Abstract
During development, inhibitor of DNA binding (Id) proteins, a subclass of the helix-loop-helix family of proteins, regulate cellular proliferation, differentiation, and apoptosis in various organs. However, a functional role of Id2a in liver development has not yet been reported. Here, using zebrafish as a model organism, we provide in vivo evidence that Id2a regulates hepatoblast proliferation and cell death during liver development. Initially, in the liver, id2a is expressed in hepatoblasts and after their differentiation, id2a expression is restricted to biliary epithelial cells. id2a knockdown in zebrafish embryos had no effect on hepatoblast specification or hepatocyte differentiation. However, liver size was greatly reduced in id2a morpholino-injected embryos, indicative of a hepatic outgrowth defect attributable to the significant decrease in proliferating hepatoblasts concomitant with the significant increase in hepatoblast cell death. Altogether, these data support the role of Id2a as an important regulator of hepatic outgrowth via modulation of hepatoblast proliferation and survival during liver development in zebrafish.
Keywords: Inhibitor of DNA binding, differentiation, biliary epithelial cell, hepatoblast, liver specification, helix-loop-helix
1. Introduction
Liver organogenesis is a multifaceted process involving hepatoblast specification from the ventral foregut endoderm, budding and outgrowth of the liver bud, and hepatoblast differentiation into either hepatocytes or biliary epithelial cells (BECs) (Zaret, 2002; Lemaigre, 2003). In both mice (Jung et al., 1999; Rossi et al., 2001) and zebrafish (Shin et al., 2007; Chung et al., 2008), inductive signals of Fibroblast Growth Factors (FGFs) and Bone Morphogenetic Proteins (BMPs) are essential for hepatoblast specification. In conjunction with the BMP and FGF signaling pathways, several homeobox transcription factors, including HHEX and PROX1, also regulate the initial stages of liver organogenesis (Si-Tayeb et al., 2010). HHEX regulates hepatoblast proliferation and delamination from the foregut endoderm as Hhex−/− mice lack a liver bud and the hepatoblasts fail to migrate into the surrounding septum transversum mesenchyme (Bort et al., 2004). PROX1 also regulates hepatoblast delamination from the liver diverticulum as hepatoblasts fail to migrate in Prox1−/− mice (Sosa-Pineda et al., 2000). Hepatocyte metabolic gene expression is altered in favor of biliary gene expression when Prox1 is ablated in post-delaminated hepatoblasts (Seth et al., 2014). hhex (Wallace et al., 2001) and prox1a also regulate liver development in zebrafish. prox1a, specifically, marks the initiation of hepatoblast specification in zebrafish (Ober et al., 2006). Besides HHEX and PROX1, zebrafish and mammals share additional transcription factors critical for liver organogenesis, such as GATA6 and hepatic nuclear factors (HNFs) (Bossard and Zaret, 1998; Matthews et al., 2004; Holtzinger and Evans, 2005; Lokmane et al., 2008). However, a comprehensive understanding of the molecular mechanisms underlying transcriptional regulation during liver development still needs to be defined.
One family of transcriptional regulators essential in developmental processes, including cell lineage commitment, proliferation and differentiation, is the helix-loop-helix (HLH) family of transcription factors (Massari and Murre, 2000; Jones, 2004). The HLH domain, essential for dimerization, is important in the formation of homo- or hetero-dimers. While some HLH proteins are ubiquitously expressed (e.g., E proteins), other HLH proteins are tissue-specific (e.g., PTF1, HES1). HES1, in particular, downstream of Notch signaling, is essential for digestive system development, especially in extrahepatic bile duct development (Sumazaki et al., 2004). In Hes1−/− mice, no tubular structures form in the ductal plate during intrahepatic bile duct development (Kodama et al., 2004). In addition, the bHLH factor, heart and neural crest derivatives expressed 2 (Hand2), is expressed in tissues that surround the liver primordium, such as the lateral plate mesoderm in zebrafish, which later contributes to the hepatic stellate cells (Yin et al., 2012). Moreover, bHLH-PAS (Per-ARNT-Sim) factors, such as the hypoxia inducible factors (HIFs), participate in hepatic disease, regeneration, fibrosis, and hepatocellular carcinoma (Nath and Szabo, 2012). Hif2α (renamed as Epas1b) binds hypoxia response elements (HREs) and regulates hepatic outgrowth in zebrafish (Lin et al., 2014). The activity of bHLH factors can be regulated by the inhibitor of DNA binding (ID) family of proteins.
ID proteins lack the basic DNA binding domain and regulate HLH factors via heterodimerization and subsequent creation of nonfunctional, dominant negative complexes that lack DNA-binding capability (Norton, 2000). By heterodimerizing and sequestering ubiquitously expressed HLH factors, such as E-proteins (E47, E2-2, HEB, E12), or tissue-restricted HLH factors, ID proteins can thereby regulate cell proliferation, differentiation and apoptosis in a cell-context dependent manner (Sikder et al., 2003). In the pancreas, for instance, by binding and sequestering NeuroD, a bHLH factor implicated in pancreatic beta cell survival and differentiation, ID2 regulates pancreatic progenitor expansion (Hua et al., 2006). Non-bHLH factors can also bind and regulate ID protein function. For example, hypophosphorylated Retinoblastoma (Rb) tumor-suppressor protein interacts with ID2 during cell cycle arrest, preventing the latter from sequestering other transcription factors and consequently allows differentiation to occur (Iavarone et al., 1994; Lasorella et al., 2002). Mice with a genetic deletion of Id2 display a reduced number of natural killer cells, lack lymph nodes and experience 25% neonatal lethality (Yokota et al., 1999). To date, no study has examined the role of ID2 in hepatogenesis.
