SUMMARY
While cilia are recognized as important signaling organelles, the extent of ciliary functions remains unknown because of difficulties in cataloguing proteins from mammalian primary cilia. We present a method that readily captures rapid snapshots of the ciliary proteome by selectively biotinylating ciliary proteins using a cilia-targeted proximity labeling enzyme (cilia-APEX). Besides identifying known ciliary proteins, cilia-APEX uncovered several ciliary signaling molecules. The kinases PKA, AMPK and LKB1 were validated as bona fide ciliary proteins and PKA was found to regulate Hedgehog signaling in primary cilia. Furthermore, proteomics profiling of Ift27/Bbs19 mutant cilia correctly detected BBSome accumulation inside Ift27−/− cilia and revealed that β-arrestin 2 and the viral receptor CAR are candidate cargoes of the BBSome. This work demonstrates that proximity labeling can be applied to proteomics of non-membrane-enclosed organelles and suggests that proteomics profiling of cilia will enable a rapid and powerful characterization of ciliopathies.
INTRODUCTION
Cilia organize and tune signal transduction cascades by dynamically concentrating signaling molecules in a specialized environment (Goetz and Anderson, 2010; Nachury, 2014). This dynamic compartmentalization of signaling by cilia is best exemplified by the Sonic Hedgehog (Hh) pathway where signal activation elicits the ciliary accumulation of the GLI transcription factors and the seven transmembrane protein Smoothened (SMO), and the ciliary removal of the pathway inhibitors Patched1 (PTC1) and GPR161, a G protein-coupled receptor (Corbit et al., 2005; Mukhopadhyay et al., 2013; Rohatgi et al., 2007). Remarkably, in unstimulated cells, the cilium is required for the conversion of GLI2 and GLI3 into transcriptional repressors even though GLI2 and GLI3 are hardly detectable inside cilia (Tukachinsky et al., 2010; Wen et al., 2010). Proteins that rapidly traverse the cilium can thus be altered within cilia. Yet tools to identify such transient visitors of the cilium are currently lacking.
Primary cilium dysfunction causes a variety of hereditary disorders collectively named ciliopathies (Bettencourt-Dias et al., 2011; Fliegauf et al., 2007). The characterization of Bardet-Biedl Syndrome (BBS) gene products led to the discovery of the BBSome, a coat complex that removes signaling molecules from the cilium (Jin et al., 2010; Lechtreck et al., 2013; Liew et al., 2014). In a landmark study, the biochemical purification of flagella from the single-cell organism Chlamydomonas reinhardtii was leveraged to identify proteins that accumulate in bbs4 mutant flagella (Lechtreck et al., 2009). Similarly, the finding that the translation factor EF1α abnormally enters cilia of cep290/mks4/nphp6 mutants was the first indication that MKS and NPHP proteins are part of a diffusion barrier at the base of cilia (Craige et al., 2010). In contrast, the comprehensive profiling of alterations of the ciliary proteome in mammalian cells is currently not feasible since isolation of primary cilia is not sufficiently reproducible and preparations are heavily contaminated by microvilli (Mayer et al., 2008). In one study, protein correlation profiling (PCP) was used to identify novel ciliary proteins (Ishikawa et al., 2012). However, the required mass-spectrometric (MS) analyses of 25 sucrose gradient fractions make PCP impractical as a routine technique. Furthermore, PCP suffers from limited sensitivity, and the number of signaling factors and membrane proteins discovered in the PCP ciliary proteome was limited.
Proximity labeling is an emerging technology that relies on enzymatic activities capable of generating free biotinyl radicals to enable the rapid and spatially restricted labeling of proteins in the vicinity of the enzyme. While proximity labeling was initially used to uncover protein-protein interactions (Firat-Karalar et al., 2014; Lambert et al., 2015; Roux et al., 2012), recent studies leveraged proximity labeling to identify the protein contents of the membrane-enclosed mitochondrial matrix and intermembrane space (Lam et al., 2015; Rhee et al., 2013). Here we show that proximity labeling can be applied to capture minute-scale snapshots of ciliary protein contents. The described method is rapid, simple to implement and uncovered several ciliary protein kinases. Most remarkably, proximity labeling is capable of identifying signaling proteins that survey the ciliary interior and whose presence inside cilia had thus far escaped detection. Finally, this method can be used to profile the proteome of ciliopathy mutant cells and rapidly uncover the underlying molecular defects.
RESULTS
Selective biotinylation of ciliary proteins by cilia-APEX
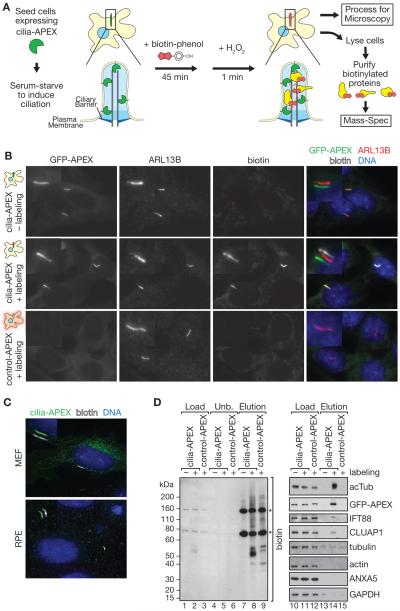
To globally biotinylate ciliary proteins (Figure 1A), we fused the ascorbate peroxidase APEX to a number of ciliary targeting signals. In the presence of H2O2, APEX catalyzes the conversion of biotin-phenol into biotin-phenoxyl radicals. Such radicals are short lived (<5 ms), have a small labeling radius (<20 nm), are membrane-impermeable, and can covalently react with electron-rich amino acids such as Tyr, Trp, His, and Cys (Rhee et al., 2013). The greatest ciliary enrichment of APEX was observed when fused to NPHP3[1-203] (Nakata et al., 2012), and the NPHP3[1-203]-GFP-APEX fusion (hereafter named cilia-APEX) was highly enriched in primary cilia of inner medullary collecting duct epithelial cells (IMCD3) (Figure 1B), mouse embryonic fibroblasts (MEFs) and retinal pigmented epithelial cells (RPE1-hTERT) (Figures 1C and S1). Staining cells with AlexaFluor647-labeled streptavidin (SA647) revealed that addition of biotin-phenol and hydrogen peroxide (H2O2) led to cilia-specific biotinylation by cilia-APEX (Figures 1B and 1C). No ciliary biotin signal was detected in the absence of H2O2, biotin-phenol or with a mislocalized control-APEX fusion in which the myristoylation site that mediates ciliary localization of NPHP3[1-203] was mutated (Figure 1B). Western Blot analyses showed similar expression of cilia-APEX and control-APEX in stable IMCD3 cell lines (Figure 1D, lanes 11 and 12), and the biotinylated proteins could be efficiently isolated by streptavidin chromatography of cell lysates (Figure 1D). Probing the isolated biotinylated proteins for ciliary markers revealed that acetylated tubulin and the IFT-B components IFT88 and CLUAP1 were specifically recovered from cilia-APEX cells subjected to labeling but not in the absence of labeling or from control-APEX cells treated with biotin-phenol and H2O2 (Figure 1D, lanes 13–15). Meanwhile, highly abundant proteins, such as tubulin, actin, GAPDH or Annexin V were either absent or greatly depleted from the streptavidin eluates, thus demonstrating the high specificity resulting from the stringent streptavidin-based purifications under denaturing conditions (Figure 1D). We conclude that cilia-APEX selectively labels ciliary proteins.
Figure 1. Cilia-APEX specifically biotinylates ciliary proteins.
