Abstract
Tauopathies are a class of neurodegenerative disorders characterized by neuronal and/or glial inclusions composed of the microtubule-binding protein, tau. Several lines of evidence suggest tau aggregation is central to the neurodegenerative process in tauopathies. First, recent animal and cell model studies find abnormally-modified tau alone may be transmitted between adjacent neurons and spread to anatomically connected brain regions to recapitulate human disease. Further, staging efforts in human autopsy cases suggest a sequential distribution of tau aggregation in the central nervous system that could reflect this observed cell-to-cell transmission of pathogenic tau species in animal models. Finally, pathogenic mutations in the MAPT gene encoding tau protein cause hereditary forms of tauopathy.
Clinically, tauopathies can present with a range of phenotypes that include both movement- and cognitive/behavioral-disorders (i.e. frontotemporal dementia spectrum disorders) or non-specific amnestic symptoms in advanced age. A major limitation is that current clinical diagnostic criteria for these disorders do not reliably differentiate underlying tauopathy from other neurodegenerative diseases, such as TDP-43 proteinopathies. Thus, current research efforts are focused on improving the ante mortem diagnosis of tauopathies, including pre-clinical stages of disease, as many therapeutic strategies for emerging disease-modifying therapies focus on preventing abnormal folding and spread of tau pathology.
Keywords: Progressive supranuclear palsy, corticobasal degeneration, corticobasal syndrome tauopathy, Pick’s disease, Frontotemporal dementia, Frontotemporal lobar degeneration, Primary progressive aphasia, MAPT mutation, Argyrophilic grain disease, primary age related tauopathy
INTRODUCTION
Age-associated neurodegenerative diseases are characterized by specific key protein inclusions, accompanied with neuronal loss and gliosis. A major class of neurodegenerative diseases, collectively known as tauopathies, are characterized by intra-cellular inclusions composed of abnormally-modified microtubule-binding protein, tau, at autopsy. Primary tauopathies are a major class of Frontotemporal Lobar Degeneration (FTLD) neuropathology (i.e. FTLD-Tau) [1] and can present clinically with several forms of Frontotemporal Dementia (FTD) clinical syndromes (i.e. behavioral-variant FTD, bvFTD [2]; primary progressive aphasia, PPA [3]), including atypical dopaminergic-resistant Parkinsonian syndromes with prominent extra-pyramidal symptoms (i.e. progressive supranuclear palsy syndrome, PSPS [4]; corticobasal syndrome CBS [5]). For a comprehensive review of clinicopathological correlations in FTLD-Tau from large autopsy series please see [6]. Other primary tauopathies can be associated with mild or poorly differentiated amnestic symptoms late in life [7, 8]. Finally, Alzheimer’s disease (AD) neuropathology includes significant neurofibrillary tau neuropathology in addition to amyloid-beta (Aβ) plaques and can be considered a secondary or non-primary tauopathy, although these classifications are currently a matter of considerable debate. AD neuropathology can also have atypical clinical presentations as FTD clinical syndromes. These discordant relationships pose a significant problem for accurate diagnosis and the gold standard is neuropathological examination at autopsy; thus, tauopathies are best viewed as clinicopathological entities, that is, the underlying neuropathological substrate and the resultant clinical syndrome.
This view is important in light of emerging evidence from in vivo models of tauopathies. Recent data from several groups find that tau may undergo a self-templating process to recruit normal soluble tau to form and propagate insoluble tau fibrils between neurons within the CNS in transgenic animals or between neurons in cell culture systems (For a comprehensive review of transmission studies please see [9]). Notably, intracerebral injections of synthetic tau fibrils alone into transgenic mice found a time- and dose-dependent sequential topographical spread of tau aggregations in the CNS [10]. These animal model findings are similar to the non-random sequential patterns of tau deposition in human diseases such as Alzheimer’s Disease (AD), originally described by Braak and colleagues in the early 1990s [11], and more recently observed in a primary tauopathy, Pick’s disease (PiD) (Irwin DJ, Brettschneider JB, McMillan CT, Cooper F, Olm C, Arnold SE, VanDeerlin VM, Seeley WW, Miller BL, Lee VMY, Grossman M and Trojanowski JQ, 2015 unpublished data). Further, intracerebral injections of tau lysates from different forms of tauopathy into transgenic mice results in similar inclusion morphologies in recipient animals to human disease, which could suggest abnormally folded tau could exist as different strains [12]. Tau transmission in these studies is similar to the observations in human prion disease, where misfolded PRPSC protein seeds fibrillization of native PRPC to cause the spread of misfolded prion throughout the CNS accompanied by neurodegeneration; however in contrast to human prion disease there is no current definitive evidence of infectivity (i.e. transmission of disease) between humans or non-human primates for AD or FTLD-Tau, even in extreme circumstances such as exposure to human CNS tissue [13].
