomach. These parameter values were refined with the urine data reported in Experiment 1 of the Kemper study and with serum data reported in Experiment 6a of the Kemper study. These parameter values were treated as sex non-specific. Initial and final calibrated values are shown in table 4.
Model Evaluation
To evaluate the performance of the calibrated model, we simulated concentrations of total PFOA in serum, urine, and feces in the adult male and female rat, and liver tissue in the adult male rat resulting from IV and oral administration of PFOA using datasets not used in model calibration. Descriptions of the experimental data used for model evaluation are presented in Table 1.
To determine the ability of the model to describe the pharmacokinetic behavior of PFOA in the adult rat, model simulations were evaluated for their ability to predict accurately peak concentrations, time-to-peak, as well as the shape of the concentration curves. The correspondence of individual data points to predicted values was evaluated visually to ensure that the model accurately predicted serum concentrations and the cumulative percentage of PFOA in the urine and feces over time. Predicted peak serum concentrations and the cumulative amount in the urine, and feces were compared to measurements in each study. The shape of the predicted serum, urine, and feces curves were examined in order to ensure that the predicted curves were consistent with the trends observed in the data. Given that feces is a very small contributor to PFOA excretion, less priority was given to feces predictions when evaluating the model.
In addition to qualitative model performance evaluation, model performance was evaluated quantitatively by calculating root mean squared errors (RMSE) for simulations of each experimental dataset with more than one experimental data point using the following equation,
where predicted is the predicted value, observed is the observed value, and n is the number of observed data points in the experimental dataset. Because RMSE is a relative parameter that exhibits dose effects, comparisons of RMSE values were made only for experiments conducted at the same dose.
Sensitivity Analysis
A time course sensitivity analysis was performed in order to determine the impact of each individual parameter on the outputs of the male and female rat models. Sensitivity coefficients were determined for the serum concentration resulting from a 1% change in the value of each parameter using the forward difference method. Sensitivity coefficients were normalized to the response variable and the parameter using the following equation,
where A is the serum concentration resulting from a 1% increase in the parameter value, B is the serum concentration resulting from the initial parameter value, C is the value of parameter increased by 1%, and D is the initial parameter value. Serum concentration predictions were run at simulations of single oral gavage administration of 1.0 and 25.0 mg/kg body weight in the male and female rat. Positive sensitivity coefficients indicate a direct association between the model output and the corresponding parameter. Negative sensitivity coefficients indicate an inverse correlation between the model output and the corresponding parameter. Parameters with absolute sensitivity coefficients greater than 0.1 using serum concentrations from either of the simulated dose levels were identified as sensitive.
Exploration of the Impact of Dose on Renal Clearance
To evaluate the impact of PFOA dose on renal clearance and determine saturating doses of PFOA on the kidney transporters, simulations over a wide range of oral doses (0.1 – 1,000 mg/kg body weight) were conducted for male and female rats. Time course data from each simulation was evaluated in order to determine the estimated half-life at each dose. The time at which the maximum serum concentration occurred, and the time at which one-half the maximum serum concentration was achieved after the maximum occurred were identified. The time required to reach the maximum concentration was subtracted from the time at which one half the maximum concentration was achieved in order to estimate the time the model predicted it would take for the peak serum concentration to be reduced by half. To evaluate the importance of the protein transporters in the kidney, simulations were repeated over the wide dose range of PFOA with the transporters effectively turned off (Vmax_baso_invitro = 0, Vmax_apical_invitro = 0).
Results
Model Calibration
IV Exposures
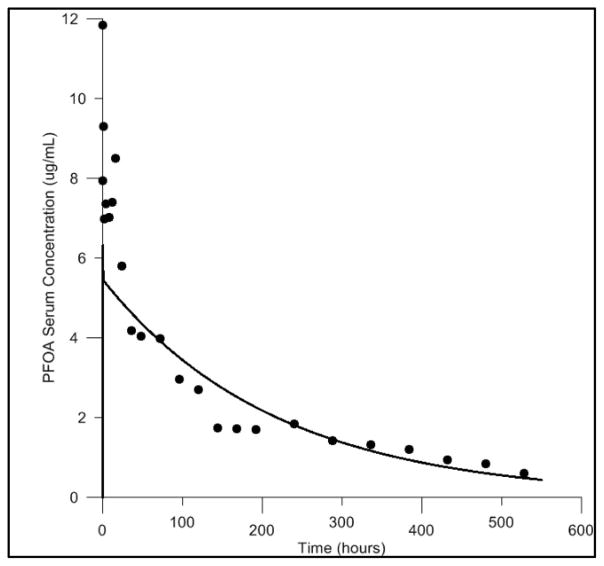
Model calibration with IV exposure data was conducted in the male rat only. This was due to a lack of experimental IV exposure data in the female rat. Comparisons of the predicted and measured serum concentrations resulting from exposure to 1.0 mg/kg body weight IV exposure in the male rat can be seen in Figure 2. A moderate underestimation of the peak serum concentration was observed in the simulations. However, at later time points the predicted serum concentrations and the overall shape of the concentration curve show good agreement with the experimental data.
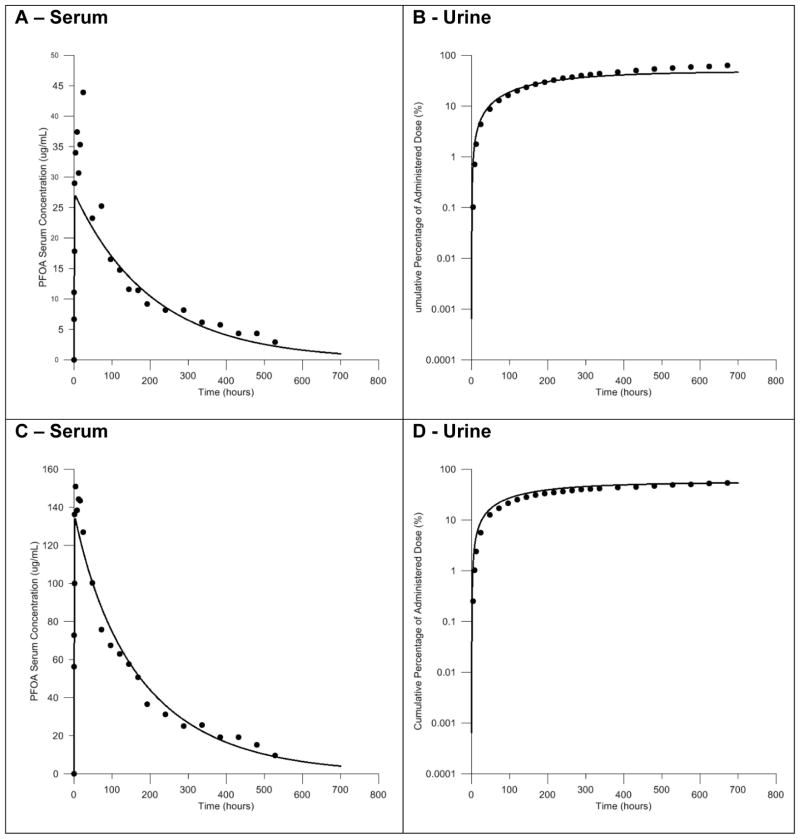
Figure 2. Model calibration of the male rat model, with IV exposure data.