(A) Diagram of the cilia-APEX labeling strategy. Cells expressing cilia-APEX (or control-APEX) were seeded at high density and ciliation induced by serum-starvation. Cells were pre-incubated with 0.5 mM biotin-phenol for 45 min, labeled for 1 min by addition of 1 mM H2O2 and the reaction terminated by washing the cells in quenching buffer. Cells were either immediately lysed or fixed for processing for immunofluorescence microscopy.
(B) Immunofluorescence of stable IMCD3 cell lines expressing control-APEX or cilia-APEX in the presence or the absence of labeling reagents. APEX fusion proteins were directly detected by GFP fluorescence, ARL13B is a cilium marker detected by antibody and biotinylated proteins were revealed by SA647. Merged insets show primary cilia with channels shifted to aid visualization. Scale bars in (B) and (C) are 5 μm (main panels) and 1 μm (insets).
(C) Immunofluorescence microscopy of mouse embryo fibroblast (MEF) and human retina pigment epithelial (RPE) cells after transient transfection with cilia-APEX (see Figure S1 for individual channels).
(D) Biotinylated proteins from cell lysates generated from control-APEX or cilia-APEX after APEX-labeling (+) or from cilia-APEX after mock-labeling (biotin-phenol was omitted; −) were isolated by Strepatvidin chromatography and samples analyzed by SDS-PAGE and Western Blotting. Biotinylated proteins were detected by streptavidin-HRP and indicated proteins by specific antibodies. Asterisks indicate endogenous biotinylated proteins. Note the slower migration of the control-APEX enzyme caused by the absence of the myristoyl moiety (lanes 11 vs. 12). Also note that the cilia-APEX enzyme is efficiently biotinylated whereas control-APEX is not, suggesting that APEX does not undergo self-biotinylation. Load and Unbound represent 0.1 % of the total lysate and Elution 10 % of the total eluate. See also Figure S1.
Cilia-APEX identifies a variety of ciliary proteins
We next conducted LC/MS-MS analyses of cilia-APEX-labeled samples. To control for the respective contributions of endogenous biotinylated proteins and of non-ciliary proteins biotinylated by APEX, we also subjected unlabeled cilia-APEX and labeled control-APEX samples to LC/MS-MS analyses (Figure 2A). By selecting proteins that were enriched at least 5-fold (estimated by spectral counts, see methods) in the cilia-APEX sample compared to either control, we identified 622 candidate ciliary proteins (Figure 2A). To identify reproducible hits, three independent replicates were carried out. We grouped the final cilia-APEX proteome in a high confidence Tier 1 and a low confidence Tier 2. Tier 1 consists of 162 candidate ciliary proteins that were identified by at least 6 spectral counts and in at least two experiments (Figure 2B and Table S1). Tier 2 consists of 208 additional proteins identified by at least 6 spectral counts in one experiment. Among the candidate ciliary proteins, we identified a large number of known ciliary proteins, such as subunits of the ciliary trafficking complexes IFT-A, IFT-B, kinesin 2 and dynein 1b; the small GTPases ARL3, ARL6 and ARL13B, as well as Septins 2 and 7 (Ghossoub et al., 2013; Hu et al., 2010) and EvC complex subunits (Pusapati et al., 2014) (Figure 2C). Several microtubule-associated proteins previously localized to cilia (Ghossoub et al., 2013; Patzke et al., 2010; Schrøder et al., 2007), such as CSPP1, MAP4 or EB1 were also identified in Tier 1. Centrosomal proteins and transition zone proteins were conspicuously absent from the cilia-APEX proteome, consistent with the highly specific localization of cilia-APEX inside cilia and the estimated 20 nm labeling radius of biotinyl radicals generated by APEX.
Figure 2. Ciliary proteins from APEX-labeling in IMCD3 cells.
(A) Venn diagram showing identified proteins after streptavidin chromatography from the indicated APEX-labeling reactions. The number in the yellow segment represents cilia-specific proteins enriched at least 5-fold in the experimental sample compared to both controls.
(B) Venn diagram showing the overlap of cilia-specific proteins between the three independent replicates. The yellow segment represents candidate ciliary proteins identified in at least 2 out of 3 experiments and with a total spectral count ≥6 (Tier 1).
(C) Candidate ciliary proteins from (B) grouped into functional categories. Asterisks denote cilia-specific proteins from Tier 2 that are known interaction partners of Tier 1 proteins. Previously known ciliary proteins are shown in grey, novel candidate ciliary proteins in black. Blue are novel ciliary proteins confirmed by independent methods in this study (see Figures 3, 5 and S2). The complete list of identified proteins is shown in Table S1.
(D) IMCD3 cells were transiently transfected with the indicated fusion proteins for 24 h, after which ciliation was induced for another 24 h before cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton-X100 and proteins detected by GFP fluorescence (LAP-SKA1), anti-FLAG or anti-HA antibodies. Cilia were counterstained using anti-ARL13B or anti-acTub antibodies as indicated. Only merged images are shown (see Figure S2 for individual channels). Merged insets show primary cilia with channels shifted to aid visualization. Scale bars represent 5 μm (main panels) and 1 μm (insets).
(E) Table comparing prominent protein categories identified in primary cilia by PCP (Ishikawa et al., 2012) vs. cilia-APEX. Note the absence of ribosomal subunits and chaperones, frequent contamination of proteomic studies in the cilia-APEX proteome. See also Figure S2.
Unexpected candidate ciliary proteins were identified by cilia-APEX. These include all subunits of the spindle and kinetochore associated (SKA) complex, four tetraspanins, ubiquitylation enzymes, as well as proteins associated with ciliary functions but not known to localize to cilia (e.g. AZI1, Dishevelled 3) (Figure 2C). To assess the cilia-APEX proteome, we transfected epitope-tagged constructs into IMCD3 cells. SKA1 was indeed found inside cilia (Figures 2D and S2A). Given that the SKA complex enables the kinetochore to track depolymerizing microtubules in mitosis (Schmidt et al., 2012), SKA might function during cilium shortening to track the axonemal tip. Similarly, the tetraspanins CD81 and CD82 were found in cilia (Figure 2D). Tetraspanins are palmitoylated 4-pass membrane proteins that shape membrane structure by influencing lipid bilayer geometry. Intriguingly, all four tetraspanins identified by cilia-APEX have been reproducibly found in extracellular vesicles (Mathivanan et al., 2010; Simpson et al., 2008), pointing to a possible role of these tetraspanins in ciliary ectosome formation, a biologically important phenomenon in C. rheinhardtii (Cao et al., 2015; Wood et al., 2013) and nematodes (Wang et al., 2014). Finally, the clathrin adaptor TOM1L2 was found in cilia (Figure 2D). TOM1 and TOM1-like 2 (TOM1L2; both Tier 1 hits) are evolutionarily conserved ESCRT-0 proteins that carry out early endocytic sorting of ubiquitinated cargoes by linking them to Myosin-VI (Tumbarello et al., 2012). The findings that the canonical ESCRT-0 complex Hrs/STAM mediates the ciliary exit and lysosomal degradation of polycystin-1 and -2 (Hu et al., 2007) and that ubiquitination enhances ciliary exit (Xu et al., 2015) suggest a possible role of TOM1/TOM1L2 in these processes.
Taking into account all Tier 1 proteins that we detected in cilia in the course of this study, the immunofluorescence validation rate of candidate ciliary proteins in the cilia-APEX proteome is 56% (Figure S2B). However, as outlined below, we believe that the sensitivity of cilia-APEX for certain proteins is greater than that of immunofluorescence, and that some proteins scored as “false positive” by microscopy may be present in cilia at very low steady state levels. Furthermore, the dataset we selected for validation by immunofluorescence did not include Tier 1 proteins that were previously known to localize to cilia. Thus, the 56% validation rate we calculate is likely underestimated. Compared to the PCP cilium proteome (Ishikawa et al., 2012), cilia-APEX identified many more transmembrane and microtubule-associated proteins, and in particular signaling-relevant proteins, such as protein kinases and predicted calcium-binding proteins (Figure 2E). Cilia-APEX thus represents a significant advance given the ease of the approach and the broad coverage of structural, trafficking and signaling components.
