Abstract
Background
There has been an increase in the use of cigarillos in the US. People who smoke cigarillos typically also regularly smoke cigarettes (dual users).
Methods
We compared puffing topography, biomarkers of acute exposure [exhaled carbon monoxide (COex) and plasma nicotine] and physiologic effects from usual brand cigarette and Black & Mild cigarillo smoking in dual users (N=23) in two laboratory sessions.
Results
Participants (21 men) smoked an average of 17.5 cigarettes/day. Cigarillo consumption varied widely from as few as 1/week to daily. Participants were highly nicotine dependent (average FTND score: 6.3). There were statistically significant differences in smoking behavior between cigarette and cigarillo smoking in time to smoke, number of puffs, and total puff volume (all P<0.001). Average puff duration, interpuff interval average puff volume, and puff velocity did not differ between cigarettes and cigarillos. Nicotine boost was similar after both cigarettes and cigarillos. COex boost was significantly greater after cigarillo smoking compared to cigarette smoking (P<0.001).
Conclusions
The smoking pattern and exposure profile indicate that dual users inhale cigarillo smoke just as they inhale cigarette smoke thereby exposing themselves to considerable amounts of nicotine and other components of tobacco smoke. COex exposure results imply that cigarillo smoking may be associated with higher exposure to smoke-delivered volatile components of mainstream cigarillo smoke including carcinogens when compared to cigarettes.
Impact
The findings that cigarillos and cigarettes are smoked similarly in dual users are relevant to health and regulatory considerations on cigar products.
Keywords: cigars, cigarillos, topography, biomarkers, exposure
Introduction
Significant progress has been made in reducing cigarette smoking among U.S. adults over the past five decades [1], however, cigar smoking has become popular recently. For example, large cigars consumption increased by 126.3% between years 2008–2011 [2]. Prevalence of cigar use was found to be highest among young adults and adolescents. In 2012, about 12.5 million (or 5.4%) adults in the U.S. reported to be cigar users, whereas 10.7% of individuals between the ages of 18–25 years reported current cigar use [3]. The 2010 National Survey on Drug Use and Health reported that the rates of past month use among young adults were 34.2% for cigarettes and 11.2% for cigars [4]. Amongst high school students, 23.3% reported use of some type of tobacco in 2012 with 12.6% reporting cigar smoking [5]. Some cigar smokers, former/current cigarette smokers, and the nonsmoking public misperceive cigar smoking to be less harmful than cigarette smoking [6–8] even though cigar consumption is associated with a risk of heart disease, pulmonary disease, and many types of cancer [9, 10]. Several recent studies reported that smokers tend to use more than one tobacco product. Between 2012 and 2013, an estimated 19.2% of U.S. adults used a combustible tobacco product every day or some days of which 72.1% have used at least one combustible tobacco product daily [1]. Richardson et al., reported 12.5% of dual users consumed both cigarettes and cigars. Dual users were more likely to be male, ages 18–29, non-Hispanic Black, of low socioeconomic status, and either unemployed or out of the work force [11]. An analysis of the 2012 National Adult Tobacco Survey showed that out of all dual users, the largest group used both cigarettes and cigars (37.0%), and multiple product use was most prevalent among young adults aged 18–24 at 62.4% [12].
The increase in cigar popularity and sales, over the past several years, may be an unintended consequence of tobacco regulation and taxation. With the reauthorization of State Children’s Health Insurance Program (S-CHIP) and the approval of the tax on little cigars, the tax rate of little cigars became equal to that of cigarettes [13]. As a result, manufacturers increased the weight of some little cigars to over 3 pounds per 1000 cigars thereby shifting their tax category from “little cigar” to the “cigar” and reducing their tax [13]. Cigarillos are typically between the weight of a little cigar and a large cigar, however there is no specific tax category and they have not been tracked systematically since there is no legal product definition [13]. Besides product cost, another reason that cigar products may appeal to youth consumers is their availability in a variety of flavors that are now prohibited from cigarettes - legislation enforced by the Family Smoking Prevention and Tobacco Control Act (FSPTCA) in 2009 [14], [15]. In April 2014, the Food and Drug Administration (FDA) proposed to extend their authority to regulate products that meet the statutory definition of a tobacco product (including cigars) [16]. The increase in cigarillo popularity, higher consumption and the implications for FDA regulation emphasizes the importance for a better understanding of these products, their toxicant delivery and addiction potential. The goals of this study were to examine toxicant delivery, smokking patterns and subjective responses to the smoking experience of cigarillos.
Materials and methods
Participants
The participants were recruited from the Baltimore, MD metropolitan area using advertisements in local newspapers, flyers, personal referrals, and a laboratory database of smokers. The eligibility of the participant was determined with an initial telephone interview conducted by an experienced recruiting specialist who gathered basic demographic, health and product use information to determine if inclusion criteria were met. The inclusion criteria of the study were: 1) adult men and women aged 18–65; 2) ability to provide study consent, attend all laboratory sessions lasting approximately 2 hours each and complete all study procedures; 3) smoke both a minimum of 10 cigarettes per day for at least 2 years and a minimum of 1 cigarillo per week; 4) absence of smoking related illness or disease; and 5) not actively trying to quit smoking. Participants were compensated $70 for each of the 2 study visits, plus an additional $25 completion bonus at the end of visit 2. Data from this study were collected between March 2013 and November 2014. The study was approved by Battelle’s Institutional Review Board (IRB).
Study design, products and procedures
At the initial laboratory visit, participants read and signed a Battelle IRB-approved consent form. They answered various questionnaires on their personal smoking history, and cigarette and cigar use patterns. A Smoking History Questionnaire (SHQ) was administered at the first visit to collect demographics and tobacco and nicotine use history information as well as the Fagerström Test for Nicotine Dependence (FTND). The Questionnaire on Smoking Urges (QSU) was self-administered pre- and post-smoking at both visits to assess urge for smoking. The Duke Sensory Questionnaire (DSQ) and Cigarette Evaluation Scale (CES) were self-administered post smoking to assess the subjective effects of the products. The NDSS, QSU, and DSQ questionnaires were modified to address eithercigars or cigarettes based on their randomized visit. Participant height and weight were recorded and a urine sample was provided. Participants were randomized to smoke either an unflavored Black & Mild (B&M) cigarillo (John Middleton Company, Limerick, PA) or their own brand of cigarette at that session; at their next visit, they smoked the other tobacco product.