These emerging findings for transmission and propagation of pathogenic tau aggregations in tauopathies suggest that slowing or halting this process may be an important strategy for therapeutic developments for tauopathies. Indeed, there are several approaches possible for potential therapies targeting tau [14] and clinical trials for tau-directed immunotherapy are currently underway (NCT02460094, NCT02494024). Thus, accurate ante mortem diagnosis of tauopathies is critical for the evaluation of these disease-modifying therapies targeting tau. This review will describe the major classes of tauopathy and key clinicopathological relationships to emphasize the importance of ante mortem diagnosis of these conditions.
NEUROPATHOLOGIC SUBTYPES AND CLINICOPATHOLOGICAL CORRELATIONS
General Features of Tauopathies
The tau protein is normally found in the cytosol of neurons and glial cells in the central nervous system (CNS) and its function is to bind microtubules to stabilize the cell cytoskeleton (For comprehensive review on tau please see [14]). Tau exists in 6 isoforms based on the presence of 0, 1 or 2 sequence inserts in the amino-terminus of the protein and inclusion or exclusion of the second of four microtubule-binding potential repeat domains (MTBD) coded by exon 10. Tauopathies are classified by the predominance of tau isoforms found in cytoplasmic inclusions: those with inclusions predominantly composed of tau with 3 MTBDs (i.e. 3R-tauopathies), those with predominantly 4 MTBDs (i.e. 4R-tauopathies) or an equal ratio of 3R:4R tau. Tau normally exists in an equal ratio of 3R:4R tau in non-disease states. Tau undergoes several post-translational modifications, most notably phosphorylation at multiple serine and threonine sites, which regulates microtubule binding under normal conditions. In pathological conditions of tauopathy tau is hyperphosphorylated. Other modifications include, acetylation, nitration, glycation, conformational change and C-terminal truncation. Some of these modifications are found only in insoluble tau aggregations associated with disease and not in healthy tissue, such as acetylation of tau at lysine 280 (ac-K280) in the second MTBD of 4R tau [15, 16]. A hypothetical sequence of tau modifications has been proposed to occur during the process of tangle formation. This sequence of modifications is based on the abundance or absence of specific tau epitopes in neurofibrillary tau pathology seen in the hippocampus across mild to severe AD cases [17, 18]. Interestingly, tauopathies universally express “early tau modifications” (i.e. phosphorylation, conformational change), while later-occurring modifications (i.e. C-terminal truncation) appear to be more prominent in in extracellular ghost tangles from degenerated neurons seen only in AD [16, 18]. Further, reactivity to amyloid-binding dyes, such as Thioflavin-S (ThS) or silver impregnation methods, which bind to more fibrillary tau deposits vary among tauopathies [16, 19, 20]. Tauopathy transmission data support these hypotheses as more mature tau pathology induced from intracerebral injections of tau fibrils are both ThS and ac-K280 positive [10]. Ultrastructural analysis finds AD tau neurofibrillary pathology is composed of tightly paired-helical filaments, while other tauopathies may have less compact straight or twisted ribbon filament [8]. Finally tauopathies vary in the cell types (i.e. neurons or glia) and anatomical regions (i.e. limbic/neocortex, basal ganglia and brainstem) most vulnerable to tau-mediated neurodegeneration.
