Abstract
OBJECTIVE
To prospectively examine the association of plasma γ’ fibrinogen with the incidence of multiple cardiovascular disease (CVD) endpoints, independent of established CVD risk factors, total fibrinogen and other inflammatory markers.
APPROACH AND RESULTS
The Atherosclerosis Risk in Communities study measured γ’ fibrinogen by enzyme-linked immunosorbent assay in stored plasma samples from 1993 to 1995 and related levels in 10,601adults to incident CVD endpoints (coronary heart disease [n = 1603], ischemic stroke [n = 548], peripheral artery disease [n=599], heart failure [n = 1411], and CVD mortality [n = 705]) through 2012 (median follow-up 18 years). In Cox models accounting for established CVD risk factors and total fibrinogen levels, γ’ fibrinogen was associated positively with peripheral artery disease (HR per 1 standard deviation (8.80 mg/dl) increment: 1.14, 1.04-1.24), heart failure (HR: 1.06, 1.01-1.13) and CVD deaths (HR: 1.12, 1.04-1.21), but not with incident coronary heart disease (HR: 1.01, 0.96-1.07) or ischemic stroke (HR: 0.98, 0.89-1.07). Additional adjustment for C-reactive protein, however, eliminated the associations with peripheral artery disease and heart failure.
CONCLUSIONS
These findings do not lend support to the hypothesis that γ’ fibrinogen influences CVD events through its pro-thrombotic properties. Rather, γ’ fibrinogen concentrations seem to reflect general inflammation that accompanies and may contribute to atherosclerotic CVD, instead of γ’ fibrinogen being a causal risk factor.
Keywords: fibrinogen, thrombosis, epidemiology
Introduction
Plasma fibrinogen is a coagulation factor and an acute-phase inflammatory marker that has been implicated in the pathophysiology of cardiovascular disease (CVD)1. Several epidemiologic studies have shown an independent, positive association between elevated levels of fibrinogen and CVD, with hypothesized mechanisms relating to increased plasma viscosity or the size and strength of thrombi 1-4. However, a study utilizing Mendelian randomization suggest the epidemiological association may not be causal 5. Fibrinogen is also associated positively with several established CVD risk factors, so elevated fibrinogen may be one pathway by which these CVD risk factors exert their influence on the cardiovascular system 4, 6, 7.
Fibrinogen is a six-chain molecule containing two copies each of the Aα, Bβ, and γ chains, with the latter having two isoforms γA and γ’ arising from alternative mRNA processing 8, 9. γ’ fibrinogen constitutes approximately 7% of plasma fibrinogen with higher levels found among individuals with pathological conditions 9.. Recent evidence suggests that higher plasma concentrations of γ’ fibrinogen yield thrombi that are very resistant to fibrinolysis 8, 9, which provides novel hypotheses to explain the relation between fibrinogen and CVD events 10. Accordingly, some studies have observed a positive association between γ’ fibrinogen and atherothrombotic events independent of total plasma fibrinogen levels 11-15. However, the retrospective or cross-sectional design of these studies which were conducted among individuals already diagnosed with CVD events hampers the determination of the temporality of this association and a prospective design could reveal new perspectives on the association of γ’ fibrinogen with CVD events.
Therefore, the aim of this study was to prospectively investigate the association of plasma γ’ fibrinogen with the incidence of multiple cardiovascular disease endpoints, independent of established CVD risk factors, total fibrinogen and other inflammatory markers, among participants enrolled in the Atherosclerosis Risk in Communities (ARIC) study, a biracial cohort of white and black men and women.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Among the 10, 601 participants free of CVD at ARIC visit 3, the mean age was 60 years, 57% were females, and 23% were African-Americans. Approximately one third of participants were on antihypertensive medications, 17% were current smokers, and 13% had diabetes. Median γ’ fibrinogen levels were modestly higher in females than males (29.8 vs. 28.6 mg/dL) and African-Americans than whites (31.6 vs. 28.7 mg/dL). The Spearman correlation coefficient between γ’ fibrinogen and total fibrinogen was 0.44. This correlation was larger for participants in whom γ’ fibrinogen and total fibrinogen were measured from the same blood draw at ARIC visit 3 (r = 0.60). The distribution of characteristics of participants stratified by quartiles of γ’ fibrinogen is presented in Table 1. The levels of γ’ fibrinogen showed positive associations with age, systolic blood pressure, BMI, current smoking status, diabetes, total fibrinogen, and CRP, and negative associations with alcohol intake, sports-related physical activity, HDL-cholesterol, and lipid-lowering medication use.
Table 1.
