Abstract
Purpose
Hypomorphic mutations in RAG1 and RAG2 are associated with significant clinical heterogeneity and symptoms of immunodeficiency or autoimmunity may be late in appearance. As a result, immunosuppressive medications may be introduced that can have life-threatening consequences. We describe a previously healthy 13-month-old girl presenting with rash and autoimmune hemolytic anemia, while highlighting the importance of vigilance and consideration of an underlying severe immunodeficiency disease prior to instituting immunosuppressive therapy.
Methods
Given clinical deterioration of the patient and a temporal association with recently administered vaccinations, virus genotyping was carried out via 4 real-time Forster Resonance Energy Transfer PCR protocols targeting vaccine-associated single nucleotide polymorphisms. Genomic DNA was extracted from whole blood and analyzed via the next-generation sequencing method of sequencing-by-synthesis. Immune function studies included immunophenotyping of peripheral blood lymphocytes, mitogen-induced proliferation and TLR ligand-induced production of TNFα. Analysis of recombination activity of wild-type and mutant RAG2 constructs was performed.
Results
Virus genotyping revealed vaccine-strain VZV, mumps, and rubella. Next-generation sequencing identified heterozygosity for RAG2 R73H and P180H mutations. Profound lymphopenia was associated with intense corticosteroid therapy, with some recovery after steroid reduction. Residual, albeit low, RAG2 protein activity was demonstrated.
Conclusions
Because of the association of RAG deficiency with late-onset presentation and autoimmunity, live virus vaccination and immunosuppressive therapies are often initiated and can result in negative consequences. Here, hypomorphic RAG2 mutations were linked to disseminated vaccine-strain virus infections following institution of corticosteroid therapy for autoimmune hemolytic anemia.
Keywords: RAG2, T cell lymphopenia, immunosuppression, vaccine-strain virus infection
INTRODUCTION
Since the introduction of whole-exome sequencing, numerous novel defects in immune-related genes have been described, resulting in the recognition of a wide variety of primary immune deficiency syndromes. Though the greatest power of next-generation sequencing may lie in isolating mutations of candidate genes to which immune dysregulation has not been ascribed, identifying novel defects in previously reported genes of immunologic importance expands the known clinical spectrum of disease. There is perhaps no greater example of an ever-growing clinically heterogeneous phenotype than that seen with mutations of recombination-activating gene 1 (RAG1) and recombination-activating gene 2 (RAG2).
RAG1 and RAG2 encode proteins that form a heterodimeric complex, which enzymatically cleaves DNA during variable (V), diversity (D), and joining (J) segment rearrangement at the T-cell receptor and immunoglobulin gene loci [1, 2]. Systematic rearrangement of antigen receptor genes via V(D)J recombination is essential for maturation of progenitor lymphocytes and thus facilitates the diverse receptor repertoire necessary for adaptive immunity. Biallelic amorphic RAG mutations interrupt V(D)J recombination by halting T and B cell development, resulting in a profound or severe combined immune deficiency (SCID) [3]. Owing to residual recombination activity, hypomorphic RAG mutations result in a much broader spectrum of disease phenotypes with manifestations including Omenn syndrome, combined immune deficiency with γδ T cell expansion and propensity for severe cytomegalovirus (CMV) infection, delayed-onset immune deficiency with diffuse granulomatous disease, isolated CD4+ lymphopenia, early-onset autoimmunity and illnesses resembling common variable immune deficiency (CVID) and selective IgA deficiency [4–13]. Lee et al. [14] and IJspeert et al. [15] recently characterized numerous RAG mutations, emphasizing the remarkable heterogeneity seen in the clinical phenotypes of RAG deficiency. Although these atypical presentations of the disease may reflect different degrees of residual recombination activity of the mutant RAG proteins [14], variability of the clinical phenotype has also been reported among patients carrying mutations with similar recombination activities [15]. Because of the broadened phenotypic variability associated with RAG mutations, often overlapping with features of autoimmune and allergic disease where immunosuppressive therapies may be instituted, clinical suspicion for these mutations is required to avoid potential life-threatening treatment-related complications.