FTLD-Tau with MAPT mutation
There are over 40 known pathogenic mutations in the MAPT tau gene (chromosome 17) which result in tauopathy (i.e. FTLD-Tau with MAPT mutation) (For comprehensive review of specific MAPT mutations please see [21]). MAPT pathogenic mutations are thought to cause disease by either 1) inhibiting the normal microtubule-binding function of tau, 2) promoting tau protein aggregation or 3) affecting the splicing of exon 10 to result in imbalances between 3R/4R tau isoforms. As such, specific neuropathological findings (e.g. tau isoform predominance, inclusion morphology/ultrastructure) vary for each specific mutation, but universally include neuronal and glial tau inclusions together with neurodegeneration throughout the CNS, with a particular propensity for frontal and temporal neocortex and limbic structures [21]. The majority of cases are negative for ThS and those with 4R tau pathology reactive for ac-K280 [19]. Clinically FTLD-Tau with MAPT mutations may present with FTD behavioral (i.e. behavioral-variant FTD; bvFTD) and/or language syndromes (i.e. primary progressive aphasia; PPA) and often times have extra-pyramidal features (Parkinsonism). The majority of mutations are inherited in an autosomal dominant pattern and usually with a high-degree of penetrance. The age of onset varies by specific mutation, but in general disease onset is between ages 45-65 with a wide variation in disease duration (average ~10 years); although cases in the second and third decade and also eighth decade may occur.
4R Tauopathies: Progressive Supranuclear Palsy
Progressive supranuclear palsy (PSP) is a 4R tauopathy characterized by globose tau inclusions in brainstem and subcortical neurons, in addition to glial “tufted astrocytes” and neuronal tangles in grey matter and oligodendrocytic “coiled bodies” in the white matter of the neocortex [8, 16] . There is prominent tau pathology in the brainstem, sub-thalamic nucleus and dentate nucleus of the cerebellum. PSP tau pathology is mildly reactive for Thioflavin-S and robustly reactive for ac-K280 [16]. The most common clinical presentation is PSP syndrome (PSPS), an age-associated atypical Parkinsonian syndrome characterized by prominent axial rigidity and poor response to dopaminergic therapy. The clinical diagnosis of PSPS, especially clinical findings of supranuclear gaze palsy and early postural instability/falls, are very specific for underlying PSP tauopathy [4]; however, PSPS is not sensitive to detect all cases of PSP neuropathology, as patients with clinical features of the non-fluent variant of PPA (naPPA), corticobasal syndrome (see below) or bvFTD (reviewed in [6]). Further, cognitive and behavioral features of PSPS are being increasingly recognized and may occur at any point during disease course. The disease is largely sporadic, but rare MAPT mutations may present with neuropathological findings indistinguishable from sporadic PSP [21]. Genetic polymorphism in the MAPT gene has been linked to increased risk for PSP; presence of a haplotype of an inverted sequence of polymorphisms in linkage disequilibrium (i.e. H1 haplotype) in MAPT has been linked to increased risk of PSPS in Caucasian populations and a recent genome-wide association study (GWAS) for autopsy-confirmed PSP found several additional common polymorphisms that conferred risk for PSP [22]. Further research into these and other genetic risk factors will help elucidate cellular mechanisms of disease.
4R Tauopathies: Corticobasal Degeneration
There is considerable clinicopathological overlap between corticobasal degeneration (CBD) and PSP. Indeed, neuropathological findings consist of 4R tauopathy with large tau-positive diffuse “astrocytic plaques” and “ballooned neurons” in grey matter of limbic and neocortical structures. CBD is also associated with a large burden of tau-positive “coiled bodies” and astrocytic tau inclusions in white matter. The basal ganglia and brainstem contain a heavy burden of inclusions as well, and sometimes the morphology be difficult to distinguish from PSP [8]. Gross atrophy is often peri-rolandic and asymmetric. CBD tauopathy is not reactive to ThS [20] but is reactive with ac-K280 [16]. The term “Corticobasal Degeneration” was previously used to describe the clinical syndrome of asymmetric Parkinsonism, apraxia, executive and parietal lobe dysfunction currently known as corticobasal syndrome (CBS) [5]. This change in nomenclature was necessitated by findings from large-scale autopsy studies in CBS that <50% of CBS patients had CBD neuropathology at autopsy; CBS can also be due to several alternative underlying neuropathologies including AD and less commonly TDP-43 proteinopathies and PSP (reviewed in [6]). The poor specificity of CBS for CBD neuropathology necessitated the development of recent clinical research criteria for CBS [5] and validation of these criteria are ongoing to help improve ante mortem identification of CBD tauopathy. CBS poses a serious clinical diagnostic challenge as patients may have mixed features of extra-pyramidal and higher cortical processing difficulties and thus, may present to either movement disorder or cognitive specialists for evaluation.
