Abstract
Objective
The study’s purpose was to develop symptoms cluster model that can describe factors of FMS associated with fatigue severity as reported by the sample. The study will also explore FMS clinical symptom sub-clusters based on varying symptom intensities.
Methods
FMS individuals (n = 120; 82% between 31–60 years of age, 90% women, 59% Caucasian) diagnosed with the 1990 or 2010 American College of Rheumatology diagnostic criteria were enrolled. Participants completed multiple validated self-report questionnaires to measure fatigue, pain, depression, anxiety, pain catastrophizing, daytime sleepiness, cognitive function, and FMS-related polysymptomatic distress. Cluster analysis using SPSS 19.0 and Structural Equation Modeling using AMOS 17.0 were used.
Results
Final Structural Equation Modeling symptoms cluster model showed good fit and revealed that FMS fatigue was associated with widespread pain, symptoms severity, pain intensity, pain interference, cognitive dysfunction, catastrophizing, anxiety, and depression (χ2 = 121.72, df = 98, p > 0.05, χ2/df = 1.242, CFI = 0.982, RMSEA = 0.045). Two distinct clinical symptom sub-clusters emerged; sub-cluster 1 (78% of total subjects) defined by widespread pain, unrefreshed waking, and somatic symptoms and sub-cluster 2 (22% of total subjects) defined by fatigue and cognitive dysfunction with pain being a less severe and less widespread complaint.
Conclusion
Overall, sub-cluster 1 had more intense symptoms than sub-cluster 2. FMS symptoms may be categorized into two clinical sub-clusters. These findings have implications for an illness whose diagnosis and management are symptom-dependent. A longitudinal study capturing the variability in symptom experience of FMS subjects is warranted.
INTRODUCTION
Fibromyalgia syndrome (FMS) is characterized by a widespread chronic bodily pain, profound fatigue, and sleep disturbance and appears to represent the end of the spectrum of polysymptomatic distress (1). Based on the 2010 diagnostic criteria, FMS affects 6% of the United State population (2). In some patients with FMS, fatigue interferes with the performance of daily activities, as much or more than bodily pain. Because most studies have primarily investigated the mechanisms and treatment of FMS-related pain, less is known about other FMS symptoms including fatigue (3–6). Patients with FMS are often unemployed and have high medical utilization rates related to their fatigue symptoms (7).
Fatigue is defined as a subjective sense of persistent tiredness that interferes with the performance of daily life activities and is not relieved by rest (6). The etiology of fatigue is unknown; however, studies agree on its multidimensionality (3, 8). Fatigue is categorized into peripheral and central components (9). In FMS and chronic fatigue syndrome, peripheral fatigue (physical fatigue) has been associated with the reduction of muscle contraction from impaired energy resources (10), while central fatigue (mental fatigue) has been associated with cognitive impairment (9, 10). In FMS, fatigue manifests within a cluster of symptoms that includes pain, sleep disorders, depression, difficulty with concentration, and worsening memory (11). Previous FMS studies reported that pain and depression are strongly associated with fatigue, while sleep quality has moderate and inverse association with fatigue (6). Psychobehavioral symptoms reported by FMS patients include depression, anxiety, and catastrophizing (12). The mutual relationship between behavioral symptoms, specifically depression and pain, have been demonstrated indicating that both share common biological pathways and neurotransmitters (13). The association between depression and fatigue has also been reported in patients who have cancer (14), arthritis (15), or FMS (16). One study found that among 839 FMS patients, fatigue was significantly associated with depression while pain was associated with anxiety (17). Pain catastrophizing was also found to be correlated with pain intensity (18). Our recent review demonstrated that catastrophizing has a large impact on fatigue severity (19); however, only one study has explored the associations among catastrophizing, pain, and fatigue, altogether in FMS patients (20).
The multidimensional model of fatigue was investigated in one study of patients with rheumatoid arthritis (RA) using structural equation modeling (SEM) and results from that study suggested that fatigue is significantly linked to disease activity, psychological factors, and sleep (21). FMS is a polysymptomatic condition that is characterized by manifest (e.g. fatigue intensity) and latent variables (e.g. cognitive vulnerabilities). The SEM approach is advantageous to use in this complex, polysymptomatic condition because it tests interrelationships among observable and latent variables. Compared to other cluster analytical strategies, the SEM approach is the only technique that can do complete and simultaneous analyses of the relationships between these variables (22).
No studies investigated the association of fatigue with other symptoms experienced by individuals with FMS using the SEM approach. This study developed a symptoms model describing the symptoms experience of FM patients based on existing literature of the relationship among polysymptomatic distress experienced by FM patients are attributed to specific symptoms such as pain, depression, anxiety, catastrophizing, cognitive dysfunction, daytime sleepiness, and fatigue. Then we utilizes the statistical approach of the previous RA study (21), to address its purposes which was to develop a symptoms cluster model that can describe the associations of fatigue with other FMS symptoms and to explore FMS symptom sub-clusters based on varying symptom intensities reported by the sample.
MATERIALS AND METHODS
Participants
This study is part of a prospective, longitudinal, observational study from an Institutional Review Board protocol. Participants diagnosed with FMS using the 1990 (self-report history of widespread pain with at least 11 out of 18 tender sites on exam) or the 2010 American College of Rheumatology criteria (Widespread Pain Index (WPI) ≥ 7/19 and Symptom Severity Scale (SSS) ≥ 5/12, or WPI = 3 – 6/19 and the SS ≥ 9/12) were included in the analyses. Based on the hypothesized model for SEM, 2 latent variables (variable that contains subscales) and 10 observed variables (variables that can be directly measured) were tested. To achieve statistical power at level of 0.80 with 0.05 significant level, sample size was calculated using a-priori sample size calculator for structural equation modeling software (23). The minimum number participant needed for model structure is 100.
