Abstract
Objective
BMP9/ALK1 and DLL4/Notch promote endothelial quiescence, and we aim to understand mechanistic interactions between the two pathways. We identify new targets that contribute to endothelial quiescence, and test whether loss of Dll4+/− in adult vasculature alters BMP signaling.
Approach and Results
Human endothelial cells respond synergistically to BMP9 and DLL4 stimulation, showing complete quiescence and induction of HEY1 and HEY2. Canonical BMP9 signaling via ALK1-Smad1/5/9 was disrupted by inhibition of Notch signaling, even in the absence of exogenous DLL4. Similarly, DLL4 activity was suppressed when the basal ALK1-Smad1/5/9 pathway was inhibited, showing these pathways are interdependent. BMP9/DLL4 required induction of P27KIP1 for quiescence, although multiple factors are involved. To understand these mechanisms, we used proteomics data to identify upregulation of thrombospondin-1, which contributes to the quiescence phenotype. To test whether Dll4 regulates BMP/Smad pathways and endothelial cell phenotype in vivo, we characterized the vasculature of Dll4+/− mice, analyzing endothelial cells in the lung, heart, and aorta. Together with changes in endothelial structure and vascular morphogenesis, we found that loss of Dll4 was associated with a significant upregulation of pSmad1/5/9 signaling in lung endothelial cells. Since steady state endothelial cell proliferation rates were not different in the Dll4+/− mice, we propose that the upregulation of pSmad1/5/9 signaling compensates to maintain endothelial cell quiescence in these mice.
Conclusions
DLL4/Notch and BMP9/ALK1 signaling rely on each other’s pathways for full activity. This represents an important mechanism of crosstalk that enhances endothelial quiescence and sensitively coordinates cellular responsiveness to soluble and cell-tethered ligands.
Keywords: endothelial cell, quiescence, DLL4/Notch, BMP9, cell cycle
Introduction
Proper function of blood vessels requires an intact endothelial monolayer and quiescent endothelial cells. Multiple cytokines activate endothelial sprouting and proliferation during angiogenesis. However, less is known about the mechanisms that maintain or restore endothelial cell quiescence. Two critical pathways are DLL4/Notch and BMP9/ALK1, which can display cooperative signal transduction1, 2, and inhibit hypersprouting to maintain appropriate capillary density3. The cooperative activity of these pathways has not been studied in quiescence or the re-establishment of endothelial homeostasis.
Recent investigations in developmental vasculogenesis and sprouting angiogenesis have provided new insights into the overlapping mechanisms of DLL4 and BMP9 in endothelial cells. These have shown that endothelial Notch and BMP pathway interactions are a complex interplay between co-regulation of target gene levels, interdependence at the signal transduction level, and variations in interactions based on anatomical distribution of vessels4–8. Most is understood about the transcription regulation of these pathways, via N1ICD/RBPJ and pSmad1/5 activation, respectively. In addition, the basic helix-loop-helix proteins of the Hey family are targets of both Notch and BMP pathways, providing the potential to further regulate up to 2000 additional mRNAs9. The ALK1 pathway was also shown to mediate the activation of Hey1 and Hey2 by serum10. However, cooperative mechanisms by which these and other pathways mediate quiescence and homeostasis of the adult endothelium remain to be elucidated.
In this study, we sought to identify new mechanisms by which DLL4/Notch and BMP9/ALK1 cooperate to regulate endothelial cell quiescence. We showed that stimulation of primary human aortic endothelial cells (HAEC) independently and cooperatively decreases their proliferation and migration and induces the expression of the target genes HEY1 and HEY2. Using siRNA-mediated knockdown, we demonstrated that suppression of either pathway induces a reversal of the anti-proliferative effects of the other. We identify P27KIP1 and thrombospondin-1 as two novel mediators of DLL4/Notch and BMP9/ALK1 crosstalk involved in HAEC quiescence. The interdependence of these pathways suggests that modulation of one might affect the other in vivo in endothelial cells. To test this, we studied the vasculature of the Dll4+/− mouse model on a genetic background that yields viable adults. We show that loss of Dll4 leads to increased endothelial cell density, impaired vascular branching morphogenesis, and a compensatory increase in pSmad1/5/9 nuclear localization. These data show that loss of Dll4 in vivo, which affects developmental vascular branching, does lead to sustained changes in BMP signaling that are maintained in the adult endothelium.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
DLL4/Notch and BMP9 cooperatively suppress HAEC proliferation and induce HEY1 and HEY2
During angiogenesis, DLL4 stimulation of Notch in endothelial cells inhibits the tip cell and promotes the stalk cell phenotype11. Similarly, BMP9 inhibits tip cell formation and angiogenic sprouting12. Although DLL4 and BMP9 work cooperatively to decrease vascular sprouting and to induce vascular stability3, little is known about the molecular mechanisms of this interaction, especially in the re-establishment of quiescence in the postnatal vasculature. To identify cell processes and targets regulated by DLL4 and BMP9, we studied their effects on primary human endothelial cells. Human aortic endothelial cells (HAEC) express ALK1 and ALK5 as primary TGFβ/BMP receptor family members, and Notch1, Notch2, and Notch4 as potential mediators of DLL4 signals. To assess effect of these ligands on human endothelial cells, we stimulated subconfluent HAEC with 20pmols coated DLL4, 5ng/mL soluble BMP9, or the combination of both ligands for 24h. The concentration of BMP9 was chosen by dose response analysis (Suppl. Fig. IA–B) and matched physiological circulating levels12. Notable changes were observed in HAEC morphology, most dramatically in the DLL4-treated and combination DLL4+BMP9-treated wells (Fig. 1A). Control cells were elongated, typical of proliferating HAEC, whereas ligand stimulation led to a cobblestone-like morphology, often associated with differentiated, confluent endothelial monolayers.
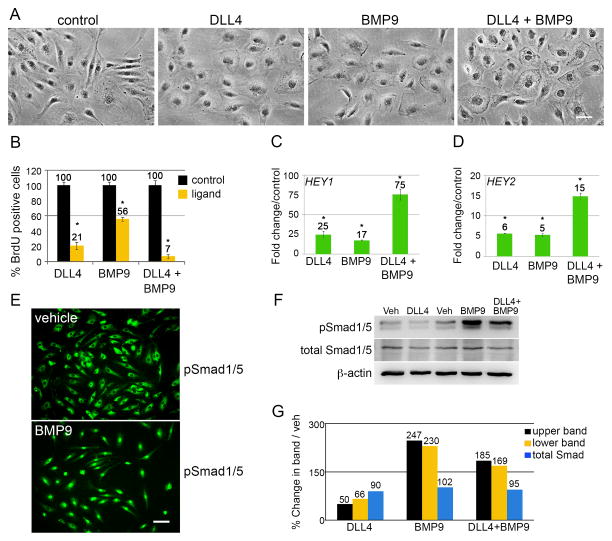
Figure 1. DLL4/Notch and BMP9 cooperatively suppress endothelial cell proliferation and induce HEY genes.