There are no established environmental risk factors for CBD and there is minimal epidemiological data on prevalence of the disorder. CBD has been shown to share genetic risk with PSP, including the H1 haplotype in MAPT [23].
4R Tauopathies: Argyrophilic Grain Disease
Argyrophilic grain disease (AGD) is a tauopathy characterized by 4R-tau predominant spindle-shaped “grains” largely restricted to the hippocampus and amygdala. Grains are largely ThS positive and react with Gallyas silver stain (i.e. argyrophilic) [8] in addition to ac-K280 [19]. These findings are accompanied by “pre-tangle” tau inclusions in the cornu ammonis of the hippocampus, “coiled bodies” in white matter and “ballooned neurons” in the amygdala. These changes are often accompanied by tau neurofibrillary tangles that are biochemically indistinguishable from AD. Less commonly grains and associated tau pathology are more wide-spread throughout the neocortex. There is no specific clinical syndrome that predicts AGD, as this pathology can be found at autopsy in late-onset amnestic syndromes and less commonly FTD spectrum disorders but large-scale clinicopathological series are lacking (reviewed in [24]).
4R Tauopathies: Globular Glial Tauopathies
Several cases in the literature have been reported to contain a predominance of globular 4R tau inclusions in oligodendorocytes accompanied by star-shaped punctate astrocytic tau inclusions reminiscent of PSP-associated tufted astrocytes (i.e. globular glial inclusions; GGIs) (reviewed in [25]). The regional distribution of GGIs and resultant neuronal loss and atrophy varies on a spectrum of prominent frontotemporal involvement with severe white matter degeneration or motor cortex and corticospinal tract degeneration, with some cases displaying both patterns of regional vulnerability. Recent nomenclature classifies this pattern of prominent oligodendrocytic tauopathy as globular glial tauopathies (GGT) and cases may present clinically with bvFTD, pyramidal motor disease or a combination of both [25].
3R Tauopathies: Pick’s Disease
Pick’s disease (PiD) is a 3R-tau predominant tauopathy that is characterized by round tau-positive intraneuronal “Pick bodies” and gial inclusions through the limbic and neocortical regions [8]. There is a predominance of disease in the frontotemporal neocortex but also significant sub-cortical tau in the basal ganglia and white matter. Pick bodies are weakly reactive to ThS [19]. PiD neuropathology most often presents with a clinical syndrome of bvFTD, but can also be associated with PPA variants and CBS. Similar to afore mentioned nomenclature shifts with CBD, the term “Pick’s Disease” is now used to exclusively as the neuropathological diagnosis of 3R tauopathy with “Pick body” inclusions above due to the poor specificity of the bvFTD clinical diagnosis for PiD tauopathy. Indeed, bvFTD patients can have other tauopathies at autopsy (e.g. PSP, CBD, AGD) and roughly half have a primary neuropathological diagnosis of TDP-43 proteinopathy (reviewed in [6]). Further, a small percentage of bvFTD are found to have AD neuropathology at autopsy as well. Thus, further refinement of the social comportment disorder of bvFTD is needed to more accurately predict PiD and other tauopathies. Autopsy studies of PPA variants also find varying frequencies of underlying tauopathies; naPPA is more commonly often due to tauopathies (e.g. CBD, PSP) while semantic variant PPA (svPPA) is largely due to TDP-43 proteinopathies but these associations are not absolute. The presence of extrampyramidal features consistent with PSPS or CBS in bvFTD/PPA may suggest an underlying tauopathy. Thus, a complex relationship between clinical phenotype and underlying neuropathology exists for tauopathies in FTD clinical syndromes.
