1. Introduction
Vascularization is one of the most important aspects to the success of tissue engineered constructs and constitutes a major hurdle facing the regenerative medicine field [1]. Tissues that have undergone significant damage exhibit an incredibly hypoxic environment due to the destruction of the local vascular network. Large bone defects that do not fully heal on their own are termed critically-sized (non-healing) bone defects and exhibit this hypoxic condition [2]. Tissue engineered constructs delivering cells or biological factors have been used to bridge these large bone defects; however, in many cases these constructs fail to perform due to a lack of vascular perfusion. Embedded cells exhibit poor survival due to the lack of oxygen, adequate nutrient supply and efficient waste removal [3]. Moreover, poor blood perfusion results in the absence of recruitment of endogenous cells to contribute to the healing process. Since biological factors included in tissue engineered constructs usually target these endogenous cells, the lack of efficient recruitment can hinder therapeutic outcomes. Various strategies aim at increasing the early vascular response following injury including delivering angiogenic growth factors, implantation of vascular cells and gene therapy. In addition to these strategies, significant efforts are directed at engineering scaffolds that work in concert with these agents to augment the vascular response. When designing an appropriate scaffold for bone vascularization, there are multiple important factors to keep in mind. The biomaterial used should allow tissue remodeling as both the mineralizing bone and neovascular networks dynamically remodel over the course of the healing process in response to biological stimuli. In addition, specifically for bone engineering, the scaffold may need to provide a biomechanically stable environment to support the load-bearing nature of the musculoskeletal system. Current materials used as bone grafts address these concerns but still lack a synergistic relationship between the scaffold itself and the embedded factors.
This review will focus on vascularization strategies currently being explored to treat critically-sized bone defects as well as provide an outlook on future generations of biomaterials engineered for this purpose. We will start with a brief introduction to bone physiology including bone development and the different growth factors and cells at work in this process. This will provide a general background for further discussion in vascularization strategies including growth factor delivery, cell delivery with stem cells, co-delivery of stem cells and vascular cells, gene therapy and combinatorial therapies. We will end by emphasizing the importance of biomaterials engineering and discussing how strategies currently used in related tissue engineering fields can apply to bone regeneration. With the wide amount of reviews present for vascularizing bone defects, we will focus on strategies currently used in vivo in animal models.
2. Mechanisms of Bone Formation
2.1 Endochondral Ossification
Depending on the location, bone develops by either of two pathways: intramembranous or endochondral ossification [4]. A common feature between these two ossification methods is that pre-vascularization is necessary for both processes to create fully functional bone. Endochondral ossification, the process through which all long and load-bearing bones in the body are generated and is characterized through development by a cartilage intermediary, initiates through migration and differentiation of mesenchymal stem cells (MSCs) into chondrocytes in part through activation and suppression of the transcription factors Sox9 and β-catenin, respectively [5]. Differentiated chondrocytes then proliferate under the control of the Sox9 transcription factor, and ultimately undergo hypertrophy through activation of Runx2 followed by apoptosis following secretion of collagen and proteoglycans. Prior to apoptosis, these hypertrophic chondrocyte cells secrete a synchronized cascade of chemokines and cytokines that recruit endothelial cells and associated vasculature. The invading vasculature then allows for recruitment of osteoclasts, which subsequently remove the cartilaginous matrix and allow for osteoprogenitors to migrate to and deposit calcium and bone matrix into the remnants of the matrix [6]. While osteoprogenitors originate from the same cell type as the initial chondrocytes that laid down the cartilaginous matrix, early activation of the transcription factor Runx2 in the absence of Sox9 followed by upregulation of osterix, alkaline phosphatase and ostepontin cause MSC differentiation down the osteogenic lineage [7]. An in-depth review of the molecular signals and pathways governing bone development can be found elsewhere [8].
Throughout this process, the spatiotemporal regulation of growth factor activity provides necessary cues for proper skeletogenesis. Notably, vascular endothelial growth factor A (VEGF) along with the family of bone morphogenetic proteins (BMPs) and fibroblast growth factors (FGFs) are central to this process. Initially, BMP 2, 4, 7 and 9 all aid in the initiation of chondrocyte differentiation and cartilage development; deletion of these growth factors leads to gross absence of most skeletal components [9, 10]. Once chondrocytes begin to undergo hypertrophy, their VEGF mRNA expression increases with subsequent secretion of the protein resulting in elevations in the proliferative capacity of nearby chondrocytes as well as inducing vascular invasion into the ischemic cartilaginous region [11–13]. Blocking the activity of VEGF through soluble VEGF receptors impairs angiogenesis, ossification and results in massive cell death in chondrocytes, whereas reverting this blocking treatment restores normal bone formation [11–13]. Coinciding with this increase of locally secreted VEGF, expression of FGF18 is increased and acts as a negative regulator of chondrocytic proliferation through FGF receptor 3 (FGFR3) [14]. Mice lacking either the Fgf18 or Fgfr3 gene exhibit heightened levels of chondrocyte proliferation and associated lower levels of osteogenic differentiation from invading MSCs into the ossification front. The fact that VEGF increases the proliferation of chondrocytes while the presence of FGF18 counteracts this process indicates a tightly regulated process in which it has been postulated that VEGF expression is controlled through activation of FGFR3 [15].
2.2 Intramembranous Ossification
In contrast to endochondral ossification, intramembranous ossification forms bone without a cartilage intermediary and is the mechanism by which all flat bones, including the cranial and clavicle bones, are formed [16]. In this process, MSCs directly differentiate into osteoblasts and secrete bone matrix into the surrounding ECM. Prior to their differentiation, MSCs destined to become osteoblasts begin to condense at the area of ossification with various FGFs being highly expressed in center of ossification [17]. Following condensation, spatiotemporal concentrations of BMP2, BMP4, and BMP7 activate the expression of Runx2 which acts as a transcription for further osteogenic differentiation [18]. Expression of late-markers for differentiation including osteocalcin and osteopontin requires the initial activation of Runx2. In regards to vascularization, the spatiotemporal expression of angiogenic factors such as VEGF and HIF-2α direct surrounding blood vessels to invade the mesenchymal condensation near the time of initial ossification [19]. As ossification occurs surrounding the blood vessels, the mesenchymal stem cells not involved in ossification at the time migrate outward. Blood vessels continue to extend toward these migrating cells with mineralization occurring near these sprouting vessels [19].
2.3 Vascular Supply of Bone
Bone is a highly dynamic and vascularized tissue undergoing constant remodeling in order to achieve its two primary tasks: structural stability and calcium homeostasis [20]. In certain long bones, the presence of bone marrow also houses a stem cell niche which is invaluable for health. To ensure bone does not lose its blood supply due to minor obstructions, numerous avenues of blood flow are present within bone. Long bones have a main diaphyseal nutrient artery as well as epiphyseal, metaphyseal and periosteal blood vessels that enter the bone and connect it to the surrounding tissue’s vascular supply. Consisting of the basic structural unit of cortical bone, the osteon has a central Haversian canal in which arteries and veins reside in. These arteries, along with blood vessels inhabiting the Volkmann canals, bring oxygen and nutrients to osteocytes embedded within the concentric mineralized lamellae as well as cells associated with the basic multicellular unit including endothelial cells, osteoblasts and osteoclasts. In short and flat bones, blood vessels run alongside the periosteal surface with certain regions having superficial periosteal arterioles that penetrate the periosteum. An in-depth overview of bone physiology including vascular supply can be found elsewhere [21].
3. Current strategies for vascularization and bone regeneration in bone defects
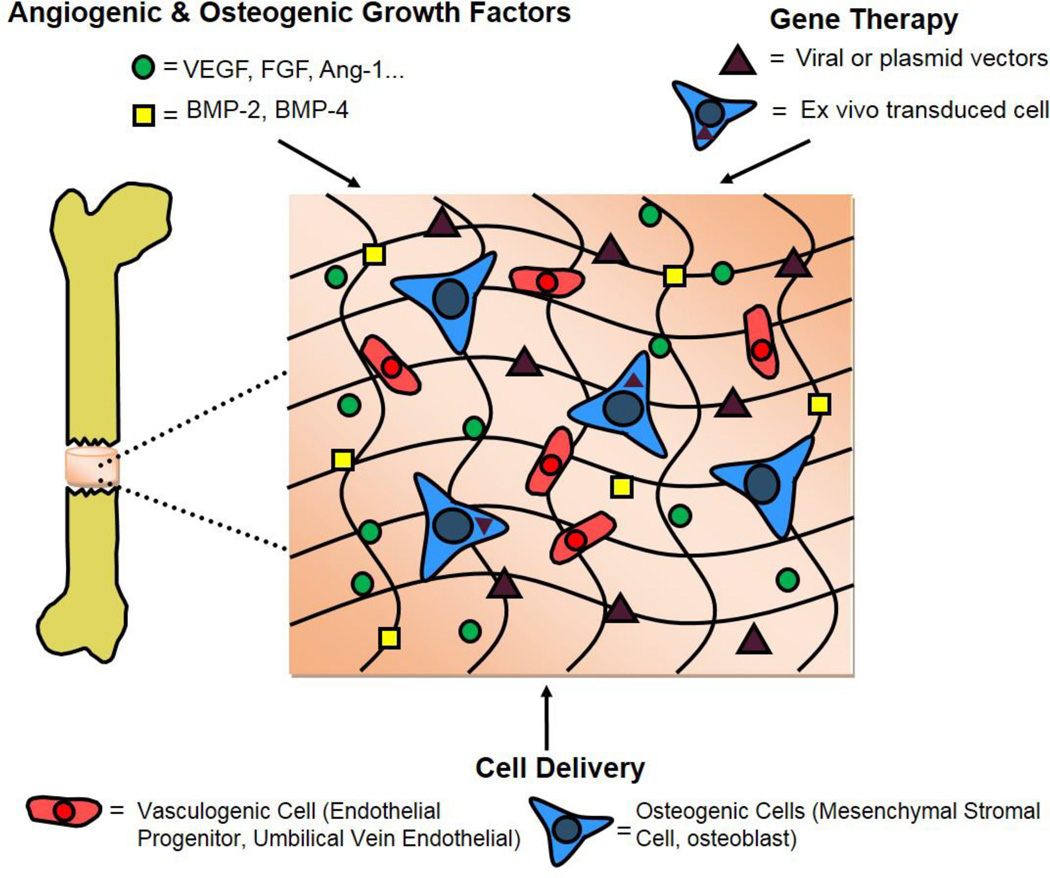
Re-vascularization of a bone defect can vastly improve the regenerative response by providing needed access to nutrients as well as a supply of stem and inflammatory cells [22, 23]. Whereas vascularized autologous bone grafts can rapidly integrate into the recipient site providing initial strength and stability to the region, the limited supply of this material and associated issues with harvesting it mandate the need for alternative strategies [24]. Various regenerative medicine approaches aimed at the restoration of vasculature and associated bone regeneration have been explored. The following sections will review current in vivo strategies that use engineered biomaterials in combination with various technologies ranging from incorporation and presentation of angiogenic growth factors, cells, and genetic strategies to induce vascularization and bone regeneration in osseous defects (Figure 1).
Figure 1.
Current biomaterial-based strategies for inducing vascularization of a critically-sized bone defect
3.1 Angiogenic and Osteogenic growth factor incorporation
One of the most widely used approaches for the induction of vascular invasion is delivery of angiogenic and osteogenic growth factors. The growth factors VEGF, FGF2, BMP2, BMP7, PDGFB and TGFB1 have all been explored to increase the early onset of vascular invasion with the most prominently utilized growth factors being VEGF and BMP2 [25]. VEGF is a potent angiogenic/vasculogenic growth factor and exerts its effects through interacting with two receptor tyrosine kinases, VEGFR1 (otherwise known as Flt1) and VEGFR2 (otherwise known as Flk1) [26]. Activation of these receptors in endothelium causes destabilization of the junctions holding endothelial cells in order to facilitate angiogenesis. Once broken down, VEGF acts as a chemotactic factor as well as signals for the proliferation of endothelial cells. One of the first studies investigating VEGF for bone regeneration showed in a fracture model in mice that treatment with a soluble VEGF receptor blocking endogenous VEGF activity impaired new bone formation [27]. Additionally, when VEGF was continuously delivered to critically-sized bone defects in rabbits over the course of seven days via a subcutaneously implanted osmotic pump, significant bone regeneration was observed in comparison to no VEGF treatments. One of the drawbacks with this study, however, was the supraphysiological doses of VEGF required to induce bone regeneration as high microenvironmental concentrations of VEGF results in the formation of aberrant and leaky neovasculature [28, 29].
