Abstract
Background
αA- and αB crystallins are principal members of the small heat shock protein family and elicit both a cell protective function and a chaperone function. α-Crystallins have been found to be prominent proteins in normal and pathological retina emphasizing the importance for in-depth understanding of their function and significance.
Scope of Review
Retinal pigment epithelial cells (RPE) play a vital role in the pathogenesis of age-related macular degeneration (AMD). This review addresses a number of cellular functions mediated by α-crystallins in the retina. Prominent expression of αB crystallin in mitochondria may serve to protect cells from oxidative injury. αB crystallin as secretory protein via exosomes can offer neuroprotection to adjacent RPE cells and photoreceptors. The availability of chaperone-containing minipeptides of αB crystallin could prove to be a valuable new tool for therapeutic treatment of retinal disorders.
Major Conclusions
α-Crystallins are expressed in cytosol and mitochondria of RPE cells and are regulated during oxygen-induced retinopathy and during development. α-Crystallins protect RPE from oxidative-and ER stress-induced injury and autophagy. αB-Crystallin is a modulator of angiogenesis and vascular endothelial growth factor. αB Crystallin is secreted via exosomal pathway. Minichaperone peptides derived from αB Crystallin prevent oxidant induced cell death and have therapeutic potential.
General Significance
Overall, this review summarizes several novel properties of α-crystallins and their relevance to maintaining normal retinal function. In particular, the use of α-crystallin derived peptides is a promising therapeutic strategy to combat retinal diseases such as AMD.
Keywords: Crystallins, retinal pigment epithelial cells, subcellular localization, stress stimuli, apoptosis, exosomes, angiogenesis, minichaperone peptide
α- Crystallins are prominent members of the small heat shock protein family. αA and αB crystallins have been shown to be present in a number of tissues. Their expression and function in the eye, particularly in the lens, has been extensively studied [1]. Our laboratory has recently summarized the findings on the expression and significance of α-crystallins in the retinal tissue and retinal pigment epithelial (RPE) cells [2]. The present review focuses on α-crystallins, particularly αB crystallin, in the RPE and their potential role in the pathogenesis and treatment of age-related macular degeneration (AMD).
Apart from the well recognized chaperone effect, a wide variety of other properties of α-crystallins have come to the fore in various tissues including the eye. These include anti-inflammatory, antifibrillar, and antiapoptotic properties, protection against ER stress and autophagy, modulation of angiogenesis as well as protein-protein interactions with a large array of proteins [2-4]. Most of the research in elucidating the above properties and their associated signaling mechanisms has been performed with αB crystallin. As will be discussed, in addition to the entire protein molecule, short chain peptides that exhibit chaperone properties (minichaperones) have also proved valuable in exploring novel beneficial functions of α-crystallins and are considered potential therapeutic agents as well.
Localization of α-Crystallins
While αA and αB crystallins are considered to be two subunits of one protein, evidence from studies in the developing ocular lens suggests that each of these two proteins exist and function independently of each other [5]. In initial work on the analysis of αA, αB (as well as β and ɣ) crystallins, Xi et al. [6] found that these crystallins were found in the inner and outer nuclear layers of the retina and the RPE. The distribution of αA crystallin and αB crystallin differed; while αB crystallin was prominent in the RPE cells, αA crystallin expression was low in RPE but was more prominent in neural tissues such as photoreceptor, astroglial and Muller cells [7-9]. Abundant expression of αB crystallin in RPE cells has been confirmed by several laboratories including ours [7,10,11-13].
Cobb and Petrash [14] found that both αA and αB complexes bound to lens membranes in a specific, saturable and partially irreversible manner the binding was both time and temperature sensitive. Retinal α-crystallins formed macromolecular multimeric complexes and were found to be abundant both in soluble and membrane associated forms and specifically bound to post-golgi membrane in the frog retina [15]. Further, αB crystallin with its chaperone properties was shown to co-localize with Golgi matrix proteins so that an important role in golgi reorganization during cell division was suggested for this protein [16].
Subcellular localization of αB crystallin has been investigated by several laboratories [7,17,18]. In our initial studies, we showed that both αA and αB crystallin were found in the mitochondrial fraction of RPE cells [7]. The role of αB crystallin in mitochondria, given its antiapoptotic function, could be to augment or maintain mitochondrial function by protein folding and to restore and prevent subsequent downstream activation of apoptotic events and transcription factors such as NF kappaB [18]. Further, Jiang et al. [19] showed that heat shock pretreatment, which upregulates sHSPs, protected cells against H2O2 induced apoptosis and its mechanism appeared to involve the inhibition of Smac release from mitochondria. αB crystallin was also shown to interact with p53 which prevented the translocation of p53 from cytoplasm to mitochondria [20]. It is well known that p53 upregulation directly promotes Bax expression which changes the integrity of mitochondria, leading to cytochrome c release, caspase 3 activation and to eventual apoptosis. Overexpression of αB crystallin blocks activation of reactive oxygen species (ROS) to inhibit ERK1/2 activation and significantly attenuated calcimycin-induced apoptosis [21]. In studies performed in the lens, a mutation of αA crystallin, R49C distributed in the cellular nucleus of cultured cells [1] and in hereditary cataracts with R49C, mislocalization of αA crystallin into the cellular nucleus was observed. A role for αA crystallin was suggested from the observation of increased polyploid cells in mouse lens epithelial cell cultures null for αB crystallin [22]. αB crystallin is associated with nuclear speckles in different cell types [23-26].
α-Crystallins are developmentally regulated. We studied the developmental expression of α-crystallins in mouse retina of postnatal days 7, 12 and 17 using posterior mouse eye cups. Expression of both αA and αB crystallins was found on postnatal days 7 to 17. We show for the first time the compartmental distribution of the two crystallins in mitochondria and cytosol during this early period of neonatal development (Figure 1). While expression of αA and αB crystallins was observed in the cytosol, only αA crystallin was expressed in significant proportion in the mitochondria. The expression of αB crystallin in mitochondria, on the other hand, was considerably lower (Figure 1A). The significance of this finding with respect to the possible differing mechanisms of action of the two crystallin isoforms during postnatal development would need further study. We also identified the expression of one of the phosphorylated forms, namely serine 59 phosphorylated αB crystallin during development. Further, we showed that mitochondrial and cytosolic αA and αB crystallin expression was higher on P12 as compared to P19 of oxygen-induced retinopathy (OIR) (Figure 1B). The ser59 phospho αB crystallin in both mitochondria and cytosol were markedly higher on P12 than on P19. This finding is consistent with the known fact that various conditions and stimuli induce phosphorylation, which in turn may regulate the α-crystallin function [27].
Figure 1. α-Crystallin expression during development and in OIR in posterior eye cups of mice.
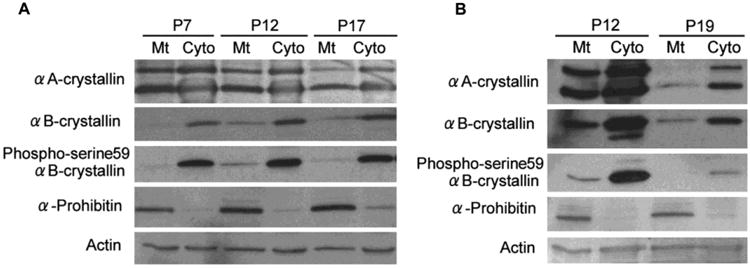
(A) Expression of αA and αB crystallin and Ser59 phosphorylated αB crystallin in neonatal posterior eye cups of mice on days 7, 12, and 17. Expression is shown in the mitochondrial and cytosolic fractions and the purity of mitochondrial fraction was verified by α-prohibitin, a mitochondrial marker. αA Crystallin shows two bands, lower molecular size one for αA crystallin and a second higher molecular weight band for αA crystallin insert. Both crystallins were localized in cytosol and mitochondria and the proportion of αA crystallin in mitochondria was significantly higher than that of αB crystallin throughout the postnatal period. (B) Expression pattern for αA and αB crystalllin and Ser59 phosphorylated αB crystallin in posterior eye cups of neonatal mouse pups in OIR. αA and αB crystallin expression was higher on day 12 as compared to day 19. Neonatal mouse pups on post natal day 7 (P7) with their nursing mothers were maintained for 5 days in 75% (75 ±3%) oxygen and then returned to room air (relative hypoxia) at P12.
