Abstract
Physical activity (PA) and sedentary behaviors (SB—i.e. activities involving low energy expenditure and a sitting/reclining posture) may each have significant implications for weight loss and other bariatric surgery outcomes. While early studies suggested that patients typically comply with clinical recommendations to adopt habitual PA, these data were based on retrospective questionnaires. Conversely, recent studies incorporating mobile health (mHealth) technologies (e.g., objective monitors), which assess PA and SB in real-time and in the natural environment, show that most patients are inactive and highly sedentary preoperatively, and only make modest changes in these behaviors postoperatively. In addition to using mHealth technologies for obtaining accurate and detailed information on PA and SB, they are increasingly being employed to intervene on patients’ PA and SB and/or evaluate intervention outcomes. Researchers and clinicians are encouraged to consider the benefits of using mHealth technology when studying and treating PA and SB in bariatric surgery patients.
Keywords: bariatric surgery, physical activity, sedentary behavior, mobile health (mHealth), objective monitors
Introduction
Habitual physical activity (PA) is an integral component of a healthy lifestyle. Increasing physical activities, particularly those performed at moderate and higher intensities and in sustained bouts [i.e. moderate-to-vigorous PA (MVPA) in bouts ≥ 10-min, a.k.a. bout-related MVPA], can help to regulate body weight, prevent and alleviate chronic disease, and lower risk of disease-specific and all-cause mortality (Bouchard, Blair & Haskell, 2007; Donnelly et al. 2009). Emerging evidence also suggests that similar health benefits, independent of PA, can be achieved via decreasing sedentary behaviors (SB—i.e. activities characterized by low energy expenditure and a sitting or reclining posture) (Dempsey, Owen, Biddle & Dunstan, 2014, Sedentary Behaviour Research, 2012).
Research also supports the importance of PA and SB in the context of bariatric surgery with higher levels of habitual PA and lower levels of SB each being associated with more favorable weight and health outcomes (Bond & King, 2014). However, these data are mostly derived from retrospective questionnaires which carry risk of bias and inaccuracies. Thus, we recently called for investigators studying PA, SB, and other behavioral aspects of bariatric surgery to begin using mobile health (mHealth) technologies such as objective activity monitors that assess behavior in real-time and in the natural environment (Thomas, Bond, Sarwer & Wing, 2011). Studies incorporating objective monitors have shown that bariatric surgery patients are both inactive and highly sedentary preoperatively (pre-op) and remain so postoperatively (postop), despite reporting otherwise (Bond & King, 2014). Given the challenges that patients face in increasing PA and decreasing SB, mHealth technologies are also being harnessed to either intervene on these behaviors and/or to accurately estimate the impact of behavioral interventions.
In this review, we first discuss the importance of PA and SB in relation to bariatric surgery outcomes (see supplementary file for more detailed information on PA and SB including the health and weight management implications of these behaviors). Next, we provide rationale for using mHealth technology to measure and intervene on PA and SB in bariatric surgery patients. Then, we summarize studies that have used objective monitors to assess PA and SB at and across pre- and post-op intervals. Finally, we describe interventions to increase habitual PA and/or decrease SB in bariatric surgery patients that have used mHealth technology to influence and/or evaluate changes in these behaviors and discuss their implications for future bariatric surgery research and clinical practice.
The Importance of PA and SB within the Context of Bariatric Surgery
PA and Bariatric Surgery Outcomes
Multiple studies have reported that habitual PA is positively associated with weight loss and other post-op outcomes. For example, Bond et al. (2009) conducted the first prospective study to evaluate the relationship of pre- to post-op PA changes with weight loss and health-related quality of life (HRQoL). Roux-en-Y gastric bypass (RYGB) patients who were inactive (<200 PA min/wk) pre-op and progressed to being active (≥200 PA min/wk) at 1-yr post-op achieved weight losses and HRQoL improvements that were greater than those experienced by patients who remained inactive and comparable to those achieved by patients who continued being active. A more recent study examined cross-sectional associations between MVPA and longer-term weight loss outcomes in adults who were on average 7-yrs post-RYGB (Herman, Carver, Christou & Andersen, 2014). Participants who reported performing one or more ≥30-min MVPA sessions/wk had both greater weight loss and less regain compared to patients who did not perform any MVPA sessions. While the above findings suggest that higher PA levels are associated with enhanced initial weight loss, weight loss maintenance, and HRQoL after bariatric surgery, both studies used retrospective questionnaires to evaluate PA. Consequently, studies that use objective monitors to assess PA levels of bariatric surgery patients provide stronger evidence.