Design
Participants’ demographic information and the symptoms scores were obtained on one initial outpatient visit. No intervention was provided in this cross-sectional study. Patients were receiving a wide array of different therapeutic interventions obtained from community physicians and practitioners at the time of the study visit.
Measures
Demographic data (gender, age, marital status, educational level and employment status) were obtained from the participants’ medical charts. Participants’ symptom experiences were assessed using methods described below.
Polysymptomatic distress was measured by the sum of the widespread pain index (WPI) and symptoms severity scale (SSS) scores. The WPI measures the number of bodily areas (total = 19) that a patient has had pain in over the past week. The SSS is a 4-item, 0–3 rating scale (total = 12) to measure severity of unrefreshed waking, cognitive problems, fatigue and other somatic symptoms (e.g., irritable bowel syndrome, numbness/tingling, dizziness, depression, constipation, nausea, nervousness, chest pain, blurred vision, fever, dry mouth, itching, wheezing, Raynaud’s phenomenon, hives/welts, ringing in ears, heartburn, oral ulcers, loss of/change in taste, seizures, dry eyes, shortness of breath, loss of appetite, sun sensitivity, hearing difficulties, easy bruising, hair loss, and frequent urination). Higher scores for both instruments indicate widespread painful bodily locations and more severe symptoms, respectively (24). These questionnaires have been validated in previous studies and are currently used as part of the 2010 FMS diagnostic criteria (24, 25).
Number of tender points reported (conducted by applying < 4 kilogram on 18 bodily areas) and the participant’s pain threshold (the average kilogram tolerated on the 18 bodily areas) were measured using dolorimetry, a reliable tool to measure tenderness in FMS (26).
The Brief Pain Inventory-Short Form (BPI-SF) measures pain intensity (4 items) and pain interference (7 items) using a numeric rating scale of 0 (no pain / interference) to 10 (pain as bad as you can imagine / complete interference) (27). The internal consistency of BPI-SF as measured by Cronbach’s alpha for pain intensity is0.88and pain interference is 0.87 (28).
Fatigue was measured by the Multidimensional Fatigue Inventory (MFI), a 20-item self-report questionnaire composed of five subscales: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue (29). Each of the five subscales is measured with 4 items using a rating scale of 1 (completely true) to 5 (no, not true), which have been found to have internal consistency reliability of Cronbach’s alpha greater than 0.80 (29).
Anxiety and depression were measured by the 14-item, two-subscale, self-report Hospital Anxiety and Depression Scale (HADS) (30). Each item is rated on a 4-point Likert scale and each subscale (anxiety and depression) has a score that ranges from 0 to 21. High scores indicate greater anxiety, and depressive symptoms. Both subscales have internal consistency with Cronbach’s alpha of 0.89 (30, 31).
Catastrophizing was measured using the Pain Catastrophizing Scale (PCS), a 13-item, self-report questionnaire consisting of three subscales: rumination, magnification and helplessness. Participants were asked to rate their thoughts and feelings on a 0 (not at all) to 4 (all the time) numeric scale. The internal consistency of PCS showed a Cronbach’s alpha of 0.87 (32, 33).
Self-perceived cognitive difficulties were measured by the Multiple Ability Self-Report Questionnaire (MASQ). This instrument evaluated five domains of cognitive difficulties based on neuropsychological evaluation that include language, visual-perceptual ability, verbal memory, visual-spatial memory, and attention/ concentration (34). MASQ contains 38 items on a 1–5 Likert rating scale. The total score for each domain ranges from 8–40, except for visual-perceptual ability, which ranges from 6–30 (35). Higher MASQ scores indicate greater perception of cognitive difficulties. The internal consistency reliability of MASQ showed a Cronbach’s alpha that ranged from 0.72–0.74 (36).
Daytime sleepiness was measured by the 8-item Epworth Sleepiness Scale (ESS). Each item was rated on a 4-point (0–3) Likert scale, with total scores ranging from 0 to 24, with higher ESS sum scores mean higher daytime sleepiness (37). The internal consistency of EES showed a Cronbach’s alpha of 0.70 (38).
Data analysis
Subjects were grouped based on their symptom scores. The agglomerative hierarchical cluster analysis with ward’s methods and squared Euclidean distances were performed using the SPSS 19.0 program. SEM analysis was used to test and estimate the relations among polysymptomatic distress to pain severity and interference, depression, anxiety, catastrophizing, cognitive dysfunction, daytime sleepiness, and fatigue. The hypothesized symptoms cluster model (Figure 1) was tested using the AMOS™ 17.0 program (39). Prior to testing the hypothesized symptoms cluster model, data were screened for normal distribution. Missing data were managed using the expectation maximization (EM) method, by finding the maximum log-likelihood parameters for the missing data (40). The hypothesized symptoms cluster model was assessed for multiple goodness of fit criteria to include: model chi-squared goodness of fit statistic, the ratio of chi squared to the degree of freedom (χ2/df), the Comparative Fit Index (CFI), and the root mean square error of approximation (RMSEA). The criterion of non-significant chi-squared statistic (p > 0.05) was suggested as a good fit between data and the tested symptoms cluster model (41). Other indications for the goodness of fit included the χ2/df less than 2 and a CFI of greater than .95 (42). The RMSEA value of less than .06 was considered acceptable to minimize type I and II errors (43). Symptoms cluster model modifications were performed to interpret the model fit. Using a previous approach (44), relationship paths that were theoretically justifiable and empirically explainable based on existing literature were added and the non-significant paths were dropped.