BMPs have also been extensively utilized in bone regeneration therapies, and BMP2 therapy is currently FDA approved for certain lumbar spinal fusions [30]. Considered an osteogenic factor, BMPs are well known to induce the osteogenic differentiation of MSCs in vitro [31, 32]. When co-delivered with stromal cells in a murine subcutaneous model, BMP2, 4, 6, 7, and 9 all exhibit robust ALP activity and elevated calcium deposition pointing to their effectiveness in vivo as well. [33]. Recent studies indicate that BMPs also regulate angiogenesis and VEGF secretion through its auto- and paracrine actions on osteoblasts and MSCs [34, 35]. When cultured in media containing BMP4, preosteoblasts increase their VEGF production in a dose-dependent manner while addition of the BMP-inhibitor Noggin causes VEGF levels to drop to non-BMP stimulated control levels [36]. BMP2 also induces proliferation of endothelial cells as well as elevates their tube-forming capacity in Matrigel angiogenesis assays [37, 38]. In clinical settings, recombinant human BMP2 (rhBMP2) is delivered via an absorbable collagen sponge under the trade name INFUSE to treat patients suffering from significant bone loss or in need of bone fusion. While having significant success in the clinic, delivery of this growth factor within a collagen sponge results in a large burst release at early time points, and this delivery vehicle requires the use of supraphysiological doses of rhBMP2 to attain significant bone formation [39, 40]. However, elevated doses of are associated with excessive amounts of ectopic bone formation and increased inflammation and neuropathies, deleterious consequences in clinical settings [41, 42]. Because of this, numerous groups are exploring various methodologies to control the release and presentation of BMP2 in order to reduce the dose delivered driving down both unwanted effects as well as total cost of therapy.
To optimally control the kinetics and distribution of therapeutic protein release and subsequently lower the total delivered dose, biomaterials have been engineered to achieve tailored and sustained delivery profiles. A common strategy is absorbing and entrapping growth factors within biomaterial scaffolds synthesized with pore sizes that would dictate growth factor release. Early reports utilizing BMPs focused on incubating biomaterials including ceramic and polymer scaffolds in growth factor solutions until saturation was reached followed by immediate implantation [43–47]. Many of these studies achieved success in terms of bone repair, but were limited by using high doses of BMP as the materials exhibit an initial burst release followed by a gradual slow release based on the scaffold structure. Whereas these studies did not specifically focus on vascularization, subsequent research employed similar biomaterial strategies with VEGF to investigate whether this factor alone can induce vascularization and bone repair. Entrapment of VEGF into β-TCP scaffolds exhibited increased invasion of microvasculature and osseointegration in a murine calvarial defect [48]. Similarly, incorporation of VEGF into a PLGA scaffold followed by coating with bioactive glass showed increased infiltration of blood vessels with an increase in bone mineral density in a rat calvarial defect compared to scaffolds without VEGF [49].
Interestingly, even though endogenous VEGF is crucially important in the development and repair of bones, many reports utilizing VEGF in bone defects show no difference between scaffolds with and without VEGF and studies have instead investigated the synergistic effects of VEGF with other proteins [50–52]. Co-delivering VEGF and BMP2 within polymer scaffolds enhances bone regeneration in critically-sized defects compared to that of the delivery of single growth factors [53, 54]. Additionally, delivering VEGF alone, but not BMP2 alone, exhibited increased blood vessels within the defect regardless of whether or not BMP2 was also delivered. Other studies have investigated the effects of temporal cascades of dual growth factors through the use of specifically engineered biomaterials. Mikos and colleagues designed a system in which BMP2 was loaded into PLGA microparticles, embedded within a poly(propylene fumarate) (PPF) scaffold which was then further embedded within a VEGF-loaded gelatin hydrogel and implanted into either a subcutaneous pocket or a critically-sized rat femoral defect [55]. The setup allows quick release of VEGF thus eliciting a vasculogenic response followed by slow release of the osteogenic BMP2 as vasculature invaded the defect. The results, however, showed that this temporally-controlled delivery had no beneficial effect on bone regeneration in the orthotopic model over the delivery of BMP2 alone. In the subcutaneous model however, increased ectopic bone and blood vessel volume was seen compared to BMP2 or VEGF only scaffolds. A similar experiment utilizing a combination of acidic and basic gelatin microparticles where the different charges result in a similar VEGF/BMP2 release profile as Mikos’ study also demonstrated that in an orthotopic model, the dual temporal control of VEGF and BMP2 produced no increase in bone formation compared to BMP2 only scaffolds [56]. The lack of bone regeneration with the temporal cascade indicates that it is possible that both growth factors need to be present at relevant microenvironmental concentrations at the same time to be effective therapeutically, although identifying what these target concentrations are and how to control them depends on the system and model being used.
A continually pervasive issue in growth factor therapy is the relatively short half-life many proteins have once scaffolds are transplanted. To circumvent this issue, novel strategies are using small pharmacological agents to promote vascularization in bone defects mainly through regulation of early inflammation [57, 58]. Delivery of FTY720, a selective agonist for the sphingosine 1-phosphate receptor, has been shown to promote the formation of new arterioles, enlarge existing arterioles, and enhance the recruitment of anti-inflammatory monocytes in a mouse skinfold window chamber [59, 60]. When used in orthotopic defect models, FTY720 increases both the number of mature blood vessels and the structural integrity of newly formed bone compared to that of scaffolds lacking the small molecule [61, 62].
3.2 Cell Delivery
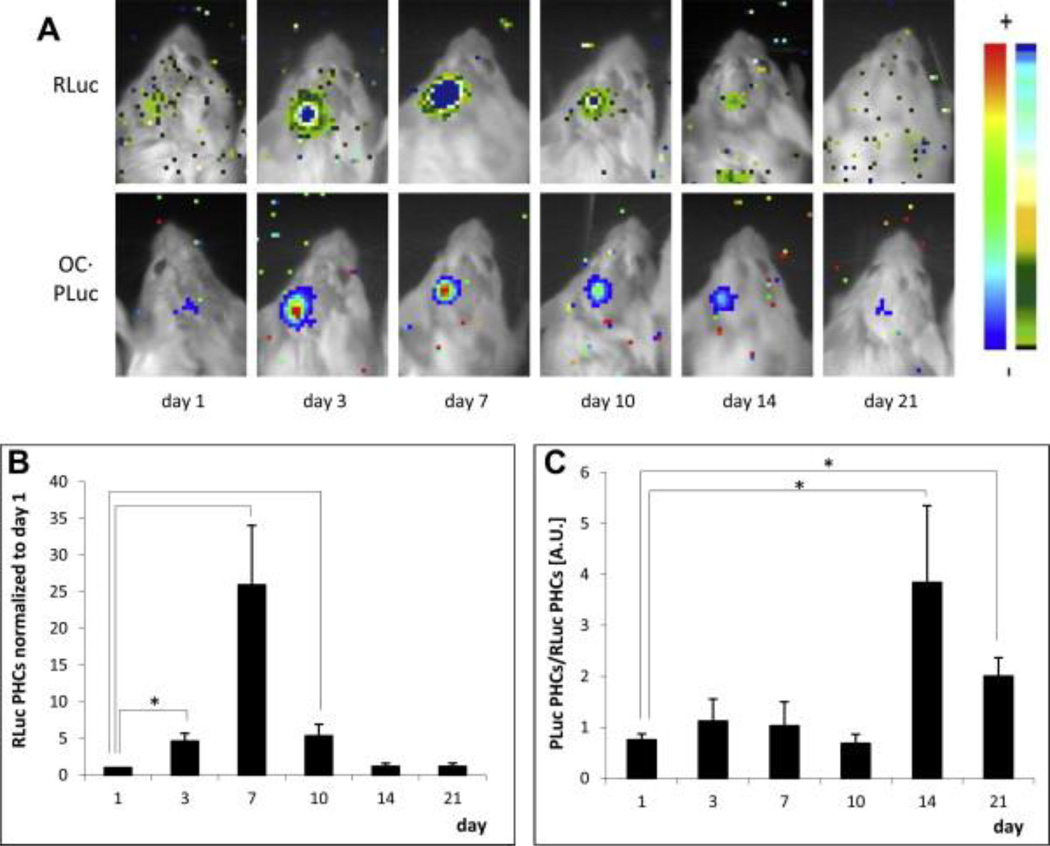
Several groups have investigated the extent to which delivering one or multiple cell types within implantable scaffolds influences vascularization and repair of bone defects, as reviewed in [63]. Initial cell-delivery studies investigating bone tissue regeneration focused on solely transplanting osteogenic cells, mainly bone marrow-derived MSCs, into bone defects [64]. MSCs have long been recognized as clinically relevant cells for bone applications due to their ability to differentiate down an osteogenic lineage not only in vitro, as is widely known, but also in vivo [65]. Vila et al. demonstrated, using a dual-luciferase tracking system in which MSCs were transduced to express luciferase driven by constitutive and osteocalcin (OC)-responsive promoters, that MSCs implanted into calvarial defects in mice greatly upregulated their expression of osteocalcin, a late-marker for osteogenic differentiation [66]. In addition to their osteogenic capabilities, the ability to isolate MSCs from either bone marrow or adipose tissue, their immunosuppressive phenotype and the potential for utilization in autologous therapies make these cells alluring for both clinicians and researchers [67, 68]. Importantly, the study from Vila et al. shows the bioluminescence from the constitutively-expressed luciferase decreased across all groups after one week reinforcing our current inability to maintain long-term cell engraftment and survival with current biomaterials (Figure 2).
Figure 2.
Bioluminescence imaging of Renilla reniformis (RLuc) luciferase under transcriptional control of the cytomegalovirus promoter and PLuc under transcriptional control of the human osteocalcin promoter in transduced human adipose-derived mesenchymal stem cells. Cells were seeded within fibrin matrices and implanted in a 3 mm calvarial defect in SCID mice. A) Representative bioluminescence images showing RLuc (top row) and PLuc (bottom row) intensities. Color bars indicate light intensities from RLuc (black=low; blue=high) and PLuc (blue=low; red=high) B) Quantification of the bioluminescence signal under the constitutive promoter RLuc normalized to day 1. C) Quantification of the bioluminescence signal under the osteocalcin promoter PLuc normalized to RLuc. While osteocalcin signal increases at 2 and 3 weeks, the total amount of cell signal decreases after week one in immunocompromised mice. Adapted from Vila et al [66].
In terms of vascularization, the potential of MSCs to differentiate into endothelial-like cells and generate capillary-like structures when cultured in specifically defined media has been reported [69–71]. Although early studies concluded that VEGF was the most important growth factor regulating this differentiation, recent reports have disputed this claim showing no evidence of dose-dependent response of VEGF on expression of endothelial cell-related genes and proteins [72]. In a similar fashion, shear stress has been implicated in inducing endothelial differentiation of MSCs [73, 74]. However, with MSCs also showing upregulation of osteogenic markers in response to shear stress, it is still unknown what specific environmental factor(s), either acting separately or in conjunction, causes the cascade resulting in apparent endothelial differentiation [75]. Nevertheless, this endothelial potential does not seem evident when these cells are implanted within a bone defect. In the same study showing positive osteocalcin expression of MSCs, Vila et al. also transduced MSCs to express a luciferase-dependent promoter for PECAM1, an endothelial-specific marker, and observed a decrease in PECAM1 expression when MSCs were implanted within a bone defect but an increased expression when implanted intra-muscularly denoting how the microenvironmental cues influence the fate of implanted cells [66]. Although MSCs do not themselves differentiate down an endothelial lineage when implanted in a bone defect, they appear to aid in recruiting endogenous endothelial cells to begin vascular repair [76]. Rather than relying on recruitment of host cells, groups have investigated delivering a second type of cell in addition to MSCs to serve as a vasculogenic cue.