Comparisons of the predicted and measured serum concentrations resulting from IV exposure to 1.0 mg/kg body weight PFOA. Predictions shown as solid line, measured data shown as black circles.
Oral Gavage Exposures
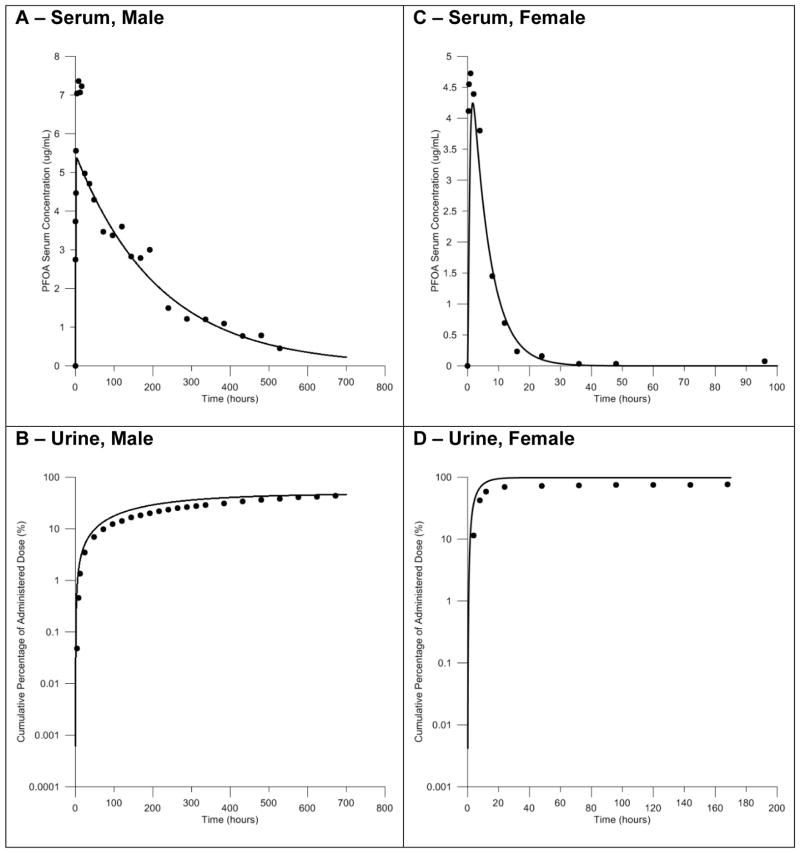
Comparisons of the simulated serum concentrations to measured serum concentrations following administration of male and female rats to a single oral dose of 1.0 mg/kg body weight showed good agreement with measured serum concentrations following calibration of model parameters. These comparisons are presented in Figure 3. A slight underestimation of the peak serum concentration was observed in simulations with the male rat model (Fig 3A). However, the overall shape of the predicted concentration curve closely matched the experimental data. Simulations of PFOA serum concentration with the female rat model were also in good agreement with the experimental data (Fig. 3C). Comparisons of the predicted and experimentally measured cumulative amount of PFOA in the urine following an oral dose of 1.0 mg/kg body weight can also be seen in Figure 3. The cumulative amount of PFOA in the urine predicted by the model accurately described the experimental data for this metric, both at individual time points and the overall shape of the curve, in the male and female rat model (Fig. 3B, 3D).
Figure 3. Model calibration of the male and female rat models with oral gavage exposure data.
Comparisons of the predicted and measured serum concentrations and the cumulative percent of the dose in the urine resulting from oral exposure to 1.0 mg/kg body weight PFOA. Simulations in the male rat model shown in panels A and B. Simulations in the female rat model shown in panels C and D. Predictions shown as solid lines, measured data shown as black circles.
Model Evaluation
Male Rat
In order to evaluate its predictive ability, the model was used to simulate time-course data for IV and oral exposures in the male rat.