A) Primary human aortic endothelial cells (HAEC) were stimulated with ligand as described for 24h. Scale bar = 50μm. B) After 18h treatment, cells were given 10μg/mL BrdU for the last 6h of growth. Cells were fixed at 24h and processed for immunostaining with anti-BrdU. The percentage of BrdU positive cells was calculated (n=10 per condition). Graphed are means ±SD. C–D) Cells under the same experimental conditions were grown for 24h, and total RNA was collected for qPCR. Primers were used to amplify HEY1 and HEY2 transcripts normalized to PPIA (cyclophilin). Graphed are fold change means compared to control ±SD. E) HAEC were synchronized by serum starvation for 8h, treated with vehicle or BMP9 for an additional 16h, then fixed and stained for phospho-Smad1/5 (pS463/465). Scale bar = 50μm. F) HAEC were treated with vehicle or ligands for 24h and whole cell lysates were immunoblotted for phospho-Smad1/5 (pSmad1/5) and total Smad1/5 (total Smad1/5). pSmad1/5 present as a doublet and the bands were quantified separately. G) Quantified immunoblot bands were normalized to β-actin and represented as a percentage of vehicle. (*) indicates a statistical significance with p<0.05.
To assess effects of the ligands on proliferation, we incubated cells with 10μg/mL 5-bromo-2′-deoxyuridine (BrdU) treatment during the final 6h of growth. BrdU incorporation was quantified (Fig. 1B). Compared to control, there was a dramatic decrease in proliferation after 24h stimulation with either DLL4 or BMP9, and additive inhibition with combined ligands. The suppression of proliferation was observed as early as 12h of ligand treatment, and further suppressed by 24h (Suppl. Fig. 1C). The effect of the ligands was reversible, as removing cells from ligand exposure re-established normal proliferation (Suppl. Fig. 1D).
HEY1 and HEY2 basic helix-loop-helix transcriptional repressors are well-established targets of both Notch and BMP signaling. Quantitative RT-PCR (qPCR) analysis of HEY1 and HEY2 transcripts demonstrated induction of both genes, and a cooperative induction with combined ligand treatment (Fig. 1C–D). To validate BMP9 activity, we treated HAEC with BMP9 and analyzed cells by immunofluorescence and clearly observed pSmad1/5 (pS463/465) translocation to the nucleus (Fig. 1E). We also immunoblotted lysates from 24h ligand-stimulated cells and observed that pSmad1/5 is highly increased by BMP9, decreased by DLL4, and significantly higher than baseline in the combined stimulation (Fig. 1F–G). We examined the protein levels of growth arrest-specific gene 1 (GAS1), since it is associated with G0 cell cycle arrest, but found no changes after DLL4 or BMP9 treatment (Suppl. Fig. 1E). To rule out that the decrease in proliferation was a secondary effect of cellular senescence, we analyzed levels of senescence-associated β-galactosidase activity, which was virtually undetected under control or ligand-treated conditions (Suppl. Fig. 1F). Finally, to determine whether ligand stimulation affects endothelial activation, we first treated HAEC with lipopolysaccharide (LPS), and found that ICAM1 was the most robustly induced inflammatory mediator (Suppl. Fig. 1G). Ligand-stimulated HAEC demonstrated a more potent induction of ICAM1 upon activation with bacterial lipopolysaccharide (LPS), and polyinosinic:polycytidylic acid (PIC), a mimic for viral infection (Suppl. Fig. 1H). Thus, while DLL4 and BMP9 induce a quiescent state in endothelial cells, they increase sensitivity to strong activation cues.
Cooperative proliferation effects of DLL4/Notch and BMP9 are reversed by knocking down components of each pathway
We next sought to clarify specific signaling mechanism required for interaction of DLL4/Notch and the BMP9/ALK1 pathways. We used siRNA knockdown to target key components of each pathway. First, to identify the Notch responsible for DLL4-mediated cellular quiescence, we knocked down the Notch1 (siN1), Notch2 (siN2), and Notch4 (siN4) receptors, which resulted in 92%, 86%, and 78% reduction of each receptor, respectively, relative to a non-targeting (NT) siRNA control (Fig. 2A–C). These are the primary Notch proteins expressed in human endothelial cells, with Notch3 present at very low levels (Suppl. Fig. 3A). SiRNA-targeted cells were stimulated with ligands, and proliferation was measured (Fig. 2A–C). We found that suppression of Notch1 rendered the cells insensitive to the quiescence signal of DLL4 and also BMP9 (Fig. 2A). However, loss of Notch2 or Notch4 did not affect the sensitivity of the cells to either ligand (Fig. 2B–C). The loss of Notch1 also significantly suppressed the induction of HEY1 in all ligand conditions (Fig. 2D). Using antibodies specific to the cleaved, activated forms of Notch1, Notch2, and Notch4 in immunoblot, we confirmed that only Notch1 was activated by DLL4 in HAEC (Fig. 2E). BMP9 did not increase activated forms of any Notch receptor, even in the presence of DLL4 (Fig. 2E–F). These results clearly identified Notch1 as the primary DLL4 receptor, and led to the unexpected finding that endothelial cells have decreased sensitivity to BMP9-mediated regulation of cell growth in the absence of Notch1. Another key component of Notch signaling is the transcriptional co-activator RBPJ (CBF1, CSL). A 90% knockdown of RBPJ (siRBPJ, Fig. 2G) led to the partial reversion of the proliferation effects of DLL4, BMP9, and the combination. These data indicate a requirement for Notch1/RBPJ signaling as a component of BMP9 initiation of quiescence.
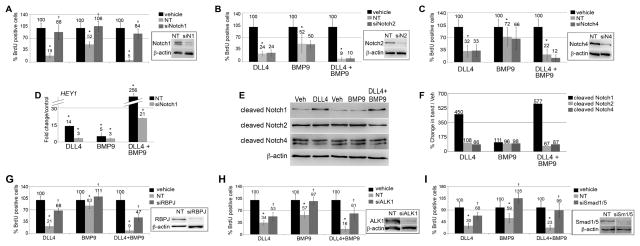
Figure 2. Notch1 and ALK1 activity are required for DLL4 and BMP9 cooperative signaling.
Primary human aortic endothelial cells (HAEC) were treated with siRNA targeting Notch or ALK pathways compared to non-targeting (NT) siRNA. A–C) Suppression of Notch1 (siN1), Notch2 (siN2), or Notch4 (siN4) was validated by immunoblot (8%, 14%, and 22% of NT, respectively). HAEC were treated with ligand as indicated, and proliferation measured by BrdU incorporation. D) HAEC targeted with siN1 or NT siRNA were treated with ligands, and HEY1 mRNA quantified by qPCR. E) HAEC were treated with vehicle or ligands for 24h and whole cell lysates were immunoblotted for cleaved, activated forms of Notch1 (V1744), Notch2 (D1733/A1734), or Notch4 (V1432), and quantified in comparison to β-actin (F). G) siRNA was used to suppress RBPJ (siRBPJ), and cell lysates were immunoblotted to confirm loss of protein (10% of NT). Proliferation was measured using BrdU incorporation. Similar knockdown experiments targeted ALK1 (H, siALK1, 17% of NT) or Smad1/5/9 (I, siSmad1/5, 19% of NT). Graphed are means ±SD. (*) signifies statistical significance with p<0.05. (†) signifies statistically significant reversal of effects (p<0.05).
The primary receptor for BMP9 on endothelial cells is ALK1, which phosphorylates Smad1/5/9 proteins, leading to interaction with Smad4 and activation of target genes. We suppressed ALK1 by siRNA (siALK1, 83% suppression, Fig. 2H), and, as expected, knockdown of ALK1 led to insensitivity to BMP9 (Fig. 2H). Suppression of ALK1 was only partially able to reverse the inhibitory effects of DLL4 on cell proliferation. When we knocked down the Smad1/5/9 proteins by 81% (siSM1/5, Fig. 2I), BMP9 effects were completely abolished. Loss of Smad1/5/9 also partially reversed the growth suppression effects of DLL4. Thus, although Notch1 signaling is critical for propagation of the BMP9 signal, interruption of the BMP9 pathway diminished, but did not eliminate, DLL4/Notch1 signaling.