3/4R Tauopathies: Alzheimer’s disease and Primary Age-Related Tauopathy
AD can be considered a secondary or non-primary tauopathy, as it is pathologically characterized by both intracellular neurofibrillary tangles composed of equal ratios of 3R and 4R tau and Aβ extracellular plaques; however, the exact relationship between amyloid-beta plaques and tau aggregation is still a matter of contention. Indeed, autopsy studies of cognitively normal individuals find pre-tangle inclusions in subcortical structures with wide-spread cortical projections, such as the locus coerulleus, in a significant number of subjects younger than 30 years old [26]. Further, neurofibrillary tangle pathology that is biochemically indistinguishable from AD can occur in the absence of significant Aβ plaque pathology and is largely restricted to the medial temporal lobe. This pathological finding was previously named tangle-predominant senile dementia (TPSD), which was classified as a form of FTLD-Tau [1], and more recently has been re-defined as primary age-related tauopathy (PART) [7]. PART appears to lack association with AD-associated risk polymorphism in apolipoprotein gene (ε4) while having a genetic risk associated with MAPT tau H1 haplotype, and can be associated with minimal or no cognitive impairment in older patients [7]. Some hypothesize that these data suggest that tau neuropathology in PART is an independent disease process from AD [7]; however, others contend that tau and AB neuropathology exist on a spectrum and that hippocampal tau neurofibrillary pathology in the absence of cortical Aβ plaque cannot be clearly separated from the pathophysiological process of AD [27, 28]. Thus, the discrepancies between AD, PART and normal aging are currently unresolved.
Geographically Isolated Tauopathies
Two isolated populations in the Pacific (i.e. Chamorro natives of Guam and inhabitants of the Kii Peninsula of Japan) display a high rate of clinical amyotrophic lateral sclerosis and/or parkinsonism with dementia (ALS-PDC) (For comprehensive review please see [29]) . At autopsy these patients are found to have widespread 3R/4R tauopathy, including in the lower motor neurons in the spinal cord, along with TDP-43 deposits. The geographic isolation of these cases and high degree of family history indicates a possible genetic etiology although thus far none has been identified. Numerous environmental factors have been examined with no clear causative agent identified. Interestingly the prevalence and incidence of ALS-PDC is dramatically declining in Guam.
There is also an abnormally high proportion of patients with atypical Parkinsonism in the island of Guadalupe in the French West Indies [30]. The majority of patients have key features of PSPS along with atypical symptoms of visual hallucinations, REM sleep-behavior disorder and rest-tremor. In the few available autopsies, tau pathology similar to PSP was found. Guadeloupian PSPS patients are from diverse ethnic backgrounds and have minimal positive family history, making a genetic cause less likely. The underlying etiology of these disorders are currently unclear.
CONCLUSIONS
The lack of disease-modifying therapies in tauopathies is a critical unmet need and with emerging evidence for the central role of tau misfolding, aggregation and propogation in disease pathogenesis for tauopathies, many current drug development efforts are directed at preventing pathological tau transmission within the CNS. A major limitation for the implementation of such therapies is the inability to readily detect tauopathies ante mortem, as the gold-standard for diagnosis is autopsy. Novel biomarkers specific for tauopathies, such as in vivo tau imaging, will be critical to address this problem and elucidate of the relationships between aging, AD and PART.
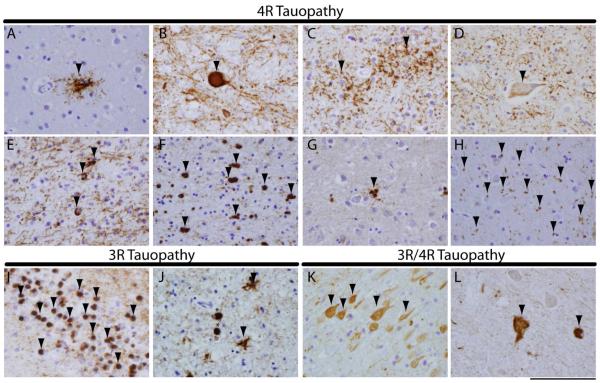
Figure 1. Tauopathy histopathological subtypes.