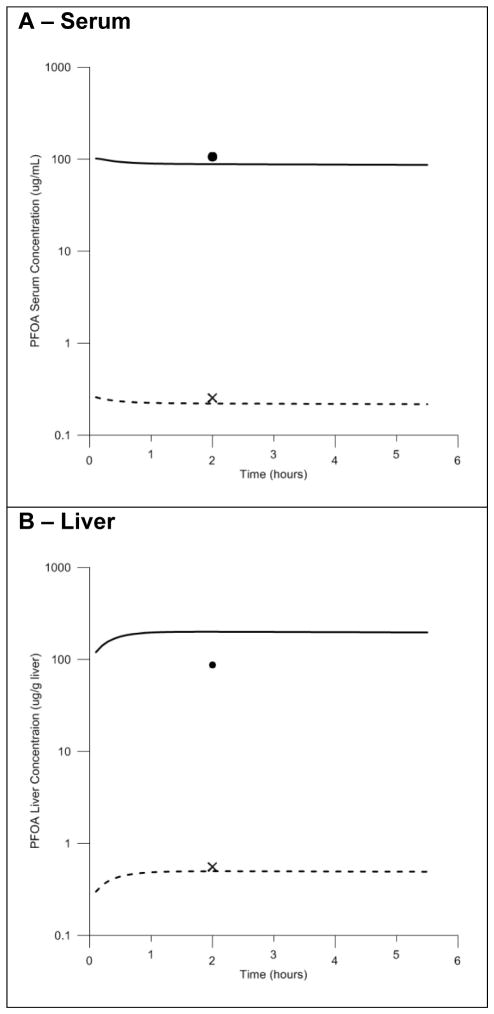
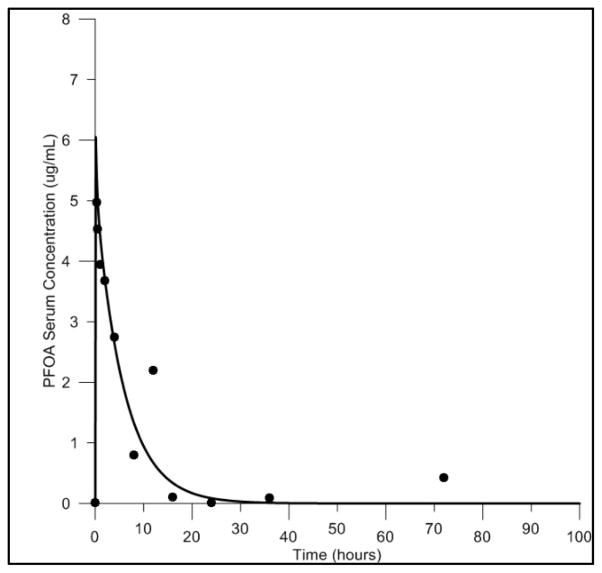
In the experiments reported by Kudo and colleagues male rats were administered a high (16.0 mg/kg body weight) or low (0.041 mg/kg body weight) bolus dose of PFOA via IV injection. Model simulations of serum and liver tissue concentrations were compared to the measured serum and liver concentrations resulting from each dose in the study (Figure 4). While data for only one time point is available for these experiments, good agreement is observed between predicted and measured serum concentrations at both high and low doses (Fig. 4A). The model slightly under predicted liver concentrations at the low dose, and slightly over predicted liver concentrations at the high dose (Fig. 4B). However, these discrepancies were within two-fold of the data. The authors of the experimental study (Kudo et al., 2007) reported that a larger portion of the administered PFOA was distributed to the serum, liver, and other tissues at the high dose compared to the low dose. The model successfully described this dose dependency.
Figure 4. Model evaluation with time course data resulting from IV exposure in the male rat.
Comparisons of the predicted and measured serum (4A) and liver (4B) concentrations resulting from high (16.0 mg/kg body weight) and low (0.041 mg/kg body weight) dose IV exposures. High exposure dose data shown as solid line (predicted) and black circle (measured). Low exposure dose data shown as dashed line (predicted) and black cross (measured).
Experimental time-course serum, urine, and feces data following oral administration of 0.1, 5.0, and 25.0 mg/kg body weight (Kemper, 2003) were also available for model evaluation (Figure 5). Model simulations of serum concentration and the cumulative percent of the dose in the urine and feces for 5.0 and 25.0 mg/kg body weight doses were compared to the data reported in the study. Simulated serum concentrations showed good agreement with measured serum concentrations (Fig. 5A, 5C). A slight underestimation of the peak was observed at the 5.0 mg/kg body weight dose level, however, the overall shape of the predicted concentration curve closely match the experimental data at all doses. The cumulative amount of PFOA in the urine predicted by the model accurately described the experimental data for this metric, both at individual time points and the overall shape of the curve (Fig. 5B, 5D). The cumulative amount of PFOA in the feces predicted was over predicted by the model (data not shown).
Figure 5. Model evaluation with time course data resulting from oral gavage exposure in the male rat.
Comparisons of the predicted and measured serum concentrations and the cumulative percent of the dose in the urine resulting from oral exposure to 5.0 (panels A and B) or 25.0 (panels C and D) mg/kg body weight PFOA. Predictions shown as solid lines, measured data shown as black circles.
Female Rat
The model was used to simulate time-course data for IV and oral exposures in the female rat.
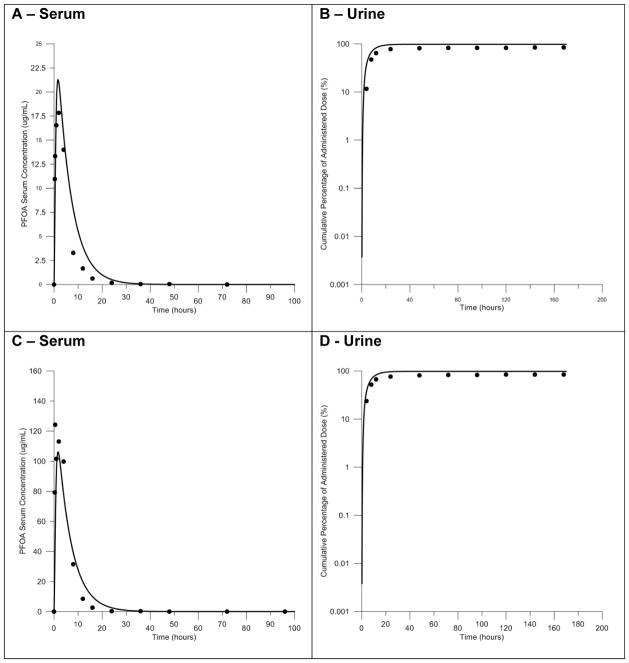
In the available IV study (Kemper, 2003) female rats were administered a single bolus dose of 1.0 mg/kg body weight and serial serum samples were collected for 72 hours following the dose. Model simulations of serum concentrations were compared to concentrations measured in the study (Figure 6). Simulations of serum concentrations were in good agreement with experimental data. Clearance of PFOA from the serum is much faster in female rats than male rats – this is seen in the experiments conducted by Kemper and elsewhere in the literature (Vanden Heuvel et al., 1991; Andersen et al., 2006; Tatum-Gibbs et al., 2011). The model was able to accurately describe this sex-specific clearance behavior following IV administration of PFOA.
Figure 6. Model evaluation with time course data resulting from IV exposure in the female rat.