ALK1 also signals in a Smad-independent manner, through pathways including TGF-beta-associated kinase 1 (TAK1). To determine if TAK1 mediates the effects of BMP9 or DLL4, we treated cells with a TAK1 inhibitor. Suppression of TAK1 activity had a global effect of suppression of proliferation (Suppl. Fig. IIA), suggesting that basal levels of TAK1 support transit through the cell cycle. These data point to pSmad signaling as the primary pathway mediating specific BMP9/ALK1 signals for endothelial quiescence.
Multiple cell cycle-related proteins are affected by DLL4 and BMP9 stimulation, and P27KIP1 is a major mediator of quiescence
To further understand the molecular regulation of endothelial quiescence, we analyzed cell cycle-associated proteins following stimulation with DLL4, BMP9, or both. Immunoblots were run using whole cell lysates of vehicle (Veh) or ligand-stimulated HAEC (Fig. 3A–B, Supp. Table IA–B). Several proteins were altered following ligand stimulation (Fig. 3A). Despite the potent decrease in proliferation, several cell cycle inhibitors were decreased, including P18INK4C, P19INK4D, and P21CIP1, the latter being a transcriptional target of p53, which also decreased as a result of ligand stimulation. Potentially contributing to the decreased proliferation, we observed decreases in cyclinD1 (by both DLL4 and BMP9) and cyclinD3 (by DLL4 only). The CDKs typically associated with the D-type cyclins also changed, albeit differentially, with CDK4 decreasing and CDK6 increasing upon stimulation with the ligands. To corroborate our BrdU measurements of proliferation, we evaluated levels of the Ki-67 marker of proliferation. Ki-67 was significantly decreased following stimulation with DLL4 and BMP9, and levels were correlated with levels of proliferation. Proteins with negligible change following ligand stimulation were the P14ARF, P15INK4B, and P16INK4A cell cycle inhibitors, cyclinE1 and E2, and CDK2 (Suppl. Fig. IIB–C).
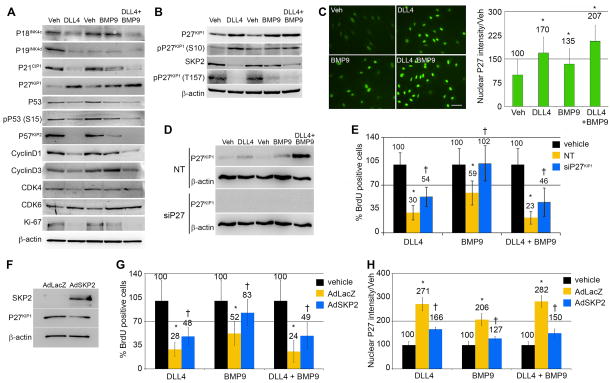
Figure 3. P27KIP1 mediates the quiescence signal of DLL4 and BMP9.
Primary human aortic endothelial cells (HAEC) were treated with ligands as indicated. A–B) Cell lysates were collected after 24h for immunoblot using antibodies against cell cycle regulatory proteins. Quantification of the immunoblot bands in panels A, B and E are in Supplementary Table I. C) Cells stimulated under the same conditions were fixed after 24h and immunostained for P27KIP1 (scale bar=100μm), and nuclear fluorescence intensity was quantified. Graphed are means relative to vehicle ±SD, N≥5 fields per condition. D) P27KIP1 was targeted using siRNA (siP27) compared to non-targeting (NT) siRNA, and protein levels detected by immunoblot. E) Proliferation following knockdown of P27KIP1 was measured by BrdU incorporation. F) Cells were transduced with adenoviral vectors with control LacZ (adLacZ) or an expression construct for SKP2 (adSKP2). Cell lysates were immunoblotted, validating the overexpression of SKP2 (2232% of control) and consequent downregulation of its target P27KIP1 (63% of control). G) Cells transduced with control AdLacZ or AdSKP2 were stimulated with ligands or vehicle control, and proliferation measured by BrdU. SKP2 overexpression reversed the proliferation effects of the ligands (G) and significantly decreased the ligand-mediated nuclear intensity of P27KIP1 (H). Graphed are means ±SD. (*) signifies statistical significance of P<0.05. (†) signifies statistically significant reversal of effects (p<0.05).
The most potently upregulated cell cycle inhibitor observed was P27KIP1 (Fig. 3A–C, Suppl. Table IA–C), whose induction correlated with decreased proliferation for each ligand, and especially the combined ligand stimulation. Potential contributors to elevated P27KIP1 were decreased levels of the SKP2 E3 ubiquitin ligase, which targets P27KIP1 for proteosomal degradation, and increased phosphorylation of the serine-10 residue of P27KIP1, which enhances the half-life and stability of the protein13 (Fig. 3B). In addition, P27KIP1 phosphorylation on threonine-157 is associated with its cytoplasmic retention, which is associated with its functional inactivation14, 15. DLL4 and BMP9 reduced the levels of pP27KIP1(T157), which is expected to increase nuclear localization (Fig. 3B), consistent with growth suppression. Indeed, immunofluorescent staining of ligand-stimulated cells revealed increased nuclear P27KIP1 intensity, inversely correlating to the trend of decreasing proliferation (Fig. 3C). We observed similarly-increased nuclear P27KIP1 immunofluorescence intensities, and corresponding decreases in proliferation when we stimulated a variety of other primary human endothelial cell types with DLL4 and BMP9 (Suppl. Fig. IIIB–H). We used siRNA knockdown to further investigate the contribution of P27KIP1 to ligand-induced changes in proliferation, and were able to suppress P27KIP1 by ~92% (Fig. 3D, Suppl. Table IC). Without P27KIP1 the effects of DLL4 and combined DLL4 and BMP9 were blunted, and BMP9 was unable to suppress proliferation (Fig. 3E).
To determine if the ligand-mediated suppression of SKP2 was also contributing to the upregulation of P27KIP1, we used an adenoviral construct to overexpress the SKP2 protein (AdSKP2). We validated the overexpression of SKP2 and the corresponding decrease in endogenous baseline expression of P27KIP1 by immunblot (Fig. 3F). AdSKP2 significantly rescued the proliferation of the cells from ligand-mediated suppression (Fig. 3G), and the nuclear P27KIP1 intensities were correspondingly decreased as well (Fig. 3H).
Endothelial cells are sensitive to cytokine stimulation, and Notch signaling influences VEGF activity by regulating VEGF receptors16–18. To test the possibility that loss of sensitivity to growth factors was responsible for the quiescence, we analyzed levels of growth factor receptors. As expected, VEGFR2 was decreased by DLL4. In addition, neuropilin1 was suppressed by treatment with DLL4 and BMP919. Moreover, EGFR1 and the FGF receptors were also suppressed by ligand treatment, and VEGFR1 was induced (Suppl. Fig. IVA–B). Because lower yet significant levels of receptors were still present, we tested whether the quiescence could be overcome by cytokine supplementation. However, despite high levels of additional growth factors, DLL4 and BMP9 still fully invoked the quiescent phenotype under serum-free conditions (Suppl. Fig. IVC), and complete medium (data not shown). In contrast, expression of constitutively active Ras (caRas, Suppl. Fig. IVD) was able to antagonize the effects of the ligands and lead to normal proliferation (Suppl. Fig. IVE).