King et al. (2012) examined associations between objectively-measured pre- to 1-yr post-op PA changes and weight loss in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Increases in PA were related to greater weight loss at 1-yr post-op, and an additional 3,000 steps/d increase above the mean change in steps was associated with additional decreases of 1.5 kg in weight and 1.1% in percent body fat. Two cross-sectional studies examined associations between time spent in objectively-measured PA of different intensities and post-op weight loss. The first study reported a positive association between MVPA min/wk and excess weight loss, after controlling for important covariates (i.e. time since surgery and caloric intake) in patients who were 2–5 yrs post-RYGB (Josbeno, Kalarchian, Sparto, Otto & Jakicic, 2011). Additionally, participants who performed ≥150 MVPA min/wk achieved significantly greater weight loss than those who performed <150 min/wk. The second study showed that LPA was inversely related to current weight in patients 6–18 mo. post-laparoscopic adjustable gastric banding (LAGB) or sleeve gastrectomy (LSG) (Chapman et al., 2014). Use of objective PA monitors in the above studies provides data of higher quality, usefulness, and potential validity on patients’ PA patterns and the relation of these patterns to weight loss and other outcomes. However, all of the studies are observational, warranting experimental studies to provide more reliable evidence of causation between PA and post-op outcomes.
Few RCTs have tested the impact of PA or physical fitness on weight loss and other postop outcomes. Shah et al. (2011) randomized post-RYGB patients to 12 wks of either exercise and dietary counseling or dietary counseling alone. Participants in the exercise + diet arm were progressed to expending ≥2000 kcal/wk via semi-supervised moderate-intensity exercise on ≥5 d/wk. After 12 wks, these participants showed significantly greater improvements in self-reported PA, cardiorespiratory fitness, and glucose control, but not weight loss, compared to the diet only group. More recently, Coen et al. (2015) randomized patients who were 1–3 mo. post-RYGB to 6 mo. of either semi-supervised moderate exercise or a health education control. Exercise produced greater improvements in insulin sensitivity, glucose effectiveness, and cardiorespiratory fitness, but not weight loss or adiposity, compared to control. These studies provide preliminary evidence that moderate-intensity exercise improves important components of metabolic health and cardiorespiratory fitness above and beyond those produced by RYGB-induced weight loss alone. While reasons for the lack of an exercise effect on post-op weight and other measures of adiposity are not clear, certain study limitations might offer a partial explanation. The Coen study was conducted during the initial post-op year when the majority of weight loss occurs, and thus the powerful surgery effect may have masked any exercise effects during the active weight loss period. Additionally, participants were progressed to ≥120 exercise min/wk, and free-living exercise participation was not objectively measured. Given that higher PA doses are recommended for weight loss, the average exercise performed by participants (185 min/wk) was possibly insufficient to enhance weight losses produced by RYGB. Similar issues accompany the Shah et al. study. Moreover, the study was small and underpowered to detect a weight loss effect, duration of participation since surgery was highly variable (i.e. 3 mo.-8.5 yrs), the drop-out rate was high, and less than half of the participants achieved the exercise goal. Future experimental studies with adequate statistical power are needed to examine the effects of higher doses of objectively-measured PA on post-op weight loss, particularly after weight has stabilized and weight regain begins to occur. Additionally, given the high rates of noncompliance and drop-out, development and testing of innovative intervention strategies to promote PA and exercise adherence is needed.
SB and Bariatric Surgery Outcomes
Given that SB is a novel behavioral focus in the bariatric surgery population, few studies have evaluated associations of SB with post-op outcomes. Vatier et al. (2012) found that decreases in reported television (TV) watching from pre- to 1-yr post-RYGB were inversely associated with changes in fat mass, independently of PA. In another study, self-reported sitting time was inversely associated, independently of MVPA, with total weight loss and weight loss maintained in patients 2–16 yrs post-RYGB (Herman et al., 2014). Only one study to date has examined the relationship between objectively-measured SB and bariatric surgery outcomes, showing a positive association between SB time and current weight in patients 6–18 mo. post-op (Chapman et al., 2014). The above studies, although few and observational in nature, provide preliminary data to suggest that SB could play an independent role in post-op weight and body composition outcomes. Additional research is needed that uses both objective and subjective measures to evaluate relationships of time spent performing SB, including specific forms of SB (e.g., TV watching), with post-op weight loss and related outcomes (e.g., waist circumference, cardiometabolic risk factors and HRQoL).
mHealth Technology to Assess PA and SB in Bariatric Surgery Patients
The tools most often used to assess PA and SB across both clinical and research settings include retrospective self-report questionnaires and interview-administered recalls that rely on patients’ ability to remember and describe their PA and/or SB over the preceding days, weeks, or months; and diaries that are intended to be used by patients to record their PA and/or SB soon after it occurs (Prince et al., 2008). Validation studies often find that retrospective measures of PA are, at best, only moderately related to actual PA (Prince et al., 2008). As described below, the few studies comparing self-report and objective measures in bariatric surgery patients suggest that they tend to substantially overestimate the degree to which they increase their PA post-op.