Protection from Apoptosis by α -Crystallins
As is well known, oxidative stress is one of the key causative factors of AMD. There is evidence that oxidative stress induced inflammation initiates AMD [28]. Most of the studies that address the antiapoptotic function and associated signaling mechanisms of α-crystallins use oxidative stress stimuli as a model for such studies. For example, αB crystallin was shown to protect from cell death induced by oxidative stress as well drugs such as staurosporine and doxorubicin [29]. Work from Arrigo's laboratory had shown that human αB crystallin and HSP27 prevented TNFα induced apoptosis in L929 cells and this property of sHSPs was associated with increased cellular glutathione which facilitated attenuating ROS generation [30]. The significance of antioxidants, particularly glutathione (GSH) in RPE protection was reported by our laboratory [31]. We showed that human RPE cells that overexpress αA or αB crystallin were resistant to H2O2 induced cell death as compared to the vector controls (Figure 2). Further, RPE cells overexpressing either αA or αB crystallin contained increased cellular GSH arising from an increase in GCLC, the regulatory subunit of the rate limiting enzyme of GSH biosynthesis. We further showed a selective increase in mitochondrial GSH compartment of oxidatively stressed RPE in αA and αB overexpressing cells provided cellular protection (Figure 2). These studies further established that the αB crystallin induced protection of cell death was mediated by the multidrug associated protein MRP1, a GSH efflux transporter.
Figure 2. Protection of RPE cells from H2O2-induced oxidative stress by αA and αB crystallin is linked to cellular GSH.
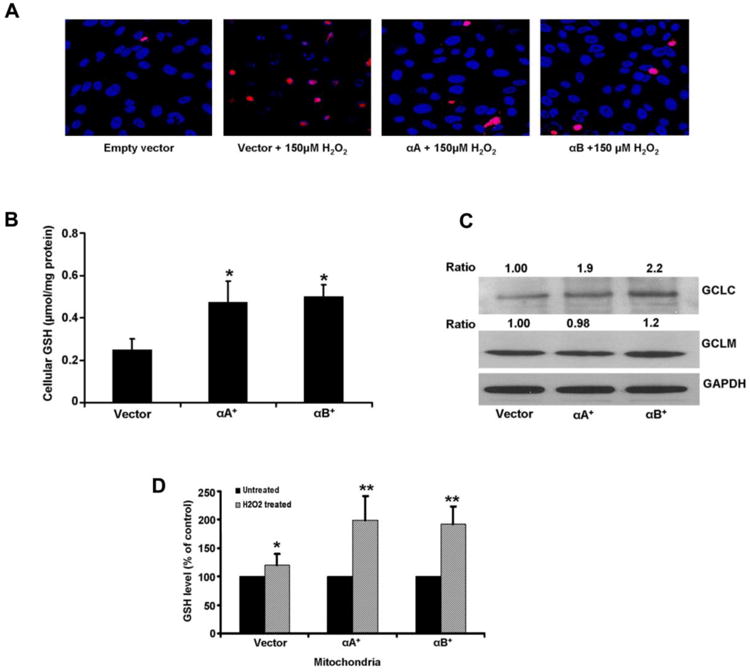
(A). α-Crystallin overexpressing RPE cells were incubated with 150 μM H2O2 for 24 h in serum free medium and cell death was analyzed by TUNEL staining. Cell death induced by H2O2 was significantly reduced in α-crystallin overexpressing cells. Blue: DAPI nuclear staining; Red: TUNEL positive cells. (B) Cellular GSH levels in ARPE-19 cells stably overexpressing αA crystallin and αB crystallin increased significantly (P<0.05) when compared to vector only cells. (C) A significant increase in the protein expression of the modifier unit (GCLC) was found with α-crystallin overexpression and no significant change in the catalytic unit (GCLM). (D) Oxidative stress causes a significant increase in mitochondrial GSH with αA and αB crystallin overexpressing cells. GSH was assayed in mitochondrial fraction of RPE cells after treatment with 150μM H2O2 for 24h. Protein bands were quantified and presented as ratio normalized to loading control, GAPDH. *, ** denote p<0.05, p<0.01, respectively. Reproduced with permission under terms of the Creative Commons Attribution License and modified from Sreekumar et al. PLoS One. 2012; 7(3):e33420.
Apoptosis is mediated by multiple signaling pathways and regulators such as the mitogen activated protein kinases (MAPKs) and or RAF/MEK/ERK or AKT kinases [21]. It was reported that αA crystallin provided higher level of protection against cell death than αB crystallin in cultured lens epithelial cells [1]. However, we found that RPE isolated from αA crystallin KO mice were as susceptible as αB crystallin KO RPE to oxidative stress despite the relatively low abundance of αA crystallin in RPE [7]. Further, RPE cells overexpressing either αA- or αB crystallin provided similar protection against oxidant induced cell death [31]. It is of interest that in vivo, in CoCl2-induced hypoxia, retinas of αA- and αB-crystallin KO mice exhibited similar, rapid and more severe degeneration as compared to WT retinas, supporting in vitro findings [32]. However, it has to be recognized that, while the two α-crystallin isoforms display similar antiapoptotic properties in the retina and RPE, the associated mechanisms of protection may differ based on the stress stimulus and experimental conditions [29, 33].
Role of α-Crystallins in Autophagy
Autophagy plays a key role in cellular homeostasis. To maintain normal cellular function, autophagy is often upregulated in response to environmental stresses and excessive organelle damage to facilitate aggregated protein removal. Among the three known autophagic mechanisms [34], chaperone-mediated autophagy (CMA) is relevant to our discussion although the autophagic systems are not completely separated from each other. Further, adverse effects of autophagy have been described in a mouse model of retinitis pigmentosa and in a rat model of ischemia [35,36]. Increase in αB crystallin expression in neurodegenerative diseases such as AMD where it is a component of drusen has been documented [10, 37, 38]. The presence of αB crystallin in drusen could be in response to toxic protein aggregation and lipofuscin accumulation. It was postulated that increased autophagy and exocytic activities in aged RPE could supply extracellular materials for the formation of drusen and indeed the authors reported the presence of autophagic and exosomal markers in drusen from AMD patients [39, 40]. Thus, autophagy may represent an important therapeutic target in AMD although the effect and interpretation is complex due to a variation in the AMD phenotypes. Recently, it was reported that autophagy proteins, autophagosomes, and autophagy were significantly reduced in tissue from human donor AMD eyes and two animal models of AMD [3]. With respect to mechanism, the autophagy regulating-kinases AMPK and MTOR can be considered potential therapeutic targets for preventing RPE cell degeneration and AMD progression either alone or as an adjunct to other treatments [41]. Mitter et al. [3] studied whether the autophagy pathway played a critical role in protecting ARPE-19 cells against oxidative stress. Acute oxidative stress led to a marked increase in autophagy whereas chronic oxidative stress reduced autophagy. The work by Robbins group with cardiomyocytes showed that the R120G mutation of αB crystallin decreased the expression Atg7, a mediator of autophagosomal biogenesis and induction of autophagy with the overexpression of Atg7 rescued the accumulation of the misfolded mutant αB crystallin [42, 43]. It is of interest that a recent report demonstrated that a member of β-crystallin family βA3/A1 crystallin, impairs phagosome degradation and results in a defect in autophagy in the RPE [44, 45].
Endoplasmic Reticulum (ER) Stress
ER is known as the cell's protein factory and is involved in the biosynthesis, post-translational modifications, folding and trafficking of proteins. The importance of ER stress and the unfolded protein response (UPR) in retinal degeneration has recently been reviewed [46]. A number of signaling pathways for UPR have been identified among which the major ones are IRE1, PERK and ATF6 pathways. While there is no direct evidence suggesting that ER stress is linked to AMD, the relationship between ER stress and inflammation, oxidative stress, apoptosis and angiogenesis suggests a strong possibility. Among these, as mentioned earlier, oxidative stress is one of the primary causes of age-related RPE damage. Several studies have shown a role for UPR in controlling oxidative stress and cell survival in RPE cells [47, 48]. Pharmacological inhibition of ER stress by chemical chaperones attenuated apoptosis and cell death. Further, inhibition of the PERK-eiF2alpha-CHOP pathway also protected RPE cells from oxidative injury and cell death. Chen et al [48] suggested that XBP1 may function as a central coordinator of oxidative and ER stress and may help in cell survival. XBP1-KO mice developed significantly increased RPE apoptosis and RPE atrophy as well as increased photoreceptor loss and inflammation. In addition, overexpression of XBP1 alleviated apoptosis and cell death induced by oxidative stress in cultured cells. Recently, targeting of IRE1/XBP1 and ATF6 branches of the UPR was found to enhance vascular endothelial growth factor (VEGF) blockade to prevent retinal and choroidal neovascularization [49].