Characteristics of participants according to quartiles of γ’ fibrinogen, the ARIC study, 1993-1995
| γ’ fibrinogen quartiles, mg/dl |
||||
|---|---|---|---|---|
| Characteristics | Q1 8.0 - 24.34 |
Q2 24.35 - 29.26 |
Q3 29.27 - 35.18 |
Q4 35.19 – 80.28 |
| N | 2651 | 2648 | 2652 | 2650 |
| Age (years) | 59.2 (5.6) | 59.5 (5.6) | 59.7 (5.7) | 60.3 (5.7) |
| Female (%) | 52.4 | 55.2 | 58.9 | 62.3 |
| Race, Black (%) | 15.1 | 20.1 | 23.6 | 31.2 |
| Education (%) | ||||
| < High school | 15.9 | 18.8 | 18.5 | 22.1 |
| High school | 42.7 | 42.2 | 41.6 | 41.4 |
| > High school | 41.4 | 39.0 | 39.9 | 36.5 |
| Smoking status (%) | ||||
| Former | 43.5 | 42.2 | 38.8 | 35.0 |
| Current | 13.4 | 14.9 | 17.9 | 22.0 |
| Alcohol intake (g/d) | 7.2 (16.0) | 5.9 (14.3) | 5.5 (16.8) | 5.0 (15.5) |
| Systolic blood pressure (mm Hg) | 122.4 (17.4) | 123.4 (18.6) | 124.0 (19.2) | 126.2 (19.9) |
| Hypertension meds (%) | 26.1 | 29.0 | 32.3 | 40.1 |
| Diabetes (%) | 10.0 | 11.7 | 13.8 | 16.8 |
| Body mass index (kg/m2) | 27.3 (4.6) | 27.9 (5.1) | 28.5 (5.4) | 29.5 (6.2) |
| Sports physical activity score | 2.6 (0.8) | 2.6 (0.8) | 2.5 (0.8) | 2.4 (0.8) |
| Total-cholesterol (mg/dl) | 207.4 (38.0) | 206.4 (36.4) | 208.8 (36.0) | 208.3 (39.1) |
| HDL-cholesterol (mg/dl) | 54.1 (18.7) | 53.6 (18.4) | 53.2 (18.3) | 51.6 (17.8) |
| Lipid-lowering meds (%) | 7.3 | 6.9 | 7.5 | 8.9 |
| hs-CRP (mg/l) | 2.7 (4.9) | 3.3 (5.1) | 4.1 (6.6) | 5.5 (8.6) |
| Total fibrinogen (mg/dL) | 264.9 (54.1) | 285.4 (49.9) | 302.5 (54.6) | 330.6 (66.6) |
Values are means (standard deviations) for continuous variables and percentages for categorical variables
HDL: High density lipoprotein; hs-CRP: High sensitive C-reactive protein.
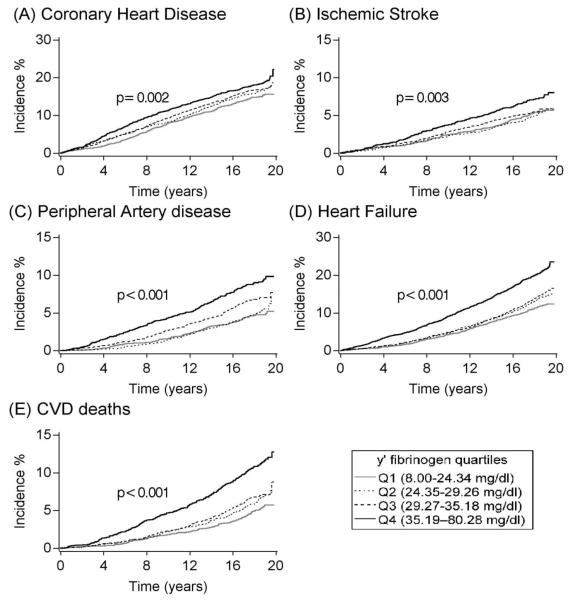
Kaplan–Meier cumulative incidence analysis showed higher incidence of all CVD outcomes with higher levels of crude γ’ fibrinogen quartiles (figure 1). The incidence of CVD endpoints and their multivariable adjusted associations with γ’ fibrinogen are shown in table 2. In models adjusted for age, sex, race, and ARIC center (model 1), compared to participants in the lowest quartile of γ’ fibrinogen (8.0 - 24.34 mg/dl), those in the highest quartile (≥ 35.19 mg/dl) had elevated incidence rates of CHD (HR: 1.40, 95% CI: 1.22-1.61), PAD (HR: 1.87; 95% CI: 1.48-2.37), HF (HR: 1.65, 95% CI: 1.42-1.92) and CVD mortality (HR: 1.96, 95% CI: 1.58-2.43) but not incident ischemic stroke (HR: 1.17, 95% CI: 0.93-1.48). These associations were attenuated but remained statistically significant when further adjustments were made for educational attainment and other established cardiovascular risk factors (model 2). Further adjustment for total fibrinogen (model 3) suggested that the positive association between γ’ fibrinogen and incident CHD was not independent of plasma concentrations of total fibrinogen (HR for highest vs. lowest γ’ fibrinogen quartiles: 1.06, 95% CI: 0.91-1.24). In contrast, the elevated risk among participants in the upper quartile of γ’ fibrinogen compared to those in the lowest quartile persisted for the other CVD endpoints after accounting for total fibrinogen, with the risk most pronounced for CVD deaths (HR: 1.39, CI: 1.10-1.76), followed by PAD (HR: 1.36, CI: 1.05-1.75) and HF (HR: 1.21, CI: 1.02-1.42). Further adjustment for CRP concentrations (model 4) slightly attenuated the associations of γ’ fibrinogen with PAD (HR for highest vs. lowest quartiles: 1.26, CI: 0.96-1.66), HF (HR: 1.18, CI: 0.99-1.41) and CVD deaths (HR: 1.36, CI: 1.05-1.75). Similar patterns were observed when HR’s were calculated using continuous values of γ’ fibrinogen.