The suboptimal performance of PA recalls is not surprising given the ample evidence that retrospective self-report measures, in general, are affected by numerous types of bias (Gorin & Stone, 2001). Most of the bias comes from the use of heuristics, or mental shortcuts, outside of conscious awareness, which are used to fill gaps in memory. For example, recency effects may lead an individual to assume that their recent behavior and/or experiences are similar to those occurring weeks or months ago, when the actual information from the more distal period cannot be accurately recalled. Even when the memory trace is adequate, bias may affect the recall process. For example, mood-congruent memory bias may lead an individual to recall more positive behaviors and experiences (e.g., performance of PA) when positive affect is high, and more negative behaviors and experience (e.g., barriers that prevented performance of PA) when negative affect is high. The effect of bias tends to be greatest when attempting to recall and describe behaviors and experiences that are habitual and/or occur frequently, such as SB (Stone, Shiffman, Atienza & Nebeling, 2007).
Diaries ostensibly avoid bias affecting retrospective self-report measures because diaries are intended to capture information related to PA and SB soon after it occurs. However, evidence suggests that paper diaries are often completed retrospectively (e.g., at the end of the diary period), despite patients’ claims to the contrary (Stone, Shiffman, Schwartz, Broderick & Hufford, 2003). Because it is typically impossible to verify when entries were made using a paper diary, it is difficult to estimate how much bias may have been introduced by retrospective recall. Furthermore, diaries can be perceived as burdensome, and there may therefore be a disincentive to report on all behaviors of interest, particularly those that occur frequently, such as SB. For these reasons, the accuracy and utility of diaries may also be limited.
mHealth approaches to assessment currently hold the greatest promise for collecting accurate and detailed information on free-living PA and SB patterns (Thomas et al., 2011). To more accurately and reliably estimate participation in PA and/or SB, objective monitors employ one or more sensors to detect changes in: 1.) acceleration of the body during movement in different dimensions; 2.) physiologic parameters (e.g., electronic thermometer to measure skin temperature); and 3.) posture (sitting, standing, prone). For example, it is possible to obtain highly accurate minute-by-minute estimates of caloric expenditure, time spent in various intensities of PA, and even to differentiate SB occurring while sitting versus lying down (Butte, Ekelund & Westerterp, 2012). In addition to requiring no recall whatsoever, monitors also reduce patient burden and it is easy to determine adherence to the measurement protocol (i.e. when the monitor was and was not worn). For these reasons, objective monitors have been recommended for measuring PA with bariatric surgery patients (Thomas et al., 2011).
The following section summarizes findings from studies using objective monitors to quantify pre- and/or post-op patterns of PA and SB in bariatric surgery patients. We focus exclusively on studies that used: 1.) accelerometer-based monitors such as the StepWatch Activity (SAM; Orthocare Innovations) (King et al., 2008, 2012, 2015), Rt3 (Stayhealthy, Inc, Monrovia, CA) (Bond et al., 2010a, 2010b) and ActiGraph (ActiGraph, LLC, Pensacola, Florida) (Berglind et al., 2015; Ramirez-Marrero, Miles, Joyner, & Curry, 2014) monitors; 2.) the SenseWear Armband (SWA; Body Media Inc., Pittsburgh PA) that integrates data from both accelerometer and physiologic sensors (Bond et al., 2011, 2015a, 2015b; Chapman et al., 2014; Langenberg, et al., 2015; Josbeno, et al., 2011); and 3.) the ActivPAL™3 accelerometer (PAL Technologies Ltd., Glasgow, UK) that uses accelerometer and inclinometer sensors to detect movement and differentiate postures (Reid, Carver, Andersen, Court, & Andersen, 2015).
Objectively-Assessed PA and SB Patterns in Bariatric Surgery Patients
Pre-op PA and SB Patterns
The first study to objectively assess pre-op PA patterns was conducted in LABS-2 study sample (King et al., 2008). Participants averaged 7569 steps/d; however, more than half (54%) of the sample were categorized as sedentary (<5,000 steps/d) or low active (5000–7499 steps/d) and only 20% were considered active (≥10,000 steps/d). Similarly, a recent study from Germany found that pre-op patients averaged 7140 steps/d, yet more than half (57.8%) were considered sedentary or low active, and only 18.3% were categorized as active (Langenberg, et al., 2015). Bond et al. (2010b) examined pre-op engagement in PA of different intensities and bout duration relative to normal-weight controls. Patients spent one-half as much time in total MVPA versus controls (26 versus 52 min/d). Additionally, 68% of patients accumulated 0 min of bout-related MVPA compared to 13% of controls, and 4.5% of patients were considered active (i.e. ≥150 MVPA bout-related min/wk) versus 40% of controls (Haskell , et al., 2007). The above studies and those more recent are consistent in showing that most pre-op patients are inactive and rarely perform PA of a moderate intensity in sustained bouts, suggesting little or no engagement in purposeful, structured exercise.