Our laboratory is interested in understanding the crosstalk between mitochondria and the ER in the RPE and the role played by αB crystallin in mediating this phenomenon. We found that RPE cells from αB crystallin KO mice, and human RPE cells transfected with αB crystallin siRNA were more vulnerable to ER stress induced by tunicamycin (Figure 3). Prolonged ER stress decreased levels of αB crystallin and exacerbated mitochondrial dysfunction [12]. Further, overexpression of αB crystallin protected RPE cells from ER-stress induced apoptosis by attenuating increases in Bax, CHOP, mitochondrial permeability transition and cleaved caspase 3. It is of interest that Mitra et al. [50] found recently that activation of αB crystallin acts as a molecular switch in modulating cardiomyocyte apoptosis by mitochondria or endoplasmic reticulum during cardiac hypertrophy and myocardial infarction.
Figure 3. Deficiency of αB crystallin leads to increased apoptosis from tunicamycin (TM) induced endoplasmic reticulum stress in RPE cells.
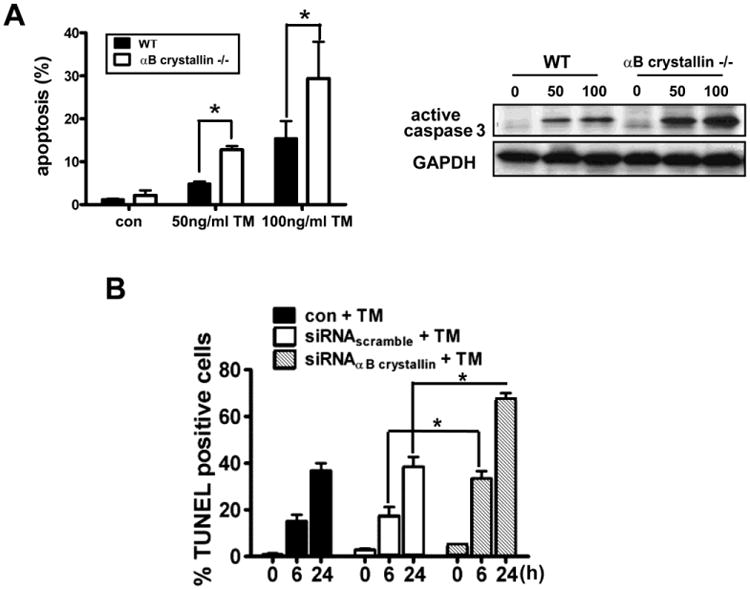
(A). RPE cells from αB crystallin knockout (KO) mice grown to confluence were treated with 50ng/ml and 100ng/ml TM for 24 h and stained for apoptosis by TUNEL (left) and caspase 3 (right panel). An increase in apoptosis and caspase 3 activation with increase in TM concentration was found. (B). Quantification of apoptotic cell death by confocal microscopy staining showed increased percentage of TUNEL positive cells in the αB crystallin siRNA group as a function of time with TM exposure. * denotes p<0.05. “Reprinted and modified from Dou et al. Free Radic Biol Med. 2012 Sep 1;53(5):1111-22, Copyright 2012), with permission from Elsevier.
Role of αB Crystallin in Angiogenesis as a VEGF Chaperone
An important area of extensive research is the interaction of αB crystallin with a wide variety of other proteins that include apoptosis related, cytoskeletal, signaling, β-amyloid associated proteins as well as several growth factors. These proteins as well as the nature of their interactions with αB crystallin have been summarized [2] and will not be elaborated here. We will focus on the interaction of αB crystallin with VEGF and regulation of angiogenesis. αB Crystallin expression predicted poor clinical outcome in breast cancer and was considered an oncoprotein [51]. It was reported that αB crystallin functions as a molecular chaperone to bind to and correct intracellular misfolded/unfolded proteins such as VEGF, preventing non-specific protein aggregations under the influence of the tumor microenvironment stress and/or anticancer treatments including bevacizumab therapy [52]. This observation is consistent with previous studies that reported the importance of promotion of tumor angiogenesis by αB crystallin by increasing vascular survival [53]. The action of αB crystalin in regulating vasculogenesis and angiogenesis is believed to be by multiple mechanisms and is dependent on the cell and tissue type. αB Crystallin acted as a chaperone for VEGF and other growth factors such as fibroblast growth factor-2 [54]. Our laboratory has utilized two murine models of intraocular disease for studying the effect of αB crystallin on angiogenesis and neovascularization namely OIR and laser-induced choroidal neovascularization (CNV) [4]. We found that α-crystallin KO resulted in attenuation of retinal neovascularization in OIR while prominent neovascularization was observed in the wild type mice. In the laser-induced CNV model, CNV lesion size was significantly reduced in αB crystallin KO mice. VEGF-A protein expression remained low in αB crystallin KO retina as compared to controls in which an eight fold increase in VEGF was found on days 3 and 7 after laser injury to Bruch membrane (Figure 4). We further found VEGF-A binding to αB crystallin by immunoprecipitation. Accordingly, αB crystallin KO RPE showed low VEGF-A secretion under serum-starved condition as compared to wild type cells. Our work also revealed that in these models locally deficient VEGF-A secretion led to a defective neovasculature with endothelial apoptosis. In in vitro studies, evidence for a prominent ubiquitination of VEGF in the cytoplasm in stressed (αB crystallin siRNA) cells was observed suggesting the involvement of αB crystallin in the ubiquitin/proteosome pathway. den Engelsman et al. [55] found that αB crystallin promoted FBX4-dependent ubiquitination in a phosphorylation and cell cycle dependent manner. It was later found that the FBX4-αB crystallin complex is an E3 ubiquitin ligase that promotes ubiquitin degradation of the 286-phosphorylated cyclin D1 [36].
Figure 4. Attenuation of laser-induced CNV in αB-crystallin knockout mice and changes in plasma VEGF levels.
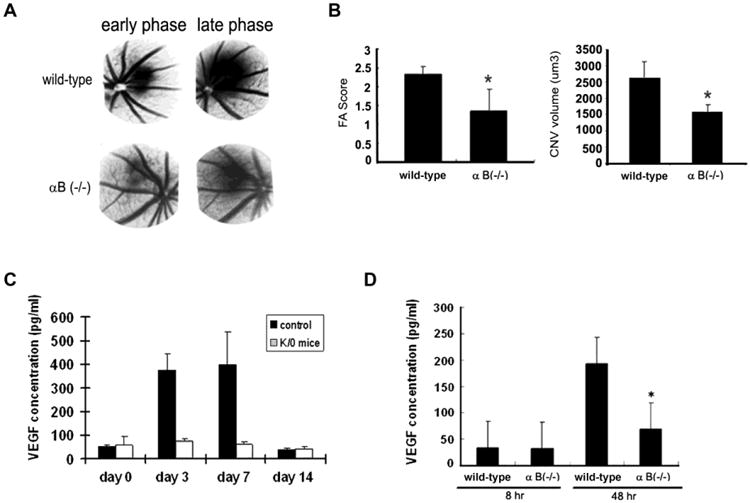
In panels A-B, CNV was induced by the use of laser photocoagulation and relative FA score and CNV volume measured 14 days after laser. (A) Representative FA in laser-induced CNV in wild-type and αB crystallin KO mice at day 14 after laser. (B) FA score and CNV volume were significantly reduced in αB crystallin KO mice compared with wild-type mice, respectively. In panel (C), VEGF-A concentrations (ELISA in pg/mL) in peripheral blood of wild-type and αB crystallin KO mice on day 0, 3, 7, and 14 after laser treatment are presented. In panel (D), VEGF concentrations in supernatants of cultured RPE cells exposed to serum-free medium for 48 hours are shown. VEGF concentrations are significantly lower in αB crystallin KO mice than that in wild-type mice. * represents P< 0.02; P<0 .05, P<0 .01, respectively. “This research was originally published in Blood, Kase et al. alphaB-crystallin regulation of angiogenesis by modulation of VEGF, Blood. 2010 115(16):3398-406. (c) The American Society of Hematology”.