Photomicrographs of illustrate characteristic tau inclusion morphologies for 4R (a-h), 3R (I,J) and 3R/4R (K,L) primary tauopathies. Characteristic PSP tauopathy including (A) a tufted astrocytes in mid-frontal cortex and (B) a globose neuronal tau tangle in midbrain. CBD characteristic tau pathology showing (C) glial astrocytic plaques in the mid-frontal cortex, (D) a ballooned neuron in the anterior cingulate gyrus and (E) oligodendrocyte coiled bodies in the mid-frontal cortex. Note ballooned neurons and coiled bodies may be present in other tauopathies to a lesser extent. GGT (F) globular oligodendrocytes in white matter and (G) astrocytic tau inclusions in grey matter of the mid-frontal cortex show distinct globular morphology from PSP and CBD tau pathology. AGD associated (H) small comma-shaped 4R tau reactive grains (arrows) in the amygdala. PiD (I) spherical “Pick bodies” seen in the hippocampal dentate gyrus neurons and (J) mid-frontal cortex (asterisks), along with glial tau inclusions (arrows). PART (K) neurofibrillary tau tangles seen in the cornu ammonis of the hippocampus. ALS-PDC spinal cord with (L) tau-reactive tangles in lower motor neurons of the spinal cord. PHF-1 stain. Scale bar=100 µm.
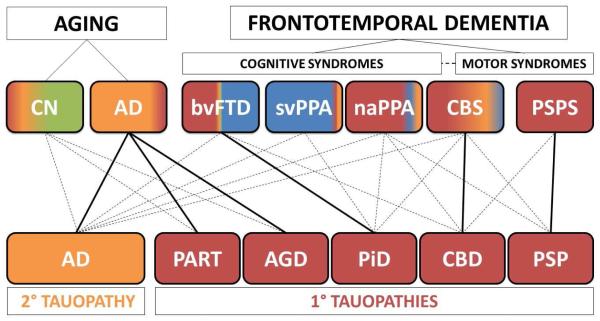
Figure 2. Clinicopathological correlations in tauopathies.
The scheme portrays (top) the relative frequencies of neuropathological subtypes of FTLD-Tau (i.e. primary tauopathies-red) and AD (i.e. secondary tauopathy-yellow) seen at autopsy in clinical phenotypes in FTD-spectrum disorders and aging by the color-coding of each box. Unremarkable neuropathological findings are shaded in green and TDP-43 protienopathies in blue (this schematic does not account for cerebrovascular changes, co-morbid pathology or less common neurodegenerative diseases). FTD clinical phenotypes are divided by cognitive and motor syndromes; the dashed line represents the clinical overlap between FTD cognitive and motor syndromes and CBS is placed intermediate to these categories as this clinical syndrome has aspects of both cognitive and motor dysfunction. AD neuropathology (yellow) is found in approximately a third of older patients who are cognitively normal (CN) and is seen in the majority amnestic AD clinical phenotype. FTLD-Tau (red) is found in virtually all PSPS cases and the majority of naPPA. FTLD-Tau is also found in a significant proportion of CBS and relatively rare in svPPA. Roughly half of bvFTD cases harbor FTLD-Tau while a small percentage can have AD neuropathology (yellow). Solid lines represent the predominant clinicopathological association for each specific tauopathy (bottom) while dashed lines represent less common clinical manifestations of each tauopathy. AD neuropathology is most commonly associated with the amnestic AD clinical phenotype but may also present as bvFTD, PPA variants and CBS. PART and AGD are largely associated with late onset (>80 years) amnestic syndrome similar to clinical AD and less commonly in CN individuals. PiD is most commonly found in association with bvFTD but can present with PPA variants or CBS. CBD tauopathy is primarily associated with CBS but also can manifest as bvFTD, naPPA or PSPS, while PSP is predominantly associated with PSPS and less commonly associated with naPPA or CBS.
Highlights.