While the mammalian genome consists of four Id genes, Id1–4 (Lasorella et al., 2014), five id genes are present in the zebrafish genome: id1, id2a, id2b, id3 and id4. In the developing zebrafish liver, we observed that among the five id genes, only id2a is restrictively expressed in BECs. This unique expression pattern prompted us to investigate the function of id2a in liver development. Here, we show that initially, id2a is expressed in hepatoblasts and later, in BECs. In addition, using the morpholino knockdown approach, we show that Id2a regulates hepatic outgrowth, without affecting hepatoblast specification or differentiation, by modulating hepatoblast proliferation and survival.
2. Results
2.1. id2a expression in the developing liver
Using whole-mount in situ hybridization (WISH), we examined id2a expression during liver development in detail. We first detected id2a expression in the liver-forming region from 30 hours post fertilization (hpf) (Fig. 1A), when hepatoblast specification has already occurred. At this stage, the liver tissue consists of hepatoblasts, which are liver progenitor cells, capable of differentiating into either hepatocytes or BECs. Following hepatoblast differentiation, around 72 hpf, we noted that id2a expression displayed a branching pattern in the liver, indicative of the intrahepatic biliary network consisting of BECs. The BEC-specific expression was maintained even at 5 dpf (Fig. 1A). To confirm id2a expression in BECs, we conducted immunostaining in conjunction with WISH utilizing the Tg(prox1a:YFP), Tg(Tp1:GFP) and Tg(kdrl:GFP) lines, which express fluorescent proteins in hepatoblasts (Bussmann and Schulte-Merker, 2011), BECs (Parsons et al., 2009), and liver endothelial cells (Beis et al., 2005), respectively. As initially observed, id2a was specifically detected in prox1a:YFP-positive (Fig. 1B) and Tp1:GFP-positive cells (Fig. 1C), but not in the endothelial cells (Fig. 1D), indicating that in the liver, id2a is initially expressed in hepatoblasts and later restricted to BECs.
Figure 1. id2a expression in the developing liver.
(A) WISH reveals id2a expression in the liver-forming region at 30 hpf (arrow) and in the liver at 48 hpf (arrow), 72 hpf and 5 dpf (dotted lines). From 72 hpf to, at least, 5 dpf, id2a expression in the liver appears to be restricted to BECs. Arrowhead points to the interrenal primordium; bracket denotes the intestinal bulb. Dorsal (30–72 hpf) or lateral (5 dpf) views, anterior to the left. (B–D) id2a in situ hybridization (red) combined with anti-GFP immunostaining (green) in Tg(prox1a:YFP) (B), Tg(Tp1:GFP) (C), or Tg(kdrl:GFP) (D) embryos reveals id2a expression in hepatoblasts at 36 hpf and BECs at 60 and 72 hpf, but not in the endothelial cells of the liver (dotted line), respectively. Asterisks mark id2a expression in the intestinal bulb. Single confocal section (B) or projections of z-stack confocal sections (C, D). Scale bars: 100 (A), 20 (B– D) μm.
Since the zebrafish genome contains five id genes, we further investigated the expression patterns of the remaining four id genes, id1, id2b, id3, and id4, in the liver during embryonic development. At 30 hpf, id2b, id3, and id4 are not expressed in the liver-forming region; however, it was not clear whether id1 is expressed in the liver-forming region due to its broad expression (Fig. 2A). Double labeling of id1 and sox17:GFP, which labels all endodermal cells (Chung and Stainier, 2008), showed id1 expression in the liver-forming region (Fig. 2E; brackets). At 48 hpf, none of the four genes are expressed in the liver. At 72 hpf, id2b and id3, but not id1 or id4, are expressed in the liver (Fig. 2B and 2C; arrows); however, their expression does not mimic the biliary branching pattern of id2a expression. Altogether, these expression data indicate that both id1 and id2a are expressed in the liver-forming region at 30 hpf and that only id2a expression is restricted to BECs.
Figure 2. The expression patterns of id1, id2b, id3, and id4 during liver development in zebrafish.
(A–D) Wild-type embryos were processed for WISH analysis with id1 (A), id2b (B), id3 (C), and id4 (D) probes. id1 appears to be ubiquitously expressed at 30 hpf, but its expression is absent in the liver at 48 and 72 hpf. id2b and id3 expression in the liver was detected at 72 hpf (arrows), but not at 30 or 48 hpf. id4 is not expressed in the liver. Dorsal views, anterior to the left. (E) id1 in situ hybridization (red), combined with anti-GFP immunostaining (green), in Tg(sox17:GFP) embryos reveals id1 expression in the liver-forming region (brackets), but not in the dorsal pancreas (dotted lines) at 30 hpf. Single confocal optical section, ventral view, anterior up. Scale bars: 100 (A–D), 20 (E) μm.