Further research will be needed to fully understand the overall role of α-crystallins and the mechanism of angiogenesis in both physiological and pathological conditions. In a model of suture or chemical burn induced corneal neovascularization, Zhu et al. [57] reported that subconjuctival injection of αA crystallin significantly attenuated corneal neovascularization. The inhibition was found to be mediated by the expression of soluble VEGFR1. One very recent study reported the inhibition of ocular neovascularization by the knockout of αA crystallin [58]. The authors found both in vitro (HUVEC cells) and in vivo (αA crystallin KO), inhibition of angiogenesis which was mediated by the suppression of VEGF secretion and the inhibition of VEGFR2 signaling pathway. These studies suggest that α-crystallin could be a novel target for the prevention of ocular neovascularization.
αB Crystallin is Released from Cells via Exosomes
Most proteins targeted for release from cells are secreted by the canonical pathway, in which they are inserted co-translationally in to the ER, progress through the golgi apparatus and are released extracellularly [59,60]. However, all secretion pathways do not follow this route and non-conventional pathways via exosomes exist for release of proteins without signal sequences such as α-crystallins. Exosomes, are non-plasma-membrane-derived vesicles (50–100 nm in diameter), initially contained within the multivesicular bodies, and also present in body fluids such as cerebrospinal fluid, blood, urine, saliva, ascitic fluid and amniotic fluid [61-66]. Originally thought as a mechanism for the release of waste products from the cells, there are now convincing data demonstrating exosomes as important mediators of extracellular signaling [66]. Exosomes have a membrane consisting of a lipid bilayer and membrane proteins, which encloses the lumen-containing proteins and RNA molecules that are protected from extracellular degradation. α-Crystallins are synthesized in the cytosol and exported to extracellular space. This secretory process for αB crystallin is not blocked by typical inhibitors of the classical ER-Golgi protein secretory pathway, such as brefeldin or tunicamycin, demonstrating a pathway independent of the classical secretory route [11]. To test the hypothesis that αB crystallin could be released via non-classical pathway, we cultured primary RPE cells in exosome-free medium, and isolated and characterized exosomes from the media [11, 67]. Our studies revealed that αB crystallin localized to exosomes, which was further confirmed by immunoblot analysis (Figure 5A, B). Our laboratory could also demonstrate mRNA of αB crystallin in exosomes isolated from primary hRPE cells (Figure 5C). When RPE cells were treated with dimethyl amiloride (DMA) that blocks the exosome protein secretory pathway, DMA selectively inhibited the secretion of αB crystallin [11] suggesting that the stability and integrity of lipid rafts is required for efficient extracellular release. Another laboratory reported similar findings using ARPE-19 cells [68]. In addition, using highly polarized human RPE monolayers we provided evidence for preferential secretion of αB crystallin toward the apical side (Figure 5) corresponding to the photoreceptor facing neural retina which supported its neuroprotective function [11]. Further, we also localized αB crystallin in the interphotoreceptor matrix, suggesting its extracellular availability. To test the hypothesis that extracellular αB crystallin is internalized into photoreceptor cells, mouse explants were incubated with full length αB crystallin in the presence of oxidative stress. A significant uptake of full length recombinant αB crystallin by the outer and inner segments of photoreceptors under stressed conditions was found [11] strongly supporting our hypothesis of neuroprotection by extracellular αB crystallin.
Figure 5. Secretion of αB crystallin from human polarized RPE monolayers.
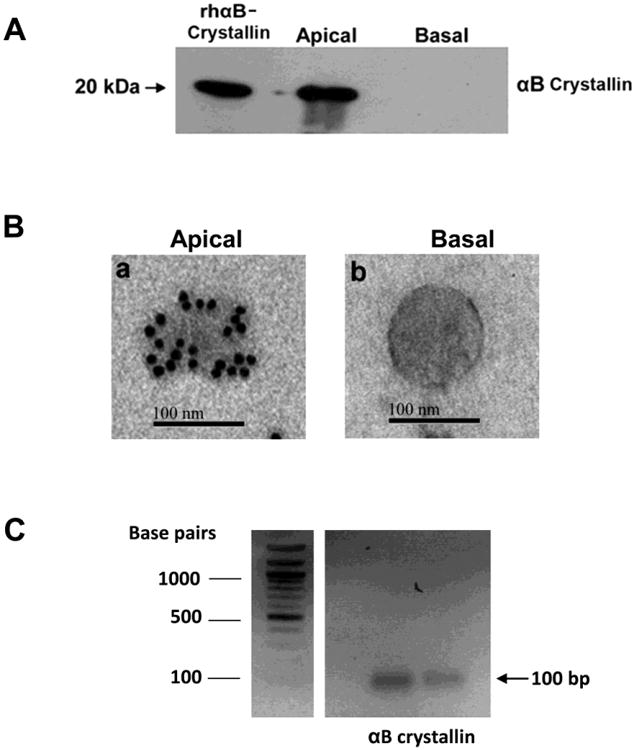
(A)Secretion of αB crystallin from human polarized RPE monolayers (mean TER 386 Ωcm2) was measured from apical and basolateral medium by Western blot analysis. αB Crystallin secretion was prominent from the apical medium and little or no secretion occurred from the basolateral medium. (B) Immunogold labeling of αB crystallin (15 nm gold particles) in the exosomes isolated from apical (panel a) and from basolateral medium (panel b). αB Crystallin was found predominantly in the apical medium. (C) αB Crystallin RNA in exosomes of RPE cells. Exosomes were isolated from human fetal RPE cells and RNA was extracted from isolated exosomes using a commercial Exosomal RNA and Protein Extraction kit. One microgram of RNA was reverse transcribed and the first-strand cDNA was amplified using a specific primer for αB crystallin. Figure 5A and 5B are reproduced with permission under the terms of the Creative Commons Attribution License and modified from Sreekumar et al. PLoS One. 2010 Oct 8;5(10):e12578.
Extracellular Vesicles for Drug Delivery
One of the major breakthroughs in this field of exosome research came in 2007 with the discovery of the presence of mRNA and microRNA (miRNA) inside exosomes [69]. Exosomes represent a novel reservoir for biomarker discovery because they contain protein, messenger RNA and microRNA that have been demonstrated to change with the disease state [70-72]. Further, exosomes are important conveyers of information between cells, through the transmission of various proteins, bioactive lipids and genetic information to alter the phenotype and function of recipient cells. One of the most exciting features of exosomes is their potential ability to transmit different RNA species between cells. Valadi et al. [69] provided evidence in vitro using high amounts of concentrated exosomes, that some mRNA present in exosomes could be translated into proteins in target cells, suggesting that exosomes can transfer genetic information. As described earlier, our studies revealed the presence of mRNA for αB crystallin in exosomes isolated from RPE cells (Figure 5C). The fact that extracellular vesicles are secreted by most cells, are rich in RNAs and are able to transfer their RNA content to recipient cells indicates that they may represent highly suitable candidates for drug delivery. Therefore, detailed studies on exosomes and exosomes circulating in blood and their possible regulation of α-crystallins are needed in order to assess their potential as diagnostic biomarkers of different neurodegenerative disorders. Given that exosomes harbor αB crystallin RNA and protein, as a next step, we would like to explore the therapeutic potential of exosomes, especially exosomes from polarized RPE cells in different models of retinal degenerative disorders. The significance of this study is that unlike other vectors which typically have the inherent risks of inducing immune activation owing to their foreign nature and systemic toxicity; extracellular vesicle-mediated delivery offers several advantages: these vesicles are biocompatible, immunologically inert provided they are derived from appropriate cells, can be patient-derived if needed and have capability to cross major biological barriers including the blood–brain barrier (BBB) [73]. Clinical trials are ongoing in different countries on the therapeutic potential of exosomes derived from multiple cells/tissues for different diseases from cancer to Type-1 diabetes mellitus (https://clinicaltrials.gov/).
α-Crystallin and its Constitutive Peptides as Therapeutic Molecules
Multiple studies have shown that endogenous α-crystallins are upregulated in neurodegenerative disorders, with some studies demonstrating the lack of α-crystallins leads to severe progression of disease, implying a protective role for these molecules. The antiapoptotic and/or anti-inflammatory effects of recombinant human αA or αB crystallin were successfully demonstrated in several animal disease models such as experimental cataracts, experimental autoimmune encephalomyelitis (EAE), stroke, cardiac ischemia-reperfusion, optic neuropathy, experimental autoimmune uveitis (EAU), and retinal degeneration [74-79]. Work from the laboratories of Sharma and Clark have identified several short chain peptides of αA and αB crystallin that elicit chaperone properties [80-82]. In recent studies, we and others used either a 19-mer peptide derived from αA or a 20-mer peptide from αB crystallin [74,79,83,84] and showed that these peptides possess chaperone and antiapoptotic properties.
