Abstract
HIV causes neural dysfunction in infected individuals. This dysfunction often manifests as cognitive symptoms and can be detected using neuroimaging. Highly active anti-retroviral therapy (HAART), in addition to providing virologic control, has reduced the number of profoundly impaired individuals but more mild forms of neurocognitive disorders remains prevalent. A potential confound in previous studies of HIV-associated cognitive dysfunction is that HAART may be neurotoxic. Thus, observed effects, attributed to HIV, may be in part due to HAART. It is unclear whether and to what extent current medications contribute to observed brain dysfunction. We studied changes in functional connectivity and cerebral blood flow in HIV uninfected (HIV−) individuals before and after being given two common antiretroviral medications: efavirenz and ritonavir. Neither drug was associated with significant changes in functional connectivity or cerebral blood flow. Our results suggests that previous changes in functional connectivity and cerebral blood flow in HIV infected individuals receiving HAART may largely due to the virus and remaining reservoirs and less due to toxic action of these anti-retroviral medications.
Introduction
HIV crosses into the brain soon after infection (Kaul et al. 2001). Although the virus does not directly infect neurons, rather it infects the microglia, HIV causes neural dysfunction often resulting in cognitive symptoms (Gonzalez-Scarano and Martin-Garcia 2005). The introduction of highly active anti-retroviral therapy (HAART) allows for virologic control and has reduced the incidence of more severe forms of HIV-associated neurological disorders (HAND), but milder forms remain common (Antinori et al. 2007). Recent observations also suggest that HAART could be neurotoxic (Robertson et al. 2012) raising the question whether some of the observed HIV-associated dysfunction may be caused by medications.
Changes in brain structure and function due to HIV can be observed using non-invasive imaging (Masters and Ances 2014). Reports using volumetric, diffusion, perfusion, and functional MRI have revealed the detrimental effects of HIV on the brain (Holt et al. 2012). In particular, brain organization can be measured using resting-state functional connectivity MRI (rs-fcMRI)(Biswal et al. 1995). Functional connectivity refers to the correlation in spontaneous brain activity across distant but functionally related brain regions. Sets of functionally connected regions form resting-state networks (RSNs)(Fox et al. 2005). We have previously demonstrated that HIV infection disrupts several RSNs (Thomas et al. 2015; Thomas et al. 2013). Similarly, brain perfusion can be studied using arterial spin labeling (ASL) (Alsop et al. 2014). Cortical and subcortical reductions in CBF are observed soon after infection and persist chronically (Ances et al. 2009). However, most studies were performed in HIV infected (HIV+) subjects on HAART which confounds the effects of HIV and HAART on brain function. Further, the HAART status of an individual is not a random variable, rather, it depends on several factors (e.g., duration of infection, severity of infection, etc.) and is thus difficult to study alone in an HIV+ population.
In this study we investigate the effects of HAART in a seronegative population using rs-fcMRI and pulsed ASL (PASL). HIV uninfected (HIV−) participants were sequentially (counterbalanced across subjects) given two HAART medications, efavirenz and ritonavir, and underwent three imaging sessions (baseline, post-efavirenz, post-ritonavir). A large effect of one or both of these medications would suggest that HAART potentially exerts a disruptive effect on functional brain organization or CBF. However, a small or absent effect would suggest that most of the observed previous effects are due to the virus and not medications.
Methods
A total of 11 subjects were recruited for participation in this study. Inclusion criteria included healthy male or female HIV− individual (ages 18–40 years old), good general health with no known major medical conditions, and body mass index < 33. Exclusion criteria included a known history of liver or kidney disease, history of major medical conditions, HIV seropositive, fasting blood glucose > 110 mg/dL, family history of type 2 diabetes, use of prescription or non-prescription medications, herbals or foods known to be metabolized by or altering pglycoprotein or CYP3A (hormonal birth control medications were acceptable if alternative means of contraception were used), females who are pregnant or nursing, and contraindications to MRI. From this cohort, 11 completed control and ritonavir scans, and 8 also completed the efavirenz scans (3 subjects dropped out or were not available for the last scan). The average age of participants within this cohort was 31.5 years of age.