2.2. id2a knockdown causes an intrahepatic biliary network defect in the developing liver
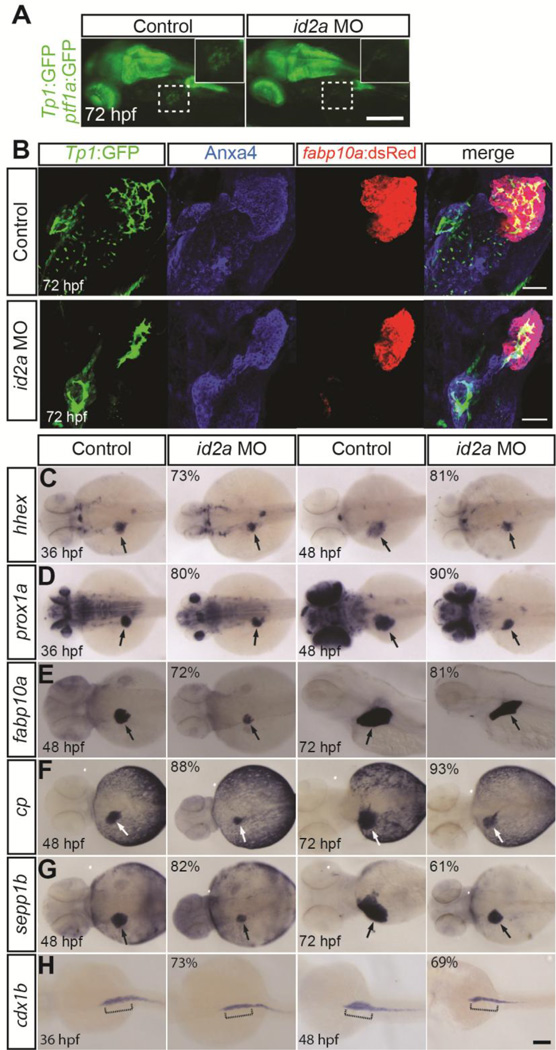
Given the restricted expression pattern of id2a in BECs, we sought to determine whether id2a is important for intrahepatic biliary development. We conducted loss-of-function analyses using published id2a morpholino oligonucleotides (MO) (Uribe and Gross, 2010; Das and Crump, 2012; Uribe et al., 2012). Consistent with previous reports, id2a MO-injected embryos were microcephalic and microphthalmic (Uribe and Gross, 2010), a phenotype also observed in Id2−/− mice (Yokota et al., 1999). Importantly, the small liver phenotype observed in id2a MO-injected embryos was partially rescued by id2a mRNA injection (Fig. S1A and S1B), further validating the id2a MO. Since id2a is expressed specifically in BECs at later stages of liver development, we used the Tg(Tp1:GFP) line to examine BECs. Using epifluorescence microscopy, we detected very few GFP-positive cells in the livers of id2a MO-injected embryos (Fig. 3A; squares), suggesting BEC number was greatly reduced. To further analyze the intrahepatic biliary structure, whole-mount immunostaining combined with confocal microscopy was used. In id2a MO-injected embryos, not only was the liver size reduced, but the intrahepatic biliary network failed to branch, appearing aggregated (Fig. 3B). Taken together, these data imply that lack of id2a results in defective biliary structure and reduced BEC number, suggesting that id2a may regulate intrahepatic biliary development.
Figure 3. id2a knockdown reduces liver size but does not block hepatoblast specification or hepatocyte differentiation.
(A) Epifluorescence images revealing a decreased number of Tp1:GFP+ BECs in the liver of id2a MO-injected embryos at 72 hpf compared with controls (squares). Higher magnification images of the square regions are shown in insets. Lateral view, anterior to the left. (B) Confocal projection images revealing fabp10a:dsRed (hepatocytes; red), Tp1:GFP (BECs; green) and Anxa4 (the hepatopancreatic ductal system; blue) expression (Zhang et al., 2014). In id2a MO-injected embryos, liver size was greatly reduced and intrahepatic BECs appeared aggregated, displaying a branching defect. (C–H) id2a MO-injected and uninjected control embryos were processed for WISH with hhex (C), prox1a (D), fabp10a (E), cp (F), sepp1b (G), and cdx1b (H) probes. Overall liver size was greatly reduced in id2a MO-injected embryos as revealed by the hepatoblast markers (hhex and prox1a) and the hepatocyte markers (fabp10a, cp, and sepp1b). However, the expression of these genes was clearly detected in the MO-injected embryos (C–G, arrows), indicating normal hepatoblast specification and hepatocyte differentiation upon id2a knockdown. The induction of the intestinal bulb as assessed by cdx1b expression appeared normal in id2a MO-injected embryos at 36 hpf; however, the intestinal bulb failed to grow at 48 hpf (H, brackets). The percentage of id2a MO-injected embryos exhibiting the representative phenotype shown is indicated in the upper left corner (n=10–20). The remaining percentage of embryos exhibited an intermediate liver/intestinal bulb phenotype: their liver/intestinal bulb size was still smaller than that of the control embryos. Arrows point to the liver. Scale bar: 250 (A), 20 (B) and 100 (C–H) μm.