Silencing of α-crystallin increases sensitivity of oxidative stress-induced cell death while its overexpression protects [7,12,13,26,29,31,85,86,87]. We showed that intravitreal cobalt chloride injection [32] or intravenous NaIO3 administration [88] to αB crystallin KO mice resulted in more severe retinal degeneration compared to wild-type animals, suggesting a protective role for the protein. Studies using αA-and/or αB crystallin KO mice have revealed increased retinal cell death in endophthalmitis and uveitis models, suggesting that α-crystallin prevents retinal cell death during inflammation [78,89]. As described earlier, absence of αB crystallin leads to significant attenuation of angiogenesis via modulation of VEGF in models of intraocular diseases [4]. In a recent study, Muraleva et al. [90] demonstrated that senescence-accelerated OXYS rat model of dry AMD develop spontaneous retinopathy because of decreased expression of αB crystallin. These above findings support the conclusion that α-crystallins may be effective targets for disease therapy.
With respect to the role of α-crystallins in mediating inflammatory response, Masilamoni et al.[91] were the first to show that purified bovine α-crystallin (αA and αB crystallin) pretreatment effectively diminished systemic inflammation induced by silver nitrate administration in mice. Subsequently, several studies with recombinant αB crystallin demonstrated its therapeutic role in different neurological animal models. Exogenous intravenous administration of human recombinant αB crystallin resulted in significant reduction of the symptoms of EAE both in αB crystallin KO and wild-type mice [75]. The same group demonstrated that αB crystallin was effective in reducing lesion size in a murine model of stroke [84]. Others have reported reduced inflammation, oxidative stress and improved heart function in a model of myocarditis [92] and increased oligodendroglial survival in the optic nerve in a model of retinal ischemia [77]. It is of interest that in EAE, the anti-inflammatory property of αB crystallin was not found to be due to influencing the adaptive immune response directly, but by binding and the subsequent modulation of the pro-inflammatory mediators in plasma [93]. In addition, intravitreal delivery of recombinant adenovirus expressing αA crystallin has been shown to protect against vascular leakage and effectively prevents pericyte loss and BRB breakdown in an experimental diabetes model [94]. Exogenous administration of αA crystallin attenuated corneal neovascularization in mouse models potentially by increasing the expression of soluble VEGFR1 [57]. Intravenous administration of recombinant αA crystallin to mice with experimental autoimmune uveoretinitis protected photoreceptors suggesting the potential for therapeutic application of αA crystallin in uveitis [78].
For a long time, one of the major aims of medicine has been to use peptides as drugs, as they mimic a variety of endogenous agents leading to their high selectivity of binding on specific targets. Peptides are involved in modulating various cell functions. These peptides could be more effective than recombinant proteins as potential drugs because of the following features: (1) smaller size, (2) easy to synthesize, optimize and evaluate, (3) no adverse immune responses, (4) easily enter the cells, and (5) perform the same functions [95]. We showed recently that a19-mer peptide from αA crystallin (DFVIFLDVKHFSPEDLTVK) and a 20-mer peptide from αB crystallin (DRFSVNLDVKHFSPEELKVK) exhibited antiapoptotic properties in primary human RPE cells [83] (Figure 6A, B). Both the αA and αB crystallin mini-chaperone peptides protected RPE from H2O2-induced cell death and inhibited caspase-3 activation. Further, unlike native αB crystallin, αB crystallin mini-chaperones exhibited prominent uptake by two related sodium-dependent oligopeptide transporters and showed time-dependent nuclear localization [83]. Independently another laboratory used the same peptide sequences of αA and αB crystallin, and demonstrated antiapoptotic properties of these peptides in lens epithelial cells [74]. The authors further provided evidence that αA-acetyl peptide was more effective than native peptide in protecting cells from oxidative stress-induced cell death. Intraperitoneal injection of the peptides inhibited cataract development in selenite-treated rats, which was accompanied by inhibition of oxidative stress, protein insolubilization, and caspase activity in the lens [74]. A selective region, corresponding to residues 73-92 of αB crystallin, was an effective therapeutic reversing paralysis in EAE [84]. A direct in vivo application of αA mini-chaperone peptide was reported in a recent study of NaIO3-induced retinal degeneration model [79] where intravitreal injection of the αA crystallin peptide rescued RPE degeneration by inhibiting apoptosis and autophagy.
Figure 6. Dose-dependent protection by αB crystall in-derived mini-chaperone or PCL–αB crystallin mini-chaperone from stressed hfRPE cells.
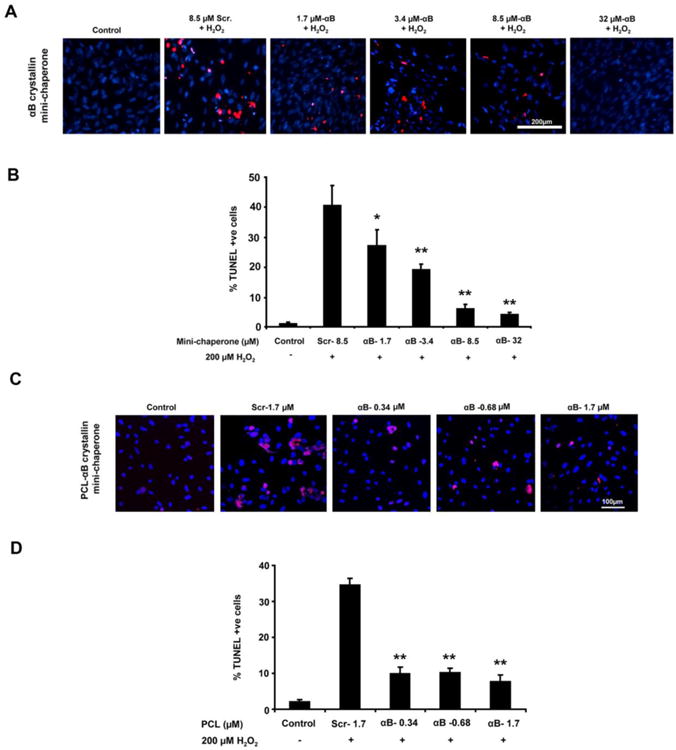
hfRPE cells were coincubated with varying doses of αB crystallin mini-chaperone and 200 μM H2O2 (A,B) or polycaprolactone (PCL)–αB crystallin mini-chaperone (C,D) and 200 μM H2O2 for 24 hours, and cell death was assessed by TUNEL assay. Confocal images of TUNEL-positive cells (red) and nuclei (blue) are shown. (B, D) Quantification of TUNEL-positive cells. Protection from cell death occurred with a much lower dose of PCL encapsulated αB crystallin minipeptide as compared to free αB crystallin minipeptide. Data are presented as percent of TUNEL-positive cells. *P < 0.05, **P < 0.01. Reproduced with permission and modified from Sreekumar et al. Invest Ophthalmol Vis Sci. 2013 Apr 17;54(4):2787-98. Copyright 2013 the Association for Research in Vision and Ophthalmology, Inc.
Peptides are readily degraded inside the human body, and thus are not ideally suited for drug development. Therefore, successful and efficient delivery of therapeutic molecules has required the development of suitable carrier systems which could allow longer retention of the peptide in bioactive form at the target area without displaying undesired immune responses. Different techniques have been developed for stabilizing the protein drugs using carriers whether in entrapped form, encapsulated in a semipermeable membrane, covalently bonded to a carrier or adsorbed to the carrier. As stated earlier, in the case of α-crystallin, specific regions within the parent proteins have similar chaperone, anti-apoptotic properties and antifibrilogenic functions [81-83]. We showed that polycaprolactone (PCL) nanoparticles loaded with either αA- or αB minichaperone peptide protected primary RPE cells from oxidative stress-induced cell death and more effectively, about 4-fold greater than nonencapsulated αB crystallin mini-chaperone peptide for the same doses (Figure 6). A dose-dependent reduction in TUNEL positive cells was found in αB minipeptide containing PCL particle compared to PCL particle alone (Figure 6). In another study, poly (lactic-co-glycolic acid) (PLGA) nanoparticles containing superoxide dismutase effectively prevented H2O2-induced neuronal cell death when compared to superoxide dismutase alone [96]. An emerging and promising method to bioengineer peptides with potent biological activity is to fuse them to protein polymers. Protein polymers can provide a platform for controlling release, multivalency, molecular weight, phase behavior, and even nanoparticle assembly [97-100]. In our recent work, one class of protein polymers known as elastin-like polypeptides (ELPs) and the αB crystallin peptide were recombinantly fused with two high molecular weight (∼40kDa) protein polymers [101]. These two ELP fusion proteins, cryS96 and crySI, retained chaperone activity and protected RPE cells from cell death, as indicated by reduced caspase 3 activation (Figure 7). Further, similar to the free mini-chaperone peptide, H2O2-induced stress markedly enhanced cellular uptake and nuclear localization of both cryS96 and crySI ELPs. Our ongoing work focuses on the study of the half life of these engineered drugs in vivo and the mechanism of uptake and efficiency in protecting retinal degeneration in different animal models. Further information on the in vivo toxicity, role in retinal neovascularization, dosage regimens, routes of injection, and assessing the optimal time of pre-treatment and post-treatment would prove to be of value in the use of α-crystallin minichaperone peptides in ocular pathology.