Tauopathies have heterogeneous underlying neuropathology
Tauopathies cause a range of clinical phenotypes including atypical Parkinsonism
Gold-standard diagnosis of tauopathies is obtained only at autopsy
Understanding clinicopathological associations will improve ante mortem diagnosis
Accurate ante mortem diagnosis is critical for evaluation of tau-directed therapies
ACKNOWLEDGEMENTS
Funding for this work was provided by NIH/NINDS grant NS088341. Photomicrographs were obtained by tissues generously provided by Drs. John Q. Trojanowski and Virginia M.Y. Lee at the Penn Center for Neurodegenerative Disease Research brain bank.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
XXI World Congress on Parkinson’s Disease and Related Disorders Session “Translational Parallel Session” 1.21 What is new in Tauopathies- December 6th 15:00-15:30.
REFERENCES
- [1].Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta neuropathologica. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- [5].Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, et al. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta neuropathologica. 2015;129:469–91. doi: 10.1007/s00401-014-1380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta neuropathologica. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dickson D. Sporadic Tauopaties: Pick's disease, corticobasal degeneration, progressive supranuclear palsy and argyrophilic grain disease. In: Esiri MLVM-Y, Trojanowski JQ, editors. The Neuropathology of Dementia. 2ed Cambridge University Press; NY, NY: 2004. [Google Scholar]
- [9].Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nature medicine. 2014;20:130–8. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1024–37. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [12].Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9535–40. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, Lee VM, et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA neurology. 2013;70:462–8. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee V, Brunden K, Hutton M, Trojanoswki JQ. Developing therapeutic approaches to tau, selected kinases, and related neuronal protein targets. In: Selkoe D, Holtzman DM, Mandelkow E, editors. The Biology of Alzheimer Disease. 1. Cold Spring Harbor Labratory Press; Cold Spring Harbor, NY: 2012. pp. 416–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX, Lee VM, et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain : a journal of neurology. 2012;135:807–18. doi: 10.1093/brain/aws013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, et al. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiology of aging. 2005;26:1015–22. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [18].Guillozet-Bongaarts AL, Glajch KE, Libson EG, Cahill ME, Bigio E, Berry RW, et al. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta neuropathologica. 2007;113:513–20. doi: 10.1007/s00401-007-0209-6. [DOI] [PubMed] [Google Scholar]
- [19].Irwin DJ, Cohen TJ, Grossman M, Arnold SE, McCarty-Wood E, Van Deerlin VM, et al. Acetylated tau neuropathology in sporadic and hereditary tauopathies. The American journal of pathology. 2013;183:344–51. doi: 10.1016/j.ajpath.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schmidt ML, Schuck T, Sheridan S, Kung MP, Kung H, Zhuang ZP, et al. The fluorescent Congo red derivative, (trans, trans)-1-bromo-2,5-bis-(3-hydroxycarbonyl-4-hydroxy)styrylbenzene (BSB), labels diverse beta-pleated sheet structures in postmortem human neurodegenerative disease brains. The American journal of pathology. 2001;159:937–43. doi: 10.1016/s0002-9440(10)61769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Forman M, Trojanoswki JQ, Lee VM-Y. Hereditary Tauopathies and Idiopathic Frontotemporal Dementias. In: Esiri MLVM-Y, Trojanowski JQ, editors. The Neuropathology of Dementia. 2ed Cambridge University Press; NY, NY: 2004. [Google Scholar]
- [22].Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature genetics. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun. 2015;6:7247. doi: 10.1038/ncomms8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain : a journal of neurology. 2008;131:1416–32. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- [25].Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B, et al. Globular glial tauopathies (GGT): consensus recommendations. Acta neuropathologica. 2013;126:537–44. doi: 10.1007/s00401-013-1171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta neuropathologica. 2011;121:171–81. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- [27].Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta neuropathologica. 2014;128:767–772. doi: 10.1007/s00401-014-1356-1. [DOI] [PubMed] [Google Scholar]
- [28].Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M, et al. PART is part of Alzheimer disease. Acta neuropathologica. 2015;129:749–56. doi: 10.1007/s00401-015-1390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70:1984–90. doi: 10.1212/01.wnl.0000312571.81091.26. [DOI] [PubMed] [Google Scholar]
- [30].Caparros-Lefebvre D, Sergeant N, Lees A, Camuzat A, Daniel S, Lannuzel A, et al. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain : a journal of neurology. 2002;125:801–11. doi: 10.1093/brain/awf086. [DOI] [PubMed] [Google Scholar]