Participants attended 2 laboratory sessions, separated by at least 24 hours without required abstinence periods. Exhaled carbon monoxide (COex) was measured and blood (10 ml) was drawn from a forearm vein using butterfly needles at baseline (before smoking). Participants were then instructed to smoke as they normally do (ad libitum): either the provided B&M cigarillo with the plastic tip removed or their own brand of cigarette through the mouthpiece of a smoking puff analyzer. Within 10 minutes post-smoking, COex was measured again. Venous blood samples were collected 5 minutes and 10 minutes post-smoking. Two post-smoking blood samples were collected to assess peak nicotine levels which may occur slightly later for cigarillo compared to cigarette smoking if significant buccal absorption occurs [17, 18]. The cigarillos and cigarettes were weighed before and after smoking to determine the amount of tobacco smoked. Acute biomarkers of exposure (COex and plasma nicotine) were normalized using two methods: 1) exposure per gram of tobacco smoked and 2) exposure per 1,000 mL of total puff volume. The procedures at the second visit were identical using the other tobacco product.
Dependent measures
A) Puff Measures
Smoking topography measures how a person puffs (brings smoke into their mouth) a tobacco article. Measures of topography include: the number of puffs, puff volume, puff duration, puff velocity, interpuff interval (IPI), and time to smoke (TTS); total puff volume is obtained by adding the individyal puff volumes. Smoking topography was measured using a SPA/D Puff Analyzer (Sodim Instruments, MebTEC, Mebane, NC). A cigarette or cigarillo was inserted into the mouthpiece. The product was smoked and data were saved to a computer. TTS was recorded by the topography unit and with handheld digital timers. The cigarillo (or cigarette) was lit by study staff to assure accurate measurement of smoking onset time. Participants were continually observed during smoking; at the end of the last puff they provided a specific visual cue to signal the end of smoking.
B) Toxicant Exposure (Tobacco Smoke Biomarkers)
Plasma nicotine
Venous blood samples were drawn to assess changes in plasma nicotine level, before and after smoking, as a biomarker of tobacco exposure. The blood samples were centrifuged and the plasma was separated and stored frozen until it was analyzed for nicotine concentration by the Bioanalytical Laboratory at Virginia Commonwealth University (VCU) School of Pharmacy. Plasma sample were analyzed using ing LC/MS/MS. With a lower limit of quantification of 2.5 ng/mL [19].
Exhaled carbon monoxide
COex is a recognized biomarker of recent tobacco smoke exposure and smoke inhalation [20, 21]. COex was collected using the BreathCO Monitor (Vitalograph Inc., Lenexa, KS) at baseline and at 2 minute post-smoking. This was used to determine the COex boost, which is the difference between the post-smoking and pre-smoking COex measurement (in parts per million; ppm).
Urinary cotinine and trans 3’-hydroxycotinine
Total cotinine, the primary metabolite of nicotine, was analyzed from urine samples taken at baseline. Cotinine has a longer half-life (16 hours) than nicotine (90 min) which provides a more stable assessment of nicotine exposure [22]. The urine samples were analyzed by Labstat International ULC (Kitchener, Ontario, Canada) using UPLC/MS/MS [23]. The lower limit of quantification for cotinine and 3-OH-cotinine assays are 8.78 ng/mL and 7.06 ng/mL, respectively. The results were corrected for creatinine.
C) Subjective Measures
Tobacco use history
Tobacco use history was attained at baseline via the SHQ. The questionnaire evaluated the participants’ demographics, current and past smoking history, and types of nicotine products used. Information was also collected regarding age of initiation, brand and flavor preference, and use of other tobacco products including modified risk tobacco products (MRTPs).
Dependence, appeal and effects
Nicotine dependence was assessed using the FTND [24] at baseline. The level of nicotine craving for cigars or cigarettes was evaluated using the brief version of the Questionnaire on Smoking Urges (QSU) [25–27]. The appeal and subjective effects of the products (cigar or cigarette) post-smoking were evaluated using the Duke Sensory Questionnaire (DSQ) [28] and Cigarette Evaluation Scale (CES) [29], which both employ a 7-point Likert scale with 1 = ‘not at all’ and 7 = ‘extremely’. The DSQ contains 9 questions related to puff liking; puff satisfaction; nicotine in puffs; puff strength on the tongue, nose, mouth and throat, windpipe, and chest; and similarity to own brand. DSQ ratings for puff strength on tongue, nose, mouth and throat, windpipe, and chest were collapsed to form an overall strength score (range 7–35). Questions on similarity to own brand were excluded when participants smoked their own cigarette and were therefore not included in statistical analyses. The CES contains 11 questions related to cigarette or cigar satisfaction, good taste, and effects (dizziness, calmness, concentration, wakefulness, hunger reduction, nausea, irritability, enjoyment of sensations in the throat and chest, and reduction of cigarette craving). Several of these items were collapsed to form composite scores of satisfaction (satisfaction and good taste), psychological reward (calmness, concentration, wakefulness, hunger reduction and irritability), and aversion (dizziness and nausea) [30].
Sample Size and Statistical Analysis
The study design involved within-subjects comparisons, which conferred varying levels of power to test differences in cigarette/cigarillo nicotine delivery, smoking topography or user’s perceptions. The study was powered to detect differences of clinical relevance between cigarettes and cigarillos. Sample size for this study was estimated at 17–25 participants. Based on our previous research, standard deviations of 5.4–18.4 of nicotine boost per mL of plasma were used to power the study [31–34]. Benowitz and Henningfield proposed products that contain more than 0.5 g tobacco and deliver 0.17g nicotine (expected plasma boost = 7 ng/mL) would support and maintain addiction [35]. For the study group of 21 people, a 6 ng/mL difference in nicotine boost between the two groups would yield a statistical difference. Statistical analyses were conducted using Stata version 13.1 and Statistica 12. Skewness and kurtosis tests and Shipiro-Wilk tests were used to determine normally distributed variables. Variables that were not normally distributed were log transformed or non-parametric tests were used for analysis. Repeated measures Analysis of Variance (ANOVA) was performed to test for within-subject differences among dependent variables. The within-subject factors were product type (cigarette vs. cigarillo) and time point (pre- vs. post-smoking). Data in tables are presented as arithmetic mean (SD) unless indicated otherwise.