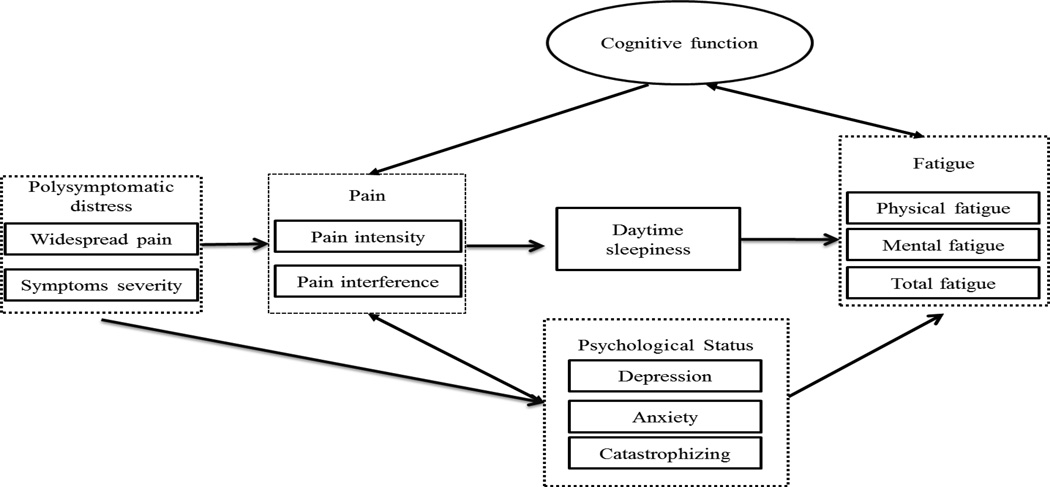
Figure 1.
Hypothesized FMS symptoms cluster model. Hypothesized structural model on the relationship between polysymptomatic distress, pain, psychological status, cognitive function, daytime sleepiness, and fatigue.
To identify the number of distinct sub-clusters from the data, the maximum percentage change in the agglomeration coefficient recorded between successive sub-cluster profiles was used. Following the formation of sub-clusters, discrimination function analysis using the SPSS19.0 program was conducted to investigate the relative weight of each predictive variable in discriminating between the sub-clusters. Multiple analyses of variance (MANOVAs) were performed to investigate differences in symptom experience between sub-clusters and to explore whether the goodness of fit of the symptoms cluster model remained unchanged between the identified FMS sub-clusters. Multiple sample structural equation modeling analyses were used to examine the differences between FMS symptoms sub-clusters 1 and 2. The chi-square difference statistic was used to test differences between the constrained model where regression weights of all relationship parameters were constrained to be equal between the two sub-clusters and the unconstrained model, where regression weights of all relationship parameters were allowed to vary. If the chi-square results revealed a significant difference between the constrained and unconstrained models, then the SEM models for sub-clusters 1 and 2 were considered unique for each FMS sub-cluster. Then, each relationship path was tested to determine if relationships between variables in that path had equal regression weights in the model for both sub-clusters by allowing one relationship path to be unequal between the two sub-clusters. Significant chi-square differences between the unconstrained and constrained models indicated that the specific relationship path significantly differed between the two FMS sub-clusters.
RESULTS
Participant characteristics
The sample included a total of 120 participants with 12 males (10%) and 108 females (90%) ages 21 to 82 years (mean age = 46.30 ± 11.00). Majority of these participants were Caucasians (59%), married (37%), and college educated (41%). About 40% were employed and 25% were on disability (Table 1).
Table 1.
Demographic and clinical characteristics of sample
| Characteristics | n | % | Range | Mean (SD) |
|---|---|---|---|---|
| Gender | ||||
| Male | 12 | 10 | ||
| Female | 108 | 90 | ||
| Age | 21.00–82.00 | 46.30 (11.00) | ||
| 21–30 | 12 | 10 | ||
| 31–40 | 26 | 21.7 | ||
| 41–50 | 35 | 29.2 | ||
| 51–60 | 38 | 31.7 | ||
| >61 | 9 | 7.5 | ||
| Race | ||||
| Caucasian/White | 71 | 59.2 | ||
| African American | 35 | 29.2 | ||
| Hispanic/Asian | 4 | 3.3 | ||
| Others | 4 | 3.3 | ||
| Missing data | 6 | 5.0 | ||
| Education | ||||
| Less than 12 grade | 6 | 5.0 | ||
| 12 grade | 20 | 16.7 | ||
| Trade School | 4 | 3.3 | ||
| College | 49 | 40.8 | ||
| Graduate School | 35 | 29.2 | ||
| Missing data | 6 | 5.0 | ||
| Marital status | ||||
| Never Married | 32 | 26.7 | ||
| First marriage | 44 | 36.7 | ||
| Divorces | 22 | 18.3 | ||
| Widowed | 3 | 2.5 | ||
| Remarried | 12 | 10.0 | ||
| Missing data | 7 | 5.8 | ||
| Employment | ||||
| Full time | 35 | 29.2 | ||
| Part time | 13 | 10.8 | ||
| Unemployed | 21 | 17.5 | ||
| Disability | 30 | 25.0 | ||
| Students | 8 | 6.7 | ||
| Retired | 6 | 5.0 | ||
| Missing data | 7 | 5.8 | ||
| Pain Threshold | ||||
| # of tender points | 120 | 0–18.0 | 14.28 (4.54) | |