Figure 1.
Kaplan-Meier cumulative incidence estimates of cardiovascular outcomes according to γ’ fibrinogen quartiles, ARIC, 1993-2012. P values for the Log-rank tests represent differences among all groups (unadjusted).
Table 2.
Hazard ratios (95% CI) of incident cardiovascular outcomes in relation to plasma γ’ fibrinogen, the ARIC study, 1993-2012
| γ’ fibrinogen quartiles, mg/dl |
Continuous |
|||||
|---|---|---|---|---|---|---|
| Q1 8.0 - 24.34 |
Q2 24.35 - 29.26 |
Q3 29.27 - 35.18 |
Q4 35.19 – 80.28 |
P TREND | 1–SD increment* | |
| CHD | ||||||
| Events, n | 359 | 393 | 407 | 444 | 1603 | |
| Incidence rate† | 8.6 (7.8-9.6) | 9.6 (8.7-10.6) | 9.9 (9.0-10.9) | 11.3 (10.3-12.4) | 9.8 (9.4-10.3) | |
| Model 1 | 1 (Referent) | 1.12 (0.97-1.30) | 1.20 (1.04-1.38) | 1.40 (1.22-1.61) | 0.001 | 1.14 (1.08-1.19) |
| Model 2 | 1 (Referent) | 1.11 (0.96-1.28) | 1.08 (0.93-1.25) | 1.18 (1.02-1.37) | 0.040 | 1.05 (1.00-1.11) |
| Model 3 | 1 (Referent) | 1.07 (0.93-1.24) | 1.01 (0.87-1.17) | 1.06 (0.91-1.24) | 0.816 | 1.01 (0.96-1.07) |
| Model 4 | 1 (Referent) | 1.10 (0.94-1.28) | 1.02 (0.88-1.20) | 1.04 (0.89-1.23) | 0.847 | 1.00 (0.94-1.06) |
| Ischemic stroke | ||||||
| Events, n | 127 | 120 | 132 | 169 | 548 | |
| Incidence rate† | 2.9 (2.5-3.5) | 2.8 (2.3-3.3) | 3.1 (2.6-3.6) | 4.1 (3.5-4.7) | 3.2 (2.9-3.5) | |
| Model 1 | 1 (Referent) | 0.90 (0.70-1.16) | 0.95 (0.75-1.22) | 1.17 (0.93-1.48) | 0.049 | 1.11 (1.02-1.20) |
| Model 2 | 1 (Referent) | 0.89 (0.69-1.15) | 0.86 (0.69-1.10) | 0.96 (0.76-1.22) | 0.884 | 1.00 (0.92-1.09) |
| Model 3 | 1 (Referent) | 0.86 (0.67-1.11) | 0.84 (0.65-1.08) | 0.89 (0.69-1.16) | 0.397 | 0.98 (0.89-1.07) |
| Model 4 | 1 (Referent) | 0.90 (0.69-1.17) | 0.82 (0.63-1.08) | 0.87 (0.66-1.15) | 0.352 | 0.97 (0.87-1.07) |
| PAD | ||||||
| Events, n | 111 | 117 | 160 | 211 | 599 | |
| Incidence rate† | 2.5 (2.1-3.1) | 2.7 (2.2-3.2) | 3.7 (3.2-4.3) | 5.1 (4.5-5.8) | 3.5 (3.2-3.8) | |
| Model 1 | 1 (Referent) | 1.02 (0.79-1.33) | 1.40 (1.10-1.78) | 1.87 (1.48-2.37) | 0.001 | 1.30 (1.20-1.40) |
| Model 2 | 1 (Referent) | 0.97 (0.74-1.26) | 1.20 (0.94-1.54) | 1.43 (1.13-1.82) | 0.001 | 1.16 (1.07-1.25) |
| Model 3 | 1 (Referent) | 0.95 (0.73-1.25) | 1.17 (0.91-1.50) | 1.36 (1.05-1.75) | 0.042 | 1.14 (1.04-1.24) |
| Model 4 | 1 (Referent) | 1.00 (0.76-1.32) | 1.16 (0.89-1.51) | 1.26 (0.96-1.66) | 0.050 | 1.09 (0.99-1.19) |
| Heart failure | ||||||
| Events, n | 272 | 317 | 336 | 486 | 1411 | |
| Incidence rate† | 6.3 (5.6-7.1) | 7.4 (6.7-8.3) | 7.9 (7.1-8.8) | 12.1 (11.0-13.2) | 8.4 (7.9-8.8) | |
| Model 1 | 1 (Referent) | 1.12 (0.95-1.32) | 1.14 (0.97-1.34) | 1.65 (1.42-1.92) | 0.001 | 1.23 (1.17-1.30) |
| Model 2 | 1 (Referent) | 1.06 (0.90-1.25) | 0.99 (0.84-1.16) | 1.30 (1.11-1.52) | 0.001 | 1.10 (1.04-1.15) |
| Model 3 | 1 (Referent) | 1.04 (0.88-1.23) | 0.93 (0.79-1.11) | 1.21 (1.02-1.42) | 0.066 | 1.06 (1.01-1.13) |
| Model 4 | 1 (Referent) | 1.08 (0.91-1.28) | 0.91 (0.76-1.09) | 1.18 (0.99-1.41) | 0.080 | 1.05 (0.99-1.12) |