To determine if the HEY genes were involved in the regulation of P27KIP1, we used adenoviral overexpression of FLAG-tagged HEY1 and HEY2 (Suppl. Fig. VA), and measured the changes in proliferation, and P27KIP1 by immunoblot and immunofluorescence (Suppl. Fig. VA, C). Overexpression of the HEY was sufficient to significantly suppress cell proliferation (Suppl. Fig. VB), but P27KIP1 levels did not significantly change (Suppl. Fig. VA, C). We also used siRNA knockdowns of HEY to investigate their contribution to ligand-mediated changes in P27KIP1. HEY knockdowns were validated by qPCR (Suppl. Fig. VD). Knocking down HEY1, HEY2, or both did not affect the capacity of either DLL4 or BMP9 to suppress proliferation (Suppl. Fig. VE), although the knockdown did decrease nuclear P27 KIP1 (Suppl. Fig. VF) and total P27 KIP1 levels (Suppl. Fig. VG).
DLL4 and BMP9 regulate extracellular matrix proteins and TGFbeta
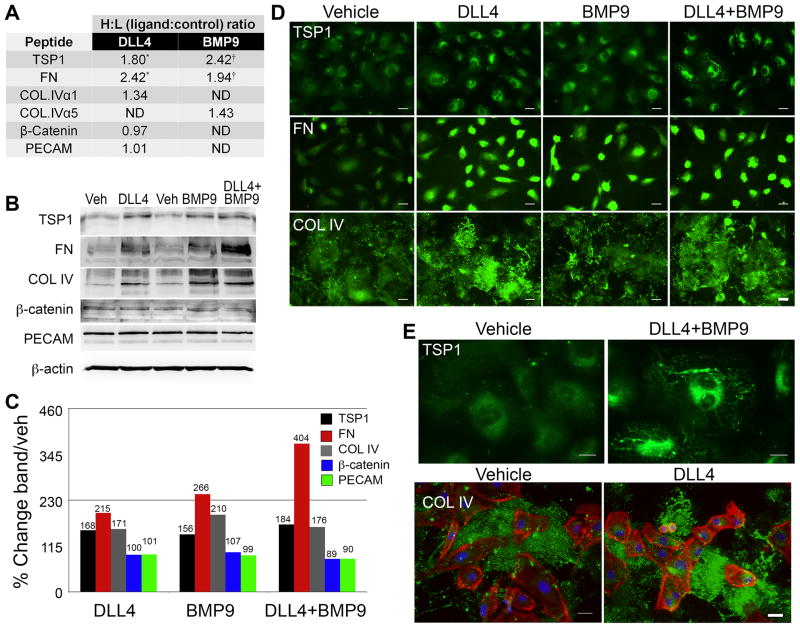
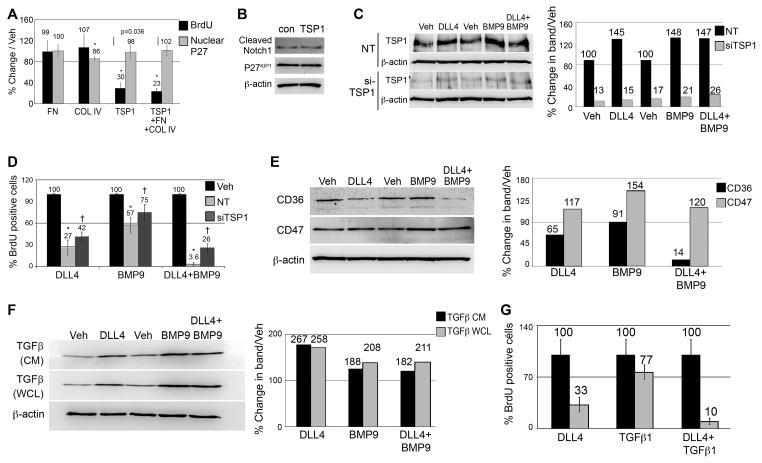
A quantitative mass spectrometric proteomic screen was performed as previously described20 to identify changes in protein profiles during DLL4 and BMP-induced endothelial cell quiescence. Interestingly, several extracellular matrix (ECM) proteins were identified as induced by ligand stimulation (Fig. 4A). Noteworthy among them, thrombospondin-1 (TSP1) is a potent inhibitor of endothelial proliferation21. Fibronectin (FN) and collagen type IV (COL IV) were also significantly increased. To validate the mass spectrometry data, immunoblots and immunofluorescence assays were performed on vehicle and ligand-stimulated cells. Both confirmed increased ECM in ligand-stimulated cells (Fig. 4B–E), as well as verified proteins that were unchanged by ligand stimulation (β-catenin and PECAM). While the majority of the increased FN was localized intracellularly, TSP1 accumulated in fibrillar networks on the surface of cells, and COL IV was secreted and deposited in a dense network between cells (Fig. 4E). To determine the contribution of each ECM component to induction of quiescence, BrdU proliferation assays were performed with cells plated on ECM-coated plates (60pmols/well, Fig. 5A). Among the individual ECM proteins, only TSP1 decreased proliferation, whereas COL IV and FN did not significantly change proliferation. However, the combination of COL IV, FN and TSP1 together (60pmols/ECM/well) had a stronger quiescence effect than that of TSP1 alone, implying that either the suppressive effects of TSP1 are amplified when combined with FN and COL IV, or a different anti-proliferative signal results as cells contact all three ECM components simultaneously. Stimulation of cells with TSP1 did not significantly affect P27KIP1 overall expression levels or nuclear intensity, nor did TSP1 independently increase the baseline activation of Notch1 (Fig. 5B). To determine if increased TSP1 contributed to DLL4 and BMP9-induced quiescence, we used siRNA, which suppressed TSP1 protein by an average 82% (Fig. 5C–D). There was a small but statistically significant rescue of proliferation when TSP1 was decreased in the presence of ligand (Fig. 5E). Two of the major receptors for TSP1 implicated in its effects on proliferation are CD36 and CD47. We probed for these receptors by immunoblot, and found them differentially regulated upon ligand stimulation, with CD36 levels decreased and CD47 levels increased (Fig. 5F–G). Although the changes in these receptors could be a direct result of regulation by the ligands, it could also be that the receptors are internalized and degraded upon binding TSP122, 23.
Figure 4. Extracellular matrix proteins accumulate following DLL4 and BMP9 stimulation.
A) Primary human aortic endothelial cells (HAEC) were stimulated with ligands as described. Proteins were collected and conjugated to isotope coded affinity tags and analyzed by mass spectrometry. Heavy (H) to light (L) isotope ratios of specific peptides reflected fold changes in proteins in ligand-stimulated conditions to control conditions. ND indicates peptides not detected. (*/†) indicate peptides detected in multiple cellular fractions. B) Cell lysates were collected 24h after ligand treatment, and immunoblotted to independently validate mass spectrometry analysis. C) Quantified immunoblot bands are represented as a percentage of vehicle. D) Cells were fixed and processed for immunofluorescence staining to detect thrombospondin-1 (TSP1), fibronectin (FN), or collagen type IV (COL IV) under the conditions indicated. Scale bar = 50μm. E) Representative high magnification views are shown to demonstrate the fibrillar, cell surface and extracellular accumulation of TSP1 and COL IV. All ECM proteins are shown in green, phalloidin-stained actin filaments are red, and DAPI-stained nuclei are blue. Scale bar = 30μm for the TSP1 panels and 50μm for the COL IV panels.
Figure 5. TSP1 increased P27KIP1 and contributes to the quiescence phenotype induced by DLL4 and BMP9.