Given that pre-op patients spend minimal time in MVPA, Bond et al. (2011) assessed the amount of time they spent in SB relative to LPA. Participants on average spent 81% (10.9 h/d), 14.8% (1.9 h/d) and 3.8% (0.5 h/d) of their waking time in SB, LPA, and MVPA, respectively. Thus, it appears pre-op patients spend the vast majority of their day in SB, and are much more sedentary compared to the general population for whom SB accounts for 57%-73% of waking hours (Matthews et al., 2008; Carson et al., 2014). Our group has since replicated these initial findings in different samples and using different monitors (Unick et al., 2012). However, much lower estimates of percentage of time spent in SB have been reported by recent studies conducted in Sweden (48%) and in the LABS-2 sample (65%) (Berglind et al., 2015; King et al., 2015). While reasons for these lower estimates are unclear, factors such as cultural differences (Berglind et al., 2015) and monitors that do not directly estimate SB (King et al., 2015) may be partially responsible. Despite this variability, the above findings indicate that most patients are both inactive and spend a large proportion of their daily time in SB. Additional studies that compare estimates from different monitors of overall time spent in SB as well as that spent in prolonged bouts are needed.
Postoperative PA and SB Patterns
Chapman et al. (2014) found that patients <18-mo. post-op engaged in 48 min/d of MVPA; however, only 4% of this amount was bout-related. Also, participants on average spent 72% (10 h/d) of waking hours in SB, nearly half (44%) of which was spent in prolonged bouts ≥30-min. Another study showed that patients who were ≥1-yr post-RYGB spent an average of 186 min/wk in MVPA (23 bout-related MVPA min/wk) and 9.4 h/d in SB (Ramirez-Marrero et al., 2014). Most recently, Reid et al. (2015) assessed PA and SB in patients who were on average 8.9 years post-RYGB. Participants on average were classified as low active (6375 steps/d), and only 11.3% were classified as active (≥10,000 steps/d). Also, participants on average spent 88% (9.7 h/d) of their waking hours in SB, specifically in a sitting or reclining posture, according to the ActivPAL™3 monitor. The above studies suggest that, similarly to pre-op, BS patients across post-op intervals are both inactive, and highly sedentary. However, given their cross-sectional nature, these studies could not determine whether PA and SB levels are changed after surgery.
Pre- to Post-Op Changes in PA and SB Patterns
Few studies have objectively evaluated pre- to post-op PA changes, and even fewer have evaluated changes in SB. Bond et al. (2010a) compared self-reported and accelerometer-based estimates of changes in MVPA min/wk from pre- to 6-mo. post-op. While self-reported MVPA min/wk increased nearly 5-fold from pre-op to post-op (from 45 to 212 min/wk), there were no significant changes in accelerometer-measured min/wk of total (186 to 151 min/wk) and bout-related (41 to 40 min/wk) MVPA. Similarly, the percentage of participants who achieved ≥150 bout-related MVPA min/wk increased markedly from pre-op to post-op based on self-report (10% to 55%), but not on the objective measure (i.e. decrease of 10% to 5%). Subsequent studies have yielded similar findings. King et al. (2012) showed that while LABS-2 study participants were significantly more active at 1-yr post-op versus pre-op, the magnitude of change was small—i.e. median steps/d increased from 7563 to 8788, MVPA min/wk increased from 72 to 112, and bout-related MVPA min/wk increased from 0 to 23. Another study from Sweden (Berglind et al., 2015) showed that while patients engaged in relatively high levels of bout-related MVPA (26 min/d) and low levels of SB (430 min/d) pre-op, these levels did not change at 9 mo. post-op (i.e. 30 min/d of bout-related MVPA and 421 min/d of SB). Most recently, King et al. (2015) examined changes in both PA and SB in LABS-2 study participants from pre- to 3-yrs post-op. From pre- to 1-yr post-op, participants made small but significant increases in steps/d (7688 to 8974), MVPA min/wk (77 to 106) and bout-related MVPA min/wk (7 to 24), along with decreases in SB min/d (573 to 544). However, both PA and SB did not change from 1- to 3-yrs post-op. The above studies indicate that patients generally perform small amounts of bout-related MVPA and spend most of their time in SB pre-op, and despite reporting otherwise, make only modest changes in these behaviors post-op. Importantly, these data also suggest that patients require additional intervention to increase PA and decrease SB given that post-op weight loss and improvements in comorbidities alone may be insufficient to produce changes in these behaviors.
mHealth Technology to Evaluate and/or Intervene in Bariatric Surgery Patients
As described above, mHealth technology is a powerful tool for evaluating free-living PA and SB in bariatric surgery patients. Likewise, it can be used to measure the effects of PA and/or SB interventions. Use of objective monitors to study intervention effects may be particularly important given the abovementioned evidence that patients’ estimation of their PA, and particularly their estimate of change in PA after surgery, may be inflated. Objective monitors reduce the likelihood of overestimating interventions effects on pre- to post-op changes in PA.