Subjects were first scanned at baseline prior to administration of either drug. Two drug conditions then followed; between the two drugs sufficient time was given for the previous drug to be washed out. The subjects were given ritonavir (200 mg orally three times per day for 1 day, 300 mg orally twice a day for one day, and then 400 mg orally on the morning of the third day), followed by a 2–3 week washout, then efavirenz (600 mg orally every night for 14 days). Subjects were scanned before, and on the last day of each drug administration. Drug doses and durations were based on prior drug interaction studies (Kharasch et al. 2008; Kharasch et al. 2012). The order of drug administration was counterbalanced across subjects. Thus, each data set is composed of a control scan, a scan while on efavirenz, and a scan while on ritonavir.
Image acquisition was performed using a 3T Siemens Trio scanner (Erlangen, Germany) equipped with a standard 12-channel head coil. A high-resolution structural scan was acquired using a 3-dimensional sagittal T1-weighted magnetization-prepared rapid gradient echo (MPRAGE). This scan was used for atlas registration. High-resolution 2-D multi-slice oblique axial spin density/T2-weighted fast spin echo (FSE) structural images were also acquired. These T2-weighted FSE data were used for rs-fcMRI atlas registration. rs-fcMRI scans were collected using a gradient spin-echo sequence sensitive to blood oxygen level dependent (BOLD) contrast (Ogawa et al. 1993). Two six minute rs-fcMRI runs (164 volumes per run) were acquired during which participants were asked to fixate on a visual cross-hair and not fall asleep. PASL was acquired using a common Siemens sequence with PICORE Q2T. Two PASL runs each containing 63 volumes (duration of 2.7 minutes) were acquired.
Initial preprocessing of rs-fMRI data followed conventional methods as previously described (Brier et al. 2015; Brier et al. 2014). Volumes strongly contaminated by head movement (Power et al. 2012) were removed. Frame censoring was computed using the DVARS measure (Smyser et al. 2010). Signals of non-interest were extracted from white matter, ventricles, and the global signal averaged over the whole brain (Fox et al. 2009). BOLD time-series were extracted from 36 previously defined regions of interest (ROI), a subset of which is sensitive to the effects of HIV (Brier et al. 2012; Thomas et al. 2013). Functional connectivity is defined as the correlation between BOLD time-series extracted from two ROIs. We have previously defined composite scores as measures of RSN integrity based on correlations averaged over ROI pairs within and between networks (Brier et al. 2012). These measures are calculated by evaluating z(r) between a priori defined region pairs and averaging these quantities over all ROI pairs within a given RSN. In the present analyses, composite scores were obtained for five RSNs. Examined RSNs included: [1] Default-Mode Network (DMN), [2] Dorsal Attention Network (DAN), [3] Control Network (CON), [4] Salience Network (SAL), [5] Sensori-motor Network (SMN).
PASL images underwent rigid registration to correct for head motion and a transformation to an atlas space. CBF was computed using a single bloodcompartment model (Chen et al. 2011):
| (1) |
Where λ represents proton density 0.9g/mL (Herscovitch and Raichle 1985); TI1, bolus duration, 0.7s; TI inversion time a measure of time between tagging the blood to slice acquisition which starts at 1.8s for the first axial slice and increases 0.0467s for the next axial slice and so on; T1b, T1 decay of labeled protons, 1.65s (Lu et al. 2004); M0, equilibrium image, the first frame of each run is used; α, labeling efficiency, 0.85 (Dai et al. 2008); Cp and Tp, control and tagged from for a PASL pair. A weighted average scheme was used to mitigate motion contamination by using temporal derivative of the frame-to-frame variance (DVARS) as a metric for motion contamination (Tanenbaum et al. 2015). Similar to the functional connectivity composite scores, CBF composite scores for each network were calculated as the mean CBF in the same ROIs belonging to a given RSN.