Clinical Case
At 13 months of age, a previously healthy girl presented acutely with fever, jaundice, cough, rhinorrhea, diarrhea and approximately 10 erythematous papules on her torso and extremities. Preliminary studies revealed direct Coombs-positive anemia and leukocytosis with granulocytes predominating. Initial treatment measures included blood transfusions, intravenous immunoglobulin (IVIG) and high dose methylprednisolone (up to 80 mg/day), leading to transient resolution of hemolysis. In addition, she developed autoimmune thrombocytopenia (nadir 25,000 platelets/µL). Three weeks after onset of illness, her rash evolved into discrete 3-mm eschars; however, very few, if any new skin lesions developed. Skin biopsy revealed intranuclear inclusion bodies typical of Herpesviridae and intravenous acyclovir was initiated. Despite antiviral therapy, which later included foscarnet, her clinical status deteriorated over subsequent weeks. Obtundation ensued, and the patient died due to complications of hepatic and renal failure, disseminated intravascular coagulation and recalcitrant autoimmune hemolytic anemia.
Three weeks before onset of illness, the patient received her first varicella, measles, mumps, rubella (MMR) and hepatitis A immunizations. She had previously received all vaccinations as per the Advisory Committee on Immunization Practices (ACIP) recommended schedule, including the rotavirus series, which were well tolerated. Prior to death, vaccine-strain varicella zoster virus (VZV) was detected in her cerebrospinal fluid (723 copies/mL), skin (137,156 copies/mL) and esophagus by PCR assays and immunohistochemistry staining, respectively. Throat swabs, also obtained prior to death, detected mumps and rubella by PCR assays. Stool samples submitted for viral electron microscopy were negative, including assessment for rotavirus. Ophthalmic examination revealed corneal dendrites with typical features of VZV infection. Autopsy demonstrated VZV dissemination in the lungs and liver as well as profound lymphoid depletion of lymph nodes and thymic tissue [16].
METHODS
Virus Genotyping
VZV isolated from the patient was analyzed via 4 real-time Forster Resonance Energy Transfer PCR protocols targeting vaccine-associated single nucleotide polymorphisms (National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA). Appropriate regions of the mumps and rubella genomes were amplified via standard RT-PCR and sequenced via Sanger sequencing. Genotype analysis was performed relative to reference sequences known for each virus, including those established for the vaccine genotypes.
Immune Studies
Lymphocyte immunophenotyping was performed on heparinized whole blood. Percentages and absolute numbers of T cells, B cells, natural killer cells, and select subpopulations were determined. Anti-human monoclonal antibodies to the following were used for staining: CD3 (UCHT1), CD4 (RPA-T4), CD8 (RPA-T8), CD16 (3G8), CD19 (HIB19), CD21 (Bu32), CD25 (BC96), CD31 (WM59), CD34 (561), CD38 (HB-7), CD45RO (UCHL1), CD45RA (HI100), CD56 (MEM-188), FOXP3 (PCH101), IGD (IA6-2), IGM (MHM-88), kappa (MHK-49), lambda (MHL-38), TCR-αβ (IP26) and the appropriate isotype controls. After cell separation, peripheral blood mononuclear cells were washed and then used in in vitro assays, including lymphocyte proliferation and toll-like receptor (TLR) ligand-induced TNF production. Lymphocyte proliferation was determined after 3 days of mitogen (phytohemagglutinin, PHA; concanavalin A, ConA; pokeweed, PWM) stimulation and 6 days stimulation with antigen (tetanus toxoid and Candida albicans) monitoring 3H-thymidine incorporation. TLR ligand-induced TNF responses were measured by ELISA 24 hours after stimulation. TLR ligands included PAM3CSK4 for TLR2-TLR1, zymosan cell wall particles from Saccharomyces cerevisiae for TLR6-TLR2, poly(I:C) for TLR3, ultra-pure S. minnesota lipopolysaccharide for TLR4, flagellin purified from S. typhimurium for TLR5 and CL097 imidazoquinoline compound for TLR7-TLR8. Total serum immunoglobulin levels were determined by nephelometry.
Genetic Testing
Genomic DNA was extracted from whole blood and analyzed via the next-generation sequencing method of sequencing-by-synthesis (GeneDx, Gaithersburg, MD). Analysis included 169 coding exons of 18 genes (ADA, AK2, CD3D, CD3E, CD3Z, CD45, DCLRE1C, IL2RG, IL7R, JAK3, LIG4, NHEJ1, PNP, RAC2, RAG1, RAG2, RMRP and ZAP70), in which deleterious mutations have resulted in SCID. Mutations were identified by comparing the patient’s DNA to published genomic reference sequences. Targeted sequencing of affected exons was performed on the father, mother, and sister to determine the inheritance pattern.