Constituting the primary cell type lining the vasculature, bonafide endothelial cells have been extensively studied for their ability to form 3-D vascular-like networks in either mono-culture or when co-cultured with a variety of cell types ranging from embryonic fibroblasts to adult MSCs [77–82]. More importantly, endothelial cells cultured in 3-D matrices exhibit the ability to undergo anastomosis with native vasculature and rapidly perfuse a tissue-engineered construct when implants are vascularized with cells prior to implantation [83–85]. When human umbilical vein endothelial cells (HUVECs) are cultured with fibroblasts within a fibrin gel for 7 days pre-implantation subcutaneously, by 5 days post-implantation, robust vasculature can be seen protruding from the surrounding tissue into the construct [83]. This strategy of pre-vascularizing implants is also applicable to endothelial progenitor cells (EPCs) as these cells have also been utilized in successful anastomosis with host vasculature and even exhibited elevated scaffold perfusion compared to scaffolds pre-vascularized with HUVECs and human dermal microvascular endothelial cells (HDMECs) [86].
Co-culturing of this ‘osteogenic’ cell type, MSCs, and the ‘vasculogenic’ cell type, endothelial cells, shows considerable synergism. Alkaline phosphatase (ALP) activity, an early marker for osteogenic differentiation, as well as gene expression for BMP2 and bone sialoprotein are all upregulated in MSCs when the cells are cultured in 2D with endothelial cells [87–89]. Co-culturing MSCs with EPCs also increases ALP activity as well as calcium deposition compared to MSC monocultures [90]. Interestingly, these increases are only observed when the two cell types are incubated in direct contact with each other rather than in cell culture inserts, indicating the need of cell-cell contact [87]. Co-culturing in 3D conditions, such as within β-TCP or polycaprolactone scaffolds, also shows similar trends with increased ALP and osteocalcin expression from MSCs compared to mono-culture conditions yet exhibiting little to no difference in other osteogenic markers such as Runx2 expression [91–93]. In addition to MSCs, co-culturing bonafide osteoblasts with endothelial cells imparts beneficial effects onto osteoblasts by increasing their proliferation while decreasing the expression of proteins involved in apoptotic pathways [94]. Osteoblasts also exhibit enhanced secretion of collagen type I and VEGF when co-cultured with endothelial cells and, similar to MSCs, only exhibit this upregulation when cultured in direct contact with endothelial cells [95].
Subsequent studies have investigated whether the synergism present between osteogenic and endothelial cells translates into enhanced vascularization and associated bone formation in both subcutaneous and orthotopic animal models. In a murine subcutaneous model, scaffolds delivering both MSCs and either EPCs or HUVECs exhibit heightened ectopic bone formation as well as capillary infiltration and anastomosis compared to those delivering MSCs or endothelial cells alone [96, 97]. Scaffolds with endothelial cells only displayed immature vascular networks which ultimately regressed while scaffolds only having MSCs had lower blood vessels overall. In a study focusing on a scaffold-free, cell-sheet technology, Ren et al. engineered a material having two concentric layers of unique cell sheets encompassing the implant [98]. The inner cell sheet consisted of either mono-cultured MSCs or co-cultured MSCs with HUVECs while the outer sheer contained osteogenically-differentiated MSCs. When implanted subcutaneously, the constructs containing the co-cultured cell sheet exhibited faster anastomosis with the host vasculature and elevated osteocalcin staining compared to those of mono-cultured sheets.
Whereas results from subcutaneous implantation studies have shown generally positive results in terms of osteogenic differentiation of MSCs when co-delivered with endothelial cells, results from more clinically relevant orthotopic studies provide a more ambiguous picture. Utilizing a decalcified porcine bone construct within a murine calvarial defect, Koob et al. saw no difference in bone formation after 6 weeks between scaffolds seeded with MSCs or those seeded with a combination of MSCs and HUVECs [99]. Although co-cultured scaffolds did display higher neovascularization and formation of more mature, α-smooth muscle actin (ACTA2) positive vessels compared to those seeded with either MSCs or HUVECs alone, this did not translate to elevated bone formation. In another study using decellularized bone allografts, Cornejo et al. found that seeding adipose-derived endothelial cells increases bone healing in a rat calvarial defect compared to allografts having either osteoblasts or a co-culture of adipose-derived osteoblasts and adipose-derived endothelial cells [100]. In addition, implantation of adipose-derived endothelial cells increased the number of blood vessels within the defect compared to osteoblasts alone, or osteoblast + adipose-derived endothelial cells. The fact that stromal-cell derived endothelial cells seemed to increase bone healing compared to delivery of osteoblasts or the combination of these two cell types is particularly interesting as this could imply that the osteogenic signals needed for proper bone regeneration may be imparted by the decellularized allograft with the endothelial cells supporting the needed angiogenic signals. In lieu of bone allografts and instead utilizing a porous titanium fiber mesh scaffold delivered into a rat cranial defect, Ma et al. observed increased bone formation in scaffolds seeded only with adipose-derived MSCs compared to those seeded with both adipose-derived MSCs and HUVECs [101].
The number of studies showing a detrimental or no-effect of co-delivery of osteogenic and vasculogenic cells in orthotopic animal models are balanced by studies showing positive effects on both neovascularization and associated bone healing. When implanted within a HA/PCL scaffold into a murine critically-sized femoral defect model, Yu et al. reported that MSCs differentiated into osteoblasts along with endothelial cells derived from EPCs increased blood vessel invasion and newly formed bone compared to mono-culture conditions after 6 weeks [102]. Pang et al. showed delivery of non-differentiated MSCs with EPCs within a decellularized bone matrix increased neovascularization at 2 weeks post implantation with an associated increase in bone healing at 12 weeks within a rabbit radial segmental defect [103]. Interestingly, an immunohistochemical analysis showed that blood vessels in grafts containing both MSCs and EPCs exhibited increased VEGF expression compared to grafts containing a single cell type. These neovascularization results have also been mirrored by studies showing delivering MSCs and EPCs seeded inside β-TCP granules within a rat femoral defect enhanced the ingrowth of vasculature within one week post-implantation with associated increases in bone regeneration at 4 and 8 weeks post implantation [104, 105]. The disparity between the various studies co-delivering osteogenic and vasculogenic cells merits further investigation as to the underlying parameters that ultimately regulate and determine whether or not bone will heal. Factors such as cell numbers, differentiation and growth protocols of implanted cells and types of models and biomaterials used can all determine the efficacy of treatment and for this therapy to move forward to the clinic, it will be necessary to fully evaluate under what conditions do MSCs and endothelial cells synergistically work to generate vascularized bone.
Currently, there are several clinical trials underway evaluating the safety and efficacy of using MSCs in bone regeneration (clinicaltrials.gov). An important aspect to keep in mind however in adapting a cellular therapy for clinical applications is the obstacles it will face in cell sourcing and regulatory approval. The decision to use either allogenic or autologous MSCs will immensely impact the nature of treatment with allogenic MSCs being simpler to expand to clinically relevant numbers but may warrant the use of immunosuppressive therapy following infusion [106]. In addition, procuring allogenic MSCs requires extensive donor and cellular screening to ensure safety of transplantation [107]. In contrast, autologous MSC therapy bypasses many of the screening platforms but is subject to multiple medical procedures related to the extraction and reinfusion of stem cells with possible expansion occurring in between. The business model for autologous therapy is also more difficult to implement as it requires individualized cell expansion for each patient. The decision is further muddled in terms of bone healing as differing cell sources exhibit different regeneration potentials [108]. Autologous and allogenic sourcing will both be subject to non-trivial cell expansion procedures which requires Good Manufacturing Practice (GMP) SOPs and facilities for FDA approval.
The need for cell expansion is a point of current debate as studies have previously shown positive bone regeneration results by extracting, purifying and re-infusing MSCs from the patient’s bone marrow into the bone defect all within the time frame of one surgery [109, 110]. While this method provides about 104–105 MSCs for transplantation, other studies have investigated the use of ex vivo expanded MSCs such that at the time of transplantation, 107–109 MSCs are delivered [68, 111, 112]. While there is no clear consensus on the optimal dose of MSCs for beneficial therapeutic outcomes, it will undoubtedly depend on the type of non-union and range on a patient-to-patient basis with traumatic injuries more than likely necessitating cell numbers only achievable through ex vivo expansion. Additionally, the exact mechanisms by which MSC delivery aids bone is still not fully elucidated with current research focusing on whether stem cells integrate and differentiate into bone or simply provide trophic and paracrine effects. The distinction between these two methods of regeneration will shed further light onto the dose of cells needed.
Sourcing clinically-relevant endothelial cells for co-delivery also brings challenges as mature endothelial cells exhibit low regenerative potential [113]. Companies offer umbilical cord tissue banking which would enable extraction of residing endothelial cells for future autologous therapies; however, the percentage of patients having access to this is relatively small at the moment and therapies using this strategy are not suitable for this generation. EPCs are currently the subject of clinical trials aimed at restoring vasculature at ischemic sites but a lack of agreement as to the biological markers and proper isolation protocols that result in bona fide EPC makes purification difficult to define and regulate [114]. Furthermore, for EPCs to show reasonable therapeutic benefits in severe ischemia, it is estimated that as much 1.0 × 109 cells are required [115]. Since on average, only 5.0 × 106 EPCs can be isolation per 100 milliliters of peripheral blood after 7 days in culture, this total number translates to 20 liters of peripheral blood, a daunting task for clinical relevance.
3.3 Combined Cell and Growth Factor Delivery
Within a physiological environment, a continuous restructuring of the ECM by surrounding cells releases a multitude of growth factors which in turn act on the cells in order to further dictate their function. To recapitulate this scenario for vascularized bone regeneration purposes, materials have been engineered to deliver both inductive molecules and cells in order to further increase the efficacy of treatments. As previously described, treatment of critically-sized bone defects with BMPs or MSCs alone has shown promising results in terms of regenerative potential. Additionally, BMPs and MSCs both exhibit a vasculogenic potential through their interactions with endothelial cells. Combining these two therapies, however, is much more complicated than a simple additive effect due to the cross-talk that exists between this growth factor and the various cells necessary for bone healing. For example, BMP9 exerts its osteogenic effects partly by increasing the expression of hypoxia-inducible factor 1α (HIF1A), a potent angiogenic transcription factor which increases VEGF secretion when activated [116, 117]. When immortalized mouse embryonic fibroblasts are injected subcutaneously, those transfected with recombinant adenoviruses expressing BMP9 show drastically increased calcium deposition compared to transplanted cells that were transfected with adenoviruses expressing both BMP9 and a HIF1A inhibitor.
In an orthotopic defect model, the combination of MSCs and BMPs shows synergism in terms of their angiogenic and osteogenic effects. Co-delivering MSCs and BMP2 in rat calvarial defects has been shown in two studies to increase bone healing at 8 weeks compared to scaffolds having either BMP2 or MSCs alone [118, 119]. Kim et al. delivered a combination of MSCs and BMP2 in a hyaluronic acid hydrogel within a rat calvarial defect and, in addition to showing enhanced bone formation compared to MSC or BMP2 treatments alone, the combinatorial therapy also exhibited increased expression of von Willebrand factor, VEGF, and endothelial-specific PECAM1, indicating increased vascularization within the defect [120]. Given the beneficial effects that BMPs have on MSCs in vivo, studies have pre-incubated MSC- and BMP2-ladened scaffolds up to 4 weeks in vitro prior to delivery to prime them for bone regeneration [121, 122]. However, these analyses show minimal improvement of pre-incubated scaffolds compared to constructs prepared immediately before implantation.