Results
Participants
The study consisted of 23 participants who met eligibility criteria and attended both smoking sessions. Participant characteristics are shown in Table 1. The sample consisted of mostly African Americans (n=18) and men (n=21) with an average age of 39.0 years. Nine participants smoked cigarillos daily and 14 participants smoked between 1 and 5 cigarillos per week. Generally, participants began smoking cigarettes at a younger age than when they began smoking cigarillos (13.9 vs 23.0, P<0.001). The number of products smoked before coming to each session was similar for both smoking conditions. On average, 3.7 and 4.3 cigarettes and 0.1 and 0.2 cigarillos were smoked prior to arrival to the lab for the cigarette and cigarillo smoking conditions, respectively. The time since last cigarette was (113 minutes (SD 219) and 142 minutes (SD 325)) and time since last cigar (1,844 minutes (SD 1,879) and 1,813 minutes (SD 1,613)) was similar for the cigarette and cigarillo smoking conditions, respectively. Participants were dependent on nicotine as shown by the number of cigarettes smoked per day (17.5) and average Fagerström Score (6.3).
Table 1.
Participant Demographics and Smoking Characteristics
| Demographics | |
|---|---|
|
| |
| Variable (n=23) | % (n) |
| Gender | |
| Male | 91.3 (21) |
| Female | 8.7 (2) |
| Race | |
| African American | 78.3 (18) |
| Caucasian | 13.0 (3) |
| Other | 8.7 (2) |
| Education | |
| Less than high school | 26.1 (6) |
| High school grad/GED | 52.2 (12) |
| More than high school | 21.7 (5) |
| Income | |
| <$20,000 | 65.2 (15) |
| $20,001–$35,000 | 17.4 (4) |
| >$35,000 | 17.4 (4) |
| Age in years | |
| Mean (SD) | 39.0 (12.5) |
|
| |
| Smoking Characteristics | |
|
| |
| Variable | % (n) |
|
| |
| Preferred Cigarette Brand | |
| Newport | 82.6 (19) |
| Marlboro | 13.0 (3) |
| American Spirit | 4.4 (1) |
| Preferred Cigarette Flavor | |
| Menthol | 87.0 (20) |
| Non-menthol | 13.0 (3) |
| Preferred Cigarillo Brand | |
| Black & Mild | 60.9 (14) |
| Dutch Masters | 30.4 (7) |
| Other | 8.7 (2) |
| Preferred Cigarillo Flavor | |
| Unflavored | 13.0 (3) |
| Flavored | 87.0 (20) |
| Cigarillos frequency of use | |
| At least one per day | 39.1 (9) |
| < one per day | 60.9 (14) |
| Cigarettes per day | |
| Mean (SD) | 17.5 (4.5) |
| Years smoked cigarettes | |
| Mean (SD) | 20.6 (11.6) |
| Years smoked cigarillos | |
| Mean (SD) | 16.1 (9.1) |
| Age of cigarette initiation | |
| Mean (SD) | 13.9 (3.9) |
| Age of cigarillo initiation | |
| Mean (SD) | 23.0 (10.3) |
| FTND | |
| Mean (SD) | 6.3 (1.5) |
Study products
The mean weight of the cigarette (1.075g) was significantly less than the cigarillo (3.097g) prior to smoking (P<0.001). Participants smoked significantly (P<0.001) more tobacco while smoking a cigarillo (1.356g; range: 0.199g – 2.423g) than a cigarette 0.669g (range: 0.322g – 0.868g). Before smoking, participants indicated how much of the cigarillo they usually smoke by drawing a line on a picture of a. On average, participants indicated they usually smoke approximately 48% of their cigarillo which was significantly correlated with the amount they actually smoked in the lab (44%; P<0.01). Fourteen participants (61%) regulary smoked the study product (B&M).
Tobacco use history
Most participants reported smoking Newport cigarettes (n=19) and B&M cigarillos (n=14). More participants smoked menthol cigarettes (n=20) compared to non-menthol (n=3). A variety of cigarillo flavors were reported as shown in Table 1. Generally, participants began smoking cigarettes before cigarillos. The average age of first cigarette (13.9) was significantly less than the average age of cigarillo (22.9, P<0.001).
Dependent measures
A) Puff Measures
The results of smoking topography measurements are summarized in Table 2. Participants took significantly more puffs during cigarillo than cigarette smoking (24 vs 15, respectively; P<0.001) and total puff volume was significantly larger (1,267 vs 698 mL, respectively; P<0.001). Moreover, for cigarillo smoking, average TTS was significantly longer than for cigarette smoking (620 vs 315s, P<0.001). Average puff volume, puff duration, interpuff interval, and puff velocity (mean) were similar in both cigarette and cigarillo smoking. The pattern of puffing for both cigarillo and cigarette smoking was similar; the initial three puffs were smoked more vigorously than the last three puffs. Specifically, puff volume and durations were significantly larger and IPI was significantly shorter (all P<0.01), however, puff velocity did not change.
Table 2.
Average Smoking topography parameters by product type
| Smoking topography parameter | Cigarette smoking | Cigarillo smoking | P |
|---|---|---|---|
|
| |||
| Mean (SD) | |||
| Number of puffs | 15 (6) | 24 (9) | <0.001 |
| Total puff volume (mL) | 698 (215) | 1267 (476) | <0.001 |
| Time to smoke (s) | 315 (79) | 620 (350) | <0.001 |
| Puff volume (mL) | 50.3 (15.4) | 56.2 (16.0) | NS |
| Interpuff interval (s) | 22.0 (8.1) | 24.4 (11.0) | NS |
| Puff velocity (mL/s) | 24.5 (6.1) | 23.2 (6.1) | NS |
| Puff duration (s) | 2.3 (0.8) | 2.8 (1.1) | NS |
P, Repeated Measures ANOVA; NS, statistically non-significant
There were no significant differences in puff topography measures between participants who normally smoke B&M (n=14) versus those who normally smoke other brands (n=9). However, some differences were observed in puff topography measures when comparing daily and non-daily cigarillo smokers. Those who smoked cigarillos daily took longer to smoke the B&M (806 vs 501s, P<0.05), took more puffs (30 vs 20, P<0.01) and had a significantly greater total puff volume (1,572 vs 1,072mL, P<0.05) compared to those who did not smoke cigarillos daily.
B) Toxicant Exposure (Tobacco Smoke Biomarkers-- Table 3)
Table 3.