| CVD deaths | ||||||
| Events, n | 123 | 151 | 166 | 265 | 705 | |
| Incidence rate† | 2.7 (2.3-3.3) | 3.4 (2.9-4.0) | 3.7 (3.2-4.3) | 6.2 (5.5-7.0) | 4.0 (3.7-4.3) | |
| Model 1 | 1 (Referent) | 1.19 (0.94-1.51) | 1.27 (1.00-1.60) | 1.96 (1.58-2.43) | 0.001 | 1.30 (1.21-1.39) |
| Model 2 | 1 (Referent) | 1.16 (0.91-1.46) | 1.17 (0.92-1.48) | 1.66 (1.33-2.08) | 0.001 | 1.19 (1.11-1.28) |
| Model 3 | 1 (Referent) | 1.10 (0.86-1.40) | 1.04 (0.81-1.32) | 1.39 (1.10-1.76) | 0.014 | 1.12 (1.04-1.21) |
| Model 4 | 1 (Referent) | 1.121 0.87-1.45) | 1.00 (0.77-1.30) | 1.36 (1.05-1.75) | 0.015 | 1.10 (1.01-1.20) |
Model 1: Cox proportional hazards model adjusted for age (continuous), sex, race (white, black), and ARIC center
Model 2: Model 1 additionally adjusted for education (< high school, high school, > high school), smoking (current, former, never), alcohol intake (continuous), sports-related physical activity (continuous), systolic blood pressure (continuous), body mass index (continuous), use of antihypertensive medications (yes, no), diabetes (yes, no), cholesterol medication (yes, no), HDL cholesterol (continuous), and total cholesterol (continuous).
Model 3: Model 2 additionally adjusted for total fibrinogen (continuous).
Model 4: Model 3 additionally adjusted for hs-CRP (continuous).
1 standard deviation (SD) = 8.80 mg/dl.
Unadjusted incidence rate per 1,000 person-years with 95% confidence intervals.
CHD: coronary heart disease; PAD: peripheral artery disease; CVD: Cardiovascular disease
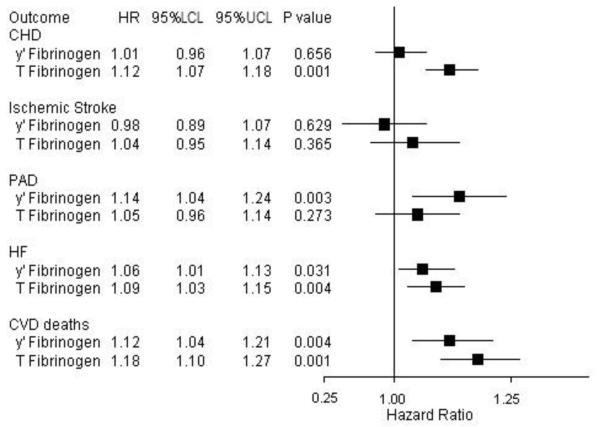
Comparison of the associations of γ’ fibrinogen and total fibrinogen, adjusted for each other, with CVD endpoints are shown in figure 2. With the exception of PAD, the HRs per 1 SD increment of total fibrinogen with each CVD endpoint were higher than those for γ’ fibrinogen. For all CVD endpoints, no significant interactions between γ’ fibrinogen and race, sex, CRP and total fibrinogen were identified. For the endpoint that seemed most strongly associated with γ’ fibrinogen (CVD deaths), the risk was particularly elevated for participants who were in the highest tertiles of both γ’ fibrinogen and total fibrinogen (figure 3). Finally, restricted cubic spline Cox regression analysis revealed that the relations between γ’ fibrinogen and CVD endpoints were approximately linear (data not shown). In sensitivity analyses, we found no appreciable differences in the associations of corrected and uncorrected γ’ fibrinogen with CVD endpoints. (Supplemental table I). In a sensitivity analysis limiting follow-up to 5 years, we found most associations were stronger, including a significant positive association between γ’ fibrinogen and incident CHD (Supplemental table II). However, in an additional sensitivity analysis of the entire 20 year follow-up that excluded events that occurred in the first 3 years, we observed attenuation of the associations between γ’ fibrinogen and all CVD endpoints (Supplemental tables III).