By and large, the majority of therapies using a combinatorial delivery of growth factors and MSCs utilize BMPs due to their osteogenic potential in the absence of any delivered cells. It is important, however, to note that in normal physiological bone repair, a multitude of growth factors are at work, even though many may not induce bone repair by themselves. It is ultimately the cross-talk between these proteins, the surrounding microenvironment and the delivered cells that will dictate the efficacy of treatment. Gao and colleagues studied the effects of a bolus injection of VEGF next to implanted MSC-collagen scaffolds in a critically-sized murine defect and noted that, whereas MSC scaffolds alone did not mineralize, the combination of a bolus VEGF treatment with the MSC scaffold increased the mineralization and integration of the scaffold with the native bone [123]. As detailed before, VEGF by itself does not reliably induce bone regeneration but its combination with seems to have beneficial effects, at least in the stated model. Due to the delivery of VEGF through a bolus injection which consequently leads to a very short half-life in vivo, this study is noteworthy as fine-tuning the VEGF dose and kinetics of secretion could significantly improve therapeutic benefits.
Eman et al. investigated the effects of delivery of stromal-cell-derived factor-1α (CXCL12) in conjunction with MSCs on vascularized ectopic bone formation [124]. CXCL12 controls numerous aspects of stem cell function including the homing capacity of both MSCs and HSCs to sites of injured tissue through local activation of the HIF1A pathway in ischemic environments [125]. Notably, CXCL12 has been shown to be essential for MSC homing following bone fractures, as treatment of a bone fracture with an anti-CXCL12 antibody resulting in suppressed bone regeneration [126]. In the study by Eman, MSC-ladened constructs were implanted subcutaneously in mice either with EPCs or CXCL12 with subsequent assessments of vascularization and ectopic bone formation performed. Similar to previous reports, the combination of MSCs with EPCs greatly enhanced early vascularization and resulted in increased ectopic bone formation. Interestingly, the implants containing MSCs with CXCL12 exhibited more elaborate vascular networks compared to MSC-EPC ladened constructs as well as showed similar levels of ectopic bone formed. The significance of this study lies in the distinction between the delivery of exogenous stem cells and mobilization of endogenous stem cells for performing the same overall task. Nevertheless, the data from studies delivering growth factors other than BMPs, including VEGF, FGF and CXCL12, all of which have been implicated in normal, non-critically sized bone repair and development, alongside cells remains inconclusive and merits further investigation.
3.4 Gene Delivery
A major limitation in delivering therapeutic proteins within a scaffold however is the inability, in most cases, to adequately control the release and spatial concentration of proteins in a manner suitable for endogenous processes to repair the damaged tissue. Too high of concentrations can cause aberrant physiologies such as leaky vasculature in the case of VEGF or ectopic bone formation in the case of BMPs. If the total dose though is insufficient, the therapy fails to produce a beneficial result. Furthermore, even with appropriate dosing and release kinetics, the short half-life of proteins once in situ results in only a transient effect with long-term benefits stemming from the initial cascade provided by the delivered factors. Similarly, growth factors needed at later time points of bone regeneration may not provide their full therapeutic value if delivered early. To circumvent these issues, various groups have studied whether forced gene expression via direct gene delivery or implantation of cells genetically-modified to overexpress the target protein to maintain sustained levels of target protein expression can induce both a vasculogenic and osteogenic response within critically-sized defects.
Genetic engineering of bone tissue generally follows two strategies: a) in vivo delivery of viral constructs or b) ex vivo genetic modification of cells followed by implantation within the defect [127]. Common gene targets included BMP2 and BMP4 as well as the osteoblastic transcription factor Runx2 [128–133]. For example, Baltzer and colleagues demonstrated that injection of adenoviral vectors carrying BMP2 cDNA enhanced bone regeneration as well as the mechanical properties of the newly formed bone in a critically-sized femoral defect in rabbits compared to control vectors [134]. Whereas exhibiting promising results, the delivery of viral vectors poses considerable risks related to safety and immunogenicity and the lack of controlled expression prompting groups to investigate using either a scaffold to sequester the viral vectors or non-viral vectors such liposomes or delivery of plasmid DNA [135–137]. Alternatively, positive results in terms of bone healing have been obtained by delivering genetically modified cells to overexpress various forms of BMP. An excellent review of these strategies has been published elsewhere [138].
While BMPs are known to play a vasculogenic role through their cross-talk with endothelial cells and osteoblasts, it is unclear whether genetically-mediated overexpression of these growth factors near bone injury results in early vascular repair or alleviates the ischemic environment. Given the potent angiogenic effects of VEGF as well as earlier studies demonstrating that a continuous supply of exogenous VEGF delivered through an osmotic pump can increase bone healing in a critically-sized defect, genetic-modification to increase the long-term delivery of VEGF has been explored [27]. Geiger and colleagues implanted a gene-activated matrix (GAM) loaded with plasmids encoding for VEGF into a radial defect within rabbits [139]. In vitro, these plasmids displayed a moderate transfection efficiency with increased VEGF secretion from osteoblasts exposed to the VEGF-encoding plasmid. In vivo, in addition to showing increased bone healing at 6 and 12 weeks post-surgery compared to GAMs loaded with control vectors, the group also noted an increased blood vessel density in groups containing the VEGF-plasmid loaded GAMs. In lieu of a direct in vivo strategy, transducing MSCs to overexpress VEGF followed by in vivo implantation also shows promising results in terms of bone regeneration and vascular repair in critically-sized defects [140–142].
In addition to VEGF, expression of other pro-angiogenic growth factors has been examined for bone tissue formation. Cao et al. investigated the use of stem cells modified to overexpress angiopoietin-1 (ANGPT1) in the repair of critically-sized defects [143]. ANGPT1 acts to stabilize blood vessels and promotes circumferential growth effectively thickening vessel walls [144]. After VEGF destabilizes existing vessels in order for capillary sprouting to occur, ANGPT1 promotes stabilization of new vessels by enhancing integration of endothelial cells to their surrounding environment. Even without proper temporal control, radial defects in rabbits receiving MSCs lentivirally transduced to express Ang1 exhibited higher levels of bone formation as well as increased mechanical strength of the regenerated bone compared to groups receiving MSCs transduced to express GFP. Although it does not directly induce osteogenic differentiation, Ang1 stabilizes nascent blood vessels infiltrating into the defect and prevents capillary recession to support proper bone formation. MSC-mediated constitutive expression of HIF1A, an important transcription factor upregulating various angiogenic proteins including VEGF and angiopoietins, also exhibits similar increases in the osteogenic and vasculogenic potential in repairing critically-sized defects [145].
4. Engineering Biomaterials for Enhancing Vascularization in Bone Repair
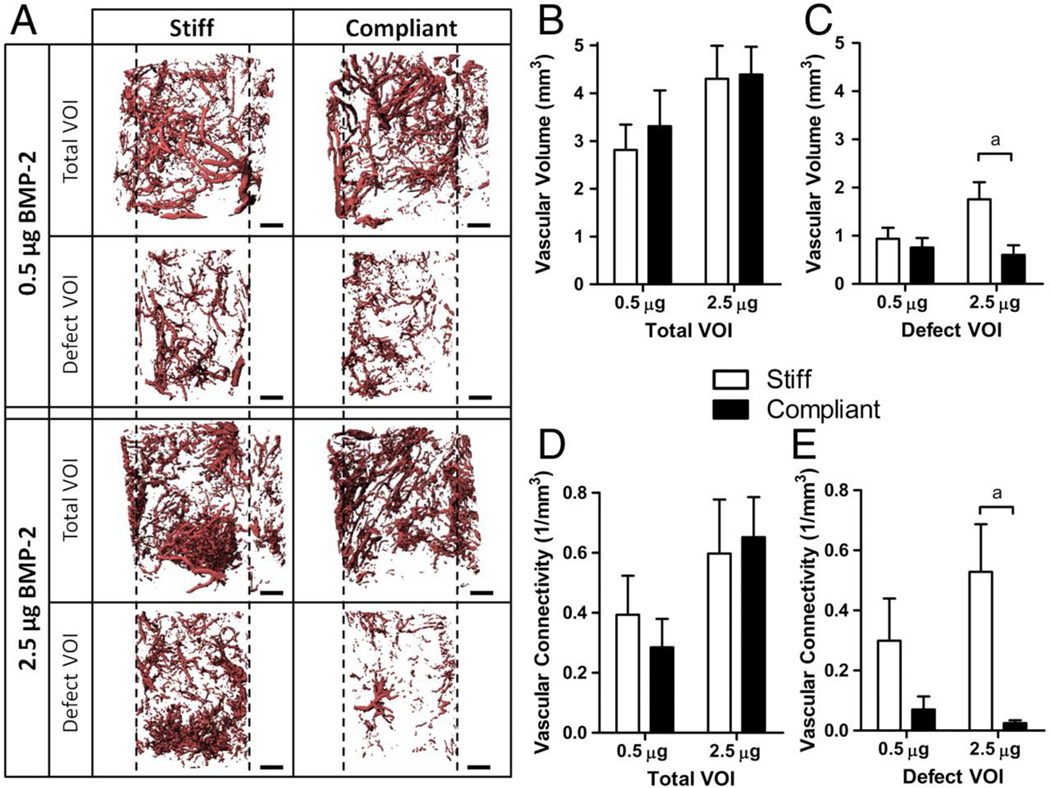
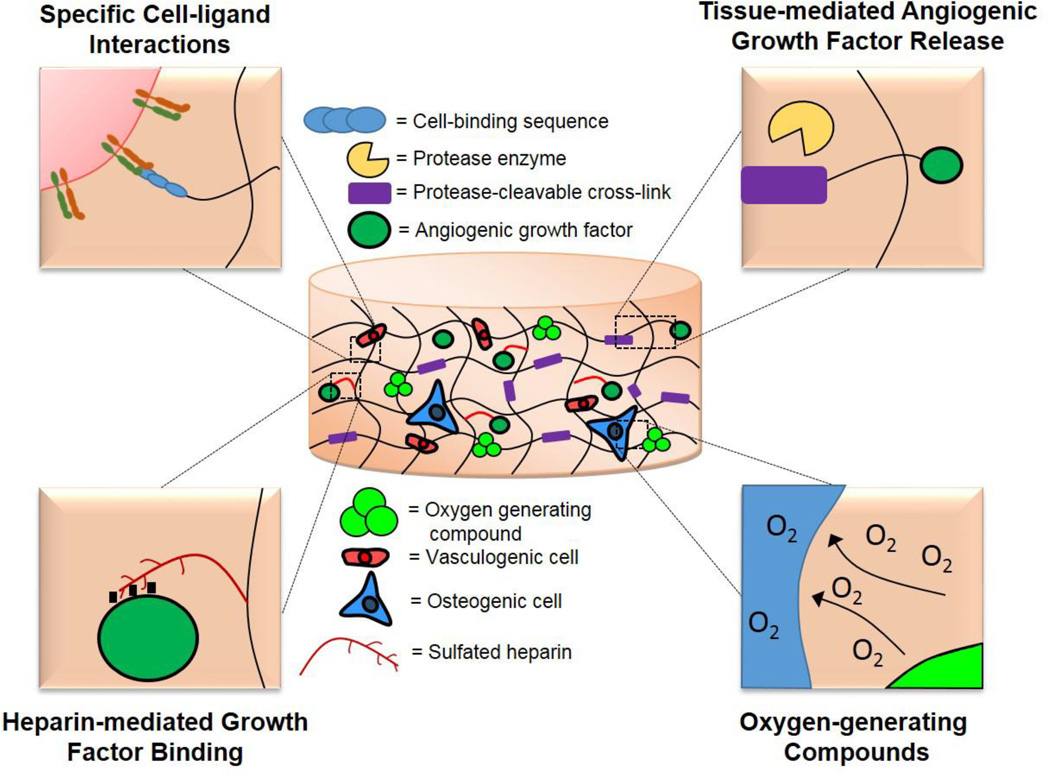
The majority of research endeavors intent on increasing vascularization and osteogenesis in bone defects focus on delivery of therapeutic proteins and genes as well as cells to enhance the healing response. Nevertheless, a key component of these strategies is the biomaterial through which these factors are delivered. Representing more than just a delivery vehicle, scaffolds can provide mechanical stability to a bone defect which can dynamically affect revascularization. Boerckel et al. discovered that fixation plates allowing early mechanical loading onto an implanted construct adversely affected vascular volume and connectivity compared to fixation plates that limited load transfer [146] (Figure 3). Additionally, biomaterials have the ability to interact synergistically with their embedded factors through innovative engineering approaches to further the vascularization response. The following sections will provide a discussion of how novel biomaterial engineering strategies can be applied to reparation of vascular networks in bone tissue constructs. (Figure 4)
Figure 3.