A) Extracellular matrix proteins were used to coat dishes at 60pmoles/well, and primary human aortic cells (HAEC) were plated on top for 24h, followed by assessment of proliferation by BrdU incorporation (black bars). Immunofluorescence staining was used to measure nuclear P27KIP1 under different conditions (gray bars). Graphed are means ±SD. B) Cell lysates were collected 24h after plating on TSP1 for immunoblot to quantify cleaved Notch1 and P27KIP1 (106%, and 93% of vehicle, respectively. C) Immunoblot was used to show efficacy of TSP1 knockdown using targeted siRNA (siTSP1) relative to non-targeting control (NT). Immunoblot results were quantified as a percentage of siTSP1 relative to NT. D) siTSP1 cells had significantly reduced sensitivity to the quiescence signals mediated by DLL4 and BMP9. Graphed are means ±SD. E) Cell lysates from ligand-treated cells were analyzed by immunoblot for the TSP1 receptors CD36 and CD47. Quantified immunoblot bands are represented in graphical form as a percentage of vehicle. F) HAEC were stimulated with ligands in serum-free and growth factor-free media for 24h. Conditioned media (CM) and whole cell lysates were collected and immunoblotted for TGFβ1. Quantified immunoblot bands are represented as a percentage of vehicle. G) HAEC were treated with ligands as indicated (1ng/ml TGFβ1) for 24h. The percentage of BrdU positive cells was calculated (n=10 per condition). Graphed are means ±SD. (*) signifies statistical significance with p<0.05. (†) signifies statistically significant reversal of effects (p<0.05).
There is some overlap in the activation of Smads with BMP family members and other TGFβ ligands, and TGFβ1 can also suppress endothelial cell proliferation and regulate ECM production. Thus, we tested whether DLL4 regulated the production of TGFβ1. We found that both DLL4 and BMP9 increased TGFβ1 in whole cell lysates as well as the conditioned medium (Fig. 5F). In addition, TGFβ1 also cooperated with DLL4 in suppressing cell proliferation (Fig. 5G).
Loss of Dll4 in Dll4+/− mice alters BMP signaling in vivo in endothelium
Our in vitro studies found that DLL4 and BMP9 signaling in endothelial cells is interdependent and requires the other pathway for full activity. Developmental angiogenesis is regulated by these pathways, and our studies in human cells show important functions in quiescence of adult endothelial cells. Our data lead to the prediction that loss of Dll4 signaling in the mouse would modify BMP signaling in endothelial cells. To test this, we used the previously developed Dll4 targeted mutation24, which has been used to show a critical role for Dll4 in embryonic development. On a C57BL/6J background, this mutation caused haploinsufficient lethality with vascular remodeling defects. However, on a congenic FVB/NJ background, some Dll4+/− mice survived to adulthood, permitting analysis of postnatal vasculature and endothelial cell phenotype. From crosses of wild type mice bred to Dll4+/− littermates, 49 litters for a total of 275 pups were genotyped. There were 195 wild type mice (71%), and 80 Dll4+/− mice (29%), rather than the expected 50% of each genotype if non-lethal. Thus, while there was still a loss of Dll4+/− mice, we could generate a sufficient number of Dll4+/− mice for study.
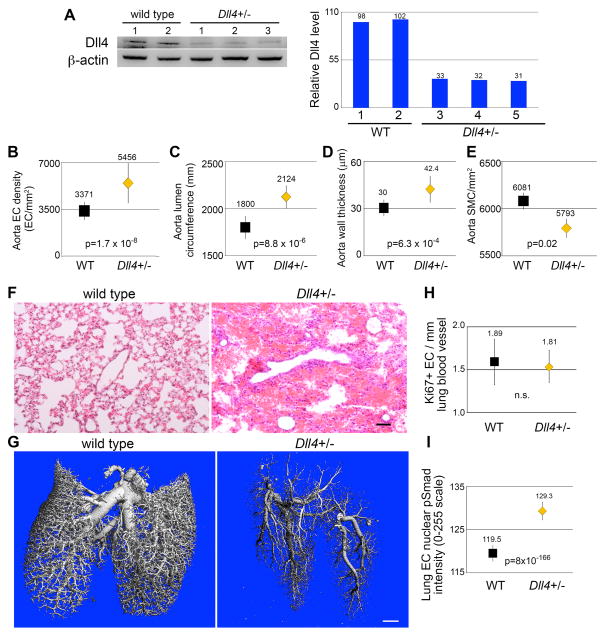
To determine the levels of Dll4 protein in the Dll4+/− mice, we collected tissue lysates from mouse aortae (Fig. 6A). We consistently saw decreased Dll4 protein compared to wild type, with vessels from Dll4+/− mice containing about one third of normal levels. Based on our in vitro results, we predicted that decreased Dll4 might lead to increased cell proliferation that would be reflected in the mature adult vessel. Thus, we performed both en face staining of aortic segments to quantify endothelial cell density, and also analyzed cross sections for vessel morphometry. We found that indeed, the density of endothelial cells per area in the aorta was significantly increased in the Dll4+/− mice (Fig. 6B), and this corresponded to an increase in vessel lumen size (Fig. 6C). The increased density of endothelial cells in the aorta was consistent in different ages and genders of mice, ranging from 3 month old to 9 month old individuals (Suppl. Fig. VIA). Although normal adult vascular smooth muscle cells do not express significant levels of Dll4 protein, endothelial cells regulate medial smooth muscle cells in a paracrine manner. Thus, we analyzed medial thickness and smooth muscle cell density in aortae. We found increased medial wall thickness in the Dll4+/− mice (Fig. 6D) without an increase in smooth muscle cell numbers, leading to a decreased density of cells/area (Fig. 6E).
Figure 6. Haploinsufficiency of DLL4 increases aortic endothelial density, and increases phospho-Smad1/5 levels in lung endothelial cells of adult mice.
A) Protein lysates collected from thoracic aortas of Dll4+/− mice and wild type (WT) littermates were immunoblotted to determine levels of DLL4. Quantified immunoblot bands are represented as a percentage of Dll4+/− relative to the average of the WT bands. B) Thoracic aortae were fixed, stained with Hoechst nuclear dye and endothelial density measured by immunofluorescent imaging. Graphed are means ± SEM. C–E) Transverse sections of paraffin embedded thoracic aortas of littermates were H&E stained, and lumen circumference (C), aorta wall thickness (D), and smooth muscle cell density (E) were measured. N=9 mice/group. Graphed are means ± SEM. F) Sections of paraffin embedded lungs from littermates were H&E stained, revealing extensive red blood cell infiltration of alveolar spaces. Scale bar = 50μm. G) Lung vasculature was perfused with radiopaque silicone rubber (Microfil), and imaged by microcomputed tomography. Irregular vascular branching patterns can be observed in Dll4+/− lung. Scale bar = 1mm. H) Sections of paraffin embedded lungs were immunohistochemically stained for the ki67 proliferation marker, and endothelial cells positive for the marker were counted, and quantified relative to total vessel lumen circumference. Graphed are means ± SEM. N=7 mice/group. I) Sections of paraffin embedded lungs were immunohistochemically stained for phospho-Smad1/5, and staining intensity of endothelial nuclei was measured. Graphed are means ± SEM. N = 7 Dll4+/− mice (31,195 nuclei) and 8 WT mice (19,101 nuclei).
We also analyzed the vasculature in the hearts of wild type versus Dll4+/− mice. After staining to detect endothelial cells, we measured and counted blood vessels. We grouped vessels based on size, from 10–50μm up to >600μm. We did not find any significant differences in the number of vessels per area when comparing hearts from wild type versus Dll4+/− mice either according to vessel size (Suppl. Fig. VIB), or averaging all sizes together (Suppl. Fig. VIC).