RAG2 Recombination Activity
Analysis of recombination activity of wild-type and mutant RAG2 constructs was performed using a similar method as previously described for RAG1 mutations [14]. A pBMN-RAG2-IREShCD2 vector was constructed by inserting the RAG2 coding region into the XhoI cutting site in the pBMN-IRES-hCD2 retroviral vector, which was derived from pBMN-IRES-GFP plasmid by replacing the GFP cDNA with hCD2 cDNA. The pBMN-RAG2–IRES-hCD2 vectors, encoding for mutant RAG2, were generated by using the Phusion Site-Directed Mutagenesis Kit (catalog no. F-541S, NEB). The pMX-RSS-GFP/IRES-hCD4 (pMX-INV) retroviral vector has been described previously. Bone marrow was harvested from Rag2−/− tg.Eµ-bcl2 mice, and cells were cultured with the pMSCV-v-abl retrovirus to generate stable v-abl-transformed A-MuLV RAG2−/− pro-B-cell lines. These cells were then transduced with a retroviral vector containing an inverted GFP cassette flanked by RSS and human CD4 (hCD4) as a reporter (pMX-INV cassette). Upon enrichment for hCD4-expressing pro-B cells using positive selection and subcloning by limiting dilution, a clone of pro-B cells containing a single integrant of the pMX-INV cassette was isolated and further expanded for analysis of expression and recombination activity of hRAG2 mutants. To this purpose, the cells were transduced with another retroviral vector (pBMN-IRES-hCD2) encoding wild-type or mutant hRAG2 and hCD2 as a reporter. In parallel, cells were also transduced with a vector encoding wild-type mRAG2 and hCD2. Retroviral transduction was carried out at a multiplicity of infection of less than 1 to minimize occurrence of multiple integrations. On magnetic cell sorting purification of hCD21 cells followed by subcloning, 3 or fewer vector integrants per cell were demonstrated by using Southern blotting, with an average vector copy number of 1.7. All A-MuLV pro-B cell lines were cultured in complete RPMI. On stimulation with 3 mmol/L STI-571/imatinib (Novartis, Basel, Switzerland) for 96 hours to maintain cells in G0/G1, recombination activity was measured by analyzing GFP expression with flow cytometry. After gating on hCD4- and hCD2-expressing cells, results were normalized to the proportion of GFP-expressing cells detected in cells expressing wild-type hRAG2. For each mutant RAG2 construct, experiments were performed three times, and results of recombination activity were expressed as mean±standard deviation (s.d.) of recombination activity detected in A-MuLV Rag2−/− pro-B-cells reconstituted with wild-type RAG2.
RESULTS
Virus Genotyping
VZV, mumps and rubella isolated from the patient were confirmed as vaccine genotypes Oka, Jeryl-Lynn and RA 27/3, respectively..
Immune Studies
Initial flow cytometric analyses of peripheral blood demonstrated a high number of mature B cells with a normal kappa:lambda light chain ratio. T cell lymphopenia with a normal CD4:CD8 ratio was detected. Though absolute lymphopenia was not evident on initial presentation, profound lymphopenia (nadir 76 cells/µL) developed after treatment with high dose methylprednisolone. Following reductions in corticosteroids, immune studies revealed elevated memory (CD45RO+) T cells, increased switched (IgD−CD27+) and unswitched (IgD+CD27+) memory B cells, low recent thymic emigrants (CD4+CD45+CD31+), poor lymphocyte stimulation, absence of γδ T cell expansion and normal cytokine responses to TLR ligand stimulation. Quantitative serum immunoglobulins were not obtained prior to administering IVIG; however, later testing revealed detectable IgA, IgM and IgE (see Table 1).
Table 1.
Laboratory results before, during and after reduction of systemic corticosteroids.