Discovery of a ciliary AC6/cAMP/PKA signaling axis that regulates Hh signaling
Adenylate cyclase 3 (AC3) serves as a specific ciliary marker of neurons and glial cells and is generally considered to be the predominant cAMP-generating enzyme in primary cilia (Bishop et al., 2007) (Figure 3A). Yet, consistent with previous findings (Kwon et al., 2010; Masyuk et al., 2008) and the absence of AC3 from the cilia-APEX proteome, we failed to detect AC3 in primary cilia of IMCD3 cells (Figure 3A). Meanwhile, AC6 was a strong Tier 1 hit in the cilia-APEX proteome (Figure 2C and Table S1) and an antibody recognizing AC5 and AC6 revealed a clear ciliary signal in IMCD3 cells (Figure 3B). In the absence of AC5- and AC6-specific antibodies, we were unable to determine which of these two proteins is present in cilia of IMCD3 cells. However, out of 36 spectral counts that could be assigned to either AC5 or AC6 in cilia-APEX, 32 were unique to AC6, 4 were common to AC5 and AC6 and none were specific to AC5. These results suggest that AC6 is the main ciliary adenylate cyclase in IMCD3 cells. The presence of three distinct adenylate cyclases in cilia poses the question of their respective functional significance. While AC3 has been detected in cilia of neurons (Bishop et al., 2007), kidney cells (Nikonova et al., 2014) and fibroblasts (Halbritter et al., 2013; Ou et al., 2009), a recent publication found AC5 and 6 to be the major regulators of Hh signaling in the chicken neural tube and in mouse cerebellar granular neuron precursors (Vuolo et al., 2015). Interestingly, while AC3, 5 and 6 are all activated by Gαs, only AC5 and 6 are inhibited by Gαi or free Ca2+(Sadana and Dessauer, 2009). Given that most ciliary GPCRs are coupled to either Gαs or Gαi, modulating the respective levels of AC3, 5 and 6 in cilia would provide a means for different cell types to tune their response to ciliary GPCR activity.
Figure 3. AC6 and PKA are present in IMCD3 cilia.
(A) Cultured mouse cortical neurons (DIV 15) and serum-starved IMCD3 cells were fixed in 4% paraformaldehyde (PFA) and analyzed by immunostaining for AC3 and glutamylated tubulin (GluTub). AC3 channels were normalized to one another.
(B) IMCD3 cells were starved for 16 h to induce ciliation and fixed in 4% PFA. After permeabilization with 0.1% saponin, cells were incubated overnight with antibodies against AC5/6 and acetylated tubulin. Images showing AC5/6 localization were deconvolved.
(C) IMCD3 cells stably expressing NG-PKARIα were processed and imaged as in (B). Insets show primary cilia, merged insets are offset to aid visualization. All scale bars are 5 μm (main panels) and 1 μm (insets).
A prominent signaling target of cAMP is the cAMP-dependent protein kinase PKA. At rest, PKA exists as an auto-inhibited complex of regulatory (R) and catalytic (C) subunits and binding of cAMP to R subunits triggers the release of active C subunits. PKA exerts a powerful inhibition on Hedgehog signaling through phosphorylation of the GLI2 and GLI3 transcription factors, and the phenotype of a PKA-C mutant mouse is as severe as that of a Ptc1−/− mouse (Tuson et al., 2011). Paradoxically, while the localization of adenylate cyclases predicts that cAMP production takes place within cilia, immunocytochemistry found some concentration of PKA-C at the base of cilia (Barzi et al., 2010; Tuson et al., 2011). It has therefore been proposed that cAMP diffusing out of cilia activates PKA at the base and that PKA deposits inhibitory phosphorylations on GLI2/3 before or after GLI2/3 have passed through cilia (Mukhopadhyay and Rohatgi, 2014). However, it is conceivable that the pools of ciliary PKA-C have remained undetectable because they are too labile for conventional fixation methods or because of low sensitivity of the immunological reagents. Furthermore, the presence of the PKA regulatory subunit Iα (PKA-RIα) in Tier 1 and of PKA-Cα and RIIβ in Tier 2 of the cilia-APEX proteome suggested that sensing of cAMP by PKA might take place inside cilia (Figure 2C). Indeed, stably expressed NeonGreen(NG)-tagged PKA-RIα can be detected in primary cilia of IMCD3 cells (Figure 3C). Congruently, (Jacob et al., 2011) have reported that genetic ablation of PKA-RIα leads to Hh signaling defects.
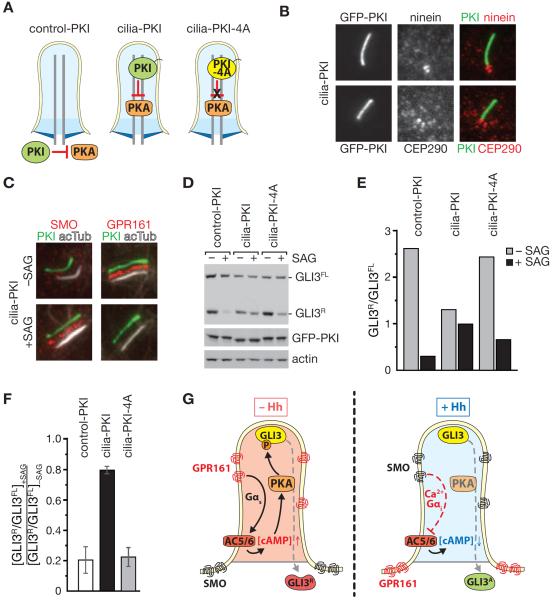
The detection of a regulatory subunit of PKA in cilia suggested that the PKA holoenzyme may enter and become activated within cilia by sensing intraciliary cAMP levels. To directly test the functional significance of ciliary localization of PKA, we turned our attention to Hedgehog signaling and in particular GLI3 processing. Upon phosphorylation by PKA, GLI3 undergoes partial proteolysis and becomes converted from a 190 kDa polypeptide (GLI3FL) into a 83 kDa transcriptional repressor (GLI3R). We reasoned if PKA phosphorylates GLI3 within cilia, then inhibiting PKA inside cilia should block GLI3 processing. To test this prediction, we directed the specific PKA inhibitor peptide PKI (Knighton et al., 1991) to cilia by expressing NPHP3[1-203]-GFP-PKI (cilia-PKI) stably and at low levels in IMCD3 cells (Figure 4A). While high level expression of GFP-PKI has been reported to inhibit Hh signaling (Iglesias-Bartolome et al., 2015), the site of PKI action has not been investigated. To control for the specificity of the PKI inhibitory peptide and the importance of ciliary targeting, we expressed cilia-PKI-4A, a 4-residue mutant that fails to block PKA activity and NPHP3[1-203;G2A]-GFP-PKI (control-PKI), a mutant that fails to target to cilia (Figures 4A and S3). All constructs were expressed at similarly low levels as evidenced by Western Blot (Figure 4D) and the extremely weak fluorescent signal of the diffusely distributed control-PKI (Figure S3). Consistent with the weak localization of GFP at the pericentriolar matrix (Breslow et al., 2013), control-PKI was slightly enriched at the ciliary base. Importantly, cilia-PKI clearly localized to the ciliary shaft, distal to the basal body (marked by ninein) and the transition zone (marked by CEP290) (Figure 4B). Moreover, all IMCD3 cell lines appropriately responded to the Smoothened agonist SAG by redistributing SMO to cilia and removing GPR161 from cilia (Figures 4C and S3). In contrast, while control-PKI and cilia-PKA-4A cells produced high levels of GLI3R at rest, cilia-PKI expression resulted in a clear reduction in GLI3R (Figure 4D), similar to the effect of genetically removing PKA catalytic activity (Tuson et al., 2011). Furthermore, Hh pathway stimulation results in decreased processing of GLI3FL into GLI3R and the GLI3R/GLI3FL ratio was appropriately decreased in control-PKI and cilia-PKA-4A cells treated with SAG (Figures 4E and 4F). In contrast, GLI3R levels and the GLI3R/GLI3FL ratio were barely altered by SAG addition to cilia-PKI cells. This demonstrates that inhibiting PKA activity within cilia leads to defective processing of GLI3 and points towards a direct function of PKA in cilia.