In addition to measuring intervention effects, mHealth technology itself can be used to modify PA and SB (Stephens & Allen, 2013). Like most interventions targeting health behaviors, attempts to improve PA and SB have historically involved face-to-face treatment. However, mHealth approaches are advantageous because they can: 1.) limit the need for clinic visits, which is important given the often poor attendance at post-op follow-up visits; 2.) provide intervention when it is most needed (i.e. as they perform the relevant health behavior), in a patients natural environment; 3.) provide intervention that is tailored to patients’ unique needs; 4.) reduce participant burden by automating routine tasks such as self-monitoring; 5.) provide immediate feedback on progress towards goals and encouragement for meeting goals; 6.) provide support and accountability in-between clinic visits; and 7.) automatically transmit key information to health providers (Thomas & Bond, 2014). The opportunity for mHealth interventions for PA and SB is growing as nearly two-thirds of individuals in the U.S. own smartphones (“Cell phone and smartphone ownership demographics,” 2013) and nearly 10% own a fitness tracking device such as a FitBit or Jawbone device (“Inside wearables,” 2014).
Just-in-time adaptive interventions (JITAIs) are some of the most sophisticated interventions for PA and SB (Nahum-Shani et al., 2014), but remain relatively uncommon due to the technical challenges involved in their development and implementation. JITAIs use mobile technology such as smartphones, sensors, and software analytics to automatically detect patient behavior and deliver intervention content that is most relevant to a patient’s needs, at the time that it is most needed and/or likely to improve health-related behaviors (Nahum-Shani et al., 2014). A JITAI targeting PA and/or SB might theoretically measure factors such as how much PA and SB an individual has performed, their location (e.g., home versus workplace, proximity to exercise facilities), the weather, and any number of other conditions to independently determine when and what type of intervention (e.g., type of PA, motivational message, reward for engaging in PA, support from a peer or coach) to supply to maximize the intervention effect. The following section summarizes findings from the few studies using mHealth technology to measure intervention effect and/or to intervene on PA and SB in the context of bariatric surgery. Special emphasis is placed on a recent JITAI aimed at reducing SB in bariatric surgery patients.
mHealth Technology to Measure PA Intervention Efficacy in Bariatric Surgery Patients
To date, only one PA intervention has used mHealth technology in the form of an objective monitor to measure efficacy. The Bari-Active study conducted by Bond et al. (2015b) was a RCT to test a 6-wk pre-op PA intervention (PAI) versus a standard pre-surgical care control condition (SC) for increasing daily bout-related MVPA. The primary aim was to compare PAI versus SC on baseline to post-intervention changes in min/d of bout-related and total MVPA, measured via the SWA monitor. The intervention was implemented pre-op as per guidelines that patients should adopt habitual PA pre-op and the notion that this period offers a “teachable moment” for focusing on motivational barriers to PA that might not be otherwise addressed post-op. Support for Bari-Active and other pre-op interventions is also provided by recent studies that demonstrate: 1) substantial changes in MVPA do not spontaneously result post-op (Berglind et al., 2015; Bond et al., 2010; King et al., 2012, 2015); and 2) pre-op MVPA is a positive and independent predictor of both post-op MVPA and weight loss (Browning et al., 2014; King et al., 2012). The intervention focused on increasing bout-related MVPA given that this level and pattern of PA is emphasized in public health guidelines and is related to improved long-term weight loss and exercise adherence. Participants in both groups wore the SWA for 7 days at baseline and post-intervention.
Outcomes analysis included 75 participants (87% women, 46 yrs old, 79% White, BMI=45 kg/m2) who were randomly assigned to 6 wks of either PAI (n=40) or SC (n=35). PAI participants were given 2 intervention goals: 1.) increasing walking exercise performed at a moderate intensity in bouts ≥10-min by 30 min/d; and 2.) increase daily step count by 5,000. To help accomplish these goals, participants attended weekly individual face-to-face counseling sessions and were provided with a pedometer and a PA monitoring log to record daily bout-related walking exercise minutes and steps. Each counseling session involved: 1.) reviewing self-monitoring records, progress towards intervention goals, and homework assignments; 2.) addressing barriers to achieving goals within a problem-solving framework; 3.) teaching behavioral and cognitive strategies to achieve goals (e.g., stimulus control, goal-setting, etc.); 4.) setting bout-related walking exercise and step goals for the next week; 5.) developing a daily action plan indicating when and where the bout-related walking exercise goal would be achieved; and 6.) discussion of a new homework assignment requiring application of strategies taught during the session. SC participants attended usual pre-op clinic visits, but were not provided any formal prescription or strategies to change PA behavior (Bond et al., 2015b).