During the course of histological analysis of tissues, we noted that some of the lungs from Dll4+/− mice had areas of hemorrhage (Fig. 6F), similar to phenotypes of some components of the Bmp9/Alk1 pathway, including loss of function of the co-receptor endoglin25, and loss of function Alk126. Because Dll4/Notch regulates branching morphogenesis, we visualized the lung vasculature by Microfil perfusion followed by microCT analysis. Although there was some variability in perfusion patterns, several Dll4+/− lungs showed significant deviation from the highly structured, organized patterning of wild type lung vasculature (Fig. 6G, supplemental video). In addition to developmental branching, other aspects such as endothelial cell growth or pruning patterns could contribute to this phenotype during the establishment of the lung vasculature. To test for changes in endothelial cell proliferation, we stained lung sections to detect the proliferation antigen Ki-67, and did comprehensive analysis of labeled endothelial cells within the lung vasculature. In these adult lungs, there was no significant difference in the proliferation rate of lung endothelium (Fig. 6H). To assess BMP/ALK signaling in lung endothelial cells, we immunostained and analyzed nuclear pSmad1/5 protein. We found a highly significant increase in pSmad1/5 nuclear staining in the lung vasculature of the Dll4+/− mice (Fig. 6I), suggesting compensatory signaling with the decrease in Dll4.
Discussion
The Notch and TGFβ/BMP signaling pathways are currently being targeted for clinical therapies related to angiogenesis. Inhibitors of ALK1 include an extracellular ALK1 domain fused to Fc (ALK1-Fc) that binds BMP9 and BMP10 and acts as a competitive inhibitor, and a humanized mAb against ALK1 (reviewed in 27). Likewise, inhibition of DLL4/Notch1 has been proposed as a method to inhibit tumor angiogenesis by increasing sprouting in angiogenic vessels, leading to reduced perfusion and thus hypoxia (reviewed in 28). However, challenges include unwanted side effects such as pathological activation of endothelium, leading to vascular tumors and disrupted organ homeostasis29 and telangiectasias30. Our results in human endothelial cells provide mechanistic insight into how blocking these pathways would negatively affect vascular quiescence and homeostasis.
Cooperative activity between Notch and TGFβ/BMP signaling is not unprecedented. Signals from multiple BMP family members lead to interaction of Notch1 intracellular domain (NICD) and the Smad proteins, potentiating their transcriptional activity on shared target genes1, 31, 32. In embryonic zebrafish, Notch and ALK1 signaling co-regulated development of certain vessels, but had differential regulation of certain target genes and cranial vessels, and distinct phenotypes of arteriovenous malformations6. In mouse endothelial cells, cooperative activity of BMP6 was shown with Notch signaling on Hey transcription factors2. Although little is known about signal interactions between DLL4/Notch and BMP9/ALK1 cascades, their high significance in angiogenesis and vascular pathology have been elegantly shown in mouse vascular developmental and disease models and venous-derived endothelial cells3. Suppression of DLL4, BMP9, or ALK1 signaling enhanced tube formation, thus coordinately regulating angiogenesis. Similarly, Dll4 deficiency in Dll4+/− mice led to increased collateral network formation following ischemia; however, tissue perfusion was not increased, suggesting the formation of poor quality vasculature33. In addition to endothelial cells, Dll4 is also involved in other cells that regulate cardiovascular diseases, including inflammatory cells. Aikawa et al. showed that Dll4 regulates macrophage activity, leading to changes in atherosclerosis and metabolic dysfunction34. During remodeling, Dll4 plays an important role in vascular maturation, and our studies would suggest that establishment of cellular quiescence is a vital part of this maturation.
Our study is the first to focus on DLL4 and BMP9 signaling in human arterial endothelial cells to study their combined effects on cellular quiescence. One important finding was related to the Notch protein that interacts with DLL4. Our findings show that Notch1 specifically mediates the DLL4-induced quiescence signal. Interestingly, Notch1 was recently implicated in endothelial cell senescence, with activation of Notch1 by Jagged1 proposed to increase cellular lifespan, corresponding to suppression of p53, p21cip1, and p1635. In our studies, we did see, separately, DLL4 and BMP9 suppression of p21 and p53, but this suppression was not associated with changes in cellular senescence. Despite very low levels of Notch3 in all of our endothelial cells, it may also have been activated by DLL4, but we did not conduct those studies due to a lack of cleaved/activated Notch3 antibody36, 37. Recent investigations have also demonstrated the antagonistic interactions of Jagged1 with DLL4 for proper angiogenesis and mural cell recruitment for vessel maturation7, 38. It was shown that DLL4 activation of Notch1 increased the levels of Jagged1, which activated Notch4 to negatively regulate DLL4, but also increased mural cell coverage of nascent vessels. This negative feedback feature of vessel maturation may also be co-regulated by BMP9 signaling, as ALK1 also maintains Jagged1 expression levels39.
Our studies also show that BMP9/ALK1 suppression of proliferation occurs primarily via Smad signaling. Suppression of Smad expression by targeted siRNA abrogates the BMP9 suppressive effect, and even leads to enhanced proliferation. This observation may be due to the fact that BMP9/ALK1 activates Smad-independent pathways such as TAK1 (TGFβ-activated kinase-1). Although predominant Smad signaling in endothelial cells overall inhibits proliferation, the downstream targets of pTAK1 (MEK1/2, MEK4/7, MEK3/6, IKKα/β) clearly activate proliferation. In fact, use of a TAK1 inhibitor completely suppressed endothelial cell proliferation, supporting its endogenous role to promote cell cycle progression. This view is supported by other studies of siRNA-mediated suppression of TAK1 in endothelial cells, which led to suppressed proliferation, migration, and tube formation40. Thus, we propose that primary BMP9 signaling in human endothelial cells is mediated via Smad activation to promote the quiescence phenotype, and when Smad is lacking, signaling via alternative pathways such as TAK1 may predominate. Although not addressed in our study, another ligand that activates ALK1 and the Smad pathway is BMP10. In zebrafish embryos, Bmp10 was shown to activate Smad1/5/9 to promote endothelial cell quiescence in response to blood flow, whereas Bmp9 was not involved for this effect41. In humans, by inference from mouse genetic phenotypes, it is expected that there may be some overlapping and some unique functions between BMP9 and BMP10, possibly depending on the tissue42, 43.
Our novel finding that DLL4 and BMP9-induced quiescence is associated with accumulation of specific extracellular matrix proteins has implications for cytokine signaling and angiogenesis. Perhaps most well characterized are the matrix effects on VEGF signaling, which in the presence of matrix proteins, causes interaction of the VEGFR and integrins and modification of signaling44. Vascular-expressed matrix proteins, such as laminin 10, signal through integrins to regulate the expression of DLL445, and we show a reciprocal regulation of DLL4 towards specifically TSP1, FN, and COL IV. TSP1 is a well-known inhibitor of endothelial proliferation46, but its regulation by Notch signaling has not been previously shown. TSP1 contributes to the mature, differentiated phenotype of adult endothelial cells47, and this is consistent with its induction by BMP9 and DLL4, which promote vascular quiescence. Although proliferation was suppressed, we did not observe any changes in P27KIP1 or activated Notch1 upon stimulation of endothelial cells with TSP1, which indicates that TSP1 contribution to vascular quiescence is likely via inhibition of growth factor signaling48–51. Although TSP-1 was the only one of these ECM proteins that was sufficient to decrease proliferation, the change in overall cellular microenvironment is expected to impact growth factor availability, cell motility, and cytokine signaling. The finding that TSP1 is induced by BMP9 and DLL4 may potentially impact the pursuit of anti-angiogenic molecules, as combined therapeutics can provide multimodal strategies52–54. Overall, our studies using human endothelial cells suggest a model where DLL4 and BMP9 pathways activate multiple regulatory nodes to maintain a quiescent phenotype (Suppl. Fig. VII).