2.3. id2a knockdown reduces liver size but does not block hepatoblast specification or hepatocyte differentiation
The main steps of liver development include hepatic competence, hepatoblast specification, hepatocyte or BEC differentiation, and hepatic outgrowth (Zaret, 2002). Since id2a is expressed in the liver-forming region from 30 hpf (Fig. 1A) after hepatoblast specification, which occurs around 22 hpf in zebrafish (Ober et al., 2006), it is unlikely that id2a is implicated in hepatic competence. Thus, we examined the expression of the following markers in id2a MO-injected embryos: the early hepatoblast markers, hhex and prox1a (Field et al., 2003; Ober et al., 2006), for hepatoblast specification and maintenance; and the hepatocyte markers, fabp10a, cp and sepp1b, for hepatocyte differentiation and hepatic outgrowth. hhex and prox1a expression was detected in the livers of the MO-injected embryos at 36 hpf (Fig.3C and 3D, arrows), suggesting that id2a does not regulate hepatoblast specification or its maintenance (Fig.3C and 3D, arrows). Hepatic fabp10a, cp and sepp1b expression was also detected in the MO-injected embryos at 48 hpf (Fig. 3E–G, arrows), suggesting that id2a does not regulate hepatocyte differentiation. However, the liver size was reduced following id2a knockdown, implicating id2a in regulating hepatic outgrowth. Additionally, since id2a is expressed strongly in the gut and intestinal regions during development (Fig. 1A; bracket), we examined the expression of cdx1b, an intestinal bulb marker (Cheng et al., 2008), expecting a similar outgrowth phenotype as observed in the liver. As expected, lack of id2a had no effect on cdx1b induction; however, the intestinal bulb failed to grow at 48 hpf in id2a MO-injected embryos (Fig. 3H, brackets), indicative of an intestinal outgrowth defect. We further sought to determine whether id2a knockdown resulted in a general outgrowth defect of all endoderm-derived organs or specific organs in which id2a is expressed. Since id2a is not expressed in the dorsal pancreas (Fig. 1A and Fig. S2A), we performed WISH to examine the expression of insulin, which marks the pancreatic beta cells of the dorsal pancreas (Argenton et al., 1999). We found no difference in the size of the dorsal pancreas between control and id2a MO-injected embryos (Fig. S2B).
Previous studies have implicated ID proteins in the maintenance of neural stem cells. Id1 and Id3 double-knockout mice exhibit precocious neuronal differentiation, whereas ID2 overexpression in the chick hindbrain inhibits neuronal differentiation (Bai et al., 2007). Thus, it is still possible that id2a knockdown may result in precocious hepatocyte differentiation. To test this possibility, we examined fabp10a expression at 36 hpf, when fabp10a expression is not yet detected in the livers of wild-type embryos. However, fabp10a expression was not detected in id2a MO-injected embryos (data not shown), ruling out this possibility. Moreover, we examined whether Id2a overexpression could increase liver size. However, liver size was not further increased in id2a mRNA-injected embryos at 72 hpf compared with controls (Fig. S1E), indicating that Id2a is not sufficient for liver outgrowth.
Altogether, these data indicate that during liver development, id2a is not required for hepatoblast specification or hepatocyte differentiation, but rather for hepatic outgrowth. Moreover, the outgrowth defect observed in id2a MO-injected embryos may also apply to the development of other organ systems, such as the intestinal bulb.
2.4. id2a knockdown reduces liver size via decreased hepatoblast proliferation and increased cell death
To determine whether the small liver observed in id2a MO-injected embryos was caused by reduced proliferation and/or enhanced cell death, we conducted anti-phospho-Histone 3 (pH3) immunostaining and EdU labeling for proliferation and TUNEL labeling for cell death. Although the percentage of pH3+ cells among prox1a:YFP+ hepatic cells in id2a MO-injected embryos at 40 hpf was not significantly different from that in controls, there was a trend of reduced pH3+ cell number in the MO-injected liver compared to the control liver (0–3 versus 3–5) (Fig.4A and 4B). EdU labeling revealed about a 40% decrease in the percentage of EdU+ cells among sox17:GFP+ hepatic cells in id2a MO-injected embryos at 40 hpf compared with controls (Fig.4C and 4D), indicating reduced proliferation. In addition, we observed TUNEL and Prox1 double-positive cells in id2a MO-injected embryos at 40 hpf, but not in controls (Fig.4E and 4F). MO-mediated knockdown can often induce apoptosis mediated via aberrant p53 activation; thus, concurrent knockdown of tp53 can ameliorate apoptosis induced by MO off-targeting (Robu et al., 2007). Therefore, we performed simultaneous knockdown of tp53 and id2a. We did not detect any differences in microphthalmic, microcephalic, or small liver phenotypes between single id2a and double id2a/tp53 MO-injected embryos at 60 hpf (Fig. S1C and S1D), indicating that id2a knockdown phenotypes are independent of the p53 pathway. Altogether, these data indicate that id2a regulates hepatic outgrowth by promoting hepatoblast proliferation and repressing cell death.
Figure 4. id2a knockdown decreases hepatoblast proliferation and increases cell death in the developing liver.
(A) Whole-mount immunostaining with anti-pH3 (red) anti-GFP (green) antibodies in Tg(prox1a:YFP) embryos. The total number of prox1a:YFP+ hepatic cells per liver is 316 ± 8.5 in controls and 156 ± 5.6 in id2a MO-injected embryos. (B) A graph showing the percentage of pH3+ cells among prox1a:YFP+ hepatic cells (n=10). (C) EdU labeling (red), combined with anti-GFP immunostaining (green), in Tg(sox17:GFP) embryos reveals a significant reduction of proliferation in the liver of id2a MO-injected embryos at 40 hpf compared with controls. Dotted lines outline the liver. The total number of sox17:GFP+ cells per liver is 220 ± 16.6 in controls and 127 ± 12.7 in id2a MO-injected embryos. (D) A graph showing the percentage of EdU+ cells among GFP+ hepatoblasts (n=10). (E) TUNEL labeling (red) combined with anti-Prox1 immunostaining (green) reveals apoptosis in the liver of id2a MO-injected embryos at 40 hpf, but not in controls. The total number of Prox1+ cells per liver is 276 ± 20.5 in controls and 140 ± 8.6 in id2a MO-injected embryos. (F) A graph showing the percentage of TUNEL+ cells among Prox1+ hepatoblasts (n=10). *p < 0.05, **p < 0.005; error bars, ± s.e.m. Scale bars: 50 μm.