| Pain threshold (kg) | 120 | 0–8.13 | 2.89 (1.56) |
| Cluster 1 Mean (SD) (N = 94) |
Cluster2 Mean (SD) (N = 26) |
Funivariate; Pvalue | ||
|---|---|---|---|---|
| Gender | Female | 85 (90.4%) | 23 (88.5%) | |
| Male | 9 (9.6%) | 3 (11.5%) | ||
| Age | 46.30 (11.3) | 46.30 (10.2) | F = 1.11; p = 0.34 | |
| Race | Caucasian | 49 (52.1%) | 22 (84.6%) | |
| African American | 33 (35.1%) | 2 (7.7%) | ||
| Other | 12 (11.8%) | 2 (7.7%) | ||
| BMI | 31.14 (8.15) | 31.39 (8.8) | F = 0.05; p = 0.82 | |
| Polysymptomatic distress | WPI | 14.15 (2.5) | 5.77 (2.4) | F = 238.4; p = 0.00** |
| SSS-fatigue | 2.45 (0.7) | 1.96 (0.8) | F = 8.87; p = 0.00** | |
| SSS- unrefreshed waking | 2.46 (0.8) | 2.04 (1.0) | F = 5.09; p = 0.03* | |
| SSS-cognitive symptoms | 1.83 (0.9) | 1.35 (1.0) | F = 5.71; p = 0.02* | |
| SSS-somatic symptoms | 2.17 (0.6) | 1.65 (0.6) | F = 16.47; p = 0.00** | |
| Tender point | Number of tender points | 14.77 (4.2) | 12.50 (5.4) | F = 5.266; p = 0.02* |
| Average pain threshold | 2.69 (1.4) | 3.60 (1.8) | F = 7.44; p = 0.01* | |
| Pain | Pain Intensity | 6.02 (1.7) | 3.62 (2.1) | F = 35.37; p = 0.00** |
| Pain Interference | 6.48 (2.0) | 4.05 (2.9) | F = 23.98; p = 0.00** | |
| Fatigue | Physical fatigue | 16.03 (3.36) | 13.77 (3.09) | F = 9.54; p = 0.00** |
| Mental fatigue | 15.25 (3.9) | 13.19 (4.1) | F = 5.57; p = 0.02* | |
| Total fatigue | 76.98 (13.4) | 64.84 (12.3) | F = 17.37; p = 0.00** | |
| Day time sleepiness | 9.28 (4.5) | 8.55 (3.67) | F = 0.59; p = 0.45 | |
| Depression | 8.39 (3.9) | 5.96 (3.8) | F = 7.88; p = 0.01* | |
| Anxiety | 9.69 (4.5) | 7.12 (4.2) | F = 6.85; p = 0.01* | |
| Catastrophizing | Rumination | 8.12 (4.7) | 4.82 (3.8) | F = 10.74; p = 0.00** |
| Magnification | 4.72 (3.2) | 2.55 (2.3) | F = 10.67; p = 0.00** | |
| Helplessness | 10.86 (6.3) | 5.65 (5.4) | F = 14.85; p = 0.00** | |
| Cognitive dysfunction | Language | 20.50 (4.6) | 17.65 (4.3) | F = 8.00; p = 0.01* |
| Visual-perceptual ability | 15.27 (5.1) | 13.73 (4.1) | F = 1.97; p = 0.16 | |
| Verbal memory | 22.82 (4.9) | 21.66 (4.7) | F = 1.07; p = 0.30 | |
| Visual-spatial memory | 18.94 (4.8) | 17.31 94.0) | F = 2.49; p = 0.12 | |
| Attention/concentration | 22.77 (5.5) | 21.10 (4.7) | F = 2.00; p = 0.16 |
p < 0.05;
p < 0.01;
SD = standard deviation; BMI: body mass index; WPI: widespread pain index; SSS: symptoms severity score.
Structural equation modeling results
Hypothesized model
Based on the goodness of fit criteria, the hypothesized model shown in Figure 1 showed a poor fit to the data (χ2 = 259.6, degrees of freedom = 113, probability level < 0.01, the χ2 /df = 2.6, CFI = 0.89, RMSEA = 0.10). The hypothesized model was modified based on the theoretical and statistical plausibility of the data using the modification indices suggested by the AMOS™ program. The daytime sleepiness variable was dropped from the FMS symptoms cluster model because it was not associated with fatigue and any of the other variables. Only statistically significant paths (p < 0.05) as shown in Figure 2 and Table 3 were included in the model. The modified model had a better fit to the data (χ2 = 121.7, df = 98, p > 0.05, χ2/df = 1.24, CFI = 0.98 and RMSEA = 0.04) than did the hypothesized model. The most striking observation from the FMS symptoms cluster model was the negligible impact of pain symptoms on the severity of fatigue, cognitive dysfunction, anxiety, and depression of FMS.
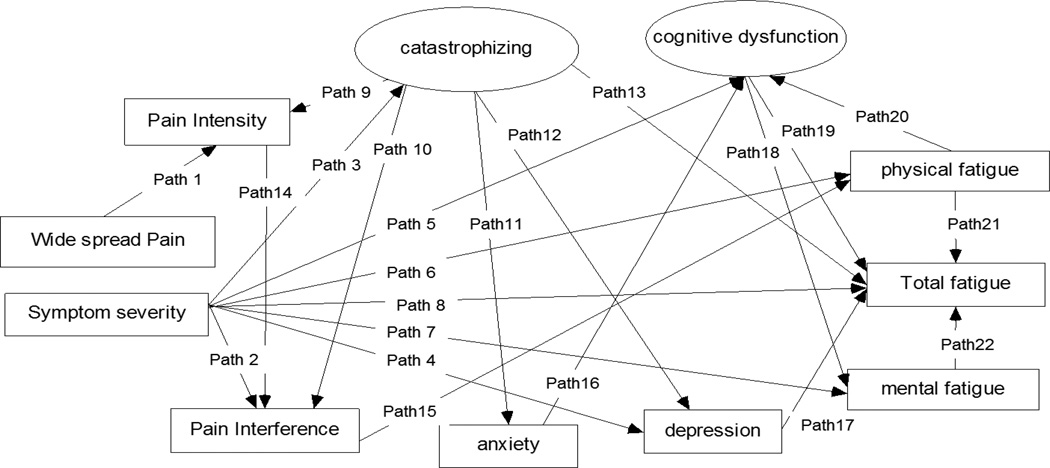
Figure 2.
Final FMS symptoms cluster model using structural equation modelling.
Table 3.