MicroCT angiography of a femoral bone defect treated with rhBMP2 and stabilized with fixation plates that were either locked together to prevent all modes of load transfer (stiff) or unlocked to allow transfer of compressive loads along the bone axis (compliant) at 3 weeks post-surgery. A) Representative 3D reconstructions of vascular networks in total or defect region volume of interest. Scale bar= 1 mm B,C) Vascular volume in total and defect volume of interest, respectively D,E) Vascular connectivity in total and defect volume of interest, respectively. Compliant fixations allowing transfer of mechanical loading exhibited significantly reduced vascular connectivity compared to stiff fixtures. Adapted from Boerckel et al. [146]
Figure 4.
Novel biomaterial strategies that hold potential for applications in vascularized bone tissue engineering
4.1 Role of Biomaterials in Delivering Angiogenic and Osteogenic Growth Factors
The effectiveness of therapeutic proteins depends heavily in the spatial and temporal microenvironmental concentrations of the protein(s). This delivery profile in turn is controlled by the total dose incorporated within a material, the kinetics of release, and the persistence and stability of the protein. In most therapies, including those in which therapeutic proteins are absorbed or incorporated into materials without any covalent or affinity-mediated tethering, release kinetics are defined by non-specific interactions between the protein and the material. Scaffold porosity, adsorption parameters, and affinity to the scaffold control the release of these proteins. In this sense, the kinetics of protein release can be thought of as a ‘materials-driven process’ with the advantage being that one can precisely engineer a material, by regulating pore size for example, to fit a distinct release profile. The issue, however, is that this process is independent on the particular protein-material pair and the surrounding biological environment. To engineer biomaterials to effectively deliver therapeutic proteins, it may be beneficial to consider how these growth factors are typically presented in tissue repair.
Following vascular injury, platelets, neutrophils, and macrophages respond to the injury and lay down a provisional fibrin-rich matrix with embedded growth factors [147]. As matrix metalloproteinases (MMPs) work to degrade this provisional matrix, ECM-bound growth factors are released and dictate the appropriate course of action for surrounding cells to heal the wound and revascularize the region [148]. It is this spatiotemporal control of MMP activity followed by the spatial regulation of growth factor concentration by ECM degradation and presentation that, among other factors, controls proper wound revascularization. Mimicking this ECM-embedding strategy can prove useful in aptly controlling angiogenic growth factor presentation within bone defects.
A ground-breaking study by Hubbell and colleagues demonstrated the engineering of a completely synthetic scaffold in which BMP2 had been entrapped within an MMP-sensitive hydrogel and used to regenerate bone within an orthotopic model [149]. In vitro, hydrogels exhibited BMP2 release kinetics which were highly dependent on gel degradation with 90% of the protein being retained in a saline solution whereas addition of the proteolytic MMP-2 induced 100% protein release. BMP2-loaded MMP-sensitive hydrogels also exhibited significantly higher bone healing in rat calvarial defects compared to hydrogels without MMP-sensitivity as well as those without BMP2. Further work has even shown that MMP-sensitive BMP2-loaded synthetic hydrogels exhibit higher bone regeneration in a critically-sized defect compared to the current clinical standard of a BMP2 loaded absorbable collagen sponge [39]. This biomaterial platform has also been used for vascularization applications through incorporation of angiogenic proteins [150, 151]. Covalently conjugating VEGF onto a PEG-diacrylate matrix results in increased tubule formation by endothelial cells cultured on top of the gels compared to conditions with VEGF in the media indicating the importance of controlled growth factor presentation [152]. In vivo, delivering VEGF through covalent tethering to a protease-degradable PEG-maleimide matrix increases therapeutic re-vascularization in both hind limb ischemia and myocardial infarction models [153, 154] (Figure 5). Importantly, the VEGF-incorporated matrix increases the survival and function of transplanted islets thus showing promise for other cellular therapies [155].
Figure 5.
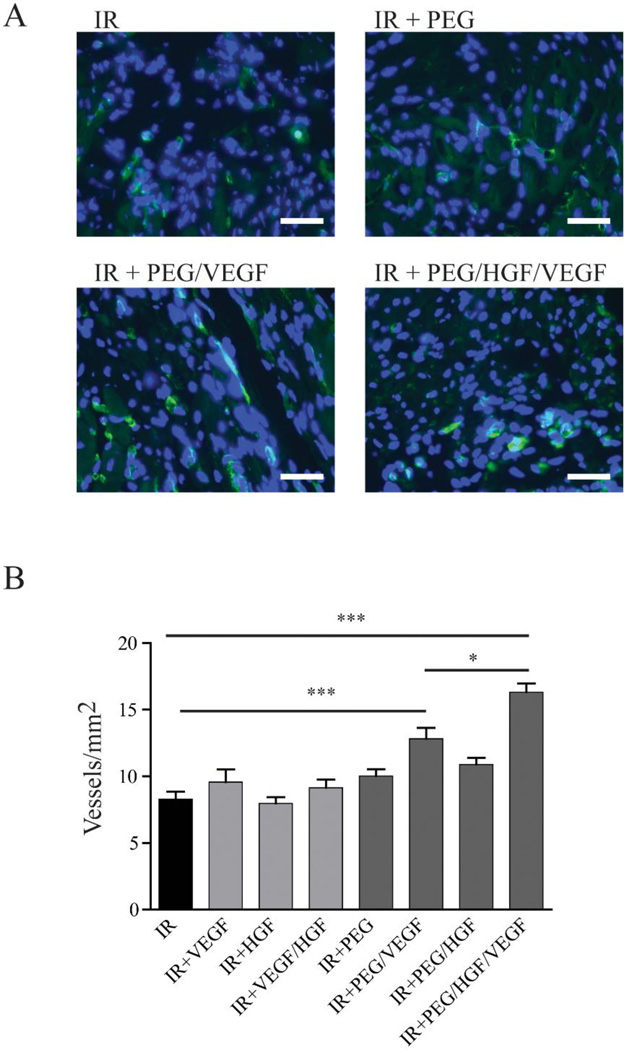
Blood vessel labelling and quantification following staining of FTIC-conjugated isolectin b4 from myocardial tissue following a myocardial infarct. A) Representative images (isolectin=green; DAPI=blue; scale bar=30 µm) of rats having either ischemic reperfusion by itself or with delivery of hepatocyte growth factor (HGF) and VEGF from either bolus injection or incorporated within a PEG-maleimide matrix B) Quantification showing increased blood vessel area in PEG/VEGF groups compared to ischemic reperfusion alone and PEG/HGF/VEGF compared to PEG/VEGF and ischemic reperfusion alone. Interestingly, there is no difference between bolus growth factor delivery coupled with ischemic reperfusion and reperfusion alone. Adapted from Salimath et al. [154]
A conceptually different strategy but one that still relates to the controlled presentation of growth factors is that of tethering heparin or other growth factor binding domains onto implantable materials. A highly negatively charged sulfated molecule, heparin exhibits the ability to bind to numerous angiogenic and osteogenic growth factors including VEGF, FGF, PDGF, and BMP2 through their heparin-binding domains. Scaffolds containing the covalently-bound heparin attempt to mimic the ECM by controlling the presentation and activity of growth factors through an affinity-based system [156, 157]. Heparin-bound BMP2 has been shown to induce elevated levels of ALP activity and increase proliferation in C2C12s compared to soluble BMP2 [158]. Taking a step further, certain materials can be chemically sulfated in order to bind to growth factors with the same or even higher affinity as heparin itself with sulfated alginate exhibiting enhanced FGF2-mediated vasculogenesis in vivo [159]. In lieu of heparin, Hubbell’s group has pioneered the use of short protein fragments having precisely controlled cell and growth factor binding sites. By engineering a recombinant fibronectin fragment to contain fibrin-binding, integrin-binding and growth-factor binding sequences, Martino et al. demonstrated that delivery of this fragment within a fibrin construct along with nominal levels of BMP2 and PDGF-BB significantly increased bone healing in a rat calvarial defect compared to fibrin constructs lacking the protein fragment [160]. Additionally, delivering VEGF and PDGF-BB within fragment-functionalized fibrin matrices enhanced skin wound healing in diabetic mice through increased angiogenesis. Further work in this area has resulted in isolation of a domain found on placenta growth-factor-2 that binds strongly to ECM proteins [161]. Inclusion of this domain onto VEGF, PDGF-BB, and BMP2 resulted in increased angiogenesis in an induced murine skin wound as well as elevated bone healing in a calvarial defect.
In addition to tissue-demanded kinetics control, covalently or affinity-based tethering of growth factors can potentially increase their half-lives compared to their unconjugated form, although this is system-dependent on the site of immobilization and the ability for interactions to still exist with specific epitopes [162–164]. To date however, no study has investigated materials in which controlled, tissue-demanded release of angiogenic growth factors have been applied in the context of bone regeneration leaving the field open to further materials engineering.
4.2 Role of Biomaterials in Delivering Stem Cells
While delivering cells and specifically stem cells has garnered an incredible amount of interest in the field of bone tissue engineering, multiple key issues including that of cell survival, proliferation and long-term engraftment remain to be addressed. Various groups have shown that within one week after orthotopic transplantation, there is a significant loss of cells [66, 165–168]. Additionally, histological analysis up to 4 weeks post implantation shows no presence of transplanted MSC markers indicating that much of the healing response imparted by the delivered cells is limited to transient paracrine signaling [167, 169]. Increasing MSC and endothelial cell survival within scaffolds could thus potentially increase the duration and activity of their associated osteogenic and vasculogenic cues in non-healing defects. An ischemic environment coupled with the lack of an early vascular network providing needed nutrients has been shown to be the main causes of implanted cell death [170, 171]. While the various strategies outlined here, including scaffold-based growth factor delivery and gene therapy, have attempted to increase cell survival, in large bone defects, the speed at which vascularization can invade and perfuse the scaffold may not be fast enough to prevent the cell death that occurs within days of implantation. To help prevent this cell death, rather than just being used as carriers, biomaterials themselves can be engineered in two specific ways: 1) to sustain cellular viability while a vascular network forms and 2) to interact directly with encapsulated cells to increase vasculogenic cues.
To sustain cellular viability before a vascular network can adequately form, biomaterials need to provide a crucial factor: oxygen. Various groups have addressed this concern through the use of oxygen-generating biomaterials. Oxygen generation is done by implanting materials infused with solid inorganic peroxides such as sodium percarbonate, calcium peroxide or magnesium peroxide [172]. Interaction of these inorganic solids with water ultimately generates oxygen through a hydrogen peroxide intermediary. Harrison et al. synthesized a PLGA film which incorporated an oxygen-generating compound, sodium percarbonate, and demonstrated that implantation of this film over a murine skin flap decreased tissue necrosis and cell apoptosis over the course of one week [173]. Follow-up studies using this material resulted in a 3D scaffold that generated considerable levels of oxygen up to ten days following synthesis [174]. Calcium peroxide has also been included within scaffolds and through its oxygen-generating capacity, has been shown to increase the metabolic activity and viability of encapsulated beta cells [175] (Figure 6). It is important to emphasize that these oxygen-generating compounds use a hydrogen peroxide intermediary which in high concentrations is harmful to encapsulated cells and the surrounding tissue; concentrations of starting peroxides and kinetics of reaction need to be controlled.
Figure 6.