Comparisons of the predicted and measured serum concentrations resulting from IV exposure to 1.0 mg/kg body weight PFOA. Predictions shown as solid line, measured data shown as black circles.
Experimental time-course serum, urine, and feces data following oral administration of 0.1, 5.0, and 25.0 mg/kg body weight (Kemper, 2003) were available for evaluation of the female rat model (Figure 7). Model simulations of serum concentration and the cumulative percent of the dose in the urine and feces for 5.0 and 25.0 mg/kg body weight doses were compared to the data reported in the study. Simulated serum concentrations were in good agreement with experimental data with respect to the peak concentrations and the overall shape of the serum concentration curve (Fig. 7A, 7C). As with the simulation of the IV data, the model was able to describe successfully the sex-specific clearance of PFOA following oral gavage exposures at all doses. Comparison of the predicted cumulative amount of PFOA in the urine to measured data also showed good agreement (Fig. 7B, 7D). The model successfully described the measured amounts in the urine and the overall kinetics of urinary clearance as indicated by the shape of the curve. The cumulative amount of PFOA in the feces was under predicted by the model in the lower dose tested (data not shown).
Figure 7. Model evaluation with time course data resulting from oral gavage exposure in the female rat.
Comparisons of the predicted and measured serum concentrations and the cumulative percent of the dose in the urine resulting from oral exposure to 5.0 (panels A and B) or 25.0 (panels C and D) mg/kg body weight PFOA. Predictions shown as solid lines, measured data shown as black circles.
Quantitative Model Evaluation
RMSE values were calculated for simulations of each experimental dataset with more than one experimental data point. Results are available in table 5. Comparison of RMSE values for simulation of experimental time-course serum data for male and female experiments conducted at the same dose were similar. This suggests that the model predicted experimental serum data in the male rat equally well as experimental data in the female rat. Comparison of RMSE values for simulation of the measured cumulative percent of the dose to appear in the urine for experiments conducted at the same dose were lower in simulation for the male rat than the female rat, suggesting that the model predictions more closely fit time-course urine data for the male than the female. Conversely, comparisons of the cumulative percent of the dose to appear in the feces for experiments conducted at the same dose suggest that the model more accurately predicted feces data for the female rat.
Table 5.
Root Mean Squared Errors for Simulations of Male and Female Rat Experiments
| Experiment | Dose (mg/kg) | Male | Female |
|---|---|---|---|
| Kemper Experiment 1 - Urine | 1.0 | 6.36 | 20.86 |
| Kemper Experiment 2 - Urine | 5.0 | 6.75 | 16.53 |
| Kemper Experiment 3 - Urine | 25.0 | 4.48 | 15.99 |
| Kemper Experiment 1 - Feces | 1.0 | 22.75 | 0.52 |
| Kemper Experiment 2 - Feces | 5.0 | 31.81 | 3.80 |
| Kemper Experiment 3 - Feces | 25.0 | 22.54 | 0.61 |
| Kemper Experiment 5 - Serum | 0.1 | 0.06 | 0.17 |
| Kemper Experiment 6 - Serum, oral | 1.0 | 0.89 | 0.78 |
| Kemper Experiment 6 - Serum, IV | 1.0 | 1.47 | 0.75 |
| Kemper Experiment 7 - Serum | 5.0 | 5.69 | 2.98 |
| Kemper Experiment 8 - Serum | 25.0 | 9.00 | 18.88 |
Impact of Dose on Renal Clearance
Male Rat
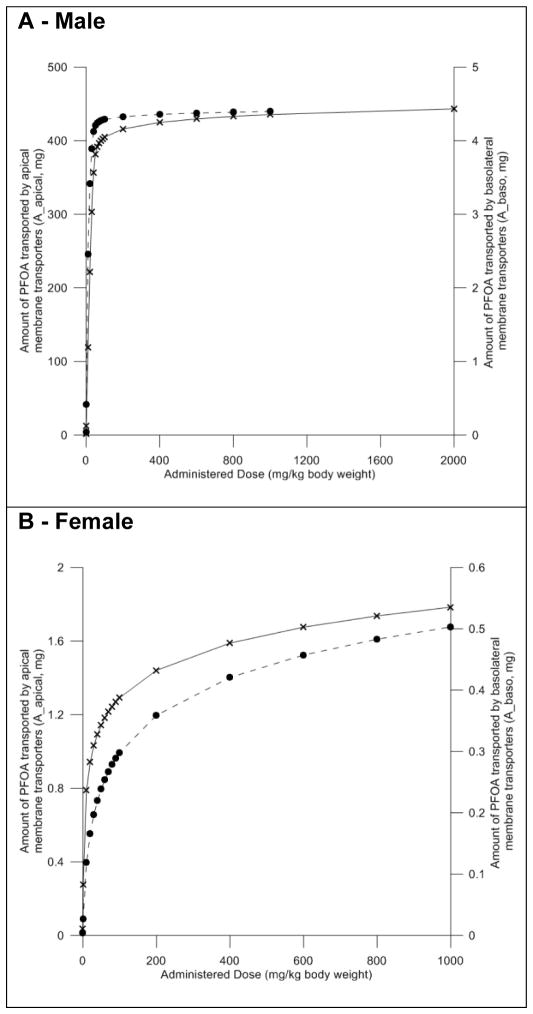
Our model predictions indicated that the amount of PFOA transported from the blood into the filtrate by the basolateral membrane transporters is linear in a dose range from 0.1 to 40 mg/kg body weight (Figure 8A). Similarly, the predicted amount of PFOA transported from the filtrate back into the proximal tubules also appears to be linear in a dose range from 0.1 to 40 mg/kg body weight. At doses higher than 40 mg/kg body weight the amount of PFOA transported by both basolateral and apical membrane transporter gradually decreases, suggesting saturation of these mechanisms.
Figure 8. Evaluation of the impact of dose on transporter activity in the male and female rat models.
Model predicted mass of PFOA transported by basolateral (dashed line – right Y-axis) and apical (solid line – left y-axis) membrane transporters. Black circles and crosses represent actual data points. Male data shown in panel A, female data shown in panel B.