Figure 4. Cilium-specific inhibition of PKA perturbs GLI3 processing during Hedgehog signaling.
(A) Diagrams of cell lines expressing cilia-PKI to inhibit PKA activity within primary cilia, the non-inhibiting mutant cilia-PKA-4A, and control-PKI expressed in the cytosol.
(B) Ciliated IMCD3-[cilia-PKI] cells stained for ninein and CEP290. Cilia-PKI was detected by GFP fluorescence. Scale bar: 1 μm. (c–f) Ciliated cells were treated with 200 mM SAG or vehicle control for 16 h.
(C) IMCD3-[cilia-PKI] cells were stained for acetylated Tubulin, GPR161 or SMO. Channels were shifted to aid visualization (see Figure S3 for individual channels and control cell lines).
(D–E) Indicated cell lines were analyzed by immunoblotting for GLI3, GFP and actin (D), the GLI3R and GLI3FL signals were quantified by densitometry (ImageJ) and the GLI3R/GLI3FL ratios plotted in (E).
(F) The effect of SAG addition on the GLI3R/GLI3FL ratios was measured. The average from 3 independent experiments is shown. Error bars depict standard deviations (n = 3).
(G) Model depicting the ciliary AC/cAMP/PKA signaling axis during Hedgehog signaling. See text for details. See also Figure S3.
Hence, consistent with the well-established functions of cAMP inside olfactory cilia (Anholt, 1989), we propose that cAMP is sensed inside cilia by the PKA holoenzyme to transmit Hh signals to the rest of the cell. In this model, cilia harbor high levels of cAMP owing to the activity of AC3, 5 and 6 and the tonic stimulation of Gαs by GPR161 (Mukhopadhyay et al., 2013). PKA thus becomes activated inside cilia where it phosphorylates GLI3FL and primes it for proteolytic processing into GLI3R. Once the Hh pathway becomes activated, GPR161 exits cilia and SMO enters cilia where it may inhibit AC5/6 either through Gαi (Riobo et al., 2006; Barzi et al., 2011) or elevated ciliary Ca2+ (DeCaen et al., 2013). These alterations result in lower levels of ciliary cAMP, decreased phosphorylation of GLI3FL by PKA inside cilia and reduced production of GLI3R (Figure 4G).
LKB1-AMPK signaling in primary cilia
Liver kinase B1 (LKB1) is a master regulator of cell growth and epithelial polarity that is frequently altered in cancer (Jansen et al., 2009). Instead of relying on phosphorylation for its activation, LKB1 is activated through formation of a heterotrimer with the pseudokinase STRAD and the scaffolding protein MO25 (Zeqiraj et al., 2009). Since vertebrates have two paralogues of STRAD (α and β) and two paralogues of MO25 (α and β), the functional diversification of the four STRAD-MO25-LKB1 complexes remains an intriguing yet unsolved question. Interestingly, STRADβ but not STRADα was found in the cilia-APEX proteome (Figure 2C) and transfection of epitope-tagged STRADβ confirmed a significant ciliary enrichment of STRADβ (Figure 5A). Since LKB1 has been localized to primary cilia of Madin-Darby canine kidney (MDCK) cells (Boehlke et al., 2010), and since LKB1 and MO25α were Tier 2 hits in the cilia-APEX proteome, we tested the contribution of STRADβ to LKB1 localization. In our experimental setup, endogenous LKB1 was undetectable in IMCD3 cilia unless exogenous STRADβ was introduced into the cell (Figure 5A), thus suggesting that STRADβ functions as a ciliary targeting subunit for the STRAD-MO25-LKB1 complex. To identify the ciliary targeting determinants of STRADβ, we compared the sequences of STRADα and STRADβ proteins from five different organisms. Whereas the N-terminal extensions of STRADα orthologues displayed significant differences in length and composition, the STRADβ N-termini were highly conserved among species and contained two conserved cysteine residues near the amino-terminus, suggestive of potential palmitoylation sites (Figure 5B). Congruent with prior evidence of acylation mediating ciliary targeting (Constantine et al., 2012; Follit et al., 2010), STRADβ[C6S,C8S] no longer localized to the primary cilium but was instead found dispersed throughout the cell, similar to the distribution of STRADα (Figure 5C). When LKB1-NG was co-expressed with the STRAD variants, STRADβ directed LKB1 to the primary cilium while STRADβ[C6S,C8S] and STRADα directed LKB1 to the cytosol and nucleus. Hence, palmitoylation of STRADβ is likely to direct the LKB1/STRADβ/MO25 holoenzyme to the primary cilium, opening the possibility of a cilia-localized LKB1 signaling cascade regulated by STRADβ palmitoylation. Alternatively, it is conceivable that the two cysteine residues at positions 6 and 8 may be directly recognized by the ciliary import machinery.
Figure 5. LKB1 localization to IMCD3 cilia is mediated by STRADβ palmitoylation.
(A) IMCD3 cells were transiently transfected with STRADβ3xFLAG, serum-starved for 16 h and fixed in 4% PFA and stained with anti-FLAG, anti-LKB1 and anti-acTub antibodies to visualize the primary cilium.
(B) Multiple sequence alignment of STRADα and STRADβ from five different organisms each using ClustalW 2.0.11. Black boxes indicate identical residues in at least 4 out of 5 species; grey boxes indicate similar amino acids. Black underlining indicates the STRAD core domain used for crystallization in (Zeqiraj et al., 2009). Only the N-terminal part of alignment is shown. Hs, Homo sapiens; Mm, Mus musculus; Bt, Bos taurus; Dr, Danio rerio; Xl, Xenopus laevis; Xt, Xenopus tropicalis.
(C) IMCD3 cells were co-transfected with LKB1-NG and indicated STRAD isoforms and mutants and analyzed as in (A).
(D) Ift27−/− and wild-type IMCD3 cells stably expressing NG-AMPKβ2 were analyzed as in Figure 3C. All scale bars are 5 μm (main panels) and 1 μm (insets).
The major downstream targets of LKB1 are AMP kinase (AMPK) family members. AMPK itself is activated when ATP is depleted and the cellular levels of AMP are increased, and the LKB1-AMPK axis integrates the metabolic state of the cell to regulate energy production (Jansen et al., 2009). Examination of the cilia-APEX dataset revealed the presence of AMPK subunits β2 in Tier 1 and α1 in Tier 2. Surprisingly, NG-AMPKβ2 stably expressed in IMCD3 cells was not detectable in primary cilia (Figure 5D). Several proteins such as SMO, GLI2 and GLI3 have been proposed to rapidly cycle in and out of cilia and are only detected in cilia under signaling conditions (Corbit et al., 2005; Kim et al., 2009; Tukachinsky et al., 2010; Wen et al., 2010) or when ciliary exit is compromised by inhibition of Dynein 1b (Kim et al., 2009; Ocbina and Anderson, 2008) or by removal of the BBSome export factor IFT27 (Eguether et al., 2014; Liew et al., 2014). Remarkably, AMPKβ2 was strongly enriched in primary cilia of Ift27−/− cells (Figure 5D), indicating that AMPK is likely to rapidly cycle into and out of cilia. Collectively, these findings suggest that IMCD3 cilia may serve as signaling hubs for energy metabolism through a STRADβ-LKB1-AMPK pathway. In this context, it is worth noting that SMO ligands that translocate SMO to cilia increase AMPK activity and alter glucose metabolism (Teperino et al., 2012). Since the effects of SMO on AMPK and metabolism occur on the scale of minutes, do not require a transcriptional response or SMO activation and depend on intact cilia, a tantalizing conclusion is that SMO translocation leads to activation of AMPK within cilia. In addition, AMPK has recently been shown to regulate Hh signaling through destabilizing phosphorylation of GLI1 (Li et al., 2015). Finally, LKB1 deletion results in increased stability of GLI3R (Jacob et al., 2011). AMPK may thus respond to Hh inputs and fine-tune the Hh response within cilia.