| Test | Before Corticosteroids |
During High Dose Corticosteroids |
After Corticosteroid Reduction |
Normal Values For Age* |
|---|---|---|---|---|
| Lymphocytes (cells/µL) | 5100 | 76 | 1479 | 1800–9000 |
| CD3 (cells/µL) | 788 | 10 | 401 | 2100–6200 |
| CD4 (cells/µL) | ND | 7 | 315 | 1300–3400 |
| CD8 (cells/µL) | ND | 2 | 70 | 620–2000 |
| CD4:CD8 Ratio | 3.7:1 | 2.9:1 | 4.5:1 | 1.2–6.2 |
| CD19 (cells/µL) | 1970 | 51 | 524 | 720–2600 |
| Kappa:Lambda Ratio | 1.3:1 | ND | ND | 1–2 |
| CD16/56 (cells/µL) | ND | 15 | 654 | 180–920 |
| CD4+CD45RO+ (%) | ND | ND | 91.1 | 9.5–41.9% of CD4+ T Cells |
| CD4+CD45RA+ (%) | ND | ND | 7.4 | 16.5–42.2% of CD4+ T Cells |
| CD4+CD45RA+CD31+ (%) | ND | ND | 2.1 | 54.1–75.3 |
| TCR αβ (%) | ND | ND | 94.2 | >85% of CD3+ T Cells |
| CD20+IgD+CD27− (%) | ND | ND | 42 | 83.3–93.7% of CD20+ B Cells |
| CD20+IgD+CD27+ (%) | ND | ND | 32.9 | 3.3–10.8% of CD20+ B Cells |
| CD20+IgD−CD27+ (%) | ND | ND | 18.8 | 1.0–5.0% of CD20+ B Cells |
| IgG (mg/dL) | ND | 505 | ND | 421–120 |
| IgA (mg/dL) | ND | 32 | ND | 15–111 |
| IgM (mg/dL) | ND | 309 | ND | 35–184 |
| IgE (IU/mL) | ND | 50 | ND | 0–49 |
| PHA stimulation | ND | Minimal | ND | - |
| ConA stimulation | ND | Minimal | ND | - |
| PWM stimulation | ND | Minimal | ND | - |
| Tetanus stimulation | ND | None | ND | - |
| Candida stimulation | ND | None | ND | - |
| TLR 1–8 signaling | ND | ND | Normal | - |
Assays were performed in different laboratories where normal values may differ.
ND: Not Done
Genetic Testing
Post-mortem analysis of the patient’s genomic DNA revealed two mutations in exon 2 of RAG2. Sequencing of family members revealed heterozygosity for the R73H mutation in the patient’s father and sister; however, the P180H mutation was not found in samples obtained from the patient’s father, mother or sister.
RAG2 Recombination Activity
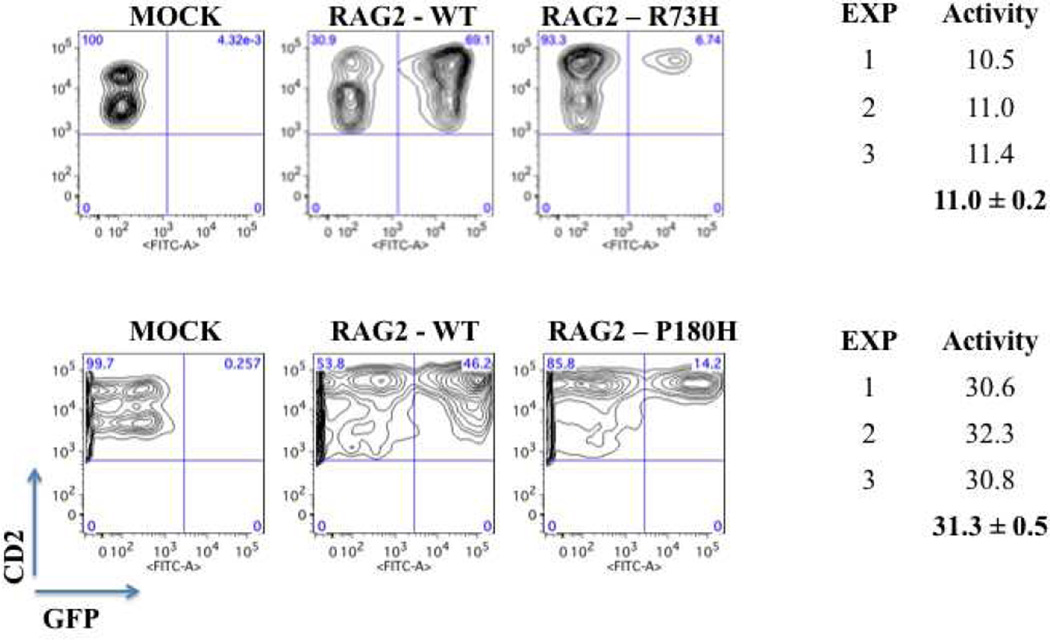
The R73H missense mutation had been previously reported to cause RAG2 deficiency, resulting in a relative activity of approximately 60% of wild-type via an in vivo V(D)J recombination assay [17]. The P180H missense mutation was novel and resulted in a non-conservative amino acid substitution. Recombination analyses of each RAG2 mutant revealed significantly depressed activity at 11.0±0.2 and 31.3±0.5% of wild-type for R73H and P180H, respectively (Fig. 1).
Figure 1.
Recombination activity associated with the patient’s mutations.