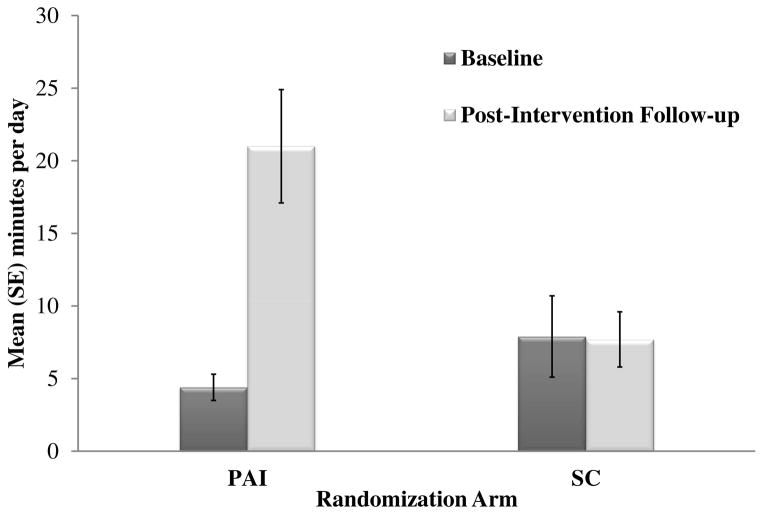
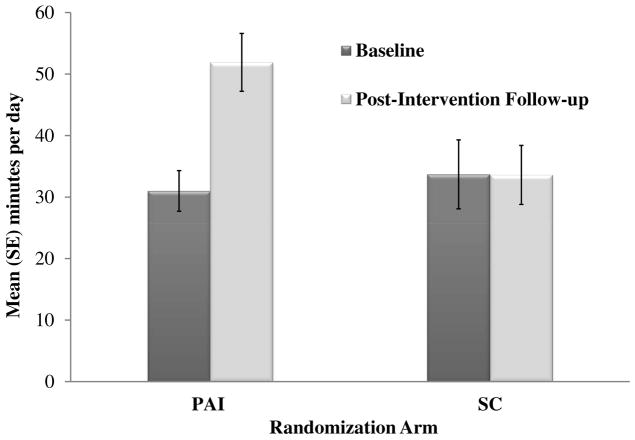
Results showed that retention was 84% at the post-intervention primary endpoint with no differences between arms. As shown in Figure 1, intent-to-treat analysis of the primary aim showed that PAI participants on average increased bout-related MVPA by 16.6 min/d from baseline to post-intervention (4.4 to 21.0 min/d) and total MVPA by 21.0 min/d (30.9 to 51.9 min/d). By contrast, SC participants on average demonstrated no change in daily bout-related (-0.3 min/d; 7.9 to 7.6 min/d) or total (-0.1 min/d; 33.7 to 33.6 min/d) MVPA. Among study completers, 26 (79%) participants in the PAI arm increased daily bout-related MVPA from baseline to post-intervention (3.6 to 29.7 min/d), 2 (6%) did not change (0 to 0 min/d), and 5 (15%) decreased (9.1 to 6.6 min/d). All PAI completers attended all 6 counseling sessions. In the SC arm, 11 (37%) participants increased daily bout-related MVPA from baseline to post-intervention (2.3 to 12.5 min/d), 8 (27%) did not change (0 to 0 min/d), and 11 (37%) decreased (18 to 7 min/d). At post-intervention, a greater proportion of study completers in the PAI arm met the guideline of 150 min/wk of bout-related MVPA compared to study completers in the SC arm (36% versus 13%) (Bond et al., 2015a, 2015b).
Figure 1.
The above results from the Bari-Active trial demonstrate that with intervention, patients can achieve significant increases in objectively-measured MVPA before undergoing bariatric surgery. Of note, PAI participants on average increased bout-related MVPA to a level (147 min/wk) that is both higher than that attained by post-op patients in non-intervention studies using the same objective monitor (Chapman et al., 2014; Josbeno et al., 2011), and consistent with public health guidelines (i.e. ≥150 min/wk). These findings challenge any notion that patients have too many barriers to adopt changes in PA pre-op and also support published recommendations that patients adopt habitual PA pre-op (Blackburn et al., 2009; Poirier et al., 2011). While additional RCTs are needed to test whether intervention-related changes in pre-op PA can be sustained post-op, an unpublished exploratory analysis of Bari-Active participants who went on to have surgery reveals that the PAI group achieved a more than two-fold increase in bout-related MVPA compared to SC at 6-months post-op (27 vs. 11 min/d). It will also be important for future studies to determine whether higher levels of PA, if sustained, can help maintain weight loss and improvements in other post-op outcomes beyond the active weight loss period. Finally, it should be acknowledged that most patients invited to participate in the Bari-Active trial refused, and not all participants who completed the PAI achieved large increases in bout-related MVPA. Thus, there is a need to identify strategies that can not only effectively attract more patients to participate in adjunctive PA and other behavioral interventions, but also help more of these patients to achieve successful outcomes. One strategy might be focusing on decreasing SB versus increasing MVPA, given that the former might be a more achievable behavioral target. Additionally, previous research suggests that LPA, which most often replaces SB, is associated with lower weight after bariatric surgery (Chapman et al., 2014). Interventions to reduce SB in the BS population are therefore warranted.
mHealth Technology to Intervene on SB in Bariatric Surgery Patients
Findings showing that patients spend a large proportion of their waking hours in SB pre-op and make only small changes in SB post-op are problematic given that higher post-op levels of SB are related to higher current weight, smaller weight losses, and weight regain (Berglind et al., 2015; Bond et al., 2011; Chapman et al., 2014; Herman et al., 2014; King et al., 2015). Despite these findings, systematic efforts to decrease SB in bariatric surgery patients have been few.