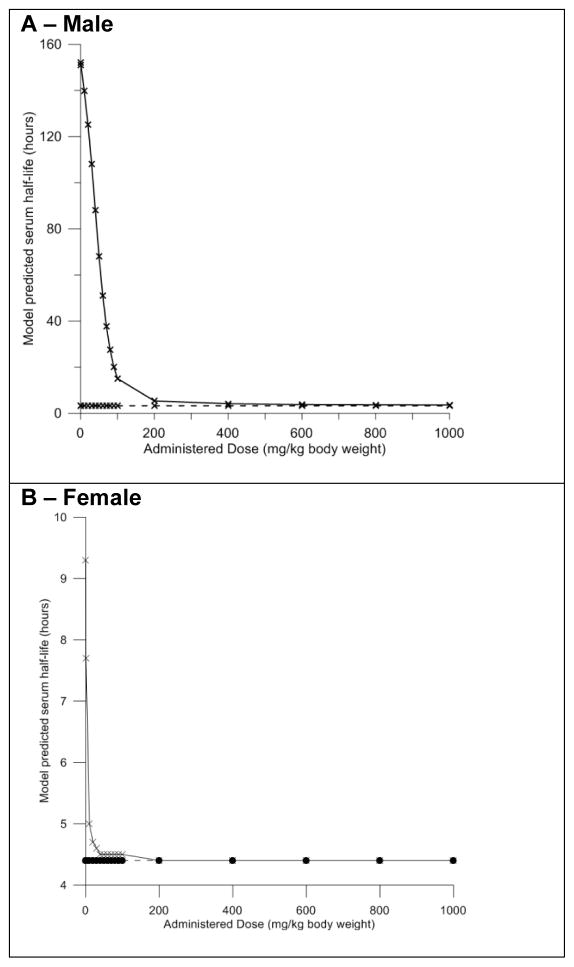
Measurements of serum half-life for PFOA in male rats are reported to range from 96 – 216 hours (Lau et al., 2004; Harada et al., 2005; Kudo et al., 2007). Our model predictions are in agreement with these estimates and with reports in the literature that suggest that serum clearance of PFOA may increase (causing half-life to decrease) as dose increases and transporters become fully saturated (Cui et al., 2010). In an exposure dose-range from 0.1 to 40 mg/kg body weight, our model predicts that half-life decreases linearly from 152 to 88 hours (Figure 9A).
Figure 9. Evaluation of the impact of dose on serum half-life in the male and female rat models.
Model predicted serum half-life. Solid line – transporters turned on, dashed line – transporters turned off. Black circles and crosses represent actual data points. Male data shown in panel A, female data shown in panel B.
When Vmax parameters for the transporters (Vmax_baso and Vmax_apical) expressed in the proximal tubule cells are set to zero, effectively turning them off, the predicted serum half-life is 3.3 hours at all doses (Figure 9A). Simulations of exposures to very high doses (greater than 200 mg/kg body weight) with transporters turned on predicted similar serum half-lives, again suggesting that transporters can be saturated at high doses.
Female Rat
Simulations with the female rat model indicated that the amount of PFOA transported from the blood into the filtrate by the basolateral membrane transporters is linear in a dose range from 0.1 to 10 mg/kg body weight and became non-linear at a lower dose than in the male rat (Figure 8B). At doses higher than 10 mg/kg body weight the amount of PFOA transported by both basolateral and apical membrane transporter gradually decreases, suggesting saturation of these mechanisms.
Serum half-life for PFOA decreased linearly from 9.3 – 5.0 hours at doses of 0.1 – 10 mg/kg body weight (Figure 9B). These predictions are in agreement with estimates from experimental studies that report the serum half-life for PFOA in the female rat to be 1.92–24 hours (Han et al., 2003; Lau et al., 2004; Harada et al., 2005). When Vmax parameters (Vmax_baso and Vmax_apical) were set to zero to turn off transporter activity, the predicted serum half-life was 4.4 hours at all doses. Similar to what was seen in the male rat, simulations of exposures to high doses (greater than 30 mg/kg body weight) with transporters turned on predicted the same serum half-life (Fig. 9B).
Sensitivity Analysis
A normalized sensitivity analysis was performed for 1.0 and 25.0 mg/kg body weight single oral doses in the male and female rat. Table 6 gives model parameters determined to be sensitive with absolute sensitivity coefficients greater than 0.1 or 1.0 in the time course of PFOA serum concentrations over a period of 530 hours following simulation of PFOA administration at either dose level.
Table 6.
Sensitive Model Parameters.
| Physiological Parameters | Partition Coefficients | Chemical Specific Model Parameters | |
|---|---|---|---|
| Male Rat | BW, GFRC, protein, VfilC, VLC, HTC, QKC, QLC, QCC | PR, PL | kurinec, kbilec, k0c, kabsc, RAFapi, Km_apical, Vmax_apical_invitro, Free, MW |
| Female Rat | BW, GFRC, protein, VPTCC, VfilC, VLC, VplasC, HTC, QKC, QLC, QCC, MKC | PR, PL | kurinec, kbilec, keffluxc, k0c, kabsc, kdif, RAFapi, Km_apical, Vmax_apical_invitro, Free, MW |
Parameters with absolute sensitivity coefficients greater than 1.0 are highlighted in bold.
In general, parameters that were found to be sensitive in the male rat model were also found to be sensitive in the female rat model. However, VPTCC, VplasC, MKC, keffluxC, and kdif were found to be sensitive in the female rat model but not in the male rat model. Additionally, a greater number of parameters in the female rat model appear to impact the PFOA serum concentrations to a large extent (absolute sensitivity coefficients greater than 1.0) than in the male rat model. Of particular note, the female rat model appears to be much more sensitive to GFRC than is the male rat model.
The calculated sensitivity coefficients were not very different across simulations at the two different doses. Parameters that were identified as sensitive (or not sensitive) in the simulation of administration of 1.0 mg/kg body weight were similarly identified in the simulation of administration of 25.0 mg/kg body weight. This analysis provides a hierarchy useful to decide which parameter values must be measured most carefully (Evans et al., 1994). Additional sensitivity analysis data is available in the supplementary materials.