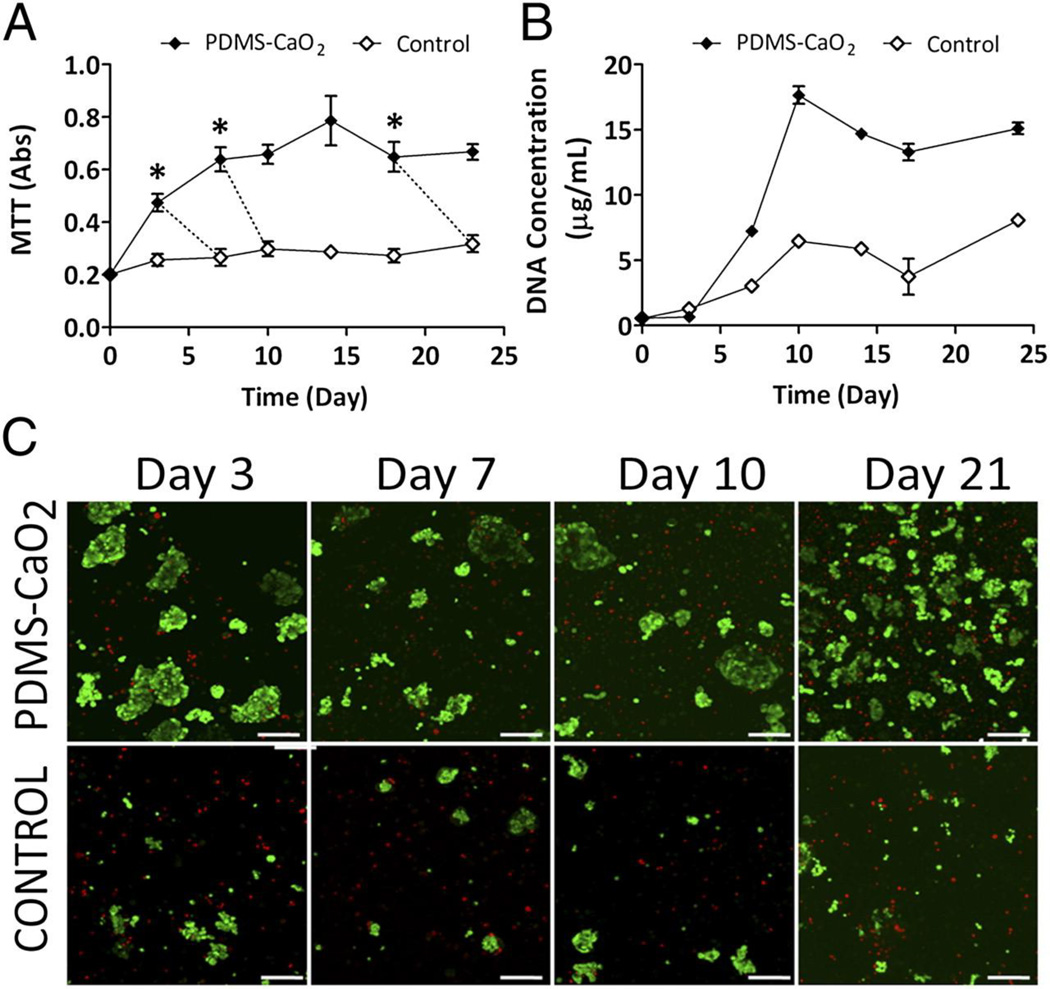
Increased pancreatic beta cell viability and metabolic activity over 3 weeks under low oxygen conditions when incubated with calcium percarbonate containing PDMS disks. A) MTT metabolic activity and B) total DNA quantification. C) Representative images of live/dead staining (green=live; red=dead) of pancreatic beta cells when incubated with or without PDMS-CaO2 materials. (Scale bar= 100 µm). Adapted from Pedraza et al. [175]
In addition to sustaining cellular viability, it is crucial for biomaterials to interact with embedded cells to increase their vasculogenic potential. Integrin binding is one of the primary ways through which the extracellular environment, including biomaterials, can influence cellular behavior [176, 177]. These transmembrane receptors consisting of alpha and beta subunits bind to extracellular ligands and mediate the attachment of cells to surfaces [178]. In addition, once activated, they are responsible for multiple signaling cascades which can decide cell fate [179]. Activation of different ligands is known to elicit different phenotypic responses and many studies have investigated how small synthetic peptides activating single integrins can increase the vasculogenic potential of endothelial cells. Modifying materials with the laminin derived peptide ‘YIGSR’ increases endothelial-specific adhesion, proliferation and migration compared to interactions with the ubiquitous ‘RGD’ ligand alone [180–182]. Binding of the α4β1 integrin through the fibronectin-derived synthetic peptide ‘REDV’ increases endothelial cell adhesion and proliferation, indicating a possible use of materials engineered to activate this integrin for vascularization purposes [183, 184]. Additionally, engineering a material to support a pro-vasculogenic phenotype could entail growth factor tethering in which case many of the engineering parameters from the previous section could be applied. Immobilization of VEGF into biomaterials enhances viability and proliferation of endothelial cells compared to freely soluble growth factors [152, 185]. While modifying materials for increased vasculogenic potential has provided needed insight, these systems are limited to in vitro studies and it will be important to test whether these same concepts apply in animal models.
5. Conclusion and Future Outlook
Because its resilient nature, bone holds the remarkable ability to repair itself in cases of minor fractures. Following a bone injury, a cascade of events occurs to repair the tissue including recruitment of inflammatory cells, vascularization, and callous formation which ultimately allow for mineralization and bone remodeling. In large bone defects however, this repair is hampered and endogenous processes are unable to bridge the bone gap thus requiring the need for outside therapy. In recent years, rapidly creating a vascular network has become evident as a crucial factor for successful bone healing in these therapies. To that end, various different strategies have been explored including growth factor delivery, cell delivery and gene therapy or a combination of the three. While the majority of studies have focused on addition of these agents to scaffolds, it is important to recognize the role of biomaterials themselves in aiding bone regeneration. Rather than just acting as a carrier, biomaterials can be engineered to mimic the natural ECM through specially designed methods of presenting growth factors as well as directing cell fate and cell behavior. Tethering growth factors through either covalent or affinity-based interactions as well as creating absorbable materials through protease-cleavable bonds can create a more ECM-like environment for surrounding cells to recognize and aid in healing. Additionally, engineering biomaterials to present cell-specific ligands on surfaces could direct cell fate and behavior granting further control over healing even after implantation.
An important factor in any of these therapies is whether or not implementation is clinically feasible. Growth factor delivery is arguably the most feasible to implement due to the ease of storage and manufacturing compared to other methodologies. The FDA-approved therapy INFUSE for example consists of an absorbable collagen sponge soaked in a recombinant human BMP2 solution. The growth factor is relatively easy to manufacture and purify due to well-established molecular biology techniques and simple to ship and store in a lyophilized or reconstituted form. In contrast, cellular therapies require extensive screening and carefully controlled GMP-environments for expansion of the cells driving up costs for both companies and patients. Additionally, the safety of these cellular therapies, especially those containing stem cells, have not been fully elucidated and constitutes the objective of multiple phase I/II clinical trials. Ex vivo gene therapy suffers from the same drawbacks as unmodified cell delivery and has even greater obstacles to overcome. In addition to screening and expansion of cells to clinically relevant numbers, strict protocols must be in place to genetically modify and subsequently purify the cell population to acceptable levels. In vivo gene therapy can bypass these obstacles but poses considerable safety concerns regarding the concentration of virus or plasmid-mediated scaffolds. The clinical relevance of therapies must however be balanced by the efficacy of their treatment and therapies that show considerable bone repair will need to find avenues to get to the clinic. Ultimately, it is these new approaches or a combination of them along with characteristics of the biomaterials scaffold itself that will dictate the healing and vascularization response and translate into the next wave of therapeutics for critical-size bone defects.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grants R01-AR062368 and R01-AR062920 (AJG). JRG was supported by the Cell and Tissue Engineering NIH Biotechnology Training Grant (T32-GM008433).
Footnotes
Conflicts of Interest
The authors report no conflict of interest
References
- 1.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4–5):300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Logeart-Avramoglou D, Anagnostou F, Bizios R, Petite H. Engineering bone: challenges and obstacles. J Cell Mol Med. 2005;9(1):72–84. doi: 10.1111/j.1582-4934.2005.tb00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10(1):12–27. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 4.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B, Kim JE. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J Bone Miner Res. 2009;24(6):1055–1065. doi: 10.1359/jbmr.081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 9.Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res. 2002;17(5):898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- 10.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189(3):275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 11.Carlevaro MF, Cermelli S, Cancedda R, Descalzi Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci. 2000;113(Pt 1):59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131(9):2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 13.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 14.Ohbayashi N, Shibayama M, Kurotaki Y, Imanishi M, Fujimori T, Itoh N, Takada S. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 2002;16(7):870–879. doi: 10.1101/gad.965702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16(7):859–869. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16(12):1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 18.Sorrell JM. Development of arteries in embryonic chick bone marrow with special reference to the appearance of periarterial granulopoietic sheaths. Anat Rec. 1988;221(3):730–736. doi: 10.1002/ar.1092210308. [DOI] [PubMed] [Google Scholar]
- 19.Percival CJ, Richtsmeier JT. Angiogenesis and intramembranous osteogenesis. Dev Dyn. 2013;242(8):909–922. doi: 10.1002/dvdy.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88(Suppl 3):4–9. doi: 10.2106/JBJS.F.00890. [DOI] [PubMed] [Google Scholar]
- 22.Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15(3):316–327. doi: 10.1016/j.joca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Zhang H, Zhang X, Lu W, Huang X, Xie H, Zhou J, Wang W, Zhang Y, Liu Y, Deng Z, Jin Y. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair. Tissue Eng Part A. 2011;17(5–6):725–739. doi: 10.1089/ten.TEA.2010.0331. [DOI] [PubMed] [Google Scholar]
- 24.Finkemeier CG. Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am. 2002;84-A(3):454–464. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Bai Y, Yin G, Huang Z, Liao X, Chen X, Yao Y, Pu X. Localized delivery of growth factors for angiogenesis and bone formation in tissue engineering. Int Immunopharmacol. 2013;16(2):214–223. doi: 10.1016/j.intimp.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 27.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karvinen H, Pasanen E, Rissanen TT, Korpisalo P, Vahakangas E, Jazwa A, Giacca M, Yla-Herttuala S. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011;18(12):1166–1172. doi: 10.1038/gt.2011.66. [DOI] [PubMed] [Google Scholar]
- 30.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31(6):729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112(12):3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D, Zhao M, Harris SE, Mi Z. Signal transduction and biological functions of bone morphogenetic proteins. Front Biosci. 2004;9:349–358. doi: 10.2741/1090. [DOI] [PubMed] [Google Scholar]
- 33.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A(8):1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87(3):305–312. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 36.Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Lowik CW. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 37.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2(3):141–149. [PubMed] [Google Scholar]
- 38.Raida M, Clement JH, Leek RD, Ameri K, Bicknell R, Niederwieser D, Harris AL. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol. 2005;131(11):741–750. doi: 10.1007/s00432-005-0024-1. [DOI] [PubMed] [Google Scholar]
- 39.Mariner PD, Wudel JM, Miller DE, Genova EE, Streubel SO, Anseth KS. Synthetic hydrogel scaffold is an effective vehicle for delivery of INFUSE (rhBMP2) to critical-sized calvaria bone defects in rats. J Orthop Res. 2013;31(3):401–406. doi: 10.1002/jor.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, Garcia AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials. 2014;35(21):5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Rihn JA, Patel R, Makda J, Hong J, Anderson DG, Vaccaro AR, Hilibrand AS, Albert TJ. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9(8):623–629. doi: 10.1016/j.spinee.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Asahina I, Watanabe M, Sakurai N, Mori M, Enomoto S. Repair of bone defect in primate mandible using a bone morphogenetic protein (BMP)-hydroxyapatite-collagen composite. J Med Dent Sci. 1997;44(3):63–70. [PubMed] [Google Scholar]
- 44.Yoneda M, Terai H, Imai Y, Okada T, Nozaki K, Inoue H, Miyamoto S, Takaoka K. Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials. 2005;26(25):5145–5152. doi: 10.1016/j.biomaterials.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 45.Hollinger JO, Schmitt JM, Buck DC, Shannon R, Joh SP, Zegzula HD, Wozney J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res. 1998;43(4):356–364. doi: 10.1002/(sici)1097-4636(199824)43:4<356::aid-jbm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 46.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81(4):710–718. doi: 10.1302/0301-620x.81b4.9311. [DOI] [PubMed] [Google Scholar]
- 47.Groeneveld EH, Burger EH. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142(1):9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 48.Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, Siebenrock KA, Hofstetter W, Klenke FM. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 49.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 50.Geuze RE, Theyse LF, Kempen DH, Hazewinkel HA, Kraak HY, Oner FC, Dhert WJ, Alblas J. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng Part A. 2012;18(19–20):2052–2062. doi: 10.1089/ten.tea.2011.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernandez A, Reyes R, Sanchez E, Rodriguez-Evora M, Delgado A, Evora C. In vivo osteogenic response to different ratios of BMP-2 and VEGF released from a biodegradable porous system. J Biomed Mater Res A. 2012;100(9):2382–2391. doi: 10.1002/jbm.a.34183. [DOI] [PubMed] [Google Scholar]