DISCUSSION
We describe a case of RAG2 deficiency in which there were no discernible clinical manifestations of immunodeficiency until acute presentation with autoimmune hemolytic anemia at 13 months of age. Symptoms began three weeks after immunization with live-attenuated varicella, measles, mumps, and rubella virus vaccines, at a time when there was no obvious contraindication to the administration of live virus vaccination. Following treatment with high dose corticosteroids for autoimmune hemolytic anemia, she became profoundly lymphopenic and eventually succumbed to disseminated VZV, with detection of mumps and rubella virus in some tissues as well. Each virus was determined to be vaccine-strain in origin. Although a direct causal relationship between sequential events could not be determined, we presume institution of corticosteroid therapy in an infant with a compromised immune system further lowered host defenses, rendering them incapable of containing live-attenuated viruses. It is important to recognize that proximity to vaccination is not necessarily a factor as recently demonstrated in a 6-year-old boy with DOCK8 deficiency who presented with vaccine-strain VZV vasculopathy and dissemination 5 years after vaccination [18]. He too manifested vaccine-strain VZV illness, but only after intensive courses of corticosteroids for severe allergic disease.
With the introduction of T cell receptor excision circle (TREC) newborn screening for severe forms of T cell deficiency, preventive actions and curative treatments can now be instituted early in life [19]. Newborn screening data compiled from 11 states (January 2008 – July 2013) identified 52 cases of SCID, which included 8 cases of RAG1 deficiency and 1 case of RAG2 deficiency [20]. Of the 8 cases of RAG1 deficiency, 4 were termed as “leaky” SCID characterized by a less prominent depression in T cell counts and lymphocyte proliferation. The case of RAG2 deficiency displayed features of typical SCID with near absence of T cells (<300 CD3+ cells/µL) and minimal lymphocyte proliferation to PHA (<10% of normal).
However, it is increasingly recognized that a number of significant primary immune deficiency diseases escape detection in the newborn period, as the presence of T cells and a normal TREC screen does not guarantee preserved immune function [21]. Thus, infants and children (potentially adults as well) with hypomorphic variants of critical immune genes or some degree of reversion in immune cells may be capable of escaping detection for a significant period of time, that is, until an event triggers suspicion and immune evaluation. Genetic testing in the patient described here revealed two mutations in exon 2 of RAG2, with assumed compound heterozygosity of paternally inherited R73H and a presumably de novo P180H mutation. Recombination analyses of each RAG2 mutant revealed residual but significantly decreased activity, consistent with hypomorphism. As described in reports of hypomorphic RAG mutations, residual recombination activity can result in autoimmunity and may also delay the development of identifiable manifestations of immune deficiency, thus providing an explanation for the apparent wellness in a 13-month-old infant and subsequent disseminated infection with autoimmune features after live virus vaccination and corticosteroid treatment.
Compared with previously reported data, the RAG2 mutant residual recombination activity levels of 11% and 31% seen in this patient would be expected to result in a combined immune deficiency phenotype with susceptibility for autoimmunity [14]. Immune dysregulation, rather than severe immunodeficiency, is the hallmark of hypomorphic RAG mutations. Initial limited lymphocyte phenotyping suggested significant T cell lymphopenia, which became profound under corticosteroid treatment. Some recovery of T cell numbers occurred with reductions in corticosteroid dosing, but few studies could be carried out to examine function at that time. Of interest, TLR ligand signaling of TNFα production appeared normal when compared to controls. Dissociation between TLR ligand-induced signaling of cytokine production and antigen-specific T cell function and T cell receptor-induced NF-κB activation has been described in a patient with a NEMO mutation [22]. Severe VZV disease has been observed in both RAG1 and RAG2 deficiencies, yet typically with wild-type strains [7, 23]. Autoimmune cytopenia in particular is an increasingly reported manifestation of RAG deficiency and may represent the first sign of RAG1 or RAG2 defects [23]. The exact cause of autoimmunity is unclear, but generation of self-reactive B cells [24] and perturbed regulatory T cell function [25] have been suggested.