3. Discussion
In this study, we sought to determine the role of Id2a in liver development. We report three important findings. First, by using WISH followed by immunostaining, we discovered that id2a is initially expressed in the liver-forming region from 30 hpf and following hepatoblast differentiation at 48 hpf, id2a expression is restricted to BECs. Second, id2a knockdown did not affect hepatocyte differentiation or hepatoblast specification, which correlates with a lack of id2a liver expression during the hepatoblast specification stage (i.e., 22 hpf). Lastly, our data revealed that id2a knockdown inhibited hepatic outgrowth during development as supported by the reduced liver size in id2a MO-injected embryos.
Similar to the phenotype observed in id2a MO-injected embryos, in which hepatic outgrowth was compromised while hepatoblast specification and hepatocyte differentiation appeared unaffected, additional genes are also implicated in regulating hepatic outgrowth in zebrafish. Classified as a tumor suppressor gene that functions as a transcriptional activator, core promoter element binding protein (copeb; renamed as klf6a) is expressed in the zebrafish digestive organs, including the liver, the pancreas, and intestine. In copeb MO-injected embryos, the expansion of the liver is impaired (Zhao et al., 2010). Moreover, the failure of hepatic outgrowth in copeb MO-injected larvae is also attributed to a decrease in cell proliferation, as observed in id2a MO-injected embryos (Zhao et al., 2010). A similar phenotype is observed in the cell cycle modulator ubiquitin-like with PHD and ring finger domains 1 (uhrf1) mutants, which exhibit smaller livers as a result of a proliferation defect during the hepatic outgrowth phase (Sadler et al., 2007). In addition, knockdown of either sfrp5 (Stuckenholz et al., 2013) or nav3a (Klein et al., 2011) also results in a defect in hepatoblast outgrowth and subsequent liver size in 40-hpf zebrafish embryos. These results correlate with the well-known role of ID proteins as master regulators of cell proliferation (Lasorella et al., 2014), especially evident during early development.
Early development is a process defined by rapid cell proliferation followed by cell differentiation, generating distinct, mature tissues. Generally, Id expression is usually upregulated during the cell proliferation phase of early tissue development, and subsequently downregulated in mature, differentiated cells (Biggs et al., 1992; Ellmeier et al., 1992). However, exceptions exist; therefore, ID protein-mediated proliferation is cell contextdependent. For example, overexpression of Id1, Id2, or Id3 in neural stem cells derived from the mouse embryonic forebrain maintains the cortical neural stem cells in a proliferative, self-renewing state, and simultaneously inhibits neuronal differentiation (Jung et al., 2010). In contrast, upon differentiation of hematopoietic progenitor cells, Id2 expression increases (Cooper et al., 1997). Previously, Uribe et al. reported on the role of id2a in zebrafish retinogenesis. Upon id2a knockdown, proliferative retinoblasts (in the S-phase) increased as mitotic retinoblasts (in the M-phase) decreased, which demonstrates a role of Id2a in regulating the S- to M-phase progression during the cell cycle (Uribe and Gross, 2010). In contrast, we noted a significant decrease in the number of proliferating hepatoblasts at 40 hpf in id2a MO-injected embryos. As aforementioned, this phenotypic difference observed in proliferative cells during retinogenesis and liver development alludes to the dependence on cellular contexts in which id genes were studied. Nonetheless, id2a appears to play an important role in regulating proliferation in diverse tissue contexts including the developing liver.
In addition to proliferation, ID proteins also regulate apoptosis in a cell-dependent manner (Florio et al., 1998; Norton and Atherton, 1998). While apoptosis is significantly increased in mammary epithelial cells of Id2−/− pregnant mice (Mori et al., 2000), overexpression of Id1, Id2, or Id3 induces apoptosis in serum-deprived rat embryonic fibroblasts (Deed et al., 1997). In id2a MO-injected embryos, we observed a significant increase in the number of TUNEL-positive hepatoblasts at 40 hpf, highlighting the role of Id2a in regulating apoptosis in the developing liver.
Although intrahepatic biliary defects were also observed in id2a MO-injected embryos, it is unclear if the defects are either attributed to (1) a primary phenotype due to id2a knockdown or (2) a secondary phenotype due to compromised hepatic outgrowth. BEC-specific knockdown or knockout of id2a should conclusively establish a direct or indirect role of id2a in intrahepatic biliary morphogenesis. Currently, however, it is a challenge to create such a tool in zebrafish. As id2a expression is restricted in BECs during liver development, it will be interesting to explore the role of id2a in biliary-driven liver regeneration. Previously, we reported on a novel hepatocyte-specific genetic ablation model in zebrafish: following severe hepatocyte loss, BECs contribute to the repopulation of the liver (Choi et al., 2014). Few reports have explored the role of ID2 in liver regeneration. In two different models of liver injury in rats, partial hepatectomy and bile duct ligation, Id2 is immediately upregulated during the hepatocyte priming phase and ID2 expression is detected in the proliferating hepatocytes, respectively (Rodriguez et al., 2006). However, the role of Id2 in liver regeneration has not been reported yet. In addition, although ID2 has been shown to interact with various factors, including MyoD during myogenesis (Langlands et al., 1997), future analysis should consider the currently unknown binding factor of Id2a in the developing zebrafish liver. Since id2a knockdown reduced the liver size, we speculate the binding partner of Id2a to function as a suppressor of hepatic outgrowth and a negative regulator of hepatoblast proliferation.