- 52.De la Riva B, Sanchez E, Hernandez A, Reyes R, Tamimi F, Lopez-Cabarcos E, Delgado A, Evora C. Local controlled release of VEGF and PDGF from a combined brushite-chitosan system enhances bone regeneration. J Control Release. 2010;143(1):45–52. doi: 10.1016/j.jconrel.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 53.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31(26):6772–6781. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJ. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 56.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M, Mikos AG. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15(9):2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez-Fernandez MP, Calvo-Guirado JL, de-Val JE, Delgado-Ruiz RA, Negri B, Pardo-Zamora G, Penarrocha D, Barona C, Granero JM, Alcaraz-Banos M. Melatonin promotes angiogenesis during repair of bone defects: a radiological and histomorphometric study in rabbit tibiae. Clin Oral Investig. 2013;17(1):147–158. doi: 10.1007/s00784-012-0684-6. [DOI] [PubMed] [Google Scholar]
- 58.Kim YH, Furuya H, Tabata Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials. 2014;35(1):214–224. doi: 10.1016/j.biomaterials.2013.09.103. [DOI] [PubMed] [Google Scholar]
- 59.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering. Biomaterials. 2008;29(19):2869–2877. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110(34):13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrie Aronin CE, Shin SJ, Naden KB, Rios PD, Jr, Sefcik LS, Zawodny SR, Bagayoko ND, Cui Q, Khan Y, Botchwey EA. The enhancement of bone allograft incorporation by the local delivery of the sphingosine 1-phosphate receptor targeted drug FTY720. Biomaterials. 2010;31(25):6417–6424. doi: 10.1016/j.biomaterials.2010.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Das A, Tanner S, Barker DA, Green D, Botchwey EA. Delivery of S1P receptor-targeted drugs via biodegradable polymer scaffolds enhances bone regeneration in a critical size cranial defect. J Biomed Mater Res A. 2014;102(4):1210–1218. doi: 10.1002/jbm.a.34779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao RR, Stegemann JP. Cell-based approaches to the engineering of vascularized bone tissue. Cytotherapy. 2013;15(11):1309–1322. doi: 10.1016/j.jcyt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32(1):160–165. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 65.Berner A, Reichert JC, Muller MB, Zellner J, Pfeifer C, Dienstknecht T, Nerlich M, Sommerville S, Dickinson IC, Schutz MA, Fuchtmeier B. Treatment of long bone defects and non-unions: from research to clinical practice. Cell Tissue Res. 2012;347(3):501–519. doi: 10.1007/s00441-011-1184-8. [DOI] [PubMed] [Google Scholar]
- 66.Vila OF, Martino MM, Nebuloni L, Kuhn G, Perez-Amodio S, Muller R, Hubbell JA, Rubio N, Blanco J. Bioluminescent and micro-computed tomography imaging of bone repair induced by fibrin-binding growth factors. Acta Biomater. 2014;10(10):4377–4389. doi: 10.1016/j.actbio.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 67.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20(5):510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 68.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 69.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 70.Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, de Boer J. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7(10):e46842. doi: 10.1371/journal.pone.0046842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25(9):1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 72.Galas RJ, Jr, Liu JC. Vascular endothelial growth factor does not accelerate endothelial differentiation of human mesenchymal stem cells. J Cell Physiol. 2014;229(1):90–96. doi: 10.1002/jcp.24421. [DOI] [PubMed] [Google Scholar]
- 73.Bai K, Huang Y, Jia X, Fan Y, Wang W. Endothelium oriented differentiation of bone marrow mesenchymal stem cells under chemical and mechanical stimulations. J Biomech. 2010;43(6):1176–1181. doi: 10.1016/j.jbiomech.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 74.Dong JD, Gu YQ, Li CM, Wang CR, Feng ZG, Qiu RX, Chen B, Li JX, Zhang SW, Wang ZG, Zhang J. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol Sin. 2009;30(5):530–536. doi: 10.1038/aps.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5(5):713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seebach E, Freischmidt H, Holschbach J, Fellenberg J, Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014;10(11):4730–4741. doi: 10.1016/j.actbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 77.Moon JJ, Saik JE, Poche RA, Leslie-Barbick JE, Lee SH, Smith AA, Dickinson ME, West JL. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials. 2010;31(14):3840–3847. doi: 10.1016/j.biomaterials.2010.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghajar CM, Blevins KS, Hughes CC, George SC, Putnam AJ. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12(10):2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen EH, Zanotelli MR, Schwartz MP, Murphy WL. Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays. Biomaterials. 2014;35(7):2149–2161. doi: 10.1016/j.biomaterials.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grainger SJ, Putnam AJ. Assessing the permeability of engineered capillary networks in a 3D culture. PLoS One. 2011;6(7):e22086. doi: 10.1371/journal.pone.0022086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15(2):253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao RR, Ceccarelli J, Vigen ML, Gudur M, Singh R, Deng CX, Putnam AJ, Stegemann JP. Effects of hydroxyapatite on endothelial network formation in collagen/fibrin composite hydrogels in vitro and in vivo. Acta Biomater. 2014;10(7):3091–3097. doi: 10.1016/j.actbio.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, George SC. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15(6):1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng G, Liao S, Kit Wong H, Lacorre DA, di Tomaso E, Au P, Fukumura D, Jain RK, Munn LL. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood. 2011;118(17):4740–4749. doi: 10.1182/blood-2011-02-338426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khan OF, Sefton MV. Endothelialized biomaterials for tissue engineering applications in vivo. Trends Biotechnol. 2011;29(8):379–387. doi: 10.1016/j.tibtech.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen X, Aledia AS, Popson SA, Him L, Hughes CC, George SC. Rapid anastomosis of endothelial progenitor cell-derived vessels with host vasculature is promoted by a high density of cotransplanted fibroblasts. Tissue Eng Part A. 2010;16(2):585–594. doi: 10.1089/ten.tea.2009.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bulnheim U, Muller P, Neumann HG, Peters K, Unger RE, Kirkpatrick CJ, Rychly J. Endothelial cells stimulate osteogenic differentiation of mesenchymal stem cells on calcium phosphate scaffolds. J Tissue Eng Regen Med. 2014;8(10):831–840. doi: 10.1002/term.1590. [DOI] [PubMed] [Google Scholar]
- 88.Wang J, Ye Y, Tian H, Yang S, Jin X, Tong W, Zhang Y. In vitro osteogenesis of human adipose-derived stem cells by coculture with human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2011;412(1):143–149. doi: 10.1016/j.bbrc.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 89.Laranjeira MS, Fernandes MH, Monteiro FJ. Reciprocal induction of human dermal microvascular endothelial cells and human mesenchymal stem cells: time-dependent profile in a co-culture system. Cell Prolif. 2012;45(4):320–334. doi: 10.1111/j.1365-2184.2012.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: an in vitro study. Arch Med Res. 2013;44(7):504–513. doi: 10.1016/j.arcmed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 91.Gershovich JG, Dahlin RL, Kasper FK, Mikos AG. Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds. Tissue Eng Part A. 2013;19(23–24):2565–2576. doi: 10.1089/ten.tea.2013.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kang Y, Kim S, Fahrenholtz M, Khademhosseini A, Yang Y. Osteogenic and angiogenic potentials of monocultured and co-cultured human-bone-marrow-derived mesenchymal stem cells and human-umbilical-vein endothelial cells on three-dimensional porous beta-tricalcium phosphate scaffold. Acta Biomater. 2013;9(1):4906–4915. doi: 10.1016/j.actbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun H, Qu Z, Guo Y, Zang G, Yang B. In vitro and in vivo effects of rat kidney vascular endothelial cells on osteogenesis of rat bone marrow mesenchymal stem cells growing on polylactide-glycoli acid (PLGA) scaffolds. Biomed Eng Online. 2007;6:41. doi: 10.1186/1475-925X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steiner D, Lampert F, Stark GB, Finkenzeller G. Effects of endothelial cells on proliferation and survival of human mesenchymal stem cells and primary osteoblasts. J Orthop Res. 2012;30(10):1682–1689. doi: 10.1002/jor.22130. [DOI] [PubMed] [Google Scholar]
- 95.Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30(26):4407–4415. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 96.Usami K, Mizuno H, Okada K, Narita Y, Aoki M, Kondo T, Mizuno D, Mase J, Nishiguchi H, Kagami H, Ueda M. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res A. 2009;90(3):730–741. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
- 97.Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O'Doherty E, Lin Y, Friedrich CC, Daheron L, Lin CP, Sundback CA, Vacanti JP, Neville C. Engineered vascularized bone grafts. Proc Natl Acad Sci U S A. 2010;107(8):3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ren L, Kang Y, Browne C, Bishop J, Yang Y. Fabrication, vascularization and osteogenic properties of a novel synthetic biomimetic induced membrane for the treatment of large bone defects. Bone. 2014;64:173–182. doi: 10.1016/j.bone.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koob S, Torio-Padron N, Stark GB, Hannig C, Stankovic Z, Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2011;17(3–4):311–321. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 100.Cornejo A, Sahar DE, Stephenson SM, Chang S, Nguyen S, Guda T, Wenke JC, Vasquez A, Michalek JE, Sharma R, Krishnegowda NK, Wang HT. Effect of adipose tissue-derived osteogenic and endothelial cells on bone allograft osteogenesis and vascularization in critical-sized calvarial defects. Tissue Eng Part A. 2012;18(15–16):1552–1561. doi: 10.1089/ten.tea.2011.0515. [DOI] [PubMed] [Google Scholar]
- 101.Ma J, Both SK, Ji W, Yang F, Prins HJ, Helder MN, Pan J, Cui FZ, Jansen JA, van den Beucken JJ. Adipose tissue-derived mesenchymal stem cells as monocultures or cocultures with human umbilical vein endothelial cells: performance in vitro and in rat cranial defects. J Biomed Mater Res A. 2014;102(4):1026–1036. doi: 10.1002/jbm.a.34775. [DOI] [PubMed] [Google Scholar]
- 102.Yu H, Vandevord PJ, Gong W, Wu B, Song Z, Matthew HW, Wooley PH, Yang SY. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res. 2008;26(8):1147–1152. doi: 10.1002/jor.20609. [DOI] [PubMed] [Google Scholar]
- 103.Pang H, Wu XH, Fu SL, Luo F, Zhang ZH, Hou TY, Li ZQ, Chang ZQ, Yu B, Xu JZ. Prevascularisation with endothelial progenitor cells improved restoration of the architectural and functional properties of newly formed bone for bone reconstruction. Int Orthop. 2013;37(4):753–759. doi: 10.1007/s00264-012-1751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seebach C, Henrich D, Kahling C, Wilhelm K, Tami AE, Alini M, Marzi I. Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng Part A. 2010;16(6):1961–1970. doi: 10.1089/ten.TEA.2009.0715. [DOI] [PubMed] [Google Scholar]
- 105.Seebach C, Henrich D, Wilhelm K, Barker JH, Marzi I. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in rats. Cell Transplant. 2012;21(8):1667–1677. doi: 10.3727/096368912X638937. [DOI] [PubMed] [Google Scholar]
- 106.Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. 2013;91(1):32–39. doi: 10.1038/icb.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sensebe L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. Mesenchymal stem cells for clinical application. Vox Sang. 2010;98(2):93–107. doi: 10.1111/j.1423-0410.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 108.Kang SH, Chung YG, Oh IH, Kim YS, Min KO, Chung JY. Bone regeneration potential of allogeneic or autogeneic mesenchymal stem cells loaded onto cancellous bone granules in a rabbit radial defect model. Cell Tissue Res. 2014;355(1):81–88. doi: 10.1007/s00441-013-1738-z. [DOI] [PubMed] [Google Scholar]
- 109.Jager M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, Hendrich C, Krauspe R. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res. 2011;29(2):173–180. doi: 10.1002/jor.21230. [DOI] [PubMed] [Google Scholar]
- 110.Hendrich C, Franz E, Waertel G, Krebs R, Jager M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev (Pavia) 2009;1(2):e32. doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone. 2007;40(2):522–528. doi: 10.1016/j.bone.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 112.Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27(6):629–634. doi: 10.1097/BPO.0b013e318093f523. [DOI] [PubMed] [Google Scholar]
- 113.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49(7):741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 114.Resch T, Pircher A, Kahler CM, Pratschke J, Hilbe W. Endothelial progenitor cells: current issues on characterization and challenging clinical applications. Stem Cell Rev. 2012;8(3):926–939. doi: 10.1007/s12015-011-9332-9. [DOI] [PubMed] [Google Scholar]