The data from both the BOLD and PASL experiments are summarized into RSN composite scores. For each RSN, or RSN-pair in the case of BOLD, each subject contributes three measurements: control, post-ritonavir, and post-efavirenz. Given this repeated measures design, statistical significance was assessed using repeated measures ANOVA with drug status as factor and subjects as the repeated factor.
Results
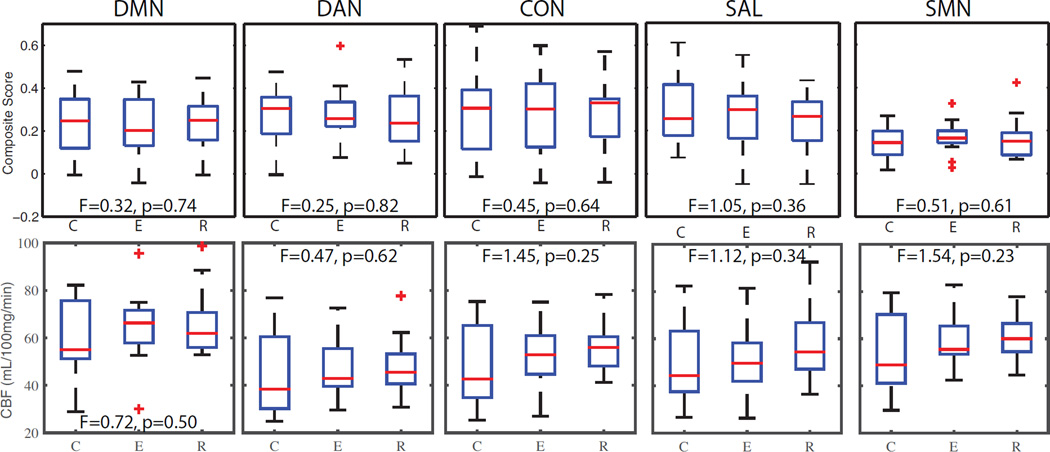
We began by examining the effect of efavirenz and ritonavir on functional connectivity composite scores. We subjected the RSN composite scores (Figure 1A) for each RSN separately to a repeated-measures ANOVA with drug condition as factor. We found no significant differences in any RSN as a function of drug condition. Further, between RSN composite scores, a measure of inter-network interaction, were also unaffected by drug condition (data not shown).
Figure 1.
Top row: Box plots of functional connectivity composite resting-state networks (RSNs) scores for control (C), efavirenz (E), and ritonavir (R) conditions. Red line represents the mean, boxes define quartiles, and red plusses are outliers. No significant differences were observed. Bottom row: Mean cerebral blood flow (CBF) within each RSN in same style. No significant differences were observed.
Finally, we investigated changes in CBF due to drug condition. We subjected CBF composite scores (Figure 1B) to repeated measures ANOVA with drug condition as factor. We found no significant difference in CBF in any network as a function of drug condition.
Discussion
In this study we investigated the effects of two HAART medications on functional connectivity and CBF in HIV− subjects. We found that short exposure to efavirenz and ritonavir was not associated with detectable changes in functional connectivity or CBF. This would suggest that previously observed disruptions in brain physiology in medicated HIV+ subjects are predominantly due to the effects of the virus, not HAART.