Figure 7. ELP fusion proteins of αB crystallin minipeptide protect RPE cells from H2O2-induced cell death.
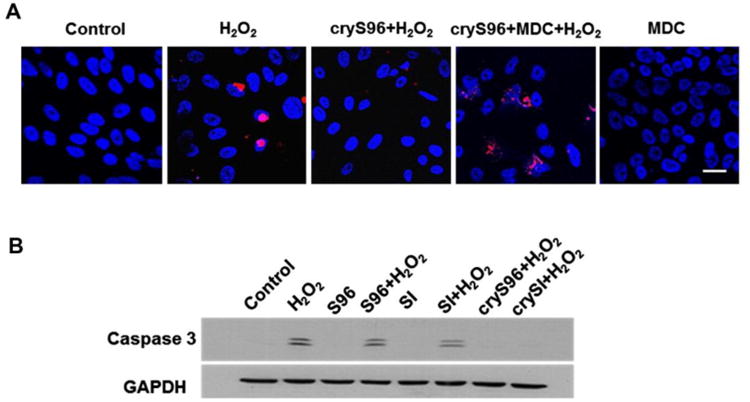
(A) Uptake and nuclear translocation is critical for elastin-like polypeptides (ELP)-αB crystallin peptide (cryS96) anti-apoptotic activity. Cells were pre-incubated with 100 μM monodansylcadaverine (MDC) for 30 min followed by co-incubation with 5 μM cryS96 for 24 h along with 200 μM H2O2. Control cells represent no treatment with either MDC or H2O2. Representative TUNEL images showing MDC significantly reduced the anti-apoptosis efficacy of cryS96 under H2O2 stress. Red: TUNEL + cells (apoptosis); blue: DAPI. Scale bar: 20 μm. (B) Representative western blot showing exogenous crySI and cryS96 protected RPE cells from oxidative stress by inhibiting activation of caspase 3. Caspase 3 activation was prominent in control cells treated with 200 μM H2O2. Unmodified ELPs SI and S96 failed to protect against caspase 3 activation. Reprinted and modified from Wang et al. J Control Release. 2014 Oct 10;191:4-14; copyright (2014) with permission from Elsevier.
Future Perspectives
Remarkable advances have been made in elucidating the function of α-crystallins in the retina and RPE in the past few years. One important aspect of αB crystallin action that is of significant interest is its possible extracellular function. Our recent discovery that human RPE cells secrete αB crystallin via exosomes is relevant in this regard. Since our studies showed that the secretion was predominantly apical and thus could provide protection of neighboring RPE and photoreceptor cells this mechanism is likely to be important for retinal protection under pathological states. Whether exosomal secretion is selective to RPE or whether other retinal cell types possess this property remains to be determined. At any rate, detailed evaluation of αB crystallin release from RPE models of retinal injury and degeneration will be of value. Further, it is not known whether αB crystallin participates in targeting of exosomal content; this is an important question that remains to be answered. Micro RNAs are also known to be secreted by exosomes and how this process is regulated and the exact role of αB crystallin in microRNA secretion and vice versa needs to be addressed.
Several reports including the work from our lab have shown a definitive role for αB crystallin in endothelial cell survival and in retinal and choroidal angiogenesis. In addition to its binding to VEGF, whether αB crystallin interacts with pro- and anti-angiogenic factors in the RPE would be of interest to study. Being a chaperone, αB crystallin may elicit additional effects on the phenotype of endothelial cells such as in the modulation of cytoskeletal rearrangement, ubiquitination of proteins and in growth factor signaling. Targeted inhibition of αB crystallin function could be considered as a novel therapeutic approach for pathologic angiogenesis; indeed, a potent small molecule has been identified that inhibits the interaction between αB crystallin and VEGF [52].
While the therapeutic role of α-crystallins in various human diseases has received attention [reviewed in 102], its therapeutic potential for retinal degeneration is only beginning to emerge. In this context, our finding that minichaperone peptides of α-crystallins have antiapoptotic activities in RPE cells that are more potent than the parent proteins suggests that delivery of these short chain minichaperones could serve a beneficial effect to injured RPE and the retina. Efficient modes of delivery of mini α-crystallins in encapsulated particles that are non-toxic and have easier penetration need to be devised. The beneficial effects of such particles in in vivo models of retinal degeneration would prove useful. Further, whether mechanisms of protection by mini α-crystallins stem from their direct effect on the retina or from upregulation of antioxidative enzymes such as SOD or catalase need to be investigated. Our work showed that αB crystallin overexpression elevates cellular GSH, particularly in the mitochondrial compartment, and the fact that αB crystallin is found prominently expressed in the mitochondria of RPE, would indicate that targeting mitochondria in drug and peptide delivery to boost its antioxidative status would prove to be a useful strategy to alleviate pathological conditions of RPE and the retina. In conclusion, better modalities for delivery of α-crystallin derived minichaperone peptides to the posterior segment of the eye is a fertile area for future research that is likely to enhance the utility of these interesting proteins in the prevention of retinal diseases.
Highlights.