Our findings validate the role of id2a in promoting hepatic outgrowth and development. Future studies should explore the mechanism of action of Id2a, including its binding partner, in liver development. Discerning the molecular mechanisms regulating liver development will improve our comprehension of the biological relevance of hepatic diseases and methods to enhance innate liver regeneration.
4. Experimental Procedures
4.1. Zebrafish Maintenance
Embryos and adult zebrafish (Danio rerio) were raised and maintained under standard laboratory conditions (Westerfield, 2000) with protocols approved by the University of Pittsburgh IACUC.
4.2. Zebrafish Strains
We used the following transgenic lines: TgBAC(prox1a:Citrine)hu338 (Bussmann and Schulte-Merker, 2011) [referred to as Tg(prox1a:YFP)], Tg(EPV.Tp1-Mmu.Hbb:EGFP)um14 (Parsons et al., 2009) [referred to as Tg(Tp1:GFP)], Tg(kdrl:EGFP)s843 (Beis et al., 2005) [referred to as Tg(kdrl:GFP)], Tg(ptf1a:EGFP)jh1 (Godinho et al., 2005) [referred to as Tg(ptf1a:GFP)], Tg(sox17:GFP)s870 (Chung and Stainier, 2008), and Tg(fabp10a:dsRed,ela31:GFP)gz12 (Korzh et al., 2008) [referred to as Tg(fabp10a:dsRed)].
4.3. Morpholino and mRNA injection
id2a MO (5’-`GCCTTCATGTTGACAGCAGGATTTC-3’) (Uribe and Gross, 2010) and tp53 MO (5’-GCGCCATTGCTTTGCAAGAATTG-3’) (Langheinrich et al., 2002) were purchased from GeneTools (Philomath, OR, USA). 3–4 ng of id2a MO or 2 ng of tp53 MO was injected into one-cell stage embryos. For rescue experiments, 3 ng of the id2a MO and 150 pg of id2a mRNA, which is resistant to the id2a MO, was sequentially injected into one-cell stage embryos. The id2a mRNA was generated using the mMessage mMachine SP6 kit (Life Technologies, Grand Island, NY, USA).
4.4. Whole-mount in situ hybridization and immunohistochemistry
Whole-mount in situ hybridization was performed as previously described (Alexander et al., 1998). cDNA from 24-hpf embryos was used as a template for PCR to amplify id1, id2a, id2b, id3, and id4 genes. We also used the following probes: hhex (Ho et al., 1999), prox1 (Glasgow and Tomarev, 1998), fabp10a (Her et al., 2003), sepp1b (Kudoh et al., 2001), cp (Korzh et al., 2001), and cdx1b (Flores et al., 2008). Whole-mount immunostaining was performed as previously described (Dong et al., 2007), using the following antibodies: chicken polyclonal anti- GFP (1:100; Aves Labs, Tigard, OR, USA), rabbit polyclonal anti-Prox1 (1:1000; Millipore, Billerica, MA, USA), mouse monoclonal anti-Anxa4 (also named as 2F11; 1:100; Abcam, Cambridge, MA USA), rabbit polyclonal anti-dsRed (1:200; Clontech, Mountain View, CA, USA), mouse monoclonal anti-phospho-Histone H3 (1:100; Cell Signaling, Danvers, MA, USA) and conjugated secondary antibodies, including Alexa Fluor 405-, 488-, 568-, and 647 (1:300; Life Technologies, Grand Island, NY, USA). Hoechst 33342 (2.5 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) was used for DNA staining. Zeiss LSM700 was used for confocal microscopy.
4.5. TUNEL and EdU assays
Apoptotic cell death was analyzed according to the protocol of the In Situ Cell Death Detection Kit, TMR Red (Roche, Switzerland). Following whole-mount immunostaining, TUNEL labeling was applied. Cell Proliferation was performed using the protocol outlined in the Click-iT EdU Alexa Fluor 647 Imaging Kit (Life Technologies, Grand Island, NY, USA). Larvae were incubated with EdU solution at 39 hpf for one hour, and at 40 hpf, they were harvested for EdU staining. Unpaired two-tailed Student’s t-tests were used for statistical analysis; p < 0.05 was considered statistically significant.
Supplementary Material
Highlights.
id2a is initially expressed in hepatoblasts of the zebrafish liver.
After hepatoblast differentiation, id2a is expressed in biliary epithelial cells.
Hepatic outgrowth is compromised upon id2a loss.
id2a is required for hepatoblast proliferation and survival.
Acknowledgements
We thank Michael Tsang for critical reading of the manuscript. This research was supported in part by grants from the NIH (T32EB001026) to M.K. and from the American Liver Foundation and the NIH (R01DK101426) to D.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mehwish Khaliq, Email: mek118@pitt.edu.
Tae-Young Choi, Email: choity@pitt.edu.
Juhoon So, Email: juhoon@pitt.edu.