Comparative cilia-APEX analyses rapidly identify alterations of the ciliary proteome
Ift27−/− cells accumulate specific signaling proteins in their cilia (Figure 5D and (Eguether et al., 2014; Liew et al., 2014) and, unlike dynein 1b or IFT-A mutants, Ift27 mutant cilia do not suffer from structural abnormalities. We therefore sought to apply the cilia-APEX technology to globally compare the protein content of Ift27−/− cilia to that of wild-type cilia. Cilia-APEX was stably expressed in Ift27−/− cells and displayed the same ciliary localization and labeling efficiency as in WT cells (Figure S4). We conducted large-scale APEX labeling experiments in triplicate in each cell line, followed by streptavidin affinity-chromatography and MS analyses, and calculated ratios of the average spectral counts for each identified protein. To correct for the variance in protein recovery between the different samples, we calculated the mean ratio of spectral counts between WT and Ift27−/− for non-ciliary contaminants (118 mitochondrial proteins). Assuming that the variability in the control group is representative of the whole data set, we set a significance threshold at 2.75 standard deviations around the mean value of the mitochondrial control group to ensure a false positive rate of less than 1%. Out of 2070 proteins identified in WT or Ift27−/− samples, only 57 showed a significant change between the two genotypes (Figure 6A and Table S2; see methods for statistical approach). Congruent with the recent finding that the BBSome accumulates in Ift27−/− cilia (Eguether et al., 2014; Liew et al., 2014), five BBSome subunits scored among the top 21 enriched proteins and all five were enriched at least 4.4-fold in the Ift27−/− cilia-APEX samples when compared to wild-type cilia-APEX samples. Moreover, LZTFL1, a BBSome interactor known to accumulate in Ift27−/− mutant cilia (Eguether et al., 2014), was also near the top of the enriched list (Figure 6A, top inset). Together, these results demonstrate the proof-of-concept of the comparative cilia-APEX approach.
Figure 6. Comparative cilia-APEX proteomics identifies proteins accumulating in or depleted from Ift27−/− cilia.
(A) Cilia-APEX labeling, streptavidin chromatography and LC-MS/MS were performed in three independent experiments in WT and Ift27−/− cells. The y-axis represents the log2-transformed ratios of average spectral counts between Ift27−/− and wild-type samples (see Methods for analytical approach). Each vertical line represents one protein. The mean (μ) and standard deviation (σ) of log2[SpC(Ift27−/−)/SpC(WT)] calculated for a control group of 118 mitochondrial proteins are 0.29 and 0.62, respectively, leading to significance thresholds of 1.98 (μ + 2.75σ) and −1.40 (μ − 2.75σ) indicated by dashed red lines. Insets show magnified views of the significantly enriched (top) and depleted (bottom) proteins. BBSome subunits and LZTFL1 are shown in green and proteins whose abundance is altered by IFT27 deletion and was confirmed by fluorescence microscopy are shown in orange.
(B) Proteins from selected functional groups are displayed. See also Figure S4.
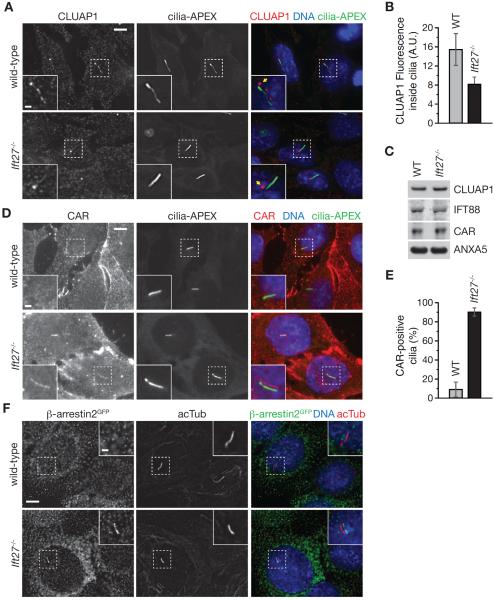
Analysis of major functional groups revealed that ciliary kinesins, most EvC zone components and most IFT components only showed non-significant changes, although a general trend of depletion was observed for IFT subunits (Figure 6B). Among the significantly depleted proteins were the microtubule-binding proteins EB2 and MAP1B, several proteins involved in clathrin-independent endocytosis (Flotillin, endophilin-A2), as well as Aurora Kinase A. Most unexpected was the presence of the IFT-B subunit CLUAP1 among the top 30 depleted proteins (Figures 6A and 6B). Consistent with previous reports (Botilde et al., 2013; Pasek et al., 2012), CLUAP1 was detected at the basal body as well as in foci along the length of WT cilia, similar to other IFT-B components (Figure 7A). While the amount of CLUAP1 at the basal body appeared unchanged in Ift27−/− cells, the levels of CLUAP1 along the length of the cilium were significantly reduced (Figures 7A and 7B). Meanwhile, the total cellular abundance of CLUAP1 was unaffected by Ift27 deletion (Figure 7C). Thus, while Ift27 deletion has been reported to not significantly affect IFT88 localization to cilia (Eguether et al., 2014; Liew et al., 2014) or the ciliary abundance of other IFT subunits (Figures 6A and 6B), CLUAP1 appears to be reduced in Ift27−/− cilia. Whether this is due to general reduction of IFT-B within Ift27−/− cilia, as suggested by the general trend of IFT depletion (see Figure 6A), or whether this defect is specific for CLUAP1 remains to be investigated. Similar to the IFT27/25 module, CLUAP1 may be considered a peripheral IFT-B subunit, since it has not been identified in initial preparations of mammalian IFT. Therefore, it is conceivable that CLUAP1 requires IFT27 for proper association with the IFT-B complex.
Figure 7. Validation of CLUAP1 depletion and CAR and β-arrestin 2 enrichment in Ift27−/− cilia.
(A) Wild-type and Ift27−/− cells expressing cilia-APEX were starved for 16 h to induce ciliation, fixed in 4% PFA, permeabilized using 0.1% saponin and stained for endogenous CLUAP1. Merged insets show primary cilia with channels shifted to aid visualization. Yellow arrows point at the basal body.
(B) Ciliary CLUAP1 signals in cilia were quantified in wild-type (n = 52) and Ift27−/−(n = 42). Error bars depict standard error of the mean.
(C) Western blots of wild-type and Ift27−/− cell lysates.
(D) Immunofluorescence microscopy as in (A) using anti-CAR antibody to stain native CAR.
(E) Number of CAR-positive (CAR+) and CAR-negative (CAR−) cilia were counted in indicated cell lines and displayed as bar graphs. Error bars depict standard deviations (n = 3).
(F) Wild-type and Ift27−/− cells stably expressing β-arrestin2-GFP were analyzed by fluorescent microscopy as in (A). All scale bars are 5 μm (main panels) and 1 μm (insets). See also Figure S5.