α-Crystallin is regulated in retinal development and in oxygen-induced retinopathy
α-Crystallins protect RPE cells from oxidative and ER stress, and autophagy
αB Crystallin regulates angiogenesis by modulation of VEGF
αB Crystallin is secreted via exosomes in RPE
αB crystallin peptides may have therapeutic potential in retinal disease
Acknowledgments
We apologize to researchers in the field whose work could not be cited due to space constraints. This work was supported by Grants EY03040 and EY01545 from the National Eye Institute; and funds from Research to Prevent Blindness, and the Arnold and Mabel Beckman Foundation. We are thankful to Dr. Satoru Kase for generating the data used in Figure 1 and to Ernesto Barron for help with preparation of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Kannan R, Sreekumar PG, Hinton DR. Novel roles for α-crystallins in retinal function and disease. Prog Retin Eye Res. 2012;31:576–604. doi: 10.1016/j.preteyeres.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Ding J, Bowes Rickman C, Boulton M. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 2010. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson ML, Overbeek PA. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 2006;37:2276–2284. [PubMed] [Google Scholar]
- 6.Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol Vis. 2003;9:410–9. [PubMed] [Google Scholar]
- 7.Yaung J, Jin M, Barron E, Spee C, Wawrousek EF, Kannan R, Hinton DR. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis. 2007;13:566–577. [PMC free article] [PubMed] [Google Scholar]
- 8.Rao NA, Saraswathy S, Wu GS, Katselis GS, Wawrousek EF, Bhat S. Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis. Invest Ophthalmol Vis Sci. 2008;49:1161–1171. doi: 10.1167/iovs.07-1259. [DOI] [PubMed] [Google Scholar]
- 9.Reddy VS, Raghu G, Reddy SS, Pasupuleti AK, Suryanarayana P, Reddy GB. Response of small heat shock proteins in diabetic rat retina. Invest Ophthalmol Vis Sci. 2013;54:7674–7682. doi: 10.1167/iovs.13-12715. [DOI] [PubMed] [Google Scholar]
- 10.De S, Rabin DM, Salero E, Lederman PL, Temple S, Stern JH. Human retinal pigment epithelium cell changes and expression of alphaB-crystallin: a biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch Ophthalmol. 2007;125:641–645. doi: 10.1001/archopht.125.5.641. [DOI] [PubMed] [Google Scholar]
- 11.Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, Hinton DR. Deficiency of αB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med. 2012;53:1111–1122. doi: 10.1016/j.freeradbiomed.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong WJ, Rho JH, Yoon YG, Yoo SH, Jeong NY, Ryu WY, Ahn HB, Park WC, Rho SH, Yoon HS, Choi YH, Yoo YH. Cytoplasmic and nuclear anti-apoptotic roles of αB-crystallin in retinal pigment epithelial cells. PLoS One. 2012;7:e45754. doi: 10.1371/journal.pone.0045754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobb BA, Petrash JM. Characterization of alpha-crystallin-plasma membrane binding. J Biol Chem. 2000;275:6664–6672. doi: 10.1074/jbc.275.9.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deretic D, Aebersold RH, Morrison HD, Papermaster DS. Alpha A- and alpha B-crystallin in the retina. Association with the post-Golgi compartment of frog retinal photoreceptors. J Biol Chem. 1994;269:16853–16861. [PubMed] [Google Scholar]
- 16.Gangalum RK, Schibler MJ, Bhat SP. Small heat shock protein alphaB-crystallin is part of cell cycle-dependent Golgi reorganization. J Biol Chem. 2004;279:43374–43377. doi: 10.1074/jbc.C400371200. [DOI] [PubMed] [Google Scholar]
- 17.Jin JK, Whittaker R, Glassy MS, Barlow SB, Gottlieb RA, Glembotski CC. Localization of phosphorylated alphaB-crystallin to heart mitochondria during ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H337–344. doi: 10.1152/ajpheart.00881.2007. [DOI] [PubMed] [Google Scholar]
- 18.McGreal RS, Kantorow WL, Chauss DC, Wei J, Brennan LA, Kantorow M. αB-crystallin/sHSP protects cytochrome c and mitochondrial function against oxidative stress in lens and retinal cells. Biochim Biophys Acta. 2012;1820:921–30. doi: 10.1016/j.bbagen.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang B, Xiao W, Shi Y, Liu M, Xiao X. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones. 2005;10:252–262. doi: 10.1379/CSC-124R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Li J, Tao Y, Xiao X. Small heat shock protein alphaB crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun. 2007;354:109–114. doi: 10.1016/j.bbrc.2006.12.152. [DOI] [PubMed] [Google Scholar]
- 21.Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, Reed JC. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005;16:4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai F, Xi JH, Wawrousek EF, Fleming TP, Andley UP. Hyperproliferation and p53 status of lens epithelial cells derived from alphaB-crystallin knockout mice. J Biol Chem. 2003;278:36876–36886. doi: 10.1074/jbc.M304010200. [DOI] [PubMed] [Google Scholar]
- 23.van den IJssel P, Wheelock R, Prescott A, Russell P, Quinlan RA. Nuclear speckle localisation of the small heat shock protein alpha B-crystallin and its inhibition by the R120G cardiomyopathy-linked mutation. Exp Cell Res. 2003;287:249–261. doi: 10.1016/s0014-4827(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 24.van Rijk AE, Stege GJ, Bennink EJ, May A, Bloemendal H. Nuclear staining for the small heat shock protein alphaB-crystallin colocalizes with splicing factor SC35. Eur J Cell Biol. 2003;82:361–368. doi: 10.1078/0171-9335-00321. [DOI] [PubMed] [Google Scholar]
- 25.Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, Mohan Rao Ch. Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell Res. 2004;299:393–403. doi: 10.1016/j.yexcr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Noh SJ, Jeong WJ, Rho JH, Shin DM, Ahn HB, Park WC, Rho SH, Soung YH, Kim TH, Park BS, Yoo YH. Sensitization of RPE cells by alphaB-crystallin siRNA to SAHA-induced stage 1 apoptosis through abolishing the association of alphaB-crystallin with HDAC1 in SC35 speckles. Invest Ophthalmol Vis Sci. 2008;49:4753–4759. doi: 10.1167/iovs.08-2166. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Kamei K, Iwamoto I, Inaguma Y, Nohara D, Kato K. Phosphorylation-induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- 28.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 30.Mehlen P, Preville X, Chareyron P, Briolay J, Klemenz R, Arrigo AP. Constitutive expression of human hsp27, Drosophila hsp27, or human alpha B-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- 31.Sreekumar PG, Spee C, Ryan SJ, Cole SP, Kannan R, Hinton DR. Mechanism of RPE cell death in α-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux. PLoS One. 2012;7:e33420. doi: 10.1371/journal.pone.0033420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaung J, Kannan R, Wawrousek EF, Spee C, Sreekumar PG, Hinton DR. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp Eye Res. 2008;86:355–365. doi: 10.1016/j.exer.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. [PubMed] [Google Scholar]
- 34.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Punzo C, Kornacker K, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piras A, Gianetto D, Conte D, Bosone A, Vercelli A. Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure. PLoS One. 2011;6:e22514. doi: 10.1371/journal.pone.0022514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch's membrane-choroid complex from AMD and age-matched donor eyes. Exp Eye Res. 2005;80:821–826. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009;5:563–564. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- 41.Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Veréb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pattison JS, Robbins J. Autophagy and proteotoxicity in cardiomyocytes. Autophagy. 2011;7:1259–1260. doi: 10.4161/auto.7.10.16882. [DOI] [PubMed] [Google Scholar]
- 43.Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–160. doi: 10.1161/CIRCRESAHA.110.237339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zigler JS, Jr, Zhang C, Grebe R, Sehrawat G, Hackler L, Jr, Adhya S, Hose S, Mc Leod DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the βA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J Cell Sci. 2011;124:523–531. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Jr, Sinha D. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang SX, Sanders E, Fliesler SJ, Wang JJ. Endoplasmic reticulum stress and the misfolded protein response in retinal degeneration. Exp Eye Res. 2014;25:30–40. doi: 10.1016/j.exer.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong Y, Li J, Wang JJ, Chen C, Tran JT, Saadi A, Yu Q, Le YZ, Mandal MN, Anderson RE, Zhang SX. X-box binding protein 1 is essential for the anti-oxidant defense and cell survival in the retinal pigment epithelium. PLoS One. 2012;7:e38616. doi: 10.1371/journal.pone.0038616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C, Cano M, Wang JJ, Li J, Huang C, Yu Q, Herbert TP, Handa JT, Zhang SX. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signal. 2014;20:2091–2106. doi: 10.1089/ars.2013.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L, Qi X, Chen Z, Shaw L, Cai J, Smith LH, Grant MB, Boulton ME. Targeting the IRE1α/XBP1 and ATF6 arms of the unfolded protein response enhances VEGF blockade to prevent retinal and choroidal neovascularization. Am J Pathol. 2013;182:1412–1424. doi: 10.1016/j.ajpath.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitra A, Basak T, Datta K, Naskar S, Sengupta S, Sarkar S. Role of α-crystallin B as a regulatory switch in modulating cardiomyocyte apoptosis by mitochondria or endoplasmic reticulum during cardiac hypertrophy and myocardial infarction. Cell Death Dis. 2013;4:e582. doi: 10.1038/cddis.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Z, Ruan Q, Han S, Xi L, Jiang W, Jiang H, Ostrov DA, Cai J. Discovery of structure-based small molecular inhibitor of αB-crystallin against basal-like/triple-negative breastcancer development in vitro and in vivo. Breast Cancer Res Treat. 2014;145:45–59. doi: 10.1007/s10549-014-2940-8. [DOI] [PubMed] [Google Scholar]