Based on our in vitro studies, we utilized a mouse model to further understand if modulating Dll4 in vivo would lead to changes in BMP9 signaling and endothelial phenotype. Previously, haploinsufficiency of Dll4+/− in the mouse was shown to be embryonic lethal, with development halting at mid-gestation due to severe defects in vascular remodeling24. However, we found heterozygosity for this Dll4 mutant allele was better tolerated on the FVB/NJ background, where we could generate some Dll4+/− mice with decreased protein levels. Although endothelial cell proliferation was not different compared to wild type mice in the adult, there was evidence of development changes in endothelial cell growth and morphogenesis. The aorta of Dll4+/− adult mice had an elevated density of endothelial cells, suggestive of an increased proliferation rate at some point during development or vascular growth. Although no changes in steady state proliferation were noted in adult endothelial cells, vessels in the lung had dramatically elevated nuclear pSmad1/5. Since we found that BMP9 quiescence signals depend on pSmad1/5, elevated pSmad signaling may be a compensatory response to decreased Dll4 that maintains endothelial homeostasis. The complex changes in lung vascular morphogenesis may be a result of developmental changes in endothelial cell proliferation, branching morphogenesis, or pruning. We found evidence in some Dll4+/− lungs for hemorrhage, which has also been described for lungs in the endoglin heterozygous mice25 and the endothelial-specific Alk1 knockout mouse26. This in vivo phenocopy of these genetic mutants again supports the cooperative nature of these signaling pathways. Current therapies incorporating Notch or BMP targets to prevent or augment various vascular processes like angiogenesis (e.g. wound healing, cancer), or treat pathologies such as hereditary hemorrhagic telangiectasia, neointimal hyperplasia, or pulmonary hypertension, can benefit from the cooperative nature of the two pathways, as they are provided a larger arsenal of targets and more nuanced strategies.
Supplementary Material
Significance.
Endothelial cells respond to environmental cues to undergo angiogenic sprouting and proliferation, but then need to re-establish quiescence. We report that two inhibitory ligands of endothelial cell growth, DLL4 and BMP9, induce quiescence through overlapping signaling networks, mediated by Notch1 and ALK1, whose signals synergize to regulate gene expression and proliferation. We used proteomics data to identify two downstream effectors of these pathways, thrombospondin-1 and P27KIP1, which contribute to endothelial quiescence. To evaluate interdependent signaling in vivo, BMP signaling and endothelial cell phenotype was analyzed in adult Dll4+/− mice. Dll4+/− mice had increased endothelial cell density in aortae, and altered lung vascular morphology, suggesting changes in developmental vascular branching and endothelial proliferation. In addition, lung endothelial cells with Dll4 loss had a significant increase in Smad1/5 activation. Thus, enhanced Smad signaling was associated with loss of Dll4, showing evidence for tight regulation of these pathways in adult endothelium in vivo.
Acknowledgments
We gratefully acknowledge the work of Caroline Suresh, Sara Fitzpatrick, Kelsey O’Neil, and Dean Darien for supportive data analysis. We also are thankful to Dr. Volkhard Lindner for providing multiple antibodies, Dr. Anyonya Guntur for providing IGF1, Dr. Igor Prudovsky for providing recombinant VEGF-A, Dr. Liangru Contois for providing collagen IV antibodies, Dr. Justin Guay for providing TAK1 inhibitor, and Drs. Xuehui Yang, Joshua Boucher, and Sumithra Urs for their guidance and tutelage.
Sources of Funding - This research was supported by 1R01HL109652 to L. Liaw. B. Rostama was supported by the Graduate School of Biomedical Sciences and Engineering, University of Maine, Orono during portions of this research. Support for the proteomics strategy came from a Pilot Project from grant 8P30GM103392, COBRE in Vascular Biology (Robert E. Friesel, PI, L. Liaw, Co-Investigator).
Abbreviations
- ALK
activin-like kinase
- BMP
bone morphogenetic protein
- DLL4
Delta-like4
- EC
endothelial cells
- HAEC
human aortic endothelial cells
- TGFβ
transforming growth factor β
- TSP
thrombospondin
- BrdU
5-bromo-2′-deoxyuridine
Footnotes
Disclosures: None
References
- 1.Takizawa T, Ochiai W, Nakashima K, Taga T. Enhanced gene activation by Notch and BMP signaling cross-talk. Nucleic Acids Res. 2003;31:5723–31. doi: 10.1093/nar/gkg778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh F, Itoh S, Goumans MJ, Valdimarsdottir G, Iso T, Dotto GP, Hamamori Y, Kedes L, Kato M, ten Dijke Pt P. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J. 2004;23:541–51. doi: 10.1038/sj.emboj.7600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R, Zhang W, Cui J, Shui W, Yin L, Wang Y, Zhang H, Wang N, Wu N, Nan G, Chen X, Wen S, Deng F, Zhang H, Zhou G, Liao Z, Zhang J, Zhang Q, Yan Z, Liu W, Zhang Z, Ye J, Deng Y, Luu HH, Haydon RC, He TC, Deng ZL. Targeting BMP9-promoted human osteosarcoma growth by inactivation of notch signaling. Curr Cancer Drug Targets. 2014;14:274–85. doi: 10.2174/1568009614666140305105805. [DOI] [PubMed] [Google Scholar]
- 5.Kerr G, Sheldon H, Chaikuad A, Alfano I, von Delft F, Bullock AN, Harris AL. A small molecule targeting ALK1 prevents Notch cooperativity and inhibits functional angiogenesis. Angiogenesis. 2015;18:209–17. doi: 10.1007/s10456-014-9457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochon ER, Wright DS, Schubert MM, Roman BL. Context-specific interactions between Notch and ALK1 cannot explain ALK1-associated arteriovenous malformations. Cardiovasc Res. 2015;107:143–52. doi: 10.1093/cvr/cvv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedrosa AR, Trindade A, Fernandes AC, Carvalho C, Gigante J, Tavares AT, Dieguez-Hurtado R, Yagita H, Adams RH, Duarte A. Endothelial Jagged1 antagonizes Dll4 regulation of endothelial branching and promotes vascular maturation downstream of Dll4/Notch1. Arterioscler Thromb Vasc Biol. 2015;35:1134–46. doi: 10.1161/ATVBAHA.114.304741. [DOI] [PubMed] [Google Scholar]
- 8.Moya IM, Umans L, Maas E, Pereira PN, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, Zwijsen A. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell. 2012;22:501–14. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heisig J, Weber D, Englberger E, Winkler A, Kneitz S, Sung WK, Wolf E, Eilers M, Wei CL, Gessler M. Target gene analysis by microarrays and chromatin immunoprecipitation identifies HEY proteins as highly redundant bHLH repressors. PLoS Genet. 2012;8:e1002728. doi: 10.1371/journal.pgen.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woltje K, Jabs M, Fischer A. Serum induces transcription of Hey1 and Hey2 genes by Alk1 but not Notch signaling in endothelial cells. PLoS One. 2015;10:e0120547. doi: 10.1371/journal.pone.0120547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–80. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 12.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–22. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida N, Kitagawa M, Hatakeyama S, Nakayama K. Phosphorylation at serine 10, a major phosphorylation site of p27(Kip1), increases its protein stability. J Biol Chem. 2000;275:25146–54. doi: 10.1074/jbc.M001144200. [DOI] [PubMed] [Google Scholar]