The top enriched protein in Ift27−/− cilia is the Coxsackie and Adenovirus Receptor (CAR), an integral component of the epithelial tight junction complex (Cohen et al., 2001). Expression of epitope-tagged proteins revealed very few cilia positive for CAR in WT cells. Similarly, CAR-positive cilia were rarely found when staining for endogenous CAR (Figures 7D and 7E). However, when analyzing Ift27−/− mutants, the majority of cilia were clearly positive for CAR, even though the total levels of CAR were unchanged (Figures 7C–7E). The possible role of the BBSome in trafficking the viral receptor CAR out of cilia remains enigmatic. CAR is normally found on the basolateral side of tight junctions where it associates with cytoskeletal and signaling proteins (Cohen et al., 2001; Coyne et al., 2004; Sollerbrant et al., 2003). One possibility is that the presence of CAR inside cilia is detrimental to the cell and that the BBSome functions as a clearing device as previously suggested for phospholipase D in Chlamydomonas (Lechtreck et al., 2013). Since CAR is typically not present on apical membranes, the entry of Coxsackie virus and Adenovirus into the organism occurs after breaching of tight junctions on respiratory and intestinal cells. However, in the absence of the clathrin adaptor AP1B, as naturally occurring in retinal pigment epithelium cells (RPE), CAR becomes misrouted to the apical membrane and adenovirus can efficiently enter cells from the apical side (Diaz et al., 2009). Similarly, when BBSome function is compromised, CAR may accumulate within cilia and permit entry of Adenovirus and Coxsackie virus from the apical side of the cells. More research will be required to test whether BBS patients and animal models are more susceptible to viral infections by Adenovirus and Coxsackie virus. Alternatively, it is conceivable that CAR performs a thus far unsuspected function inside cilia. Similar to several ciliopathy mouse models, CAR knockout mice die between E11 and E13 from insufficient heart function and display cardiac pericardial edema (Dorner et al., 2005).
Another top hit in our comparative proteomics was β-arrestin 2 (Figure 6A), and we observed increased levels of β-arrestin 2 in Ift27−/− cilia by fluorescence microscopy (Figures 7F and S5). β-arrestins mediate clathrin-dependent internalization of activated GPCRs by binding to the phosphorylated cytoplasmic tails of GPCRs (Reiter and Lefkowitz, 2006). β-arrestin 2 has been previously detected inside cilia (Molla-Herman et al., 2008) and was suggested to participate in SMO ciliary trafficking (Kovacs et al., 2008). Since the BBSome also functions in the trafficking of SMO (Zhang et al., 2011; 2012), β-arrestin 2 may cooperate with the BBSome to remove SMO from cilia.
Thus, the comparative cilia-APEX analyses have uncovered an unexpected candidate cargo of the BBSome (CAR) and a putative adaptor for BBSome-mediated export (β-arrestin 2), further highlighting the power of the comparative cilia-APEX method.
DISCUSSION
Work in the past decade has established the importance of the primary cilium in signaling and in human diseases characterized by obesity, retinal degeneration and kidney cysts. However, our understanding of the physico-chemical environment of cilia and how it endows cilia with signaling properties remains fragmentary. A major technological limitation to the understanding of events that take place inside cilia lies in our inability to obtain reproducible and pure fractions of primary cilia for sensitive analysis of the ciliary proteome by MS. Here we show that the APEX labeling technology is a versatile, readily applicable and highly effective approach to study the protein content of primary cilia. The cilia-APEX fusion functions in a variety of cell types and enables a quantitative analysis of the alterations in ciliary protein contents resulting from a specific mutation. This technology promises a comprehensive and quantitative assessment of differences in the ciliary proteomes between healthy and disease states, between different cell types, between different genotypes and upon exposure to specific drugs and other small molecules. The highly specific information provided by the comparative cilia-APEX approach allows for the generation of precise molecular hypotheses as exemplified by the identification of most BBSome subunits as well as the BBSome associated protein LZTFL1 as proteins that accumulate in Ift27−/− cilia. Within a few weeks, the comparative cilia-APEX study of Ift27−/− vs. WT cells produced a definitive molecular hypothesis that had taken over a year to be developed by traditional methods of TAP tagging and searching for the physiologically relevant interactors of IFT27 (Liew et al., 2014). Furthermore, the comparative cilia-APEX study uncovered entirely unexpected candidate BBSome cargoes and adaptors that had escaped detection by traditional biochemical association methods. Our proof-of-concept study of Ift27−/− vs. WT cells thus suggests that cilia-APEX profiling may constitute a rapid and powerful method for the characterization of ciliopathy mutants.
Interestingly, AMPKβ2 which was clearly identified by cilia-APEX was initially not detected inside cilia by immunohistochemistry. Since AMPKβ2 becomes clearly detectable inside cilia once exit is impaired, this suggests that AMPKβ2 rapidly traverses the cilium. Considering that the APEX labeling reaction is performed for 1 min, the extent of biotinylation of a given protein integrates the number of molecules that visited the cilium –either transiently or stably– within this timeframe. In theory, a cilium-resident protein present at 600 copies in the cilium at steady-state will be biotinylated by cilia-APEX as efficiently as a protein present at 10 copies in the cilium at steady-state but whose ciliary turn-over rate is one second. This interpretation is congruent with the extremely low number (150 copies) of known ciliary signaling molecules such as the ciliary Ca2+ channel PKD1L1/PKD1L2 in cilia (DeCaen et al., 2013) and suggests that other proteins with enzymatic activities that function inside cilia may have escaped detection by traditional methods. The accumulation of signaling proteins in Ift27−/− cilia highlights the Ift27−/− mutant as a promising tool to identify ciliary proteins whose steady-state levels have remained below the detection limit of immunological reagents. Other mutants defective in exit from cilia such as IFT-A and dynein-1b may prove similarly useful.
In light of the ability of cilia-APEX to detect proteins transiently visiting cilia, it is likely that several of the “false positives” that we have been unable to detect in cilia by immunofluorescence (Figure S2B) may only become detectable by standard imaging methods once studied in the appropriate export mutant. The absence of common contaminants such as chaperones and ribosomal proteins from the cilia-APEX proteome suggests a very low rate of true false positives. Meanwhile, the absence of several known IFT polypeptides from the cilia-APEX dataset is likely to represent true negatives and may reflect the selectivity of the proximity biotinylation method towards electron-rich amino acids exposed on the surface of proteins. Consistently, most of the missing IFT subunits in the cilia-APEX dataset are small proteins that likely bear no surface-exposed amino acids reactive with biotin-phenoxyl radicals.
A unique feature of the APEX technology is its ability to provide temporal snapshots of the ciliary proteome with minute-scale resolution, and cilia-APEX has the potential to reveal dynamic changes in the ciliary protein content after exposure to signaling ligands such as Hh. Moreover, the success of cilia-APEX generalizes the applicability of proximity labeling for proteomics of cellular subcompartments or domains beyond membrane-enclosed organelles such as mitochondria (Lam et al., 2015; Rhee et al., 2013). In particular, the absence of transition zone and basal body proteins from the cilia-APEX proteome suggests that the rapid and highly localized labeling provided by the APEX enzyme may be leveraged to capture snapshots of the proteome of other protrusions such as lamellipodia, filopodia and microvilli.
EXPERIMENTAL PROCEDURES
Detailed information about the generation of cell lines and plasmids, as well as antibodies and reagents used in this study can be found in the Supplemental Materials together with detailed descriptions of instrumentation and software used for immunofluorescence microscopy, image analyses as well as mass spectrometric analyses.