- 53.Dimberg A, Rylova S, Dieterich LC, Olsson AK, Schiller P, Wikner C, Bohman S, Botling J, Lukinius A, Wawrousek EF, Claesson-Welsh L. alphaB-crystallin promotes tumor angiogenesis by increasing vascular survival during tube morphogenesis. Blood. 2008;111:2015–2023. doi: 10.1182/blood-2007-04-087841. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh JC, Shenoy AK, Jr, Clark JI. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry. 2007;46:6308–6317. doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- 55.den Engelsman J, Keijsers V, de Jong WW, Boelens WC. The small heat-shock protein_alpha B-crystallin_promotes FBX4-dependent ubiquitination. J Biol Chem 2003. 2003;278:4699–4704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- 56.Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, Rustgi A, Fuchs SY, Diehl JA. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu W, Qi X, Ren S, Jia C, Song Z, Wang Y. αA-crystallin in the pathogenesis and intervention of experimental murine corneal neovascularization. Exp Eye Res. 2012;98:44–51. doi: 10.1016/j.exer.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Xu Q, Bai Y, Huang L, Zhou P, Yu W, Zhao M. Knockout of αA-Crystallin Inhibits Ocular Neovascularization. Invest Ophthalmol Vis Sci. 2015;56:816–826. doi: 10.1167/iovs.14-14734. [DOI] [PubMed] [Google Scholar]
- 59.Halban PA, Irminger JC. Sorting and processing of secretory proteins. Biochem J. 1994;299:1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanley AC, Lacy P. Pathways for cytokine secretion. Physiology (Bethesda) 2010;25:218–229. doi: 10.1152/physiol.00017.2010. [DOI] [PubMed] [Google Scholar]
- 61.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 62.Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5. doi: 10.1186/1479-5876-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao H, Wong DT. Proteomic analysis of microvesicles in human saliva by gel electrophoresis with liquid chromatography-mass spectrometry. Anal Chim Acta. 2012;723:61–67. doi: 10.1016/j.aca.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Alvarez ML, Khosroheidari M, Kanchi Ravi R, Di Stefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- 65.Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, Marmé F, Umansky L, Umansky V, Eigenbrod T, Sammar M, Altevogt P. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691–36702. doi: 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lässer C. Exosomes in diagnostic and therapeutic applications: biomarker, vaccine and RNA interference delivery vehicle. Expert Opin Biol Ther. 2015;15:103–117. doi: 10.1517/14712598.2015.977250. [DOI] [PubMed] [Google Scholar]
- 67.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 68.Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286:3261–3219. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 70.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 71.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou H, Cheruvanky A, Hu X, Matsumoto T, Hiramatsu N, Cho ME, Berger A, Leelahavanichkul A, Doi K, Chawla LS, Illei GG, Kopp JB, Balow JE, Austin HA, Yuen PS, Star RA. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74:613–621. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El Andaloussi S, Lakhal S, Mäger I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH. Chaperone peptides of α-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts. J Biol Chem. 2013;288:13022–13035. doi: 10.1074/jbc.M112.440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O'Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 76.Velotta JB, Kimura N, Chang SH, Chung J, Itoh S, Rothbard J, Yang PCL, Robbins RC, Fischbein MP. αB-crystallin improves murine cardiac function and attenuates apoptosis in human endothelial cells exposed to ischemia-reperfusion. Ann Thorac Surg. 2011;91:1907–913. doi: 10.1016/j.athoracsur.2011.02.072. [DOI] [PubMed] [Google Scholar]
- 77.Pangratz-Fuehrer S, Kaur K, Ousman SS, Steinman L, Liao YJ. Functional rescue of experimental ischemic optic neuropathy with αB-crystallin. Eye (Lond) 2011;25:809–817. doi: 10.1038/eye.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao NA, Saraswathy S, Pararajasegaram G, Bhat SP. Small heat shock protein αA-crystallin prevents photoreceptor degeneration in experimental autoimmune uveitis. PLoS One. 2012;7:e33582. doi: 10.1371/journal.pone.0033582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Zhao X, Cai Y, Li Y, Yu X, Lu L. Protection of Retina by Mini-αA in NaIO3-Induced Retinal Pigment Epithelium Degeneration Mice. Int J Mol Sci. 2015;16:1644–656. doi: 10.3390/ijms16011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 81.Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-αB-crystallin: a functional element of αB-crystallin with chaperone-like activity. Biochemistry. 2006;45:3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghosh JG, Estrada MR, Clark JI. Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry. 2005;44:14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- 83.Sreekumar PG, Chothe P, Sharma KK, Baid R, Kompella U, Spee C, Kannan N, Manh C, Ryan SJ, Ganapathy V, Kannan R, Hinton DR. Antiapoptotic properties of α-crystallin-derived peptide chaperones and characterization of their uptake transporters in human RPE cells. Invest Ophthalmol Vis Sci. 2013;54:2787–2798. doi: 10.1167/iovs.12-11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurnellas MP, Brownell SE, Su L, Malkovskiy AV, Rajadas J, Dolganov G, Chopra S, Schoolnik GK, Sobel RA, Webster J, Ousman SS, Becker RA, Steinman I, Rothbard JB. Chaperone activity of small heat shock proteins underlies therapeutic efficacy in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:36423–36434. doi: 10.1074/jbc.M112.371229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43:3575–3582. [PubMed] [Google Scholar]
- 86.Chis R, Sharma P, Bousette N, Miyake T, Wilson A, Backx PH, Gramolini AO. α-Crystallin B prevents apoptosis after H2O2 exposure in mouse neonatal cardiomyocytes. Am J Physiol Heart Circ Physiol. 2012;303:H967–978. doi: 10.1152/ajpheart.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu F, Yu H, Liu J, Cheng L. αB-crystallin regulates oxidative stress-induced apoptosis in cardiac H9c2 cells via the PI3K/AKT pathway. Mol Biol Rep. 2013;40:2517–2526. doi: 10.1007/s11033-012-2332-2. [DOI] [PubMed] [Google Scholar]
- 88.Zhou P, Kannan R, Spee C, Sreekumar PG, Dou G, Hinton DR. Protection of retina by αB crystallin in sodium iodate induced retinal degeneration. PLoS One. 2014;9:e98275. doi: 10.1371/journal.pone.0098275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whiston EA, Sugi N, Kamradt MC, Sack C, Heimer SR, Engelbert M, Wawrousek EF, Gilmore MS, Ksander BR, Gregory MS. alphaB-crystallin protects retinal tissue during Staphylococcus aureus-induced endophthalmitis. Infect Immun. 2008;76:1781–1790. doi: 10.1128/IAI.01285-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muraleva NA, Kozhevnikova OS, Zhdankina AA, Stefanov NA, Karamysheva TV, Fursova AZ, Kolosova NG. The mitochondria-targeted antioxidant SkQ1 restores αB-crystallin expression and protects against AMD-like retinopathy in OXYS rats. Cell Cycle. 2014;13:3499–3505. doi: 10.4161/15384101.2014.958393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masilamoni JG, Jesudason EP, Baben B, Jebaraj CE, Dhandayuthapani S, Jayakumar R. Molecular chaperone alpha-crystall in prevents detrimental effects of neuroinflammation. Biochim Biophys Acta. 2006;1762:284–293. doi: 10.1016/j.bbadis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Park H, Park H, Hwang HJ, Hwang HS, Kim H, Choi BR, Pak HN, Lee MH, Chung JH, Joung B. Alpha B-crystallin prevents ventricular arrhythmia by attenuating inflammation and oxidative stress in rat with autoimmune myocarditis. Int J Cardiol. 2014;182C:399–402. doi: 10.1016/j.ijcard.2014.12.152. [DOI] [PubMed] [Google Scholar]
- 93.Rothbard JB, Kurnellas MP, Brownell S, Adams CM, Su L, Axtell RC, Chen R, Fathman CG, Robinson WH, Steinman L. Therapeutic effects of systemic administration of chaperone αB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem. 2012;287:9708–97021. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim YH, Park SY, Park J, Kim YS, Hwang EM, Park JY, Roh GS, Kim HJ, Kang SS, Cho GJ, Choi WS. Reduction of experimental diabetic vascular leakage and pericyte apoptosis in mice by delivery of αA-crystallin with a recombinant adenovirus. Diabetologia. 2012;55:2835–2844. doi: 10.1007/s00125-012-2625-y. [DOI] [PubMed] [Google Scholar]
- 95.Vaishya R, Khurana V, Patel S, Mitra AK. Long-term delivery of protein therapeutics. Expert Opin Drug Deliv. 2015;12:415–440. doi: 10.1517/17425247.2015.961420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reddy MK, Wu L, Kou W, Ghorpade A, Labhasetwar V. Superoxide dismutase loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl Biochem Biotechnol. 2008;151:565–577. doi: 10.1007/s12010-008-8232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2001;46:125–148. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 98.Yang YY, Chung TS, Ng NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22:231–241. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 99.Stuart MA, Huck WT, Genzer J, Müller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 100.Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and applications. Adv Drug Deliv Rev. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 101.Wang W, Sreekumar PG, Valluripalli V, Shi P, Wang J, Lin YA, Cui H, Kannan R, Hinton DR, MacKay JA. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J Control Release. 2014;191:4–14. doi: 10.1016/j.jconrel.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reddy VS, Reddy GB. Emerging role for αB-crystallin as a therapeutic agent: pros and cons. Curr Mol Med. 2015;15:47–61. doi: 10.2174/1566524015666150114112853. [DOI] [PubMed] [Google Scholar]