References
- Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. doi: 10.1002/(SICI)1520-6408(1998)22:3<288::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Argenton F, Zecchin E, Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech Dev. 1999;87:217–221. doi: 10.1016/s0925-4773(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Bai G, Sheng N, Xie Z, Bian W, Yokota Y, Benezra R, Kageyama R, Guillemot F, Jing N. Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev Cell. 2007;13:283–297. doi: 10.1016/j.devcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Beis D, Bartman T, Jin SW, Scott IC, D'Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, Baier H, Tallafuss A, Bally-Cuif L, Chen JN, Stainier DY, Jungblut B. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Biggs J, Murphy EV, Israel MA. A human Id-like helix-loop-helix protein expressed during early development. Proc Natl Acad Sci U S A. 1992;89:1512–1516. doi: 10.1073/pnas.89.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R, Martinez-Barbera JP, Beddington RS, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125:4909–4917. doi: 10.1242/dev.125.24.4909. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Schulte-Merker S. Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development. 2011;138:4327–4332. doi: 10.1242/dev.068080. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Lin CC, Wu CS, Lu YF, Lin CY, Chung CC, Chu CY, Huang CJ, Tsai CY, Korzh S, Wu JL, Hwang SP. Zebrafish cdx1b regulates expression of downstream factors of Nodal signaling during early endoderm formation. Development. 2008;135:941–952. doi: 10.1242/dev.010595. [DOI] [PubMed] [Google Scholar]
- Choi TY, Ninov N, Stainier DYR, Shin D. Extensive Conversion of Hepatic Biliary Epithelial Cells to Hepatocytes After Near Total Loss of Hepatocytes in Zebrafish. Gastroenterology. 2014;146:776–788. doi: 10.1053/j.gastro.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Shin CH, Stainier DYR. Bmp2 Signaling Regulates the Hepatic versus Pancreatic Fate Decision. Developmental Cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Stainier DYR. Intra-endodermal interactions are required for pancreatic beta cell induction. Developmental Cell. 2008;14:582–593. doi: 10.1016/j.devcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Brady G, Bilia F, Iscove NN, Quesenberry PJ. Expression of the Id family helix-loop-helix regulators during growth and development in the hematopoietic system. Blood. 1997;89:3155–3165. [PubMed] [Google Scholar]
- Das A, Crump JG. Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deed RW, Hara E, Atherton GT, Peters G, Norton JD. Regulation of Id3 cell cycle function by Cdk-2-dependent phosphorylation. Mol Cell Biol. 1997;17:6815–6821. doi: 10.1128/mcb.17.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- Ellmeier W, Aguzzi A, Kleiner E, Kurzbauer R, Weith A. Mutually exclusive expression of a helix-loop-helix gene and N-myc in human neuroblastomas and in normal development. EMBO. J. 1992;11:2563–2571. doi: 10.1002/j.1460-2075.1992.tb05321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field HA, Ober EA, Roeser T, Stainier DY. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Flores MVC, Hall CJ, Davidson AJ, Singh PP, Mahagaonkar AA, Zon LI, Crosier KE, Crosier PS. Intestinal Differentiation in Zebrafish Requires Cdx1b, a Functional Equivalent of Mammalian Cdx2. Gastroenterology. 2008;135:1665–1675. doi: 10.1053/j.gastro.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Florio M, Hernandez MC, Yang H, Shu HK, Cleveland JL, Israel MA. Id2 promotes apoptosis by a novel mechanism independent of dimerization to basic helixloop- helix factors. Mol Cell Biol. 1998;18:5435–5444. doi: 10.1128/mcb.18.9.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow E, Tomarev SI. Restricted expression of the homeobox gene prox 1 in developing zebrafish. Mech Dev. 1998;76:175–178. doi: 10.1016/s0925-4773(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Her GM, Yeh YH, Wu JL. 435-bp liver regulatory sequence in the liver fatty acid binding protein (L-FABP) gene is sufficient to modulate liver regional expression in transgenic zebrafish. Dev Dyn. 2003;227:347–356. doi: 10.1002/dvdy.10324. [DOI] [PubMed] [Google Scholar]
- Ho CY, Houart C, Wilson SW, Stainier DY. A role for the extraembryonic yolk syncytial layer in patterning the zebrafish embryo suggested by properties of the hex gene. Curr Biol. 1999;9:1131–1134. doi: 10.1016/s0960-9822(99)80485-0. [DOI] [PubMed] [Google Scholar]
- Holtzinger A, Evans T. Gata4 regulates the formation of multiple organs. Development. 2005;132:4005–4014. doi: 10.1242/dev.01978. [DOI] [PubMed] [Google Scholar]
- Hua H, Zhang YQ, Dabernat S, Kritzik M, Dietz D, Sterling L, Sarvetnick N. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem. 2006;281:13574–13580. doi: 10.1074/jbc.M600526200. [DOI] [PubMed] [Google Scholar]
- Iavarone A, Garg P, Lasorella A, Hsu J, Israel MA. The helix-loop-helix protein Id-2 enhances cell proliferation and binds to the retinoblastoma protein. Genes Dev. 1994;8:1270–1284. doi: 10.1101/gad.8.11.1270. [DOI] [PubMed] [Google Scholar]
- Jones S. An overview of the basic helix-loop-helix proteins. Genome Biol. 2004;5:226. doi: 10.1186/gb-2004-5-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Jung S, Park RH, Kim S, Jeon YJ, Ham DS, Jung MY, Kim SS, Lee YD, Park CH, Suh-Kim H. Id proteins facilitate self-renewal and proliferation of neural stem cells. Stem Cells Dev. 2010;19:831–841. doi: 10.1089/scd.2009.0093. [DOI] [PubMed] [Google Scholar]