HAART may be neurotoxic (Schweinsburg et al. 2005) in particular systemic and peripheral nervous system effects have been well documented (Wright 2009). However, these medications may also affect the brain. Discontinuation of HAART in previously medicated HIV + subjects improved cognition with changes not attributable to practice effects. Additionally, subjects who re-initiated HAART after discontinuation did not experience cognitive improvement (Robertson et al. 2010). HAART neurotoxicity could be responsible for these unexpected results in HIV+ subjects. Subsequently, a large retrospective study (~20,000 patients) observed worse neuropsychological performance scores in HIV+ patients taking HAART (Committee et al. 2011). Neurotoxicity due to HAART has been demonstrated in animal models. Rat cortical neurons exposed to antiretrovirals had significant increase in synaptodendritic beading and pruning (Robertson et al. 2012). The median toxic dose for most of the antiretrovirals was well within the therapeutic concentration range in plasma of HIV+ subjects. However, the effects of HAART on brain function have not been assessed in the absence of confounding effects of HIV-associated dysfunction.
The interpretation of our negative results occurs in the context of two limitations. First, the sample size in this study is relatively small. However, if HAART accounted for a sizable fraction of the extant HIV effect it should be detected. As with any null finding, it is not possible to state that there is no effect of HAART on brain organization. Second, HIV− subjects in this study were only given medications for a relatively short period of time. This limitation is primarily practical in nature but limits generalizability of these results to a population that takes multiple antiretrovirals in clinical practice for their entire life. A potential interaction between HIV and HAART can also not be ruled out.
In summary, we present data demonstrating that HAART is not associated with dramatic changes in brain organization as measured with functional connectivity or CBF. This would suggest that most of the extant HIV effect in the literature is due to the virus and not HAART. These results suggest early initiation of HAART soon after seroconversion and suggest that these medications are not acutely neurotoxic.
Acknowledgements
Supported by National Institutes of Health grants R01DA14211 (EK), R01DA25931 (EK), K24-DA00417 (EK), R01NR012657 (BMA), R01NR012907 (BMA), R01NR014449 (BMA), and R21MH099979 (BMA). Funding was also provided by the Alzheimer’s Association NIRP-12-257747 (BMA).
Footnotes
The authors declare that they have no conflict of interest.
References
- Alsop DC, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–708. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin Z, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar. MRI Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brier MR, Mitra A, McCarthy JE, Ances BM, Snyder AZ. Partial covariance based functional connectivity computation using Ledoit-Wolf covariance regularization. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, et al. Unrecognized preclinical Alzheimer disease confounds rsfcMRI studies of normal aging. Neurology. 2014;83:1613–1619. doi: 10.1212/WNL.0000000000000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. Journal of magnetic resonance imaging : JMRI. 2011;33:940–949. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee UKCHCSS et al. HIV-associated central nervous system diseases in the recent combination antiretroviral therapy era. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011;18:527–534. doi: 10.1111/j.1468-1331.2010.03291.x. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2008;60:1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. What is the correct value for the brain-blood partition coefficient for water. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1985;5:65–69. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. Journal of neurovirology. 2012;18:291–302. doi: 10.1007/s13365-012-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–512. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch ED, et al. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2012;91:673–684. doi: 10.1038/clpt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X, van Zijl PC. Determining the longitudinal relaxation time (T1) of blood at 3.0. Tesla Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52:679–682. doi: 10.1002/mrm.20178. [DOI] [PubMed] [Google Scholar]
- Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders Semin. Neurol. 2014;34:89–102. doi: 10.1055/s-0034-1372346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. Journal of neurovirology. 2012;18:388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, et al. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. Journal of neurovirology. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cerebral cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum AB, Snyder AZ, Brier MR, Ances BM. A method for reducing the effects of motion contamination in arterial spin labeling magnetic resonance imaging. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1038/jcbfm.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Ortega M, Benzinger TL, Ances BM. Weighted brain networks in disease: centrality and entropy in human immunodeficiency virus and aging. Neurobiol Aging. 2015;36:401–412. doi: 10.1016/j.neurobiolaging.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80:1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EJ. Neurological disease: the effects of HIV and antiretroviral therapy and the implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4:447–452. doi: 10.1097/COH.0b013e32832dd0c2. [DOI] [PubMed] [Google Scholar]