Biomarkers of Exposure
| Acute Biomarkers | Cigarette Smoking | Cigarillo Smoking |
|---|---|---|
|
| ||
| Mean (SD) | ||
| Plasma nicotine (ng/mL; n=21) | ||
| Pre-smoking | 10.6 (8.4) | 10.2 (10.4) |
| Post-smoking | 35.1 (14.8) | 31.3 (21.0) |
| Boost | 24.5 (9.0) | 21.1 (19.1) |
| COex (ppm; n=23) | ||
| Pre-smoking | 16 (9) | 17 (12) |
| Post-smokinga | 25 (11) | 43 (20) |
| Boosta | 9 (5) | 25 (16) |
| Plasma nicotine (ng/mL/g; n=21)c | ||
| Boostc (ng/mL/g)a | 38.3 (19.6) | 14.4 (8.7) |
| Boostd (ng/mL/1,000mL)a | 39.1 (26.4) | 15.9 (9.7) |
| COex (ppm/g; n=23)c | ||
| Boostc (ng/mL/g)a | 12 (6) | 18 (7) |
| Boostd (ng/mL/1,000mL)a | 12 (6) | 19 (8) |
|
| ||
| (n=21) | ||
|
|
||
| Chronic Biomarkers | Mean (SD) | |
|
| ||
| Total urinary cotininee | 849.9 (1,021.0) | |
| Total 3-OH-cotininee | 2,662.9 (2,349.8) | |
Repeated Measures ANOVA P < 0.001 for cigarette smoking vs. cigarillo smoking
Repeated Measures ANOVA P < 0.01 for cigarette smoking vs. cigarillo smoking
Per gram of tobacco consumed
Per 1,000mL of total puff volume
Per gram of creatinine
Plasma nicotine
Though we did not control for the time of day of smoking, there were no significant differences in time since last cigarette/cigar product or baseline nicotine concentration between the cigarette and cigarillo smoking conditions (10.6 and 10.2 ng/mL, respectively). The peak plasma nicotine concentration occurred at 5 minutes post-smoking compared to 10 minutes and was used to determine nicotine boost. Smoking cigarettes and cigarillos significantly increased plasma nicotine concentration from baseline (10.6 to 35.1 ng/mL and 10.2 to 31.3 ng/mL, respectively; P<0.001). Plasma nicotine boost was similar in cigarette and cigarillo smoking conditions (24.5 and 21.1 ng/mL, respectively). There was a small, but significant correlation between baseline plasma nicotine concentration and nicotine boost in the cigarette smoking condition (r=0.45, P<0.05), but not in the cigarillo smoking condition. There were no within-subject correlations for nicotine boost, CO boost, or any puff topography parameters. Plasma nicotine boost was significantly different between daily and non-daily cigarillo smokers (30.4 vs 14.2ng/mL, respectively). However, there were still no statistically significant difference in plasma nicotine boost between cigarette and cigarillo smoking when non-daily cigarillo smokers were removed from the analysis. After normalizing toxicant exposure by the weight of tobacco consumed there was a significantly greater nicotine boost per gram of tobacco consumed after cigarette smoking compared to cigarillo smoking (38.3 vs 14.4ng/mL/g, respectively; P<0.001). Similar results were found after normalizing toxicant exposure by total puff volume. Plasma nicotine boost per 1,000mL of total puff volume was significantly greater after cigarette smoking compared to cigarillo smoking (39.1 vs 15.9ng/mL/1,000mL, respectively; P<0.01)
Exhaled carbon monoxide
Pre-smoking COex levels were similar before the cigarette and cigarillo smoking conditions (16 vs 17ppm, respectively). However, there was a significantly greater increase in COex after smoking cigarillos compared to cigarettes (25 vs 9ppm, respectively; P<0.001). After normalizing CO exposure by the weight of tobacco consumed, there remained a significantly greater increase after cigarillo smoking compared to cigarette smoking (18 vs 12ppm/g, respectively; P<0.001). Similar results were found after normalizing COex exposure by total puff volume. CO boost per 1,000mL was significantly greater after cigarillo smoking compared to cigarette smoking (19 vs 12ppm/1,000mL, respectively; P<0.001).
Urinary cotinine and 3-OH-cotinine
Urinary cotinine and 3-OH-cotinine were measured and corrected for grams of creatinine. In this population, urinary cotinine averaged 1,229.3ng/mL or 849.9μg/g of creatinine; 3-OH-cotinine measured 5,278.7ng/mL or 2,662.9μg/g of creatinine.
C) Subjective Measures
The appeal and effects
The QSU scores before and after smoking are shown in Table 4. Similar baseline scores of QSU Total and Factors 1 and 2 were evident before smoking and after smoking. Although both products reduced QSU scores, there were no significant differences between cigarettes and cigarillos in the reduction of smoking urges scores (Factor 1, Factor 2, and Total). Participants rated cigarettes and cigarillos similarly as measured by the DSQ and CES. Despite no difference in smoking urge as measured by the QSU, participants rated cigarettes as significantly greater than cigarillos in immediate reduction of craving in the CES (P<0.05).
Table 4.
Subjective Measures
| Subjective Variable | Cigarette Smoking | Cigarillo Smoking |
|---|---|---|
|
| ||
| M (SD) | M (SD) | |
| Pre-Smoking Questionnaire on Smoking Urges (QSU) | ||
| Total | 45 (19)b | 42 (20)b |
| Factor 1 | 25 (10)b | 23 (10)c |
| Factor 2 | 20 (10)b | 19 (10)b |
| Post-Smoking QSU | ||
| Total | 28 (18) | 26 (17) |
| Factor 1 | 15 (9) | 14 (10) |
| Factor 2 | 13 (9) | 12 (8) |
| Difference in QSU (Post-Pre) | ||
| Total | −16 (12) | −16 (19) |
| Factor 1 | −10 (7) | −9 (11) |
| Factor 2 | −6 (6) | −7 (9) |
| Duke Sensory Questionnaire (DSQ); post smoking | ||
| Puff Liking | 5 (1) | 5 (1) |
| Puff Satisfaction | 5 (1) | 5 (1) |
| Nicotine in Puffs | 5 (1) | 5 (2) |
| Strength | 22 (7) | 22 (7) |
| Cigarette (Cigar) Evaluation Scale (CES); post smoking | ||
| Sensation | 4 (2) | 5 (2) |
| Craving Reductiona | 6 (2) | 4 (2) |
| Satisfaction | 6 (1) | 5 (1) |
| Psychological Reward | 4 (1) | 4 (1) |
| Aversion | 2 (1) | 2 (1) |
Repeated Measures ANOVA P < 0.05 for cigarette smoking vs. cigarillo smoking
Repeated Measures ANOVA P < 0.001 for pre-smoking QSU vs post-smoking QSU
Repeated Measures ANOVA P < 0.01 for pre-smoking QSU vs post-smoking QSU
Discussion
The epidemiology of cigar smoking has changed dramatically over the past few decades. Formerly cigar smokers were typically older, affluent males, who smoked large cigars [36] and did not smoke cigarettes. Most such smokers did not inhale the cigar smoke into their lungs unless they were former cigarette smokers [37]. Current use pattern indicates the popularity of cigar use among young, urban African American men and women who typically use both cigars and cigarettes. Furthermore, the characteristics of the cigars themselves have changed. In contrast to the large, unflavored, unfiltered cigar sold singly, little cigars and cigarillos are sold in packages of 5–20, they are often tipped, sometimes filtered, and available in a wide variety of flavors.