Standardized path coefficient, standard error, unstandardized path coefficient of significant paths in final FMS symptoms cluster model (N = 120)
| Path | Predictors | Dependent variables | β | SE | Unstandardized path coefficient |
|---|---|---|---|---|---|
| 1 | Widespread Pain index | Pain Intensity | .201 | .036 | 5.550*** |
| 2 | Symptom severity | Pain Interference | .258 | .058 | 4.449*** |
| 3 | Catastrophizing | .502 | .169 | 2.978** | |
| 4 | Depression | .470 | .115 | 4.079*** | |
| 5 | Cognitive dysfunction | .311 | .145 | 2.150* | |
| 6 | Physical fatigue | .433 | .128 | 3.381*** | |
| 7 | Mental fatigue | .546 | .127 | 4.291*** | |
| 8 | Total fatigue | 1.038 | .270 | 3.848*** | |
| 9 | Catastrophizing | Pain Intensity | .164 | .039 | 4.174*** |
| 10 | Pain Interference | .146 | .036 | 4.039*** | |
| 11 | Anxiety | .690 | .088 | 7.861*** | |
| 12 | Depression | .422 | .082 | 5.146*** | |
| 13 | Total fatigue | .311 | .150 | 2.076* | |
| 14 | Pain Intensity | Pain Interference | .665 | .068 | 9.723*** |
| 15 | Pain Interference | Physical fatigue | .485 | .125 | 3.887*** |
| 16 | Anxiety | Cognitive dysfunction | .268 | .068 | 3.921*** |
| 17 | Depression | Total fatigue | .337 | .153 | 0.199* |
| 18 | Cognitive dysfunction | Mental fatigue | .589 | .098 | 5.994*** |
| 19 | Total fatigue | −.653 | .222 | −2.943** | |
| 20 | Physical fatigue | Cognitive dysfunction | .303 | .099 | 3.056** |
| 21 | Total fatigue | 2.077 | .171 | 12.174*** | |
| 22 | Mental fatigue | Total fatigue | 1.619 | .181 | 8.965*** |
p < 0.05,
p < 0.01,
p < 0.001;
β: Standardized path coefficient; WPI: Widespread Pain Index; SSS: Symptoms Severity Scores
Cluster analysis and discriminant function analysis
Two FMS sub-clusters were identified as being a good fit to the data based on the largest agglomeration coefficient difference of 1431.6. The two FMS sub-clusters had approximately equal percentages of male (9.6% in sub-cluster 1 and 11.5% in sub-cluster 2) and were similar (p > 0.05) in age, BMI, daytime sleepiness, and self-perceived cognitive dysfunction on visual-perceptual ability, verbal memory, visual-spatial memory, and attention/concentration. Sub-cluster 1 included 94 FMS subjects (78%) and sub-cluster 2 had 26 subjects (22%). Comparing symptom severities, sub-cluster 1 subjects complained of more intense symptoms compared to sub-cluster 2 and these symptoms include higher (p < 0.05) Widespread Pain Index (mean ± SD sub-cluster 1 = 14.18 ± 2.5; sub-cluster 2 = 5.77 ± 2.4), higher tender point count (sub-cluster 1 = 14.77 ± 4.2; sub-cluster 2 = 12.50 ± 5.4), and lower pain threshold as measured by dolorimetry (sub-cluster 1 = 2.69 ± 1.4; sub-cluster 2 = 3.60 ± 1.8; Table 1).
The discriminant function analysis results shown in Table 2 indicated that the two FMS sub-clusters were significantly distinct from each other (χ2 (df) = 130.990 (5), p < 0.001), suggesting that each sub-cluster has its own distinct symptoms characteristics. Widespread pain and somatic symptoms were important in separating the 2 FMS sub-clusters. Based on function loading (table 2), FMS sub-cluster 1 was distinguished from FMS sub-cluster 2 by widespread pain, unrefreshed waking, and somatic symptoms, while FMS sub-cluster 2 had fatigue and cognitive symptoms coupled with less intense and widespread pain that was distinct from FMS sub-cluster 1. About 94% of sub-cluster 1 subjects (widespread pain cluster) and 42% of sub-cluster 2 subjects (fatigue cluster) met the 2010 FMS diagnostic criteria of WPI ≥ 7 and SSS ≥ 5, while no sub-cluster 1 subject and about 23% of sub-cluster 2 subjects met the 2010 FMS diagnostic criteria of WPI = 3–6 and SSS ≥ 9. A small proportion of patients (6% in sub-cluster 1, 35% in sub-cluster 2) met only the 1990 criteria, reminding that clinically substantial tenderness can occur in the absence of self-reported widespread pain when measured in a clinical setting.
Table 2.