Discussion
The model described here aims to explore the role of kidney transporters on the renal reabsorption and excretion of PFOA in the adult rat. This work builds upon past modeling efforts that have explored similar questions. A biologically-based compartmental model for PFOA administration in Cynomolgus monkeys (Andersen et al., 2006) and a PBPK model for PFOA administration in the adult rat (Loccisano et al., 2012) have demonstrated the importance of saturable renal reabsorption in the pharmacokinetic behavior of PFOA in the rat. The PBPK model developed by Loccisano and colleagues used in vitro derived Km values for Oatp1a1 to begin to describe transporter kinetics; however, sufficient data were not available to support the inclusion of complete physiological descriptions of transporter-mediated PFOA transport. The availability of new in vitro data describing the kinetics of Oatp1a1, Oat1, and Oat3 mediated PFOA uptake has made it possible to use in vitro to in vivo extrapolation to include physiologically-based descriptions of renal excretion and reabsorption. This has allowed for a deeper exploration of the sex-specific serum clearance observed in the adult rat.
In vitro studies are frequently used to evaluate the uptake of compounds in various cellular systems (Giacomini et al., 2010; Feng et al., 2014; Hsu et al., 2014). In vitro protein transporter kinetic data can be scaled to approximate in vivo pharmacokinetic parameters (Bosgra et al., 2014). This in vitro to in vivo extrapolation of transporter data has been shown to be useful for deriving pharmacokinetic parameters for use in PBPK models elsewhere in the literature (Bosgra et al., 2014). The results of our model evaluation indicate that in vitro to in vivo extrapolated descriptions of the apical and basolateral membrane transporters are able to successfully describe and predict time-course PFOA-serum concentrations, liver concentrations, as well as the cumulative percent of the administered dose in the urine in the male and female rat.
The model over predicted the cumulative percentage of PFOA in the feces in some instances and under predicted in others. However, feces is a very small contributor to PFOA excretion, thus, less priority was given to feces predictions when evaluating the model. Johnson et al, previously suggested that hepatic accumulation of PFOA may be related to enterohepatic recirculation based on observations that treatment of rats with cholestyramine increased fecal elimination (Johnson, 1984). However, cholestyramine was later shown to increase bile excretion into the gut, and thereby increase fecal elimination (Trautwein et al., 1999). Given that the observed increase in fecal elimination was likely a result of increased biliary excretion rather than enterohepatic recirculation, enterohepatic recirculation was not considered a significant contributor to PFOA kinetics in the rat and was not included in the model.
In the male rat model, where PFOA is thought to be more readily reabsorbed into the systemic circulation, the amount of PFOA moved from the circulation to the filtrate via GFR is comparable to the amount of PFOA moved from the filtrate back into the proximal tubule cells via the apical transporters. In the female rat model, where the apical membrane transporters are thought to be less active, the amount of PFOA moved into the filtrate via GFR is much larger than the amount of PFOA moved from the circulation into the proximal tubules via the basolateral transporters. This suggests that, in the female rat, without significant renal reabsorption of PFOA, GFR is an important driver of PFOA excretion. This analysis is supported by the sensitivity analysis, which suggests that the female rat model is more sensitive to GFRC than is the male rat model.
The model was able to predict serum half-lives in both the male and female rat that correspond to estimates reported in experimental studies. These findings support the conclusions of previous computational models for PFOA in the rat that saturable renal reabsorption of PFOA is an important driver of serum concentrations in the male and female rat. In addition, our results suggest that in vitro to in vivo extrapolation of the parameters that describe transporter kinetics is a viable method for parameterization of PBPK models. Additional exploration of transporter kinetics in in vitro systems is needed to expand on this work.
Structurally, our male and female rat models are identical. We have shown that by modifying only sex-specific physiological parameters (GFR and BW) and the parameters that describes the activity of the apical and basolateral membrane transporters (RAFapi and RAFbaso), our model was able to successfully describe sex-specific serum clearance in the rat. Further, our model was able to accurately predict the serum half-life in both the male and female rat. These results support the hypothesis that the observed sex differences in serum clearance and half-life are a product of differential expression and activity of the transporters that mediate PFOA excretion and reabsorption – specifically, Oat1, Oat3, and Oatp1a1.
Evaluation of serum half-life following simulation of administration of a wide range of doses reveals that the model is also capable of describing transporter saturation. In both the male and female rat models, comparison of predicted serum half-life at very high doses, when transporters would presumably be saturated are very similar to predicted serum half-lives when model simulations are run with Vmax_baso and Vmax_apical equal to zero. Essentially, our model predicts that the serum half-life when transporters are saturated is the same as when the transporters are turned off. This non-linear pharmacokinetic response has also been observed in the mouse (Lou et al., 2009). Further, our model demonstrates that when the transporters involved in renal excretion and reabsorption are turned off or saturated, serum half-life is no longer sex specific. In addition to the previously mentioned results, these data support the hypothesis that the saturable renal reabsorption and renal clearance in the rat is driven by the expression and activity of the apical and basolateral membrane transporters in the proximal tubule cells. This data also supports the hypothesis that sex-specific pharmacokinetic behavior in the rat is primarily a product of sex-specific OAT expression and activity in the proximal tubule cells.
Historically, PBPK models have not been parameterized to specifically include expansive descriptions of transporter kinetics; however, recent models are beginning to be developed to predict transporter-mediated chemical disposition (Feng et al., 2014). Given the role that transporters play in the ultimate distribution and excretion of chemicals in the body, incorporation of data driven, physiologically-based descriptions of these processes has the potential to improve the utility of PBPK models for sex, species, and life-stage extrapolation, especially for compounds like PFOA.
Ultimately, this work could be improved with in vitro studies more specifically tailored to generate data for PBPK modeling purposes. For example, relative activity factors were only available for the human orthologues of basolateral transporters. Data collected using the same cell type (HEK293 cell) as those used in the kinetic studies of these transporters and in rat kidney slices would have resulted in greater certainty in this parameter value.
Transporter expression and activity in the proximal tubule cells have been shown to be the primary driver of sex-specific and species-specific serum clearance of PFOA (Andersen et al., 2006). Our model successfully incorporates sex-specific descriptions of these transporters. With additional in vitro data describing the kinetics of the transporters that are expressed in human proximal tubule cells, this model can be scaled up to describe transporter-mediated renal secretion and reabsorption of PFOA in humans. A human PBPK model for PFOA with transporter-specific descriptions could be used to evaluate the impact of polymorphic variability of OATs in human populations on PFOA-serum concentrations using Monte Carlo analysis. This rat model and the future planned work to expand upon these efforts have the potential to contribute to the understanding of the effects of exposure to PFOA.