Relative to the vast literature on the behavior of cigarette smoking very few studies have looked at contemporary patterns of cigar smoking behavior. Although epidemiologic data have documented increase use of cigar products, especially little cigars and cigarillos [38], there has not been published research on the puffing profile, use characteristics, and acute and chronic exposure to toxicants from cigar smoking. The present study compares cigarette and cigarillo smoking in a laboratory setting in people who regularly smoke cigarillos and cigarettes (dual users). The study was designed to measure puffing behavior and subsequent toxicant exposure. The results of the study indicate there were differences and similarities between cigarette and cigarillo smoking. Smoking either cigarettes or cigarillos exposes the users to substantial quantities of nicotine, carbon monoxide and presumably other components of tobacco smoke.
In this study comparing laboratory smoking of cigarillos and cigarettes we found similar smoking patterns by participants who ordinarily smoke both cigarettes and cigarillos. In spite of differences in size, flavoring and filtration, cigarillos and cigarettes yielded a similar nicotine boost but there was more CO delivery from the cigarillo. As with other alternative or novel tobacco products – roll your own cigarettes [21], bidis [39], cloves [40], and little cigars [41] – smokers extract familiar amounts of nicotine but may expose themselves to larger amounts of other smoke delivered toxicants.
The participants of the present study were largely African American males which is reflective of the user population of cigarillo smokers in America [11]. Because of the documented importance of topography measures in predicating smoking abstinence [42] and the preponderance of African Americans in the sample the generalizability of the results deserves comment. Several studies have investigated differences in smoking topography of cigarettes as a function of race, sex and age [43], however, there are very few studies of smoking topography in cigarillo smoking [20, 44] . Among cigarette smokers the effects of race and sex are mixed and complex [43]. For example, Ahijevych and Gillespie (1997) [45] reported that African American women had significantly higher CO boost than white women but no difference in smoking topography. Other studies have not identified significant differences between adolescent smokers [46] and adult smokers [47]. Furthermore topography differences between African and Caucasians are often confounded by the preponderance of menthol smoking among African Americans [48, 49]. It remains uncertain whether differences in topography between sex and race are significant and whether the results of cigarette smoking topography apply to cigarillo smoking but this is an important area of research because of the associations between topography and exposure to smoke toxicants [50].
Smoking topography
Puff topography measures were used to assess the behavior of cigarillo smoking. Puffing is the process of bringing mainstream smoke from the product to the mouth. Subsequently, the smoke may be inhaled into the lungs as is often the case in cigarette smoking or it may be held in the oral cavity. Some studies have suggested that cigar smoking is usually associated with puffing but not inhalation and the nicotine and other components of tobacco smoke are absorbed from the oral mucosa [51]. Puff topography is widely used to study smoking behavior associated with cigarettes [42, 52–54] and alternative tobacco products [21, 39, 55, 56]. Puff topography can also be used as a tool to measure compensatory behavior in cigarette smoking. As an illustration, smokers of commercial, factory made cigarettes take bigger and more frequent puffs when they are tobacco abstinent (plasma levels of nicotine are low) [57] or when the product delivers less nicotine than the smoker is accustomed [58]. These behavioral changes in puffing may affect carcinogen exposure [59], however, puffing and compensatory smoking have not been studied during cigarillo smoking.
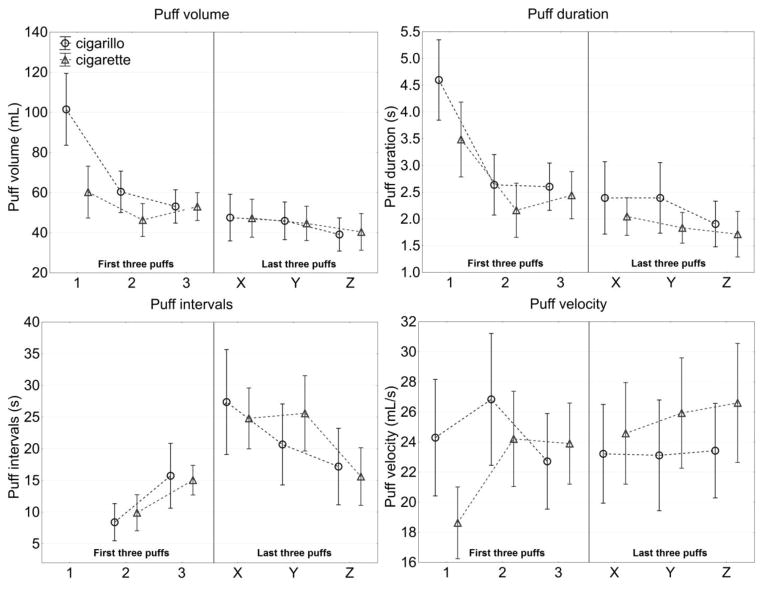
Smoking topography data indicate that the cigarillos and cigarettes were puffed similarly (Figure 1). There were no significant differences in puff volume, puff duration, puff velocity or interpuff interval. Furthermore the pattern of cigarillo and cigarette smoking was similar over the course of the smoking session. Specifically, puff volume and puff duration were larger and interpuff interval was shorter in the first three puffs than in the last three puffs. This use pattern has been reported in cigarette smoking of conventional and roll your own cigarettes [21]. More intense smoking in the beginning of a cigarette is thought to indicate that the smoker is trying to satisfy an immediate need for nicotine (or other components of smoke or smoking behavior) and that this need diminishes as the article is consumed [21]. However, on puff variables (e.g. TTS, number of puffs and total puff volume) that are related to the size of the product the significant differences observed were largely accounted for by the significant differences in the size of the article and in the amount of tobacco consumed.
Figure 1. Puff by puff analysis.