Discriminant function analysis for sub-cluster characteristics
| Saturation loading |
Classification function coefficients | |||
|---|---|---|---|---|
| FM Sub-cluster 1 | FM Sub-cluster 2 | |||
| Widespread pain | 0.979 | 2.217 | 0.815 | |
| Symptom severity | Fatigue | 0.189 | 1.167 | 1.293 |
| Unrefreshed waking | 0.143 | 2.078 | 1.674 | |
| Cognitive symptoms | 0.152 | −1.092 | −0.547 | |
| Somatic symptoms | 0.257 | 5.185 | 3.960 | |
| Constant | −24.990 | −8.926 | ||
χ2 (df) = 130.990 (5), p < 0.001; Eigenvalue of function 1 = 2.108; 100% variance
Multiple-sample SEM analysis
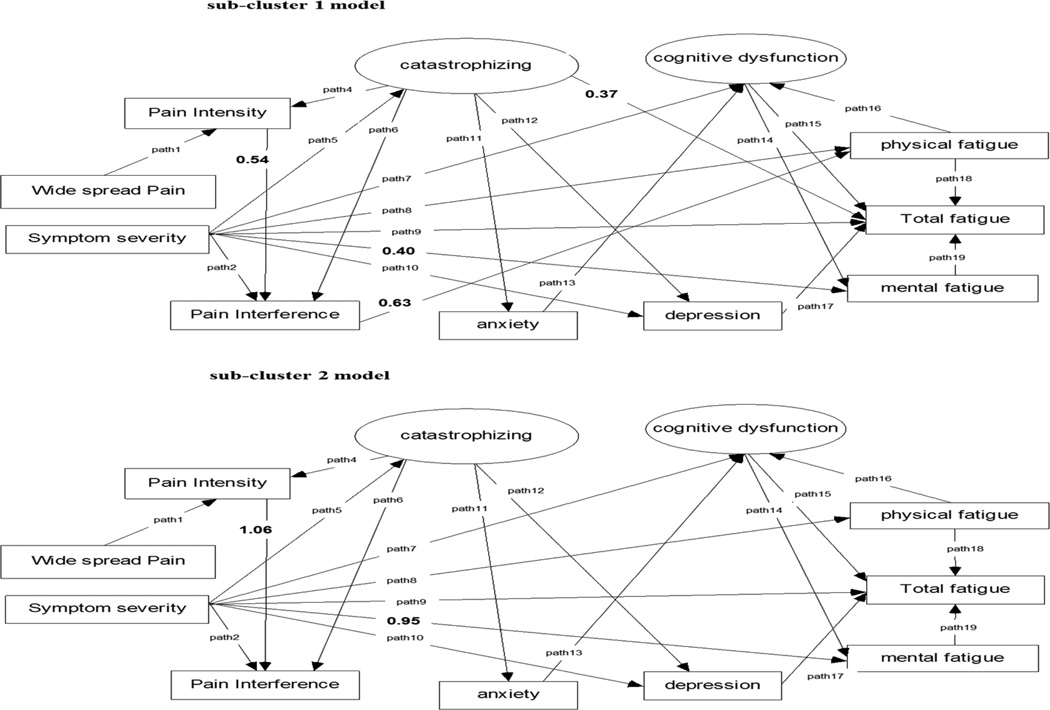
To examine the conformity of the FMS symptoms cluster model for the two identified FMS sub-clusters, a multiple-sample SEM analysis was conducted to compare the fully constrained FMS symptoms cluster model (all parameter estimates in the model between the two samples were the same) and the unconstrained FMS symptoms cluster model (all parameter estimates in the model between two samples varied). The results showed a significant difference between the fully constrained and unconstrained models (Δχ2 = 44.3, Δdf = 28, p = 0.03), indicating that each FMS sub-cluster has a distinct symptom sub-cluster model.
The significant paths that differed between the 2 sub-clusters using multiple sample SEM analyses are presented in Figure 3. The paths between pain intensity and pain interference (sub-cluster 1: β = 0.54, p < 0.05, sub-cluster 2: β = 1.06, p < 0.05) and between symptoms severity and mental fatigue (sub-cluster 1: β = 0.40, p < 0.05, sub-cluster 2: β = 0.95, p < 0.05) were significantly different in FMS sub-cluster 2, compared to the FMS sub-cluster 1 model. These FMS symptoms sub-cluster models suggest that pain appears to only influence physical fatigue, and only in FMS patients whose symptom experience is primarily defined by pain. The paths between catastrophizing and total fatigue and between pain interference and physical fatigue became non-significant (p > 0.05) in FMS sub-cluster 2.
Figure 3.
FMS symptoms sub-cluster models. Error terms not shown. In symptom sub-cluster model 2, the path from pain interference to physical fatigue and the path from catastrophizing to total fatigue were not significant.
DISCUSSION
The FMS symptoms cluster model developed in this study using structural equation modeling showed that fatigued FMS subjects have high pain severity, cognitive dysfunction, depression, anxiety, and catastrophizing, a consistent finding from previous studies (44, 45). Further investigation of this FMS symptoms cluster model revealed clinical sub-clusters of FMS patients. Previous studies identified heterogeneous clinical subgroups of patients with FMS based on their symptoms using the Multidimensional Pain Inventory (46), the Short Form 36 Health Survey (47), and the Visual Analog subscale of the Fibromyalgia Impact Questionnaire (48). One study categorized FMS subjects into three subtypes based on mood, cognition, and hyperalgesia (49). Our study reported 2 FMS clinical sub-clusters, sub-cluster 1 (defined by pain) and sub-cluster 2 (defined by fatigue). This provides further evidence that FMS is best considered an illness of polysymptomatic distress rather than a primary pain disorder.
One distinguishing feature between the FMS sub-cluster models points to how increasing pain intensity had stronger impact on pain interference in FMS sub-cluster 2 subjects (sub-cluster defined by fatigue) compared to FMS sub-cluster 1 subjects (defined by pain), suggesting that FMS sub-cluster 1 subjects may have adapted to their daily, persistent and more intense widespread pain compared to FMS sub-cluster 2 subjects. Further, FMS sub-cluster 2 subjects also complain of worst mental fatigue with increasing symptom severity, compared to FMS sub-cluster 1 subjects, confirming that fatigue is a more bothersome symptom for FMS sub-cluster 2 subjects with increasing symptom severity, compared to FMS sub-cluster 1 subjects.
The observation that 35% of the subjects in sub-cluster 2 did not met the 2010 FMS diagnostic criteria reminds that the 2010 FMS diagnostic criteria is most sensitive in capturing moderate to severe widespread pain symptoms. These results also demonstrate that a minority of persons can demonstrate the substantial widespread tenderness, as indicated by meeting the 1990 ACR criteria, despite not having substantial widespread pain within the last week. While there is a close relationship between clinical pain reporting and tenderness, it is not absolute.