A link between corticosteroid use and severe VZV disease has been described in several publications. In a retrospective study by Dowell and Bresee [26], the odds of corticosteroid use in otherwise immunocompetent children who contracted severe VZV was 178 times greater than in the general population. Furthermore, the risk was even higher in those patients with an underlying immunocompromised condition. Studies performed in the clinical development of VZV vaccines excluded individuals receiving systemic corticosteroid therapy at doses greater than required for physiologic replacement, limiting data on safety in this population. Recently, Russell et al. [27] completed a randomized, placebo-controlled study assessing the safety, tolerability and immunogenicity of live-attenuated zoster vaccination in adults receiving chronic or maintenance systemic corticosteroid therapy (daily dose equivalent of 5–20 mg of prednisone) for ≥2 weeks prior to vaccination and ≥6 weeks post-vaccination. No statistically significant difference was observed between the treatment and placebo groups for systemic or serious adverse events. Six out of the 309 subjects had fatal serious adverse events over the duration of the study, with 3 in each group; however, no deaths were determined to be vaccine-related. Notably, subjects were excluded from this study if they suffered from immune dysfunction (other than the condition requiring corticosteroid use), received additional immunosuppressive medications or required a corticosteroid daily dose >20 mg of prednisone (contraindication to vaccination) or equivalent within 8 weeks prior to vaccination or expected for 6 weeks postvaccination. Though inclusive to patients receiving systemic corticosteroid therapy at doses greater than required for physiologic replacement, higher dosing for acute management of autoimmunity or allergic disease was not assessed. For the RAG2 deficient patient described here, use of corticosteroids for management of autoimmune phenomena resulted in a profound depression of circulating lymphocytes to nearly undetectable levels.
Three fatal cases of confirmed vaccine-strain VZV in children have been reported: a 13-month-old female with SCID due to adenosine deaminase deficiency, a 15-month-old female with T cell lymphopenia, and a child receiving cytotoxic chemotherapy for acute lymphoblastic leukemia [28–30]. Two additional cases, the DOCK8-deficient patient previously described and an 11-year-old girl with NK T cell deficiency, died less than a year after vaccine-strain VZV illness. [18, 31]. Either due to birth before TREC screening was developed or because of birth in a location that did not perform such testing at that time, none of these children, including the RAG2 deficient patient described here, underwent newborn screening for SCID.
Given her presenting CD3+ count of 788 cells/µL, the patient described here likely would have escaped detection on newborn screening. Unfortunately, retrospective TREC testing of the Guthrie card used for her newborn screen was not possible as the specimen was discarded prior to her onset of illness. Potentially supporting her suspected evasion of an abnormal TREC screen, her absolute lymphocyte count rose above 8,000 cells/µL prior to institution of corticosteroids. However, as the exact composition of this lymphocyte population was not analyzed beyond T and B cell quantification, we were unable to extrapolate the proportion of T and B cell subpopulations or NK cells at that time. Definitive therapy for RAG1 or RAG2 deficiency is hematopoietic stem cell transplantation (HSCT) [32].
Acknowledgments
Declaration of all sources of funding: C.D. was supported by a fellowship from Grifols. This work was partially supported by a grant from the National Institutes of Health (grant 5R01AI100887 to L.D.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
ABBREVIATIONS
- ACIP
Advisory Committee on Immunization Practices
- CMV
Cytomegalovirus
- Con
A Concanavalin A
- CVID
Common variable immune deficiency
- D
Diversity
- hCD4
Human CD4
- HSCT
Hematopoietic stem cell transplantation
- IVIG
Intravenous immunoglobulin
- J
Joining
- PHA
Phytohemagglutinin
- RAG
Recombination-activating gene
- SCID
Severe combined immune deficiency
- SD
Standard deviation
- TLR
Toll-like receptor
- TREC
T cell receptor excision circle
- V
Variable
- VZV
Varicella zoster virus
REFERENCES