Figure 2.
Hazard ratios (per 1 SD) and 95% CIs of γ’ fibrinogen (8.80 mg/dl) and total fibrinogen (60.9 mg/dL) with CVD endpoints, ARIC, 1993-2012. Hazard ratios adjusted for age, sex, race, center, education, smoking, alcohol intake, sports index, systolic BP, BMI, antihypertensive meds use, HDL-c, total cholesterol, lipid-lowering meds and diabetes.
CHD: coronary heart disease; PAD: peripheral artery disease; HF: heart failure; CVD: Cardiovascular disease; HR: Hazard ratio; LCL: Lower confidence limit; UCL: Upper confidence limit.
Figure 3.
The joint associations of γ’ fibrinogen and total fibrinogen with CVD deaths, ARIC, 1993-2012. Hazard ratios adjusted for age, sex, race, center, education, smoking, alcohol intake, sports index, systolic BP, BMI, antihypertensive meds use, HDL-c, total cholesterol, lipid-lowering meds, and diabetes.
Discussions
In this prospective observational cohort of middle-aged whites and African Americans enrolled in the ARIC study, we found no independent association between plasma γ’ fibrinogen concentrations and the incidence of coronary heart disease and ischemic stroke. However, higher levels of γ’ fibrinogen were positively though modestly associated with peripheral artery disease, heart failure and CVD deaths, which seems to reflect a general contribution of inflammation to CVD, rather than a specific γ’ fibrinogen effect. Our findings suggest that γ’ fibrinogen is an inflammatory marker that adds little information to CVD prediction beyond total fibrinogen and/or hs-CRP levels. To our knowledge, this is the first epidemiologic prospective investigation of the association between γ’ fibrinogen and incident CVD events.
The underlying mechanism by which γ’ fibrinogen might affect cardiovascular health is debated. Previous experimental studies have suggested that γ’ fibrinogen promotes thrombosis by forming fibrin blood clots that have altered clot architecture that makes them mechanically stronger, and highly resistant to fibrinolysis 8, 9, 14, 16. However, other studies suggest that γ’ fibrinogen is antithrombotic and exhibits anticoagulant properties due to its ability to sequester thrombin 17. Recent studies observed that that γ’ fibrinogen has high affinity binding sites for thrombin exosite II, which inhibits thrombin-mediated platelet activation and reduces fibrinopeptide B cleavage and factor VIII activation 17-21. Accordingly, Walton et al 17 reported that γ’ fibrinogen did not promote acute arterial thrombosis in mice and revealed that the more dominant isoform of the fibrinogen γ chain, γA/γA, increased fibrin formation rates and shortened the time to carotid artery occlusion thereby promoting thrombosis to a greater extent than γA/γ’. Mosesson et al also observed that γ’ fibrinogen are constituents of a fibrin-dependent thrombin inhibitory system and suggested that lower levels on γ’ fibrinogen may be associated with thrombotic events18, 22. These seemingly conflicting biochemical properties of γ’ fibrinogen make its role in the etiology of CVD events unclear. In the present study, we did not identify any association between γ’ fibrinogen and the two major arterial thrombotic events, CHD and ischemic stroke, after accounting for the effect of total fibrinogen concentrations. This suggests that γ’ fibrinogen does not influence major atherothrombotic diseases by means of unique pro-thrombotic properties.
Fibrinogen is an acute phase reactant that increases in response to inflammation, and the inflammatory response has been reported to affect alternative splicing of the fibrinogen γ gene 9, 23. Levels of γ’ fibrinogen are associated positively with inflammatory markers 9, 23, 24. Rein-Smith et al 24 reported that interleukin-6 preferentially upregulates hepatocyte production of γ’ fibrinogen, and CRP influences levels of γ’ fibrinogen 13, 25. Cheng et al 26 reported that CRP levels were positively correlated with the ratio of γ’ fibrinogen to total fibrinogen in the acute phase of ischemic stroke, providing further evidence that the mRNA processing of γ’ fibrinogen is altered in the presence of inflammation. We corroborated that higher levels of CRP are associated with higher γ’ fibrinogen. Since adjusting for the inflammatory makers, total fibrinogen and CRP, eliminated the associations of γ’ fibrinogen with CHD, ischemic stroke, PAD and HF, γ’ fibrinogen’s associations seem to reflect a general contribution of inflammation to CVD, rather than a specific γ’ fibrinogen effect. Additionally, the significant positive association of γ’ fibrinogen with the broad outcome of CVD deaths supports a nonspecific effect. It also suggests that the increased levels of γ’ fibrinogen found in individuals with CVD compared with controls in prior studies may rather be a consequence of CVD rather than a cause 17.