Interventions to decrease SB may require different approaches than those to increase PA. SB, unlike PA, accounts for most waking hours, is often accumulated in longer, interrupted bouts, occurs in multiple settings, and requires minimal thought or planning. Thus, interventions to decrease SB ideally should be simple, usable across different settings, require little forethought, automatically monitor SB, and prompt movement upon meeting a clinically relevant SB threshold (Rutten, Savelberg, Biddle & Kremers, 2013; Bond et al., 2014a). The previously described JITAI approach that incorporates use of mHealth technology is particularly well-suited to satisfy the above ideal SB intervention requirements. To this end, we developed B-MOBILE, a smartphone-based JITAI to decrease SB (Bond et al., 2014a). The B-MOBILE intervention combines a smartphone device with an onboard accelerometer and a smartphone application to: 1) target SB across all settings; 2) automatically monitor SB; 3) issue automatic prompts (i.e. audible tone/vibration with motivational message) based on monitored SB data to interrupt prolonged SB with brief PA (i.e. walking breaks); 4) provide continuous real-time feedback on SB and PA accumulated throughout the day and the amount of time until the next PA break; and 5) deliver immediate reinforcement for adherence to prompts and performing PA breaks.
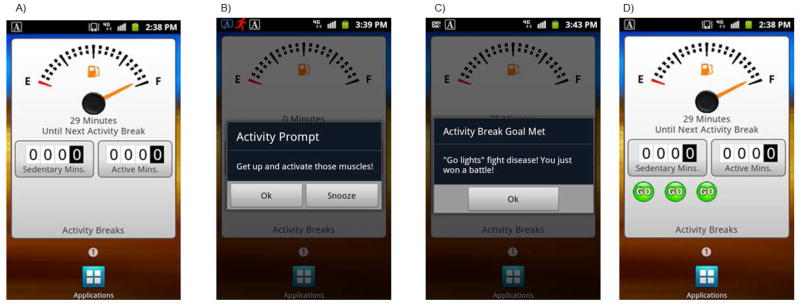
We recently completed a study to evaluate preliminary efficacy of the B-MOBILE intervention in 24 bariatric surgery patients who were on average 3 yrs post-op (Bond et al., 2014b). Participants wore the SWA monitor for 7 days at baseline, and for 3 subsequent wks during which 3 separate 7-day prompting and PA (i.e. walking) break conditions were administered in counterbalanced order to determine the optimal behavioral goal prescription for decreasing time spent in SB: 1) 3-min PA break after 30 SB min; 2) 6-min PA break after 60 SB min; and 3) 12-min PA break after 120 SB min. The smartphone’s accelerometer was programmed to monitor SB in 1-min increments. Monitored SB data were available to the investigators in real-time via the smartphone’s always-on Internet connection and used to inform the automated intervention goal-setting, prompting and feedback intervention components. As shown in Figure 2, these components were presented within the context of an automobile dashboard metaphor, with the fuel gauge depicting the number of SB min remaining until the next PA break and two odometers tracking SB and PA min accumulated during the day. The 3 PA break goals (listed above) were used in combination with the smartphone-based accelerometry data to prompt PA breaks when the maximum number of SB min was reached. For example, when the smartphone accelerometer detected that a participant had spent 30 continuous min in SB without taking a PA break of ≥3 min, the smartphone produced an audible prompt with an on-screen reminder text of the PA break goal and encouragement to take the PA break. Smartphone-based real-time accelerometery data were also used to determine whether participants achieved the PA break goal. When participants achieved the PA break goal, they received a message and a bright green “go” light (that appeared on the dashboard) to praise and symbolize this achievement. The smartphone screen display was also updated to reflect that the fuel gauge had been “refilled.”
Figure 2.
B-MOBILE Smartphone Dashboard for Intervention and Feedback on Sedentary and Non-Sedentary Time
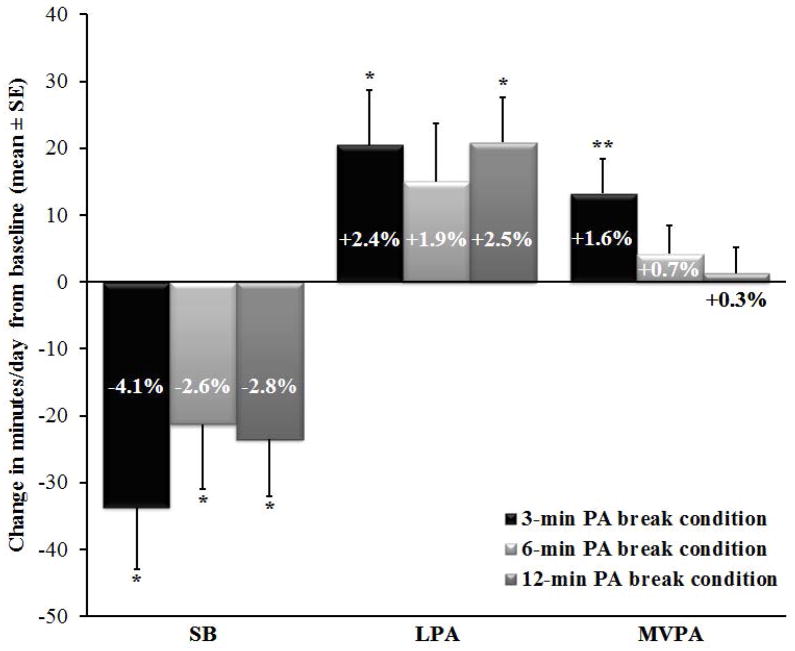
At baseline, participants on average spent about 10 h/d or 74% of waking hours in SB, 3 h/d or 21% in LPA, and 48 min/d or 5% in MVPA. Figure 3 shows that all 3 PA break conditions produced significant decreases in SB compared to baseline. The 3-min condition produced the largest decrease (-4.1% or -33 min/d), although this was not significantly different from the other 2 conditions. Conversely, LPA increased significantly during both the 3- and 12-min conditions compared to baseline and MVPA increased significantly during the 3-min condition compared to baseline and the 12-min condition. Results also revealed changes in time spent in prolonged SB bouts ≥30-min. At baseline, over half (52%) of daily SB time was spent in bouts ≥30-min: this was significantly reduced to 44% and 47% in the 3- and 6-minute conditions, respectively. Additionally, the decrease produced by the 3-min condition was significantly greater compared to the 12-min condition (decrease to 49%).