- 115.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 116.Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, Kim SH, Chen X, Gao JL, Zhang H, Zhang W, Kong YH, Zhang J, Wang J, Shui W, Luo X, Liu B, Cui J, Rogers MR, Shen J, Zhao C, Wang N, Wu N, Luu HH, Haydon RC, He TC, Huang W. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2013;126(Pt 2):532–541. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jo S, Kim S, Cho TH, Shin E, Hwang SJ, Noh I. Effects of recombinant human bone morphogenic protein-2 and human bone marrow-derived stromal cells on in vivo bone regeneration of chitosan-poly(ethylene oxide) hydrogel. J Biomed Mater Res A. 2013;101(3):892–901. doi: 10.1002/jbm.a.34354. [DOI] [PubMed] [Google Scholar]
- 119.He X, Liu Y, Yuan X, Lu L. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS One. 2014;9(8):e104061. doi: 10.1371/journal.pone.0104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28(10):1830–1837. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 121.Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, Chen J, Vunjak-Novakovic G, Kaplan DL. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J Biomed Mater Res A. 2006;78(2):324–334. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- 122.Kirker-Head C, Karageorgiou V, Hofmann S, Fajardo R, Betz O, Merkle HP, Hilbe M, von Rechenberg B, McCool J, Abrahamsen L, Nazarian A, Cory E, Curtis M, Kaplan D, Meinel L. BMP-silk composite matrices heal critically sized femoral defects. Bone. 2007;41(2):247–255. doi: 10.1016/j.bone.2007.04.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao C, Harvey EJ, Chua M, Chen BP, Jiang F, Liu Y, Li A, Wang H, Henderson JE. MSC-seeded dense collagen scaffolds with a bolus dose of VEGF promote healing of large bone defects. Eur Cell Mater. 2013;26:195–207. doi: 10.22203/ecm.v026a14. discussion 207. [DOI] [PubMed] [Google Scholar]
- 124.Eman RM, Hoorntje ET, Oner FC, Kruyt MC, Dhert WJ, Alblas J. C-X-C Motif Ligand 12/Stromal-Cell-Derived Factor-1 Effectively Replaces Endothelial Progenitor Cells to Induce Vascularized Ectopic Bone. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 126.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60(3):813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 127.Hutmacher DW, Garcia AJ. Scaffold-based bone engineering by using genetically modified cells. Gene. 2005;347(1):1–10. doi: 10.1016/j.gene.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 128.Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, Kelley P, Stumm N, Mi S, Muller R, Zilberman Y, Gazit D. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3(4):449–461. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 129.Baltzer AW, Lattermann C, Whalen JD, Braunstein S, Robbins PD, Evans CH. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- 130.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, Robbins P, Fu FH, Huard J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A(7):1032–1039. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 131.Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J Bone Miner Res. 2002;17(11):1931–1944. doi: 10.1359/jbmr.2002.17.11.1931. [DOI] [PubMed] [Google Scholar]
- 132.Byers BA, Guldberg RE, Hutmacher DW, Garcia AJ. Effects of Runx2 genetic engineering and in vitro maturation of tissue-engineered constructs on the repair of critical size bone defects. J Biomed Mater Res A. 2006;76(3):646–655. doi: 10.1002/jbm.a.30549. [DOI] [PubMed] [Google Scholar]
- 133.Yang S, Wei D, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. J Bone Miner Res. 2003;18(4):705–715. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7(9):734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 135.Ono I, Yamashita T, Jin HY, Ito Y, Hamada H, Akasaka Y, Nakasu M, Ogawa T, Jimbow K. Combination of porous hydroxyapatite and cationic liposomes as a vector for BMP-2 gene therapy. Biomaterials. 2004;25(19):4709–4718. doi: 10.1016/j.biomaterials.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 136.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12(5):418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 137.Valimaki VV, Yrjans JJ, Vuorio EI, Aro HT. Molecular biological evaluation of bioactive glass microspheres and adjunct bone morphogenetic protein 2 gene transfer in the enhancement of new bone formation. Tissue Eng. 2005;11(3–4):387–394. doi: 10.1089/ten.2005.11.387. [DOI] [PubMed] [Google Scholar]
- 138.Kimelman Bleich N, Kallai I, Lieberman JR, Schwarz EM, Pelled G, Gazit D. Gene therapy approaches to regenerating bone. Adv Drug Deliv Rev. 2012;64(12):1320–1330. doi: 10.1016/j.addr.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank HG, Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20(11):2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 140.Duan C, Liu J, Yuan Z, Meng G, Yang X, Jia S, Zhang J, Chen S. Adenovirus-mediated transfer of VEGF into marrow stromal cells combined with PLGA/TCP scaffold increases vascularization and promotes bone repair in vivo. Arch Med Sci. 2014;10(1):174–181. doi: 10.5114/aoms.2012.30950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Feng L, Wu H, E L, Wang D, Feng F, Dong Y, Liu H, Wang L. Effects of vascular endothelial growth factor 165 on bone tissue engineering. PLoS One. 2013;8(12):e82945. doi: 10.1371/journal.pone.0082945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2009;27(1):8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 143.Cao L, Liu X, Liu S, Jiang Y, Zhang X, Zhang C, Zeng B. Experimental repair of segmental bone defects in rabbits by angiopoietin-1 gene transfected MSCs seeded on porous beta-TCP scaffolds. J Biomed Mater Res B Appl Biomater. 2012;100(5):1229–1236. doi: 10.1002/jbm.b.32687. [DOI] [PubMed] [Google Scholar]
- 144.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 145.Zou D, Zhang Z, Ye D, Tang A, Deng L, Han W, Zhao J, Wang S, Zhang W, Zhu C, Zhou J, He J, Wang Y, Xu F, Huang Y, Jiang X. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1alpha. Stem Cells. 2011;29(9):1380–1390. doi: 10.1002/stem.693. [DOI] [PubMed] [Google Scholar]
- 146.Boerckel JD, Uhrig BA, Willett NJ, Huebsch N, Guldberg RE. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci U S A. 2011;108(37):E674–E680. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6(4):391–407. [PubMed] [Google Scholar]
- 149.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 150.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmokel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17(15):2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 151.Lee TT, Garcia JR, Paez JI, Singh A, Phelps EA, Weis S, Shafiq Z, Shekaran A, Del Campo A, Garcia AJ. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2014 doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed. 2009;20(12):1763–1779. doi: 10.1163/156856208X386381. [DOI] [PubMed] [Google Scholar]
- 153.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci U S A. 2010;107(8):3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Salimath AS, Phelps EA, Boopathy AV, Che PL, Brown M, Garcia AJ, Davis ME. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS One. 2012;7(11):e50980. doi: 10.1371/journal.pone.0050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Phelps EA, Headen DM, Taylor WR, Thule PM, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34(19):4602–4611. doi: 10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zieris A, Prokoph S, Levental KR, Welzel PB, Grimmer M, Freudenberg U, Werner C. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31(31):7985–7994. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 157.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials. 2004;25(4):699–706. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 158.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35(25):7228–7238. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29(22):3260–3268. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 160.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3(100):100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 161.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Muller R, Swartz MA, Hubbell JA. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343(6173):885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 162.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21(32–33):3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 163.Nguyen TH, Kim SH, Decker CG, Wong DY, Loo JA, Maynard HD. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat Chem. 2013;5(3):221–227. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McCall JD, Luoma JE, Anseth KS. Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv Transl Res. 2012;2(5):305–312. doi: 10.1007/s13346-012-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Degano IR, Vilalta M, Bago JR, Matthies AM, Hubbell JA, Dimitriou H, Bianco P, Rubio N, Blanco J. Bioluminescence imaging of calvarial bone repair using bone marrow and adipose tissue-derived mesenchymal stem cells. Biomaterials. 2008;29(4):427–437. doi: 10.1016/j.biomaterials.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 166.Geuze RE, Prins HJ, Oner FC, van der Helm YJ, Schuijff LS, Martens AC, Kruyt MC, Alblas J, Dhert WJ. Luciferase labeling for multipotent stromal cell tracking in spinal fusion versus ectopic bone tissue engineering in mice and rats. Tissue Eng Part A. 2010;16(11):3343–3351. doi: 10.1089/ten.TEA.2009.0774. [DOI] [PubMed] [Google Scholar]
- 167.Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5(6):e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dupont KM, Sharma K, Stevens HY, Boerckel JD, Garcia AJ, Guldberg RE. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci U S A. 2010;107(8):3305–3310. doi: 10.1073/pnas.0905444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang ZY, Teoh SH, Chong MS, Lee ES, Tan LG, Mattar CN, Fisk NM, Choolani M, Chan J. Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials. 2010;31(4):608–620. doi: 10.1016/j.biomaterials.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 170.Becquart P, Cambon-Binder A, Monfoulet LE, Bourguignon M, Vandamme K, Bensidhoum M, Petite H, Logeart-Avramoglou D. Ischemia is the prime but not the only cause of human multipotent stromal cell death in tissue-engineered constructs in vivo. Tissue Eng Part A. 2012;18(19–20):2084–2094. doi: 10.1089/ten.TEA.2011.0690. [DOI] [PubMed] [Google Scholar]
- 171.Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M, Logeart-Avramoglou D, Petite H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. 2011;15(7):1505–1514. doi: 10.1111/j.1582-4934.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Camci-Unal G, Alemdar N, Annabi N, Khademhosseini A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym Int. 2013;62(6):843–848. doi: 10.1002/pi.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28(31):4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 174.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 175.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A. 2012;109(11):4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13(6):558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 177.Garcia JR, Garcia AJ. Cellular mechanotransduction: sensing rigidity. Nat Mater. 2014;13(6):539–540. doi: 10.1038/nmat3996. [DOI] [PubMed] [Google Scholar]
- 178.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Integrins in mechanotransduction. Curr Opin Cell Biol. 2013;25(5):613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012;119(1):26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fittkau MH, Zilla P, Bezuidenhout D, Lutolf MP, Human P, Hubbell JA, Davies N. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26(2):167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 181.Taite LJ, Yang P, Jun HW, West JL. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J Biomed Mater Res B Appl Biomater. 2008;84(1):108–116. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 182.Maeda T, Titani K, Sekiguchi K. Cell-adhesive activity and receptor-binding specificity of the laminin-derived YIGSR sequence grafted onto Staphylococcal protein A. J Biochem. 1994;115(2):182–189. doi: 10.1093/oxfordjournals.jbchem.a124315. [DOI] [PubMed] [Google Scholar]
- 183.Massia SP, Hubbell JA. Vascular endothelial cell adhesion and spreading promoted by the peptide REDV of the IIICS region of plasma fibronectin is mediated by integrin alpha 4 beta 1. J Biol Chem. 1992;267(20):14019–14026. [PubMed] [Google Scholar]
- 184.Hubbell JA, Massia SP, Desai NP, Drumheller PD. Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Biotechnology (N Y) 1991;9(6):568–572. doi: 10.1038/nbt0691-568. [DOI] [PubMed] [Google Scholar]
- 185.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4(3):477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]