It was interesting to note, in this present study, that when we limited follow-up to 5 years, we found a significant positive association between γ’ fibrinogen and incident CHD. These short-term (5-year) associations especially for CHD may have been due to a reverse causal association; that is, subclinical disease elevating γ’ fibrinogen levels which resulted in participants with subclinical disease having elevated CHD risk. Alternatively, the stronger short-term associations could also mean that over time, a single value of γ’ fibrinogen becomes a less accurate representation of an individual’s risk.
Our results contrast with prior epidemiologic studies. Lovely et al 12 reported, cross-sectionally, sevenfold higher odds of coronary artery disease (CAD) for the highest versus lowest quartile of γ’ fibrinogen, adjusting only for age and gender, in a small case-control study of 133 patients undergoing elective, outpatient diagnostic cardiac catheterization. Another case-control study 13 comprised of 387 post-myocardial infarction patients and 387 healthy individuals from the Stockholm Coronary Artery Risk study reported a statistically significant 24% higher odds of MI per 1 SD increment in γ’ fibrinogen after adjusting for traditional CVD risk factors and total fibrinogen. Similarly, cross-sectional results from the Framingham Heart Study Offspring Cohort 14 showed a statistically significant 76% higher odds of prevalent MI among participants in the highest tertile of γ’ fibrinogen compared to the lowest tertile after adjusting for established CVD risk factors. Data on the relation between γ’ fibrinogen and ischemic stroke are sparse and conflicting. Van den Herik et al 27 observed that individuals with ischemic stroke had elevated levels of γ’ fibrinogen than age- and sex-matched stroke-free controls, and each unit increase in γ’ fibrinogen was associated with 48% higher odds for unfavorable stroke outcome. Cheung et al 15 identified elevated levels of γ’ fibrinogen in the acute phase of ischemic stroke that reduced to pre-stroke levels after 3 months. However the Framingham Heart Study Offspring Cohort 14 found no association between γ’ fibrinogen and prevalent stroke (OR: 1.42, CI: 0.68 to 2.95).
Possible explanations for these disparate results may relate to their study designs and inadequate control of the influence of total fibrinogen and other inflammatory markers. For instance, in the Framingham Heart Study Offspring Cohort 14, γ’ fibrinogen was positively associated with prevalent CVD in models adjusted for established CVD risk factors, but additional control for total fibrinogen levels rendered the association nonsignificant. Furthermore, the cross-sectional or retrospective designs employed by prior studies have greater likelihood of selection bias, as persons who died of disease before the inception of such studies may have had different γ’ fibrinogens concentrations compared to survivors who were enrolled. Moreover, cross-sectional/retrospective studies lack the ability to determine whether elevated γ’ fibrinogen preceded or followed the CVD event, as it was measured among cases who already had CVD.
This study has several notable strengths, including the use of a large population-based biracial sample, extensive assessment of CVD risk factors, physician-adjudicated CHD and stroke events using standardized criteria, and 20 years of follow-up with low attrition. Additionally, validated procedures to measure γ’ fibrinogen levels were used.
Limitations of this study should be considered when interpreting our results. First, analyses were based on a single measure of γ’ fibrinogen. Second, our quality control data showed that our γ’ fibrinogen measurements for whites had some downward drift, requiring us to adjust those γ’ fibrinogen values to be comparable to the stable and precise levels observed for African Americans. Our corrections in γ’ fibrinogen were an attempt to adjust for the drift seen in the normal data. Sensitivity analyses revealed that corrected and uncorrected results were similar. Since the ARIC study, had only a single measure of γ’ fibrinogen on participants and there is no published long-term reliability coefficient in the literature, we could not address γ’ fibrinogen variation over time. Any misclassification of γ’ fibrinogen levels is likely to have been non-differential with respect to our outcomes. Such errors would have biased our effect estimates toward the null (regression dilution), in expectation, and is one possible explanation for our findings. Third, we measured total fibrinogen and CRP from blood samples obtained six and three years respectively before the assessment of γ’ fibrinogen and these may not represent actual levels at visit 3. Since γ’ fibrinogen is moderately correlated with total fibrinogen and CRP, we deemed it necessarily to adjust for these inflammatory markers in our models to enhance our understanding of any potential underlying mechanisms for the association of γ’ fibrinogen with CVD endpoints. Fourth, the statistically non-significant findings for CHD and ischemic stroke may be due to inadequate power to detect a small effect. However, our study had high power (>0.8) for hazard ratios of 1.2 for CHD and 1.4 for stroke. Finally, some CVD endpoints (e.g., HF, PAD, and CVD mortality) relied on ICD codes. However, in ARIC, a high validity for ICD codes in identifying these CVD endpoints has been demonstrated 28.