Puff parameters for cigarettes and cigarillos showing the first 3 puffs (1,2,3) and the last 3 puffs (X,Y,Z) from participants (N=23) smoking. Comparisons of the first 3 and last 3 puffs indicated significant (p<0.01) decreases in puff volume, puff duration and an increase in inter puff interval from cigarette and cigarillo smoking; puff velocity did not significantly change after either cigarettes or cigarillos.
Exposure to nicotine
Both cigarillo and cigarette significantly increased plasma levels of nicotine above baseline levels but the increase in plasma nicotine levels (boost) was not significantly different. This result suggests that the ad lib smoking was directed to achieve a similar and familiar level of nicotine when either product was consumed. This interpretation is supported by the observation that both products equally and significantly reduced cigarette craving. When the amount of tobacco consumed and the total puff volume was factored into the nicotine exposure, it appears that cigarette consumption was more efficient than cigarillo consumption by significantly increasing plasma nicotine per gram of tobacco consumed and per 1,000mL puffed. Plasma levels of nicotine after both cigarette and cigarillos were maximum at 5 min after smoking; at 10 min after smoking the nicotine plasma levels were similar or slightly smaller. We tentatively interpret these data to indicate the nicotine absorption from both cigarillo and cigarette smoking largely occurs in the lungs and suggest that the smoke from the cigarillo was inhaled. If nicotine absorption occurred after buccal absorption the peak plasma levels would be slightly delayed. For example, after smokeless tobacco use peak plasma levels of nicotine rise and are sustained throughout the time the products are in the mouth and higher levels occur a few minutes later [17]. Others have shown delays in nicotine absorption after the smoking large cigars that are interpreted to indicate buccal absorption of nicotine [60] however, we recognize that a combination of lung and buccal absorption could have occurred in the present study. The present study investigated cigarillo and cigarette in non-abstinent (no restrictions as to the time from last smoking. It is possible that the topography variables observed in the present report may have differed from those recorded in abstinent conditions, however, tobacco abstinence is not the normal condition of smoking—usually occurring only before the first cigarette of the day after variable periods of overnight abstinence. Furthermore, in a study of commercial and self-made cigarettes we compared nicotine boost and puff parameters between conditions of overnight abstinence, intense smoking immediately before the experimental cigarette and no restriction (as in the present study). There were no differences in puff topography as a function of condition and nicotine boost was similar in all abstinent vs, non-restricted conditions [21].
Exposure to carbon monoxide
Cigarillo smoking caused significantly larger increases in CO boost than cigarette smoking and this difference remained significant even after accounting for differences in tobacco consumed and total puff volume. The increase in CO is an indication of pulmonary exposure to tobacco smoke [61] and is another indication that the cigarillo smoke was inhaled. CO is generated from incomplete combustion of tobacco and it is known that cigars deliver more CO than cigarettes because of differences in the porosity of the wrapper and the size of the article [51]. The finding of greater CO exposure implies that cigarillo smoking may expose users to higher amounts of other volatile components of mainstream cigarillo smoke including the known carcinogens benzene, 1,2-butadiene, and acetaldehyde. The overall risk assessment by product is complex since among dual users more cigarettes are smoked than cigarillos and cigarettes are still a major source of toxicants and health risk.
The participants in the present study were dual users of cigarettes and cigarillos. Dual users of cigarillos and cigarettes were chosen for this study because they are much more prevalent and more representative than exclusive cigarillo smokers [38]. However, their pattern of smoking may differ from exclusive cigarillo smokers. There are reports in the literature that former cigarette smokers are more likely to inhale cigar smoke – as they would cigarette smoke – than cigar smokers who have never smoked cigarettes [51]. Tiffany has pointed out the importance of the development of automatic and non-automatic processes in drug use behavior [62]. The constancy of puff behavior in cigarette smoking has been demonstrated in adolescents [63] and adults [33, 64]. We also noted constancy within individuals in use patterns of cigarette smoking in conditions of tobacco abstinence and after experimentally induced tobacco satiation and in people who were smoking either conventional or self-made cigarettes [21]. Taken together these studies show that a pattern of smoking is established rather early in a person’s smoking career and that once established the pattern persists regardless of the tobacco article or the circumstances of its use.
Summary
This study was an initial investigation of the use behavior and toxicant exposure from a single popular cigarillo compared to the use behavior and exposure of the participant’s own brand of cigarette. In the analysis, the cigarette smoking and exposure variables were aggregated even though several different cigarettes were used. Furthermore, the brand of cigarillo smoked in the lab was not the usual cigarillo chosen by 9 of the 23 participants. Their usual cigarillo may have differed in size, flavor and design from the experimental product. Finally we compared of smoking a single cigarette and a single cigarillo in a laboratory study. There are acknowledged differences between lab and natural smoking and there are differences in the number of cigarettes and cigarillos smoked in a single day by dual users. In spite of these limitations, our study indicates that B&M cigarillo smoking is associated with equal or greater exposure to smoke-delivered carcinogens than cigarette smoking. Because cigarettes deliver nicotine more efficiently than cigarillos, cigarillo consumption may expose users to relatively greater toxicants in mainstream smoke, including carcinogens than cigarette smoking. The higher toxicant exposure per unit of nicotine in cigarillo smoke, suggest a significant public health threat in youth and adult cigarillo smokers.
What this paper adds.
What this paper adds
This study provides the first head to head comparison of cigarillos and cigarettes on nicotine and carbon monoxide exposure that follow their use.
The higher toxicant exposure per unit of nicotine in cigarillo smoke, pose a significant public health threat in youth and adult smokers who initiatite cigarillo use.
The data collcted contributes to the development of a comprehensive knowledge base and understanding of the dual use of cigar and cigarette products. This information is crucial for making regulatory decisions surrounding increasingly popular cigar products.
What is already known on this subject
Cigarillos are becoming an increasgly popular tobacco articles especially among youth.
The usual pattern is dual use, meaning that most cigarillo users are also cigarette smokers.
What important gaps in knowledge exist on this topic
Although there has been an increase in cigarillo use, very little is known about the toxicant exposure from them or their use patterns.
What this study adds
Our study shows that dual users of cigarillos smoke these products similarly to cigarettes – specifically, the smoke is inhaled and the topography (more intense smoking in the first few puffs) is similar for each product.
The nicotine exposure is similar from each but CO exposure is greater after cigarillo use - even after accounting for grams of tobacco consumed and total puff volume.
Highlights.