- 14.Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, Fusco A, Santoro M. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–44. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 15.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–52. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 16.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–9. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–53. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 18.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–4. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 19.Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizan P, Geudens I, Collins RT, Franco CA, Abrahams CL, Thurston G, Fruttiger M, Rosewell I, Eichmann A, Gerhardt H. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun. 2015;6:7264. doi: 10.1038/ncomms8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young K, Conley B, Romero D, Tweedie E, O’Neill C, Pinz I, Brogan L, Lindner V, Liaw L, Vary CP. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood. 2012;120:4263–73. doi: 10.1182/blood-2012-07-440784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med. 2012;2:a006627. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood. 2006;108:3112–20. doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- 23.Heit B, Kim H, Cosio G, Castano D, Collins R, Lowell CA, Kain KC, Trimble WS, Grinstein S. Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization. Dev Cell. 2013;24:372–83. doi: 10.1016/j.devcel.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–73. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourdeau A, Faughnan ME, McDonald ML, Paterson AD, Wanless IR, Letarte M. Potential role of modifier genes influencing transforming growth factor-beta1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am J Pathol. 2001 Jun;158:2011–20. doi: 10.1016/s0002-9440(10)64673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tual-Chalot S, Mahmoud M, Allinson KR, Redgrave RE, Zhai Z, Oh SP, Fruttiger M, Arthur HM. Endothelial depletion of Acvrl1 in mice leads to arteriovenous malformations associated with reduced endoglin expression. PLoS One. 2014;9:e98646. doi: 10.1371/journal.pone.0098646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt RS, Atkins MB. Molecular pathways: can activin-like kinase pathway inhibition enhance the limited efficacy of VEGF inhibitors? Clin Cancer Res. 2014;20:2838–45. doi: 10.1158/1078-0432.CCR-13-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan M. Therapeutic promise and challenges of targeting DLL4/NOTCH1. Vasc Cell. 2011;3:17. doi: 10.1186/2045-824X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 30.Simonelli M, Zucali PA, Thomas MB, Brisendine A, Berlin J, Noberasco C, Denlinger CS, Kim TY, Santoro A, Gallo-Stampino C, Carpentieri M, Wang E, Williams JA, De Braud FG. Phase I study of PF-03446962 (anti-ALK-1 mAb) in hepatocellular carcinoma (HCC) Journal of Clinical Oncology. 2013;31:4121. [Google Scholar]
- 31.Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibanez CF. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163:723–8. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–99. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- 33.Cristofaro B, Shi Y, Faria M, Suchting S, Leroyer AS, Trindade A, Duarte A, Zovein AC, Iruela-Arispe ML, Nih LR, Kubis N, Henrion D, Loufrani L, Todiras M, Schleifenbaum J, Gollasch M, Zhuang ZW, Simons M, Eichmann A, le Noble F. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development. 2013;140:1720–9. doi: 10.1242/dev.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda D, Aikawa E, Swirski FK, Novobrantseva TI, Kotelianski V, Gorgun CZ, Chudnovskiy A, Yamazaki H, Croce K, Weissleder R, Aster JC, Hotamisligil GS, Yagita H, Aikawa M. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci U S A. 2012;109:E1868–77. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida Y, Hayashi Y, Suda M, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Yamashita M, Kobayashi Y, Shimizu I, Minamino T. Notch Signaling Regulates the Lifespan of Vascular Endothelial Cells via a p16-Dependent Pathway. PLoS One. 2014;9:e100359. doi: 10.1371/journal.pone.0100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Walle I, Waegemans E, De Medts J, De Smet G, De Smedt M, Snauwaert S, Vandekerckhove B, Kerre T, Leclercq G, Plum J, Gridley T, Wang T, Koch U, Radtke F, Taghon T. Specific Notch receptor-ligand interactions control human TCR-alphabeta/gammadelta development by inducing differential Notch signal strength. J Exp Med. 2013;210:683–97. doi: 10.1084/jem.20121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fung E, Tang SM, Canner JP, Morishige K, Arboleda-Velasquez JF, Cardoso AA, Carlesso N, Aster JC, Aikawa M. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007;115:2948–56. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 38.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–35. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Morikawa M, Koinuma D, Tsutsumi S, Vasilaki E, Kanki Y, Heldin CH, Aburatani H, Miyazono K. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Research. 2011;39:8712–8727. doi: 10.1093/nar/gkr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zippel N, Malik RA, Fromel T, Popp R, Bess E, Strilic B, Wettschureck N, Fleming I, Fisslthaler B. Transforming growth factor-beta-activated kinase 1 regulates angiogenesis via AMP-activated protein kinase-alpha1 and redox balance in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:2792–9. doi: 10.1161/ATVBAHA.113.301848. [DOI] [PubMed] [Google Scholar]
- 41.Laux DW, Young S, Donovan JP, Mansfield CJ, Upton PD, Roman BL. Circulating Bmp10 acts through endothelial Alk1 to mediate flow-dependent arterial quiescence. Development. 2013;140:3403–12. doi: 10.1242/dev.095307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–71. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Brady Ridgway J, Sai T, Lai J, Warming S, Chen H, Roose-Girma M, Zhang G, Shou W, Yan M. Context-dependent signaling defines roles of BMP9 and BMP10 in embryonic and postnatal development. Proc Natl Acad Sci U S A. 2013;110:11887–92. doi: 10.1073/pnas.1306074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mettouchi A. The role of extracellular matrix in vascular branching morphogenesis. Cell Adh Migr. 2012;6:528–34. doi: 10.4161/cam.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estrach S, Cailleteau L, Franco CA, Gerhardt H, Stefani C, Lemichez E, Gagnoux-Palacios L, Meneguzzi G, Mettouchi A. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res. 2011;109:172–82. doi: 10.1161/CIRCRESAHA.111.240622. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–42. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 48.Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210:807–18. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu LY, Ramakrishnan DP, Silverstein RL. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood. 2013;122:1822–32. doi: 10.1182/blood-2013-01-482315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colombo G, Margosio B, Ragona L, Neves M, Bonifacio S, Annis DS, Stravalaci M, Tomaselli S, Giavazzi R, Rusnati M, Presta M, Zetta L, Mosher DF, Ribatti D, Gobbi M, Taraboletti G. Non-peptidic thrombospondin-1 mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J Biol Chem. 2010;285:8733–42. doi: 10.1074/jbc.M109.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–76. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutierrez LS, Ling J, Nye D, Papathomas K, Dickinson C. Thrombospondin peptide ABT-898 inhibits inflammation and angiogenesis in a colitis model. World J Gastroenterol. 2015;21:6157–66. doi: 10.3748/wjg.v21.i20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell D, Pobre EG, Mulivor AW, Grinberg AV, Castonguay R, Monnell TE, Solban N, Ucran JA, Pearsall RS, Underwood KW, Seehra J, Kumar R. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Mol Cancer Ther. 2010;9:379–88. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 54.Trindade A, Djokovic D, Gigante J, Badenes M, Pedrosa AR, Fernandes AC, Lopes-da-Costa L, Krasnoperov V, Liu R, Gill PS, Duarte A. Low-dosage inhibition of Dll4 signaling promotes wound healing by inducing functional neo-angiogenesis. PLoS One. 2012;7:e29863. doi: 10.1371/journal.pone.0029863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.