In summary, γ’ fibrinogen was associated positively with peripheral artery disease, heart failure and CVD deaths, but not independently with incident coronary heart disease and ischemic stroke, after accounting for total fibrinogen levels. With the exception of CVD deaths, these associations were attenuated to marginal statistical significance when hs-CRP was added to the model. Our findings are consistent with γ’ fibrinogen concentration reflecting the inflammation that accompanies and may contribute to atherosclerotic CVD, rather than γ’ fibrinogen being a risk factor for CVD events.
Supplementary Material
Significance.
Cross-sectional and retrospective investigations have suggested that gamma prime (γ’) fibrinogen, a fibrinogen γ chain variant generated via alternative mRNA processing, is positively associated with atherothrombotic events. However, results from the Atherosclerosis Risk in Communities (ARIC) study, the first prospective study to assess this association does not lend support to the hypothesis that γ’ fibrinogen influences CVD events through its pro-thrombotic properties. Rather, γ’ fibrinogen concentrations seem to reflect general inflammation that accompanies and may contribute to atherosclerotic CVD, instead of γ’ fibrinogen being a causal risk factor.
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions, and Elaine Cornell for supervising γ’ fibrinogen measurements.
Funding sources:
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr. Appiah was supported by NHLBI training grant T32HL007779.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- CVD
Cardiovascular Diseases
- CHD
Coronary Heart Disease
- HF
Heart Failure
- PAD
Peripheral Artery Disease
- HDL
High-Density Lipoproteins
- HR
Hazard Ratio
- Hs-CRP
High Sensitivity C-Reactive Protein
- SD
Standard Deviation
- ICD
International Classification of Disease
Footnotes
Disclosures: None
References
- 1.Fibrinogen Studies Collaboration. Danesh J, Lewington S, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA : the journal of the American Medical Association. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 2.Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: The atherosclerosis risk in communities (aric) study. Circulation. 1997;96:1102–1108. doi: 10.1161/01.cir.96.4.1102. [DOI] [PubMed] [Google Scholar]
- 3.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, Rasmussen ML, Wu KK. Prospective study of markers of hemostatic function with risk of ischemic stroke. The atherosclerosis risk in communities (aric) study investigators. Circulation. 1999;100:736–742. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 4.Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, Lipinska I, Massaro JM, Wilson PF, Muller JE, D'Agostino RB., Sr. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the framingham offspring population. Circulation. 2000;102:1634–1638. doi: 10.1161/01.cir.102.14.1634. [DOI] [PubMed] [Google Scholar]
- 5.Keavney B, Danesh J, Parish S, Palmer A, Clark S, Youngman L, Delepine M, Lathrop M, Peto R, Collins R. Fibrinogen and coronary heart disease: Test of causality by 'mendelian randomization'. International journal of epidemiology. 2006;35:935–943. doi: 10.1093/ije/dyl114. [DOI] [PubMed] [Google Scholar]
- 6.Krobot K, Hense HW, Cremer P, Eberle E, Keil U. Determinants of plasma fibrinogen: Relation to body weight, waist-to-hip ratio, smoking, alcohol, age, and sex. Results from the second monica augsburg survey 1989-1990. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1992;12:780–788. doi: 10.1161/01.atv.12.7.780. [DOI] [PubMed] [Google Scholar]
- 7.Folsom AR, Wu KK, Davis CE, Conlan MG, Sorlie PD, Szklo M. Population correlates of plasma fibrinogen and factor vii, putative cardiovascular risk factors. Atherosclerosis. 1991;91:191–205. doi: 10.1016/0021-9150(91)90167-2. [DOI] [PubMed] [Google Scholar]
- 8.Lovely RS, Kazmierczak SC, Massaro JM, D'Agostino RB, Sr., O'Donnell CJ, Farrell DH. Gamma' fibrinogen: Evaluation of a new assay for study of associations with cardiovascular disease. Clinical chemistry. 2010;56:781–788. doi: 10.1373/clinchem.2009.138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell DH. Gamma' fibrinogen as a novel marker of thrombotic disease. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2012;50:1903–1909. doi: 10.1515/cclm-2012-0005. [DOI] [PubMed] [Google Scholar]
- 10.Kim PY, Stewart RJ, Lipson SM, Nesheim ME. The relative kinetics of clotting and lysis provide a biochemical rationale for the correlation between elevated fibrinogen and cardiovascular disease. Journal of thrombosis and haemostasis : JTH. 2007;5:1250–1256. doi: 10.1111/j.1538-7836.2007.02426.x. [DOI] [PubMed] [Google Scholar]