This evidence-based model supports the hypothesis that sex-specific serum half-lives for PFOA are driven by the expression and activity of transporters in the kidney and demonstrates that in vitro to in vivo extrapolation can be used to incorporate transporter-specific descriptions in PBPK models.
Acknowledgments
We would like to thank Dr. Eva McLanahan and Dr. Clement Welsh at the Agency for Toxic Substances and Disease Registry and Dr. Xiaoxia Yang, Dr. Luisa Camacho, and Dr. Fred Beland at the National Center for Toxicological Research for their helpful discussion and comments. Partial financial support was from the University of Georgia’s Graduate School and Interdisciplinary Toxicology Program.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Agency for Toxic Substances and Disease Registry.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Addis T, Poo LJ, Lew W. The quantities of protein lost by the various organs and tissues of the body during a fast. J Biol Chem. 1936;115:111–116. [Google Scholar]
- Andersen ME, Clewell HJ, 3rd, Tan YM, Butenhoff JL, Olsen GW. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys--probing the determinants of long plasma half-lives. Toxicology. 2006;227:156–164. doi: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Anton FM, Garcia Puig J, Ramos T, Gonzalez P, Ordas J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35:343–348. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Perfluoroalkyls. Division of Toxicology and Human Health Sciences; 2009. [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environmental health perspectives. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgra S, van de Steeg E, Vlaming ML, Verhoeckx KC, Huisman MT, Verwei M, Wortelboer HM. Predicting carrier-mediated hepatic disposition of rosuvastatin in man by scaling from individual transfected cell-lines in vitro using absolute transporter protein quantification and PBPK modeling. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2014;65:156–166. doi: 10.1016/j.ejps.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological Parameter Values for Physiologically Based Pharmacokinetic Models. Toxicology and Industrial Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. Gender-specific and developmental influences on the expression of rat organic anion transporters. The Journal of pharmacology and experimental therapeutics. 2002;301:145–151. doi: 10.1124/jpet.301.1.145. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Kennedy GL, Jr, Frame SR, O’Connor JC, York RG. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology. 2004;196:95–116. doi: 10.1016/j.tox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environmental science & technology. 2006;40:2128–2134. doi: 10.1021/es0517973. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environmental health perspectives. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AL, Stratman FW. Sex steroid regulation of urinary excretion of carnitine in rats. J Steroid Biochem. 1982;17:211–216. doi: 10.1016/0022-4731(82)90124-8. [DOI] [PubMed] [Google Scholar]
- Corley RA, Bartels MJ, Carney EW, Weitz KK, Soelberg JJ, Gies RA, Thrall KD. Development of a physiologically based pharmacokinetic model for ethylene glycol and its metabolite, glycolic Acid, in rats and humans. Toxicological sciences : an official journal of the Society of Toxicology. 2005;85:476–490. doi: 10.1093/toxsci/kfi119. [DOI] [PubMed] [Google Scholar]
- Cui L, Liao CY, Zhou QF, Xia TM, Yun ZJ, Jiang GB. Excretion of PFOA and PFOS in male rats during a subchronic exposure. Archives of environmental contamination and toxicology. 2010;58:205–213. doi: 10.1007/s00244-009-9336-5. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Archives of environmental contamination and toxicology. 2009;56:338–349. doi: 10.1007/s00244-008-9194-6. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharmaceutical research. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Evans MV, Crank WD, Yang HM, Simmons JE. Applications of sensitivity analysis to a physiologically based pharmacokinetic model for carbon tetrachloride in rats. Toxicology and applied pharmacology. 1994;128:36–44. doi: 10.1006/taap.1994.1177. [DOI] [PubMed] [Google Scholar]
- Feng B, Varma MV, Costales C, Zhang H, Tremaine L. In vitro and in vivo approaches to characterize transporter-mediated disposition in drug discovery. Expert opinion on drug discovery. 2014;9:873–890. doi: 10.1517/17460441.2014.922540. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nature reviews. Drug discovery. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, Jones PD, Yamashita N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA) Toxicological sciences : an official journal of the Society of Toxicology. 2006;89:93–107. doi: 10.1093/toxsci/kfj011. [DOI] [PubMed] [Google Scholar]
- Han X, Snow T, Kemper R, Jepson G. Binding of PFOA to rat and human plasm proteins. Chemical research in toxicology. 2003;16:775–781. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environmental research. 2005;99:253–261. doi: 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Hsu V, de LTVM, Zhao P, Zhang L, Zheng JH, Nordmark A, Berglund EG, Giacomini KM, Huang SM. Towards quantitation of the effects of renal impairment and probenecid inhibition on kidney uptake and efflux transporters, using physiologically based pharmacokinetic modelling and simulations. Clinical pharmacokinetics. 2014;53:283–293. doi: 10.1007/s40262-013-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Aiba K, Fukuda K, Tanaka M. The induction of peroxisome proliferation in rat liver by perfluorinated fatty acids, metabolically inert derivatives of fatty acids. Journal of biochemistry. 1985;98:475–482. doi: 10.1093/oxfordjournals.jbchem.a135302. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Gibson SJ, Laboratories R. Extent and Route of Excretion and Tissue Distribution of Total Carbon-14 in Rats After Single Intravenous Dose of FC-95-14C. Riker Laboratories, Incorporated; 1979. [Google Scholar]
- Johnson JD, Gibson SJ, Ober EE. Cholestyramine-Enhanced Fecal Elimination of Carbon-14 in Rats after Administration of Ammonium [14C]Perfluorooctanoate or Potassium [14c]Perfluorooctanesulfonate. Fundamental and Applied Toxicology. 1984;4:972–976. doi: 10.1016/0272-0590(84)90235-5. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Kobayashi H, Miura H, Kozuka H. Characterization of hepatic responses of rat to administration of perfluorooctanoic and perfluorodecanoic acids at low levels. Toxicology. 1995;99:169–178. doi: 10.1016/0300-483x(95)03027-d. [DOI] [PubMed] [Google Scholar]