- 1.Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232. doi: 10.1016/s0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 2.Kim MS, Lapkouski M, Yang W, Gellert M. Crystal structure of the V(D)J recombinase RAG1–RAG2. Nature. 2015;518:507–511. doi: 10.1038/nature14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, Friedrich W, Seger RA, Hansen-Hagge TE, Desiderio S, Lieber MR, Bartram CR. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–99. doi: 10.1126/science.274.5284.97. [DOI] [PubMed] [Google Scholar]
- 4.Avila EM, Uzel G, Hsu A, Milner JD, Holland SM. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics. 2010;126:1248–1252. doi: 10.1542/peds.2009-3171. [DOI] [PubMed] [Google Scholar]
- 5.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 6.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Déchanet-Merville J, Coumau-Gatbois E, Gougeon ML, Lemainque A, Eidenschenk C, Jouanguy E, Abel L, Casanova JL, Fischer A, Le Deist F. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115(11):3291–3299. doi: 10.1172/JCI25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, Schneider DT, Manfras B, Pannicke U, Willemze R, Knüchel R, Göbel U, Schulz A, Borkhardt A, Friedrich W, Schwarz K, Niehues T. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med. 2008;358:2030–2038. doi: 10.1056/NEJMoa073966. [DOI] [PubMed] [Google Scholar]
- 8.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, Douek DC, Pittaluga S, Poliani PL, Lee YN, Notarangelo LD, Wang L, Alt FW, Kang EM, Milner JD, Niemela JE, Fontana-Penn M, Sinal SH, Malech HL. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–1271. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuijpers TW, IJspeert H, van Leeuwen EM, Jansen MH, Hazenberg MD, Weijer KC, van Lier RA, van der Burg M. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG. Blood. 2011;117:5892–5896. doi: 10.1182/blood-2011-01-329052. [DOI] [PubMed] [Google Scholar]
- 10.Abraham RS, Recher M, Giliani S, Walter JE, Lee YN, Frugoni F, Maddox DE, Kirmani S, Notarangelo LD. Adult-onset manifestation of idiopathic T-cell lymphopenia due to a heterozygous RAG1 mutation. J Allergy Clin Immunol. 2013;131:1421–1423. doi: 10.1016/j.jaci.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson LA, Frugoni F, Hopkins G, de Boer H, Pai SY, Lee YN, Walter JE, Hazen MM, Notarangelo LD. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. J Allergy Clin Immunol. 2013;132:969–971. doi: 10.1016/j.jaci.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abolhassani H, Wang N, Aghamohammadi A, Rezaei N, Lee YN, Frugoni F, Notarangelo LD, Pan-Hammarström Q, Hammarström L. A hypomorphic recombination-activating gene 1 (RAG1) mutation resulting in a phenotype resembling common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:1375–1380. doi: 10.1016/j.jaci.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato T, Crestani E, Kamae C, Honma K, Yokosuka T, Ikegawa T, Nishida N, Kanegane H, Wada T, Yachie A, Ohara O, Morio T, Notarangelo LD, Imai K, Nonoyama S. RAG1 deficiency may present clinically as selective IgA deficiency. J Clin Immunol. 2015;35:280–288. doi: 10.1007/s10875-015-0146-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee YN, Frugoni F, Dobbs K, Walter JE, Giliani S, Gennery AR, Al-Herz W, Haddad E, LeDeist F, Bleesing JH, Henderson LA, Pai SY, Nelson RP, El-Ghoneimy DH, El-Feky RA, Reda SM, Hossny E, Soler-Palacin P, Fuleihan RL, Patel NC, Massaad MJ, Geha RS, Puck JM, Palma P, Cancrini C, Chen K, Vihinen M, Alt FW, Notarangelo LD. A systematic analysis of recombination activity and genotype-phenotype correlation in human recombination-activating gene 1 deficiency. J Allergy Clin Immunol. 2014;133:1099–1108. doi: 10.1016/j.jaci.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IJspeert H, Driessen GJ, Moorhouse MJ, Hartwig NG, Wolska-Kusnierz B, Kalwak K, Pituch-Noworolska A, Kondratenko I, van Montfrans JM, Mejstrikova E, Lankester AC, Langerak AW, van Gent DC, Stubbs AP, van Dongen JJ, van der Burg M. Similar recombination-activating gene (RAG) mutations result in similar immunobiological effects but in different clinical phenotypes. J Allergy Clin Immunol. 2014;133:1124–1133. doi: 10.1016/j.jaci.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith C, Dutmer CM, Schmid DS, Bellini WJ, Gelfand EW, Asturias EJ. A toddler with rash, encephalopathy, and hemolytic anemia. J Ped Infect Dis. 2015 doi: 10.1093/jpids/piv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asai E, Wada T, Sakakibara Y, Toga A, Toma T, Shimizu T, Nampoothiri S, Imai K, Nonoyama S, Morio T, Muramatsu H, Kamachi Y, Ohara O, Yachie A. Analysis of mutations and recombination activity in RAG-deficient patients. Clin Immunol. 2011;138:172–177. doi: 10.1016/j.clim.