- 11.Drouet L, Paolucci F, Pasqualini N, Laprade M, Ripoll L, Mazoyer E, Bal dit Sollier C, Vanhove N. Plasma gamma'/gamma fibrinogen ratio, a marker of arterial thrombotic activity: A new potential cardiovascular risk factor? Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 1999;10(Suppl 1):S35–39. [PubMed] [Google Scholar]
- 12.Lovely RS, Falls LA, Al-Mondhiry HA, Chambers CE, Sexton GJ, Ni H, Farrell DH. Association of gammaa/gamma' fibrinogen levels and coronary artery disease. Thrombosis and haemostasis. 2002;88:26–31. [PubMed] [Google Scholar]
- 13.Mannila MN, Lovely RS, Kazmierczak SC, Eriksson P, Samnegard A, Farrell DH, Hamsten A, Silveira A. Elevated plasma fibrinogen gamma' concentration is associated with myocardial infarction: Effects of variation in fibrinogen genes and environmental factors. Journal of thrombosis and haemostasis : JTH. 2007;5:766–773. doi: 10.1111/j.1538-7836.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- 14.Lovely RS, Yang Q, Massaro JM, Wang J, D'Agostino RB, Sr., O'Donnell CJ, Shannon J, Farrell DH. Assessment of genetic determinants of the association of gamma' fibrinogen in relation to cardiovascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2345–2352. doi: 10.1161/ATVBAHA.111.232710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung EY, Uitte de Willige S, Vos HL, Leebeek FW, Dippel DW, Bertina RM, de Maat MP. Fibrinogen gamma' in ischemic stroke: A case-control study. Stroke; a journal of cerebral circulation. 2008;39:1033–1035. doi: 10.1161/STROKEAHA.107.495499. [DOI] [PubMed] [Google Scholar]
- 16.Uitte de Willige S, Standeven KF, Philippou H, Ariens RA. The pleiotropic role of the fibrinogen gamma' chain in hemostasis. Blood. 2009;114:3994–4001. doi: 10.1182/blood-2009-05-217968. [DOI] [PubMed] [Google Scholar]
- 17.Walton BL, Getz TM, Bergmeier W, Lin FC, Uitte de Willige S, Wolberg AS. The fibrinogen gammaa/gamma' isoform does not promote acute arterial thrombosis in mice. Journal of thrombosis and haemostasis : JTH. 2014;12:680–689. doi: 10.1111/jth.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosesson MW. Update on antithrombin i (fibrin) Thrombosis and haemostasis. 2007;98:105–108. [PubMed] [Google Scholar]
- 19.Cooper AV, Standeven KF, Ariens RA. Fibrinogen gamma-chain splice variant gamma' alters fibrin formation and structure. Blood. 2003;102:535–540. doi: 10.1182/blood-2002-10-3150. [DOI] [PubMed] [Google Scholar]
- 20.Lovely RS, Rein CM, White TC, Jouihan SA, Boshkov LK, Bakke AC, McCarty OJ, Farrell DH. Gammaa/gamma' fibrinogen inhibits thrombin-induced platelet aggregation. Thrombosis and haemostasis. 2008;100:837–846. [PubMed] [Google Scholar]
- 21.Lovely RS, Boshkov LK, Marzec UM, Hanson SR, Farrell DH. Fibrinogen gamma' chain carboxy terminal peptide selectively inhibits the intrinsic coagulation pathway. Br J Haematol. 2007;139:494–503. doi: 10.1111/j.1365-2141.2007.06825.x. [DOI] [PubMed] [Google Scholar]
- 22.Mosesson MW, Hernandez I, Raife TJ, Medved L, Yakovlev S, Simpson-Haidaris PJ, Uitte DEWS, Bertina RM. Plasma fibrinogen gamma' chain content in the thrombotic microangiopathy syndrome. Journal of thrombosis and haemostasis : JTH. 2007;5:62–69. doi: 10.1111/j.1538-7836.2006.02270.x. [DOI] [PubMed] [Google Scholar]
- 23.Alexander KS, Madden TE, Farrell DH. Association between gamma' fibrinogen levels and inflammation. Thrombosis and haemostasis. 2011;105:605–609. doi: 10.1160/TH10-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rein-Smith CM, Anderson NW, Farrell DH. Differential regulation of fibrinogen gamma chain splice isoforms by interleukin-6. Thromb Res. 2013;131:89–93. doi: 10.1016/j.thromres.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotze RC, Ariens RA, de Lange Z, Pieters M. Cvd risk factors are related to plasma fibrin clot properties independent of total and or gamma' fibrinogen concentration. Thromb Res. 2014;134:963–969. doi: 10.1016/j.thromres.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Cheung EY, Vos HL, Kruip MJ, den Hertog HM, Jukema JW, de Maat MP. Elevated fibrinogen gamma' ratio is associated with cardiovascular diseases and acute phase reaction but not with clinical outcome. Blood. 2009;114:4603–4604. doi: 10.1182/blood-2009-08-236240. author reply 4604-4605. [DOI] [PubMed] [Google Scholar]
- 27.van den Herik EG, Cheung EY, de Lau LM, den Hertog HM, Leebeek FW, Dippel DW, Koudstaal PJ, de Maat MP. Gamma'/total fibrinogen ratio is associated with short-term outcome in ischaemic stroke. Thrombosis and haemostasis. 2011;105:430–434. doi: 10.1160/TH10-09-0569. [DOI] [PubMed] [Google Scholar]
- 28.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (aric) study: A comparison of diagnostic criteria. Circulation. Heart failure. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.