- Kemper RA. Perfluorooctanoic acid: toxicokinetics in the rat. 2003 Project ID: DuPont 7473, pp. [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of PFOA. Chemico-Biological Interactions. 2002;139:301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Kudo N, Sakai A, Mitsumoto A, Hibino Y, Tsuda T, Kawashima Y. Tissue distribution and hepatic subcellular distribution of perfluorooctanoic acid at low dose are different from those at high dose in rats. Biological & pharmaceutical bulletin. 2007;30:1535–1540. doi: 10.1248/bpb.30.1535. [DOI] [PubMed] [Google Scholar]
- Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicology and applied pharmacology. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Lindstrom AB, Strynar MJ. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicological sciences : an official journal of the Society of Toxicology. 2006;90:510–518. doi: 10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- Loccisano AE, Campbell JL, Jr, Butenhoff JL, Andersen ME, Clewell HJ., 3rd Comparison and evaluation of pharmacokinetics of PFOA and PFOS in the adult rat using a physiologically based pharmacokinetic model. Reproductive toxicology. 2012;33:452–467. doi: 10.1016/j.reprotox.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Lou I, Wambaugh JF, Lau C, Hanson RG, Lindstrom AB, Strynar MJ, Zehr RD, Setzer RW, Barton HA. Modeling single and repeated dose pharmacokinetics of PFOA in mice. Toxicological sciences : an official journal of the Society of Toxicology. 2009;107:331–341. doi: 10.1093/toxsci/kfn234. [DOI] [PubMed] [Google Scholar]
- Milo R, Jorgensen P, Moran U, Weber G, Springer M. BioNumbers--the database of key numbers in molecular and cell biology. Nucleic acids research. 2010;38:D750–753. doi: 10.1093/nar/gkp889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Lee HJ, Predko LM. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol Rev. 2003;55:229–240. doi: 10.1124/pr.55.2.1. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Hirata T, Terada T, Promsuk J, Miura D, Harada K, Inoue K, Anzai N, Endou H, Inui K-i, Kanai Y, Koizumi A. Roles of OATs in the renal excretion of PFOA. Basic and Clinical Pharamcology and Toxicology. 2007;103:1–8. doi: 10.1111/j.1742-7843.2007.00155.x. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Burlew MM, Mandel JH. Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers. Drug and chemical toxicology. 2000;23:603–620. doi: 10.1081/dct-100101973. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA, Reagen WK, Zobel LR. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environmental science & technology. 2012;46:6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- Reyes JL, Melendez E, Alegria A, Jaramillo-Juarez F. Influence of sex differences on the renal secretion of organic anions. Endocrinology. 1998;139:1581–1587. doi: 10.1210/endo.139.4.5930. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Kreckmann KH, Green JW, Gillies PJ, Reynolds JL, Leonard RC. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2007a;49:1086–1096. doi: 10.1097/JOM.0b013e318156eca3. [DOI] [PubMed] [Google Scholar]
- Sakr CJ, Leonard RC, Kreckmann KH, Slade MD, Cullen MR. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2007b;49:872–879. doi: 10.1097/JOM.0b013e318124a93f. [DOI] [PubMed] [Google Scholar]
- Smith AG, Francis JE. Evidence for the active renal secretion of S-pentachlorophenyl-N-acetyl-L-cysteine by female rats. Biochem Pharmacol. 1983;32:3797–3801. doi: 10.1016/0006-2952(83)90152-1. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Deguchi Y, Ishii I, Terai T. Sex differences in excretion of zenarestat in rat. Xenobiotica. 1991;21:1119–1125. doi: 10.3109/00498259109039552. [DOI] [PubMed] [Google Scholar]
- Tatum-Gibbs K, Wambaugh JF, Das KP, Zehr RD, Strynar MJ, Lindstrom AB, Delinsky A, Lau C. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology. 2011;281:48–55. doi: 10.1016/j.tox.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Terashita S, Sawamoto T, Deguchi S, Tokuma Y, Hata T. Sex-dependent and independent renal excretion of nilvadipine metabolites in rat: evidence for a sex-dependent active secretion in kidney. Xenobiotica. 1995;25:37–47. doi: 10.3109/00498259509061831. [DOI] [PubMed] [Google Scholar]
- Trautwein EA, Kunath-Rau A, Erbersdobler HF. Increased fecal bile acid excretion and changes in the circulating bile acid pool are involved in the hypocholesterolemic and gallstone-preventive actions of psyllium in hamsters. The Journal of nutrition. 1999;129:896–902. doi: 10.1093/jn/129.4.896. [DOI] [PubMed] [Google Scholar]
- USEPA. Summary Report of Decatur, AL Water Sample Analyses. National Exposure Research Laboratory; 2008. [Google Scholar]
- USEPA. Human Exposure and Atmospheric Sciences Division. 2009. Results of the Analyses of Screening Surface and Well Water Samples from Decatur, Alabama for Selected Perfluorinated Compounds. [Google Scholar]
- USEPA. Contaminants of Emerging Concern. 2014a. [Google Scholar]
- USEPA. Public Comment Draft Health Effects Document for Perfluorooctanoic Acid (PFOA) Office of Water; 2014b. [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. Journal of biochemical toxicology. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Vaughn B, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environmental health perspectives. 2013;121:1313–1318. doi: 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicological sciences : an official journal of the Society of Toxicology. 2010;113:305–314. doi: 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Maeda K, Kamiyama E, Sugiyama D, Kondo T, Shiroyanagi Y, Nakazawa H, Okano T, Adachi M, Schuetz JD, Adachi Y, Hu Z, Kusuhara H, Sugiyama Y. Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:2166–2176. doi: 10.1124/dmd.107.017459. [DOI] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. Organic anion transporting polypeptide (Oatp) 1a1-mediated perfluorooctanoate transport and evidence for a renal reabsorption mechanism of Oatp1a1 in renal elimination of perfluorocarboxylates in rats. Toxicology letters. 2009;190:163–171. doi: 10.1016/j.toxlet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicological sciences : an official journal of the Society of Toxicology. 2010;117:294–302. doi: 10.1093/toxsci/kfq219. [DOI] [PubMed] [Google Scholar]