- Klein C, Mikutta J, Krueger J, Scholz K, Brinkmann J, Liu D, Veerkamp J, Siegel D, Abdelilah-Seyfried S, le Noble F. Neuron navigator 3a regulates liver organogenesis during zebrafish embryogenesis. Development. 2011;138:1935–1945. doi: 10.1242/dev.056861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Korzh S, Emelyanov A, Korzh V. Developmental analysis of ceruloplasmin gene and liver formation in zebrafish. Mech Dev. 2001;103:137–139. doi: 10.1016/s0925-4773(01)00330-6. [DOI] [PubMed] [Google Scholar]
- Korzh S, Pan X, Garcia-Lecea M, Winata CL, Pan X, Wohland T, Korzh V, Gong Z. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish. BMC Dev Biol. 2008;8:84. doi: 10.1186/1471-213X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T, Tsang M, Hukriede NA, Chen X, Dedekian M, Clarke CJ, Kiang A, Schultz S, Epstein JA, Toyama R, Dawid IB. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001;11:1979–1887. doi: 10.1101/gr.209601. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol. 2002;12:2023–2028. doi: 10.1016/s0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- Langlands K, Yin X, Anand G, Prochownik EV. Differential interactions of Id proteins with basic-helix-loop-helix transcription factors. J Biol Chem. 1997;272:19785–19793. doi: 10.1074/jbc.272.32.19785. [DOI] [PubMed] [Google Scholar]
- Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- Lasorella A, Boldrini R, Dominici C, Donfrancesco A, Yokota Y, Inserra A, Iavarone A. Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 2002;62:301–306. [PubMed] [Google Scholar]
- Lemaigre FP. Development of the biliary tract. Mech Dev. 2003;120:81–87. doi: 10.1016/s0925-4773(02)00334-9. [DOI] [PubMed] [Google Scholar]
- Lin TY, Chou CF, Chung HY, Chiang CY, Li CH, Wu JL, Lin HJ, Pai TW, Hu CH, Tzou WS. Hypoxia-inducible factor 2 alpha is essential for hepatic outgrowth and functions via the regulation of leg1 transcription in the zebrafish embryo. PLoS One. 2014;9:e101980. doi: 10.1371/journal.pone.0101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokmane L, Haumaitre C, Garcia-Villalba P, Anselme I, Schneider-Maunoury S, Cereghini S. Crucial role of vHNF1 in vertebrate hepatic specification. Development. 2008;135:2777–2786. doi: 10.1242/dev.023010. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RP, Lorent K, Russo P, Pack M. The zebrafish onecut gene hnf-6 functions in an evolutionarily conserved genetic pathway that regulates vertebrate biliary development. Dev Biol. 2004;274:245–259. doi: 10.1016/j.ydbio.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Mori S, Nishikawa SI, Yokota Y. Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO. J. 2000;19:5772–5781. doi: 10.1093/emboj/19.21.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622–633. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol. 1998;18:2371–2381. doi: 10.1128/mcb.18.4.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JL, Sandoval J, Serviddio G, Sastre J, Morante M, Perrelli MG, Martinez-Chantar ML, Vina J, Vina JR, Mato JM, Avila MA, Franco L, Lopez-Rodas G, Torres L. Id2 leaves the chromatin of the E2F4-p130-controlled c-myc promoter during hepatocyte priming for liver regeneration. Biochem. J. 2006;398:431–437. doi: 10.1042/BJ20060380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler KC, Krahn KN, Gaur NA, Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1570–1575. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Ye J, Yu N, Guez F, Bedford DC, Neale GA, Cordi S, Brindle PK, Lemaigre FP, Kaestner KH, Sosa-Pineda B. Prox1 ablation in hepatic progenitors causes defective hepatocyte specification and increases biliary cell commitment. Development. 2014;141:538–547. doi: 10.1242/dev.099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, Stainier DY. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell. 2003;3:525–530. doi: 10.1016/s1535-6108(03)00141-7. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- Stuckenholz C, Lu L, Thakur PC, Choi TY, Shin D, Bahary N. Sfrp5 modulates both Wnt and BMP signaling and regulates gastrointestinal organogenesis [corrected] in the zebrafish, Danio rerio. PLoS One. 2013;8:e62470. doi: 10.1371/journal.pone.0062470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Uribe RA, Gross JM. Id2a influences neuron and glia formation in the zebrafish retina by modulating retinoblast cell cycle kinetics. Development. 2010;137:3763–3774. doi: 10.1242/dev.050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe RA, Kwon T, Marcotte EM, Gross JM. Id2a functions to limit Notch pathway activity and thereby influence the transition from proliferation to differentiation of retinoblasts during zebrafish retinogenesis. Dev Biol. 2012;371:280–292. doi: 10.1016/j.ydbio.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KN, Yusuff S, Sonntag JM, Chin AJ, Pack M. Zebrafish hhex regulates liver development and digestive organ chirality. Genesis. 2001;30:141–143. doi: 10.1002/gene.1050. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; 2000. [Google Scholar]
- Yin CY, Evason KJ, Maher JJ, Stainier DY. The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: Analysis of stellate cell entry into the developing liver. Hepatology. 2012;56:1958–1970. doi: 10.1002/hep.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- Zhao X, Monson C, Gao C, Gouon-Evans V, Matsumoto N, Sadler KC, Friedman SL. Klf6/copeb is required for hepatic outgrowth in zebrafish and for hepatocyte specification in mouse ES cells. Dev Biol. 2010;344:79–93. doi: 10.1016/j.ydbio.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.