Our FMS symptom sub-cluster models revealed that high pain intensity was not significantly associated with fatigue severity, but high depression was, which is consistent with previous findings in FMS (45) and other chronic conditions (e.g., rheumatoid arthritis, cancer) (14, 50). This important observation suggests that pain is not the main driver of the other symptoms experienced by FMS subjects. In fact, we also found in this study that depression is significantly associated with total fatigue, pain interference with physical fatigue, and cognitive dysfunction with mental fatigue. Previous studies in FMS and chronic fatigue syndrome reported a similar significant association between cognitive dysfunction and fatigue (51). The influence of cognition and the perception of fatigue support the proposed central mechanism of fatigue, suggesting that the sensation of fatigue is controlled by the combination of the afferent feedback from the periphery, current knowledge of the external environment, and prior experiences (51).
The study findings cannot be generalized because of limitations. First, this is a cross sectional study that attempted to identify relationships among self-reported symptoms. It is commonly known that the intensities of FMS symptoms change frequently. Therefore, FMS patients might shift from one sub-cluster to another based on the varying intensities of symptoms, day after day. Although this structural equation analysis precludes an interpretation of causality or directionality among symptoms, our FMS symptoms model was examined based on hypothesized relationships of symptoms from existing literature. Other plausible linkages among these variables may need to be investigated, because their indirect and reciprocal relationships were not tested. Therefore, a longitudinal study is warranted to explore causal relationships between the variables being investigated in this study. Additional investigations to include physiological measurements such as physical performance assessments, cognitive function tests, autonomic dysfunction measures, and biological profiling such as genetic and pro-inflammatory markers from a larger number of subjects, will be useful.
Conclusions
Our study suggested two heterogeneous categories of patients with FMS based on their symptom experiences. These symptoms sub-cluster models provide relevant information that can be useful for clinical diagnosis and management of FMS. A longitudinal study capturing the variability in symptom experience of FMS subjects and comparing the symptom sub-cluster models reported in our cross-sectional study will be informative.
Significance and Innovation.
-
-
This study investigated the symptoms cluster experienced by FMS patients using the score from FMS 2010 diagnostic tool with sophisticated statistical analysis.
-
-
The result of this study is a first step to help clinicians classify and provide personalized interventions for FMS patients.
-
-
Our study suggested 2 FMS sub-clusters and demonstrated the differences between the 2 sub-clusters
Acknowledgments
Source of Funding: This study is part of a collaborative activity of NINR and the Medstar Research Institute as approved by the Office of Human Subjects Research (OHSR) of the National Institutes of Health (OHSR IRB-Exempt #4966) and is funded in part by a MedStar grant (NCT00888563) and the Division of Intramural Research of the NINR.
Footnotes
Financial/nonfinancial disclosures: I and co-authors have no conflicts of interest to report.
REFERENCES
- 1.Wolfe F. New American College of Rheumatology criteria for fibromyalgia: a twenty-year journey. Arthritis Care Res (Hoboken) 2010;62:583–584. doi: 10.1002/acr.20156. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, Oh TH, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology project. Arthritis Care Res (Hoboken) 2012;65:786–792. doi: 10.1002/acr.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruera E. Cancer-related fatigue: a multidimensional syndrome. J Support Oncol. 2010;8:175–176. [PubMed] [Google Scholar]
- 4.Da Costa D, Dritsa M, Ring A, Fitzcharles MA. Mental health status and leisure-time physical activity contribute to fatigue intensity in patients with spondylarthropathy. Arthritis Rheumatol. 2004;51:1004–1008. doi: 10.1002/art.20841. [DOI] [PubMed] [Google Scholar]
- 5.Echteld MA, Passchier J, Teunissen S, Claessen S, de Wit R, van der Rijt CC. Multidimensional fatigue and its correlates in hospitalised advanced cancer patients. Eur J Canc. 2007;43:1030–1036. doi: 10.1016/j.ejca.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 6.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Canc Res Treat. 2008;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 7.Jason LA, Jordan KM, Richman JA, Rademaker AW, Huang CF, McCready W, et al. A Community-based study of prolonged fatigue and chronic fatigue. J Health Psychol. 1999;4:9–26. doi: 10.1177/135910539900400103. [DOI] [PubMed] [Google Scholar]
- 8.Echteld MA, Passchier J, Teunissen S, Claessen S, de Wit R, van der Rijt CC. Multidimensional fatigue and its correlates in hospitalised advanced cancer patients. Eur J Cancer. 2007;43:1030–1036. doi: 10.1016/j.ejca.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 10.Light AR, Vierck CJ, Light KC. In: Translational pain research: from mouse to man. Kruger L, Light A, editors. Boca Raton (FL): CRC Press; 2010. pp. 251–282. [PubMed] [Google Scholar]
- 11.Maes M. "Functional" or "psychosomatic" symptoms, e.g. a flu-like malaise, aches and pain and fatigue, are major features of major and in particular of melancholic depression. Neuroendocrinol Lett. 2009;30:564–573. [PubMed] [Google Scholar]
- 12.Garcia-Campayo J, Serrano-Blanco A, Rodero B, Magallon R, Alda M, Andres E, et al. Effectiveness of the psychological and pharmacological treatment of catastrophization in patients with fibromyalgia: a randomized controlled trial. Trials. 2009;10:24. doi: 10.1186/1745-6215-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abeles AM, Pillinger MH, Solitar BM, Abeles M. Narrative review: the pathophysiology of fibromyalgia. Ann Intern Med. 2007;146:726–734. doi: 10.7326/0003-4819-146-10-200705150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Brown LF, Rand KL, Bigatti SM, Stewart JC, Theobald DE, Wu J, et al. Longitudinal relationships between fatigue and depression in cancer patients with depression and/or pain. Health Psychol. 2013;32:1199–1208. doi: 10.1037/a0029773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolaus S, Bode C, Taal E, van de Laar MA. Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 2013;65:1128–1146. doi: 10.1002/acr.21949. [DOI] [PubMed] [Google Scholar]