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Sabry A, Hauk PJ, Jing H, Su HC, Stence NV, Mirsky DM, Nagel MA, Abbott JK, Dragone LL, Armstrong-Wells J, Curtis DJ, Cohrs R, Schmid DS, Gilden D, Gelfand EW. Vaccine strain varicella-zoster virus-induced central nervous system vasculopathy as the presenting feature of DOCK8 deficiency. J Allergy Clin Immunol. 2014;133:1225–1227. doi: 10.1016/j.jaci.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, Cogley MF, Litsheim TJ, Katcher ML, Routes JM. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124:522–527. doi: 10.1016/j.jaci.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, Abbott JK, Baker M, Ballow M, Bartoshesky LE, Bonilla FA, Brokopp C, Brooks E, Caggana M, Celestin J, Church JA, Comeau AM, Connelly JA, Cowan MJ, Cunningham-Rundles C, Dasu T, Dave N, De La Morena MT, Duffner U, Fong CT, Forbes L, Freedenberg D, Gelfand EW, Hale JE, Hanson IC, Hay BN, Hu D, Infante A, Johnson D, Kapoor N, Kay DM, Kohn DB, Lee R, Lehman H, Lin Z, Lorey F, Abdel-Mageed A, Manning A, McGhee S, Moore TB, Naides SJ, Notarangelo LD, Orange JS, Pai SY, Porteus M, Rodriguez R, Romberg N, Routes J, Ruehle M, Rubenstein A, Saavedra-Matiz CA, Scott G, Scott PM, Secord E, Seroogy C, Shearer WT, Siegel S, Silvers SK, Stiehm ER, Sugerman RW, Sullivan JL, Tanksley S, Tierce ML, 4th, Verbsky J, Vogel B, Walker R, Walkovich K, Walter JE, Wasserman RL, Watson MS, Weinberg GA, Weiner LB, Wood H, Yates AB, Puck JM, Bonagura VR. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–738. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan A, Puck JM. History and current status of newborn screening for severe combined immunodeficiency. Semin Perinatol. 2015;39:194–205. doi: 10.1053/j.semperi.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salt BH, Niemela JE, Pandey R, Hanson EP, Deering RP, Quinones R, Jain A, Orange JS, Gelfand EW. IKBKG (nuclear factor-kappa B essential modulator) mutation can be associated with opportunistic infection without impairing Toll-like receptor function. J Allergy Clin Immunol. 2008;121(4):976–982. doi: 10.1016/j.jaci.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Wu W, Mathew D, Zhang Y, Browne SK, Rosen LB, McManus MP, Pulsipher MA, Yandell M, Bohnsack JF, Jorde LB, Notarangelo LD, Walter JE. Autoimmunity due to RAG deficiency and estimated disease incidence in RAG1/2 mutations. J Allergy Clin Immunol. 2014;133:880–882. doi: 10.1016/j.jaci.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 25.Cassani B, Poliani PL, Moratto D, Sobacchi C, Marrella V, Imperatori L, Vairo D, Plebani A, Giliani S, Vezzoni P, Facchetti F, Porta F, Notarangelo LD, Villa A, Badolato R. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol. 2010;125:209–216. doi: 10.1016/j.jaci.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Dowell SF, Bresee JS. Severe varicella associated with steroid use. Pediatrics. 1993;92:223–228. [PubMed] [Google Scholar]
- 27.Russell AF, Parrino J, Fisher CL, Jr, Spieler W, Stek JE, Coll KE, Su SC, Xu J, Li X, Schlienger K, Silber JL. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine. 2015;33:3129–3134. doi: 10.1016/j.vaccine.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 28.Leung J, Siegel S, Jones JF, Schulte C, Blog D, Schmid DS, Bialek SR, Marin M. Fatal varicella due to the vaccine strain varicella-zoster virus. Hum Vaccin Immunother. 2014;10:146–149. doi: 10.4161/hv.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrauder A, Henke-Gendo C, Seidemann K, Sasse M, Cario G, Moericke A, Schrappe M, Heim A, Wessel A. Varicella vaccination in a child with acute lymphoblastic leukaemia. Lancet. 2007;369:1232. doi: 10.1016/S0140-6736(07)60567-4. [DOI] [PubMed] [Google Scholar]
- 30.Woo EJ. Letter to the Editor: Fatal varicella due to the vaccine-strain varicella-zoster virus. Hum Vaccin Immunother. 2015;11:679. doi: 10.1080/21645515.2014.1004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy O, Orange JS, Hibberd P, Steinberg S, LaRussa P, Weinberg A, Wilson SB, Shaulov A, Fleisher G, Geha RS, Bonilla FA, Exley M. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J Infect Dis. 2003;188:948–953. doi: 10.1086/378503. [DOI] [PubMed] [Google Scholar]
- 32.Schuetz C, Neven B, Dvorak CC, Leroy S, Ege MJ, Pannicke U, Schwarz K, Schulz AS, Hoenig M, Sparber-Sauer M, Gatz SA, Denzer C, Blanche S, Moshous D, Picard C, Horn BN, de Villartay JP, Cavazzana M, Debatin KM, Friedrich W, Fischer A, Cowan MJ. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123:281–289. doi: 10.1182/blood-2013-01-476432. [DOI] [PMC free article] [PubMed] [Google Scholar]