Puffing topography, exhaled CO, plasma nicotine, and physiologic effects from usual brand cigarette and Black & Mild cigarillo were tested (n=23)
Time to smoke, number of puffs, and total puff volume differed for cigarette and cigarillo smoking
Nicotine boost after cigarettes and cigarillos was similar; cigarillo smoking caused higher CO exposure
Exposure to nicotine and other toxicants form cigarillo use are relevant to health and regulatory considerations
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number 1R01CA158045-01A1 to W.B. Pickworth. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interest statement:
The authors report no competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agaku IT, King BA, Husten CG, Bunnell R, Ambrose BK, Hu SS, Holder-Hayes E, Day HR. Tobacco product use among adults - United States, 2012–2013. MMWR Morb Mortal Wkly Rep. 2014;63:542–547. [PMC free article] [PubMed] [Google Scholar]
- 2.Consumption of cigarettes and combustible tobacco--United States, 2000–2011. MMWR Morb Mortal Wkly Rep. 2012;61:565–569. [PubMed] [Google Scholar]
- 3.US Department of Health and Human Service. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. Rockville, MD: 2014. [Google Scholar]
- 4.Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S.Department of Health and Human Services and RTI International. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. 2011. 7-8-2014. [Google Scholar]
- 5.Tobacco product use among middle and high school students--United States, 2011 and 2012. MMWR Morb Mortal Wkly Rep. 2013;62:893–897. [PMC free article] [PubMed] [Google Scholar]
- 6.Malone RE, Yerger V, Pearson C. Cigar risk perceptions in focus groups of urban African American youth. J Subst Abuse. 2001;13:549–561. doi: 10.1016/s0899-3289(01)00092-x. [DOI] [PubMed] [Google Scholar]
- 7.Nyman AL, Taylor TM, Biener L. Trends in cigar smoking and perceptions of health risks among Massachusetts adults. Tob Control. 2002;11(Suppl 2):ii25–ii28. doi: 10.1136/tc.11.suppl_2.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SY, Curbow B, Stillman FA. 0Harm perception of nicotine products in college freshmen. Nicotine Tob Res. 2007;9:977–982. doi: 10.1080/14622200701540796. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. 2010. 7-8-2014. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Fact sheets. Atlanta, GA: [Accessed: 09/24/2015]. Smoking & Tobacco Use: Cigars. www.ccd.gov. [Google Scholar]
- 11.Richardson A, Xiao H, Vallone DM. Primary and dual users of cigars and cigarettes: profiles, tobacco use patterns and relevance to policy. Nicotine Tob Res. 2012;14:927–932. doi: 10.1093/ntr/ntr306. [DOI] [PubMed] [Google Scholar]
- 12.Lee YO, Hebert CJ, Nonnemaker JM, Kim AE. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med. 2014;62:14–19. doi: 10.1016/j.ypmed.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Cullen J, Mowery P, Delnevo C, Allen JA, Sokol N, Byron MJ, Thornton-Bullock A. Seven-year patterns in US cigar use epidemiology among young adults aged 18–25 years: a focus on race/ethnicity and brand. Am J Public Health. 2011;101:1955–1962. doi: 10.2105/AJPH.2011.300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S.Food and Drug Administration (U.S.FDA) The Family Smoking Prevention and Tobacco Control Act (FSPTCA) 2009. Public Law 111–31. [Google Scholar]
- 15.US Department of Health and Human Service, Centers for Disease Control and Prevention and Office on Smoking and Health Division of Adolescent and School Health. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: 2012. [PubMed] [Google Scholar]
- 16.U.S.Food and Drug Administration. FDA proposes to extend its tobacco authority to additional tobacco products, including e-cigarettes. [Accessed: 9/24/2015];FDA News Release. 2014 Apr 24; www.fda.gov.
- 17.Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tob Control. 1999;8:387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blank M, Nasim A, KAHETAustin J. Toxicant exposure, cardiovascular response, and subjective effects of cigarillo smoking. Conference Proceeding: Society for Nicotine and Tobacco Research Annual Meeting; Toronto, Canada. 2011. [Google Scholar]
- 19.Cappendijk SL, Pirvan DF, Miller GL, Rodriguez MI, Chalise P, Halquist MS, James JR. In vivo nicotine exposure in the zebra finch: a promising innovative animal model to use in neurodegenerative disorders related research. Pharmacol Biochem Behav. 2010;96:152–159. doi: 10.1016/j.pbb.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Blank MD, Nasim A, Hart A, Jr, Eissenberg T. Acute effects of cigarillo smoking. Nicotine Tob Res. 2011;13:874–879. doi: 10.1093/ntr/ntr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koszowski B, Rosenberry ZR, Viray LC, Potts JL, Pickworth WB. Make Your Own Cigarettes: Smoking Topography, Toxicant Exposure and Subjective Effects. Cancer Epidemiol Biomarkers Prev. 2014;23:1793–803. doi: 10.1158/1055-9965.EPI-14-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa M, Pacifici R, Altieri I, Pichini S, Ottaviani G, Zuccaro P. How the steady-state cotinine concentration in cigarette smokers is directly related to nicotine intake. Clin Pharmacol Ther. 1992;52:324–329. doi: 10.1038/clpt.1992.149. [DOI] [PubMed] [Google Scholar]
- 23.Meger M, Meger-Kossien I, Schuler-Metz A, Janket D, Scherer G. Simultaneous determination of nicotine and eight nicotine metabolites in urine of smokers using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:251–261. doi: 10.1016/s0378-4347(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 25.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 26.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 27.West R, Ussher M. Is the ten-item Questionnaire of Smoking Urges (QSU-brief) more sensitive to abstinence than shorter craving measures? Psychopharmacology (Berl) 2010;208:427–432. doi: 10.1007/s00213-009-1742-x. [DOI] [PubMed] [Google Scholar]
- 28.Behm FM, Rose JE. Reducing craving for cigarettes while decreasing smoke intake using capsaicin-enhanced low-tar cigarettes. Exp Clin Psychopharmacol. 1994;2:143–153. [Google Scholar]
- 29.Westman EC, Levin ED, Rose JE. Smoking while wearing the nicotine patch: is smoking satisfying or harmful? Clin Res. 1992;40:871A. [Google Scholar]
- 30.Brauer LH, Behm FM, Westman EC, Patel P, Rose JE. Naltrexone blockade of nicotine effects in cigarette smokers. Psychopharmacology (Berl ) 1999;143:339–346. doi: 10.1007/s002130050957. [DOI] [PubMed] [Google Scholar]