APEX-labeling experiments
Serum-starved cells were incubated in the presence of 0.5 mM biotin-phenol for 45 min, after which hydrogen peroxide (H2O2) was added to a final concentration of 1 mM and the plates immediately swirled to ensure equal distribution. After 1 min, the biotin-phenol- and H2O2-containing medium was aspirated and cells were washed 3 times with quenching buffer (PBS supplemented with 10 mM sodium ascorbate, 10 mM sodium azide and 5 mM Trolox). For microscopic analysis cells were immediately fixed. For proteomics and Western Blot analyses, lysis buffer (6M urea, 0.3 M NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM sodium ascorbate, 10 mM sodium azide, 5 mM Trolox, 10% glycerol, 25 mM Tris/HCl pH 7.5) was immediately added and cells lyzed by scraping them off the culture dish. The cell lysate was cleared by centrifugation (16,000 × g for 20 min), and the supernatant used immediately for further analyses or stored at −80°C.
Image analysis
Analysis of imaging data was performed using ImageJ software (National Institutes of Health). For measurement of the amounts of CLUAP1 in cilia, 3 planes from z-stacks acquired at 0.3 μm interval were projected, and the cilia defined by segmented lines (width 3 pixels) based on cilia-APEX signal. The mean signal was subtracted by the mean background and multiplied by cilium length. The average of at least 20 cilia was calculated for three independent experiments and standard errors were calculated. For CAR, at least 60 cilia were counted in 3 independent experiments and percentages of positive cilia and standard errors calculated.
Streptavidin chromatography
Protein concentrations of lysates prepared from APEX-labeling experiments were determined by Bradford assay and lysates adjusted to equal protein concentrations. Lysates were diluted 10-fold with wash buffer (lysis buffer without urea supplemented with 0.5% Triton-X100 and 0.1% SDS), from which a sample was taken as loading control. Diluted lysates were added onto streptavidin-sepharose beads (Thermo Scientific) and biotinylated proteins were captured for 1 h at room temperature. Beads were washed extensively first with wash buffer, then with 4 M urea 10 mM Tris/HCl pH 7.5, and finally with 4 M urea, 10 mM Tris/HCl pH 7.5, 50 μM biotin. For SDS-PAGE analyses, bound material was eluted by boiling beads in SDS sample buffer supplemented with 2 mM biotin. For proteomics analyses, bound proteins were eluted by boiling beads in FASP buffer (4% SDS, 0.1 mM DTT, 0.1M Tris/HCl pH 7.5), and SDS was replaced with 2M urea by filter-assisted sample preparation (FASP) before reduction, alkylation and trypsin digestion as described (Wiśniewski et al., 2009). For comparative proteomics of WT and Ift27−/− cells, beads were directly subjected to alkylation and elution by trypsin digest.
Hedgehog signaling experiments
To visualize trafficking events during Hh signaling, 50.000 cells were seeded onto glass coverslips in 24well plates, grown for 24 h, and serum starved for 16 h in the presence of 200 μM SAG or DMSO as vehicle control before fixation in 4% paraformaldehyde. For Western Blot analyses, 300.000 cells were seeded into 6well plates before treatments as above. Cells were washed in 1xPBS and lysed in buffer (0.3 M NaCl, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 25 mM Tris/HCl pH 7.5, 0.5% Triton-X100, 0.1% SDS, protease inhibitors) for 15 min. Lysates were cleared by centrifugation and protein concentrations determined by Bradford assay. 8 μg of total protein was separated by SDS-PAGE and analyzed by Western Blot using indicated antibodies.
Mass spectrometric analyses
Tryptic peptides were desalted and separated by liquid chromatography before MS-MS analyses. Mass spectra were processed and analyzed to identify proteins according to the mouse Uniprot database. Data was validated at a 2% or 1% false discovery rate. See Supplementary Experimental Procedures for details.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Chris Jacobs, Hiroshi Hamada, Jeff Bergelson, Carsten Janke, Tamara Caspary, Kathryn Anderson, Saikat Mukhopadhyay, Suzie Scales, Michel Bornens and Sophie Saunier for the gifts of antibodies, Iain Cheeseman and Mark Scott for the gifts of cDNAs, the DuBois lab for help with synthesis of biotin-phenol, David Martinelli for the gift of mouse cortical neurons, Anna Okumu, Krysta D. Wyatt, and Jaclyn Lee for technical assistance and Bianca Schrul and the Nachury lab for helpful comments, careful reading of the manuscript and discussions. This work was supported in part by NIH grants to M.V.N (GM089933) and S.P.G (GM67945) and a Stanford Dean of Research-SUMS Seed Grant to M.V.N. D.U.M. was supported by an EMBO long-term fellowship and an A.P. Giannini Foundation fellowship. SUMS acknowledges support from NCRR (S10RR027425).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS D.U.M. and M.V.N. conceived the project and wrote the paper with input from the other authors. D.U.M. established the cilia-APEX workflow, designed and performed the experiments, and analyzed the data. R.D.L, C.M.A. and A.S.C. helped to establish the mass spectrometry workflow and performed and analyzed the mass spectrometry experiments to define the cilia-APEX proteome. R.B.R, R.D.L, and S.P.G. performed the mass spectrometry experiments and analyzed the data for the comparative cilia-APEX proteomics.
The authors declare no conflict of interest.
REFERENCES
- Anholt RR. Molecular physiology of olfaction. Am J Physiol. 1989;257:C1043–C1054. doi: 10.1152/ajpcell.1989.257.6.C1043. [DOI] [PubMed] [Google Scholar]
- Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S. Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J Cell Sci. 2010;123:62–69. doi: 10.1242/jcs.060020. [DOI] [PubMed] [Google Scholar]
- Barzi M, Kostrz D, Menendez A, Pons S. Sonic Hedgehog-induced proliferation requires specific Gα inhibitory proteins. Journal of Biological Chemistry. 2011;286:8067–8074. doi: 10.1074/jbc.M110.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias MB, Hildebrandt F, Pellman D, Woods G, Godinho SA. Centrosomes and cilia in human disease. Trends Genet. 2011;27:307–315. doi: 10.1016/j.tig.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Gödel M, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botilde Y, Yoshiba S, Shinohara K, Hasegawa T, Nishimura H, Shiratori H, Hamada H. Cluap1 localizes preferentially to the base and tip of cilia and is required for ciliogenesis in the mouse embryo. Dev Biol. 2013;381:203–212. doi: 10.1016/j.ydbio.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Breslow D, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol. 2013;203:129–147. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife. 2015;4 doi: 10.7554/eLife.05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine R, Zhang H, Gerstner CD, Frederick JM, Baehr W. Uncoordinated (UNC)119: coordinating the trafficking of myristoylated proteins. Vision Res. 2012;75:26–32. doi: 10.1016/j.visres.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein-1 (MUPP-1) within the tight junction. J Biol Chem. 2004;279:48079–48084. doi: 10.1074/jbc.M409061200. [DOI] [PubMed] [Google Scholar]
- Craige B, Tsao C-C, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc Natl Acad Sci U S A. 2009;106:11143–11148. doi: 10.1073/pnas.0811227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner AA, Wegmann F, Butz S, Wolburg-Buchholz K, Wolburg H, Mack A, Nasdala I, August B, Westermann J, Rathjen FG, et al. Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development. J Cell Sci. 2005;118:3509–3521. doi: 10.1242/jcs.02476. [DOI] [PubMed] [Google Scholar]
- Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW, et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell. 2014;31:279–290. doi: 10.1016/j.devcel.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat-Karalar EN, Rauniyar N, Yates JR, Stearns T. Proximity interactions among centrosome components identify regulators of centriole duplication. Curr Biol. 2014;24:664–670. doi: 10.1016/j.cub.2014.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghossoub R, Hu Q, Failler M, Rouyez M-C, Spitzbarth B, Mostowy S, Wolfrum U, Saunier S, Saunier S, Cossart P, et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J Cell Sci. 2013;126:2583–2594. doi: 10.1242/jcs.111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney-Leo AM, Krug P, Filhol E, Davis EE, et al. Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am J Hum Genet. 2013;93:915–925. doi: 10.1016/j.ajhg.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wittekind SG, Barr MM. STAM and Hrs down-regulate ciliary TRP receptors. Mol Biol Cell. 2007;18:3277–3289. doi: 10.1091/mbc.E07-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, et al. Inactivation of a Gα(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Thompson J, Yates JR, Marshall WF. Proteomic analysis of mammalian primary cilia. Curr Biol. 2012;22:414–419. doi: 10.1016/j.cub.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob LS, Wu X, Dodge ME, Fan C-W, Kulak O, Chen B, Tang W, Wang B, Amatruda JF, Lum L. Genome-Wide RNAi Screen Reveals Disease-Associated Genes That Are Common to Hedgehog and Wnt Signaling. Science Signaling. 2011;4:ra4. doi: 10.1126/scisignal.2001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Klooster, ten JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiological Reviews. 2009;89:777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton DR, Zheng JH, Eyck, Ten LF, Xuong NH, Taylor SS, Sowadski JM. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. Faseb J. 2010;24:2859–2868. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat Methods. 2015;12:51–54. doi: 10.1038/nmeth.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J-P, Tucholska M, Go C, Knight JDR, Gingras A-C. Proximity biotinylation and affinity purification are complementary approaches for the interactome mapping of chromatin-associated protein complexes. J Proteomics. 2015;118:81–94. doi: 10.1016/j.jprot.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Brown JM, Sampaio JL, Craft JM, Shevchenko A, Evans JE, Witman GB. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J Cell Biol. 2013;201:249–261. doi: 10.1083/jcb.201207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-H, Luo J, Mosley Y-YC, Hedrick VE, Paul LN, Chang J, Zhang G, Wang Y-K, Banko MR, Brunet A, et al. AMP-Activated Protein Kinase Directly Phosphorylates and Destabilizes Hedgehog Pathway Transcription Factor GLI1 in Medulloblastoma. Cell Rep. 2015;12:599–609. doi: 10.1016/j.celrep.2015.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew GM, Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow D, Gygi SP, et al. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell. 2014;31:265–278. doi: 10.1016/j.devcel.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee S-O, Splinter PL, Stroope AJ, LaRusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Mayer U, Ungerer N, Klimmeck D, Warnken U, Schnölzer M, Frings S, Möhrlen F. Proteomic analysis of a membrane preparation from rat olfactory sensory cilia. Chem Senses. 2008;33:145–162. doi: 10.1093/chemse/bjm073. [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, Boularan C, Ghossoub R, Scott MGH, Burtey A, Zarka M, Saunier S, Concordet J-P, Marullo S, Benmerah A. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS ONE. 2008;3:e3728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Rohatgi R. G-protein-coupled receptors, Hedgehog signaling and primary cilia. Semin Cell Dev Biol. 2014;33:63–72. doi: 10.1016/j.semcdb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Rangell L, Scales SJ, Jackson PK. The Ciliary G-Protein-Coupled Receptor Gpr161 Negatively Regulates the Sonic Hedgehog Pathway via cAMP Signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Nachury MV. How do cilia organize signalling cascades? Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2014;369:20130465–20130465. doi: 10.1098/rstb.2013.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Shiba D, Kobayashi D, Yokoyama T. Targeting of Nphp3 to the primary cilia is controlled by an N-terminal myristoylation site and coiled-coil domains. Cytoskeleton. 2012;69:221–234. doi: 10.1002/cm.21014. [DOI] [PubMed] [Google Scholar]
- Nikonova AS, Plotnikova OV, Serzhanova V, Efimov A, Bogush I, Cai KQ, Hensley HH, Egleston BL, Klein-Szanto A, Seeger-Nukpezah T, et al. Nedd9 restrains renal cystogenesis in Pkd1−/− mice. Proc Natl Acad Sci U S A. 2014;111:12859–12864. doi: 10.1073/pnas.1405362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocbina PJR, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res. 2009;315:2802–2817. doi: 10.1016/j.yexcr.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek RC, Berbari NF, Lewis WR, Kesterson RA, Yoder BK. Mammalian Clusterin associated protein 1 is an evolutionarily conserved protein required for ciliogenesis. Cilia. 2012;1:20. doi: 10.1186/2046-2530-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzke S, Redick S, Warsame A, Murga-Zamalloa CA, Khanna H, Doxsey S, Stokke T. CSPP is a ciliary protein interacting with Nephrocystin 8 and required for cilia formation. Mol Biol Cell. 2010;21:2555–2567. doi: 10.1091/mbc.E09-06-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusapati GV, Hughes CE, Dorn KV, Zhang D, Sugianto P, Aravind L, Rohatgi R. EFCAB7 and IQCE regulate hedgehog signaling by tethering the EVC-EVC2 complex to the base of primary cilia. Dev Cell. 2014;28:483–496. doi: 10.1016/j.devcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rhee H-W, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, Ting AY. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339:1328–1331. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci USA. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. doi:10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JC, Arthanari H, Boeszoermenyi A, Dashkevich NM, Wilson-Kubalek EM, Monnier N, Markus M, Oberer M, Milligan RA, Bathe M, et al. The kinetochore-bound Ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev Cell. 2012;23:968–980. doi: 10.1016/j.devcel.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrøder JM, Christensen S, Schrøder JM, Schneider L, Schneider L, Christensen ST, Pedersen LB. EB1 is required for primary cilia assembly in fibroblasts. Curr Biol. 2007;17:1134–1139. doi: 10.1016/j.cub.2007.05.055. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Jensen SS, Lim JWE. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- Sollerbrant K, Raschperger E, Mirza M, Engström U, Philipson L, Ljungdahl PO, Pettersson RF. The Coxsackievirus and adenovirus receptor (CAR) forms a complex with the PDZ domain-containing protein ligand-of-numb protein-X (LNX) J Biol Chem. 2003;278:7439–7444. doi: 10.1074/jbc.M205927200. [DOI] [PubMed] [Google Scholar]
- Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello DA, Waxse BJ, Arden SD, Bright NA, Kendrick-Jones J, Buss F. Autophagy receptors link myosin VI to autophagosomes to mediate Tom1-dependent autophagosome maturation and fusion with the lysosome. Nat Cell Biol. 2012;14:1024–1035. doi: 10.1038/ncb2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuson M, He M, Anderson KV. Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development. 2011;138:4921–4930. doi: 10.1242/dev.070805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuolo L, Herrera A, Torroba B, Menendez A, Pons S. Ciliary adenylyl cyclases control the Hedgehog pathway. J Cell Sci. 2015;128:2928–2937. doi: 10.1242/jcs.172635. [DOI] [PubMed] [Google Scholar]
- Wang J, Silva M, Haas LA, Morsci NS, Nguyen KCQ, Hall DH, Barr MM. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014;24:519–525. doi: 10.1016/j.cub.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Lai CK, Evangelista M, Hongo J-A, de Sauvage FJ, Scales SJ. Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol. 2010;30:1910–1922. doi: 10.1128/MCB.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr Biol. 2013;23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Zhang Y, Wei Q, Huang Y, Li Y, Ling K, Hu J. BBS4 and BBS5 show functional redundancy in the BBSome to regulate the degradative sorting of ciliary sensory receptors. Sci Rep. 2015;5:11855. doi: 10.1038/srep11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DMF. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326:1707–1711. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC. Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci U S A. 2011;108:20678–20683. doi: 10.1073/pnas.1113220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum Mol Genet. 2012;21:1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.