- 16.Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain. 2002;100:271–279. doi: 10.1016/S0304-3959(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 17.Kurtze N, Svebak S. Fatigue and patterns of pain in fibromyalgia: correlations with anxiety, depression and co-morbidity in a female county sample. Br J Med Psychol. 2001;74:523–537. doi: 10.1348/000711201161163. [DOI] [PubMed] [Google Scholar]
- 18.Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain. 2005;21:439–445. doi: 10.1097/01.ajp.0000135236.12705.2d. [DOI] [PubMed] [Google Scholar]
- 19.Lukkahatai N, Saligan LN. Association of catastrophizing and fatigue: a systematic review. J Psychosom Res. 2013;74:100–109. doi: 10.1016/j.jpsychores.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaron LA. The moderating effects of catastrophizing as a response to daily pain in patients with fibromyalgia [thesis] Birmingham: University of Alabama; 1999. [Google Scholar]
- 21.Nicassio PM, Ormseth SR, Custodio MK, Irwin MR, Olmstead R, Weisman MH. A multidimensional model of fatigue in patients with rheumatoid arthritis. J Rheumatol. 2012;39:1807–1813. doi: 10.3899/jrheum.111068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanuar F, Ibrahim K, Jemain AA. On the application of structural equation modeling for the construct of a health index. Environ Health Prev Med. 2010;15:285–291. doi: 10.1007/s12199-010-0140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westland JC. Lower bounds on sample size in structural equation modeling. Electronic Commerce Research and Application. 2010;9:476–487. [Google Scholar]
- 24.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 26.Tunks E, McCain GA, Hart LE, Teasell RW, Goldsmith CH, Rollman GB, et al. The reliability of examination for tenderness in patients with myofascial pain, chronic fibromyalgia and controls. J Rheumatol. 1995;22:944–952. [PubMed] [Google Scholar]
- 27.Cleeland CS, Ladinsky JL, Serlin RC, Nugyen CT. Multidimensional measurement of cancer pain: comparisons of US and Vietnamese patients. J Pain Symptom Manag. 1988;3:23–27. doi: 10.1016/0885-3924(88)90134-0. [DOI] [PubMed] [Google Scholar]
- 28.Kapstad H, Rokne B, Stavem K. Psychometric properties of the Brief Pain Inventory among patients with osteoarthritis undergoing total hip replacement surgery. Health Qual Life Outcome. 2010;8:148. doi: 10.1186/1477-7525-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Savard J, Laberge B, Gauthier JG, Ivers H, Bergeron MG. Evaluating anxiety and depression in HIV-infected patients. J Pers Assess. 1998;71:349–367. doi: 10.1207/s15327752jpa7103_5. [DOI] [PubMed] [Google Scholar]
- 32.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20:589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 33.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 34.Banos JH, LaGory J, Sawrie S, Faught E, Knowlton R, Prasad A, et al. Self-report of cognitive abilities in temporal lobe epilepsy: cognitive, psychosocial, and emotional factors. Epilepsy Behav. 2004;5:575–579. doi: 10.1016/j.yebeh.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Williams DA, Arnold LM. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) Sleep Scale, and Multiple Ability Self-Report Questionnaire (MASQ) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S86–S97. doi: 10.1002/acr.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 38.Spira AP, Beaudreau SA, Stone KL, Kezirian EJ, Lui LY, Redline S, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol Biol Med Sci. 2012;67:433–439. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arbuckle JL. Amos 17.0 user's guide. Crawfordville (FL): Amos Development Corporation; 2009. [Google Scholar]
- 40.Do CB, Batzoglou S. What is expectation maximization algorithm? Nat Biotechnol. 2008;26:897–899. doi: 10.1038/nbt1406. [DOI] [PubMed] [Google Scholar]
- 41.Barrett P. Structural equation modeling: adjudging model fit. Pers Indiv Differ. 2007;42:815–824. [Google Scholar]
- 42.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alteratives. Struct Equat Model. 1999;16:1–55. [Google Scholar]
- 43.Chou CP, Bentler P. Model modification in covariance structural modeling: a comparison among likelihood ratio, Lagrange Multiplier, and Wald tests. Multivariate Behav Res. 1990;25:115–136. doi: 10.1207/s15327906mbr2501_13. [DOI] [PubMed] [Google Scholar]
- 44.Suhr JA. Neuropsychological impairment in fibromyalgia: relation to depression, fatigue, and pain. J Psychosom Res. 2003;55:321–329. doi: 10.1016/s0022-3999(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 45.Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29:S92–S96. [PubMed] [Google Scholar]
- 46.Turk DC, Okifuji A, Sinclair JD, Starz TW. Pain, disability, and physical functioning in subgroups of patients with fibromyalgia. J Rheumatol. 1996;23:1255–1262. [PubMed] [Google Scholar]
- 47.Oswald J, Salemi S, Michel BA, Sprott H. Use of the Short-Form-36 Health Survey to detect a subgroup of fibromyalgia patients with psychological dysfunction. Clin Rheumatol. 2008;27:919–921. doi: 10.1007/s10067-008-0874-4. [DOI] [PubMed] [Google Scholar]
- 48.de Souza JB, Goffaux P, Julien N, Potvin S, Charest J, Marchand S. Fibromyalgia subgroups: profiling distinct subgroups using the Fibromyalgia Impact Questionnaire. A preliminary study. Rheumatol Int. 2009;29:509–515. doi: 10.1007/s00296-008-0722-5. [DOI] [PubMed] [Google Scholar]
- 49.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheumatol. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 50.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537–543. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ickmans K, Meeus M, Kos D, Clarys P, Meersdom G, Lambrecht L, et al. Cognitive performance is of clinical importance, but is unrelated to pain severity in women with chronic fatigue syndrome. Clin Rheumatol. 2013;32:1475–1485. doi: 10.1007/s10067-013-2308-1. [DOI] [PubMed] [Google Scholar]