- 31.Malson JL, Lee EM, Moolchan ET, Pickworth WB. Nicotine delivery from smoking bidis and an additive-free cigarette. Nicotine Tob Res. 2002;4:485–490. doi: 10.1080/1462220021000018498. [DOI] [PubMed] [Google Scholar]
- 32.Lee EM, Malson JL, Moolchan ET, Pickworth WB. Quantitative comparisons between a nicotine delivery device (Eclipse) and conventional cigarette smoking. Nicotine Tob Res. 2004;6:95–102. doi: 10.1080/14622200310001656911. [DOI] [PubMed] [Google Scholar]
- 33.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5:673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 34.Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- 35.Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach D, Cummings KM, Hyland A, Gilpin E, Johnson MD, Pierce JP. Trends in Cigar Consumption and Smoking Prevalance. Smoking and Tobacco Control. Monograph No. 9: Cigars Health Effects and Trends. 1998:21–54. [Google Scholar]
- 37.Shanks TG, Burns DM. Disease Consequences of Cigar Smoking. Smoking and Tobacco Control. Monograph No. 9: Cigars Health Effects and Trends. 1998:105–160. [Google Scholar]
- 38.Richardson A, Rath J, Ganz O, Xiao H, Vallone D. Primary and dual users of little cigars/cigarillos and large cigars: demographic and tobacco use profiles. Nicotine Tob Res. 2013;15:1729–1736. doi: 10.1093/ntr/ntt053. [DOI] [PubMed] [Google Scholar]
- 39.Malson JL, Lee EM, Moolchan ET, Pickworth WB. Nicotine delivery from smoking bidis and an additive-free cigarette. Nicotine Tob Res. 2002;4:485–490. doi: 10.1080/1462220021000018498. [DOI] [PubMed] [Google Scholar]
- 40.Malson JL, Lee EM, Murty R, Moolchan ET, Pickworth WB. Clove cigarette smoking: biochemical, physiological, and subjective effects. Pharmacol Biochem Behav. 2003;74:739–745. doi: 10.1016/s0091-3057(02)01076-6. [DOI] [PubMed] [Google Scholar]
- 41.Koszowski B, Potts JL, Viray LC, Rosenberry ZR, Pickworth WB. Smoking Patterns and Effects among Little Cigar and Cigarette Dual Users. Conference Proceeding: Society for Nicotine and Tobacco Research Annual Meeting; Seattle, WA, USA. 2014. [Google Scholar]
- 42.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2004;13:1800–1804. [PubMed] [Google Scholar]
- 43.Mickens L, Ameringer K, Brightman M, Leventhal AM. Epidemiology, determinants, and consequences of cigarette smoking in African American women: an integrative review. Addict Behav. 2010;35:383–391. doi: 10.1016/j.addbeh.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fabian LA, Canlas LL, Potts J, Pickworth WB. Ad lib smoking of Black & Mild cigarillos and cigarettes. Nicotine Tob Res. 2012;14:368–371. doi: 10.1093/ntr/ntr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahijevych K, Gillespie J. Nicotine dependence and smoking topography among black and white women. Res Nurs Health. 1997;20:505–514. doi: 10.1002/(sici)1098-240x(199712)20:6<505::aid-nur5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 46.Aung AT, Pickworth WB, Moolchan ET. History of marijuana use and tobacco smoking topography in tobacco-dependent adolescents. Addict Behav. 2004;29:699–706. doi: 10.1016/j.addbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Patterson F, Benowitz N, Shields P, Kaufmann V, Jepson C, Wileyto P, Kucharski S, Lerman C. Individual differences in nicotine intake per cigarette. Cancer Epidemiol Biomarkers Prev. 2003;12:468–471. [PubMed] [Google Scholar]
- 48.Pickworth WB, Moolchan ET, Berlin I, Murty R. Sensory and physiologic effects of menthol and non-menthol cigarettes with differing nicotine delivery. Pharmacol Biochem Behav. 2002;71:55–61. doi: 10.1016/s0091-3057(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 49.Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiol Biomarkers Prev. 2013;22:382–389. doi: 10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melikian AA, Djordjevic MV, Chen S, Richie J, Jr, Stellman SD. Effect of delivered dosage of cigarette smoke toxins on the levels of urinary biomarkers of exposure. Cancer Epidemiol Biomarkers Prev. 2007;16:1408–1415. doi: 10.1158/1055-9965.EPI-06-1097. [DOI] [PubMed] [Google Scholar]
- 51.Burns DM. Smoking and Tobacco Control. Monograph No. 9: Cigars Health Effects and Trends. U.S. DHHS National Institutes of Health; 1998. Cigar Smoking: Overview and Current State of the Science; pp. 1–20. [Google Scholar]
- 52.Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005;82:320–329. doi: 10.1016/j.pbb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Pickworth WB, Houlgate P, Schorp M, Dixon M, Borgerding M, Zaatari G. A review of human smoking behaviour data and recommendations for a new ISO standard for the machine smoking of ciagrettes. 2005 A Report of the Ad Hoc WG9 Smoking Behaviour Review Team to ISO/TC 126 WG9. [Google Scholar]
- 54.Czogala J, Goniewicz ML, Czubek A, Koszowski B, Sobczak A. “How does smoker really smoke?”--preliminary report on smoking topography among Polish smokers. Przegl Lek. 2008;65:657–662. [PMC free article] [PubMed] [Google Scholar]
- 55.Pickworth WB, Malson JL. Laboratory studies of bidi smoking to estimate health consequences and addiction liability. In: Gupta PC, Asma S, editors. Bidi Smoking: A Comprehensive Review. Jaypee Brothers Medical Publishers; Daryagang, New Delhi: 2008. [Google Scholar]
- 56.Lee EM, Malson JL, Moolchan ET, Pickworth WB. Quantitative comparisons between a nicotine delivery device (Eclipse) and conventional cigarette smoking. Nicotine Tob Res. 2004;6:95–102. doi: 10.1080/14622200310001656911. [DOI] [PubMed] [Google Scholar]
- 57.Kozlowski LT, Dollar KM, Giovino GA. Cigar/cigarillo surveillance: limitations of the U.S. Department of Agriculture system. Am J Prev Med. 2008;34:424–426. doi: 10.1016/j.amepre.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 58.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107(Suppl 2):349–355. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control. 2004;13(Suppl 1):i48–i56. doi: 10.1136/tc.2002.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fant RV, Henningfield JE. Pharmacology and Abuse Potential of Cigars. Smoking and Tobacco Control. Monograph No. 9: Cigars Health Effects and Trends. 1998:181–194. [Google Scholar]
- 61.Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987;240:554–564. [PubMed] [Google Scholar]
- 62.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 63.Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2006;15:154–157. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- 64.Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]