Figure 3.
The above preliminary data suggest that the B-MOBILE intervention can produce significant decreases in daily SB among post-op patients. While all 3 PA break conditions yielded significant decreases in SB, only the 3-min condition replaced SB with both LPA and MVPA. Additionally, the 3-min condition was superior to the 12-min condition for substituting MVPA for SB and decreasing prolonged SB bouts. Consequently, interrupting BS patients’ SB more frequently with shorter breaks may be more effective than interrupting SB less frequently with longer breaks for replacing SB with PA and decreasing prolonged SB bouts. Future studies are needed to determine if interventions that focus on decreasing SB via frequent, short PA breaks of at least a light intensity might be a more attainable target than those focused only on increasing MVPA, for optimizing overall energy expenditure and weight and health-related outcomes after BS. Additionally, studies are needed to determine whether B-MOBILE and future mHealth interventions can produce longer-term reductions in SB and/or PA in the BS population.
Conclusion
Recent research indicates that most bariatric surgery patients fail to achieve recommended PA levels and engage in excessive SB, both pre- and post-op, which has important implications for surgical outcomes. Using mHealth technology, we can more accurately measure PA and SB in bariatric surgery patients (a population where self-report often does not agree with objective measures) to better understand how PA and SB affect post-op outcomes, and to identify opportunities for intervention. Likewise, mHealth technology has advantages for delivering intervention to improve PA and SB patterns in patients who often do not adhere to scheduled follow-up visits with the surgical team and therefore are not consistently available for face-to-face treatment. However, additional research is needed to determine how best to design and implement these programs.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant R03 DK095740 (PI: Bond)
References
- Berglind D, Willmer M, Eriksson U, Thorell A, Sundbom M, Uddén J, Rasmussen F. Longitudinal Assessment of Physical Activity in Women Undergoing Roux-en-Y Gastric Bypass. Obesity Surgery. 2015;25:119–125. doi: 10.1007/s11695-014-1331-x. [DOI] [PubMed] [Google Scholar]
- Blackburn GL, Hutter MM, Harvey AM, Apovian CM, Boulton HR, Cummings S, Annas CL. Expert panel on weight loss surgery: executive report update. Obesity. 2009;17:842–862. doi: 10.1038/oby.2008.578. [DOI] [PubMed] [Google Scholar]
- Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, Wing RR. Pre-to Postoperative Physical Activity Changes in Bariatric Surgery Patients: Self Report vs. Objective Measures. Obesity (Silver Spring) 2010a;18:2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DS, Jakicic JM, Vithiananthan S, Thomas JG, Leahey TM, Sax HC, Wing RR. Objective quantification of physical activity in bariatric surgery candidates and normal-weight controls. Surgery for Obesity and Related Diseases. 2010b;6:72–78. doi: 10.1016/j.soard.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DS, King WC. The role of physical activity optimizing bariatric surgery outcomes. In: Still C, Sarwer DB, Blankenship J, editors. The ASMBS textbook of bariatric surgery. New York: Springer Science+Business Media; 2014. pp. 217–229. [Google Scholar]
- Bond DS, Phelan S, Wolfe LG, Evans RK, Meador JG, Kellum JM, Wing RR. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity (Silver Spring) 2009;17:78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]
- Bond DS, Thomas JG, King WC, Vithiananthan S, Trautvetter J, Unick JL, Wing RR. Exercise improves quality of life in bariatric surgery candidates: Results from the Bari-Active trial. Obesity (Silver Spring) 2015a;23:536–542. doi: 10.1002/oby.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DS, Thomas JG, Raynor HA, Moon J, Sieling J, Trautvetter J, Wing RR. B-mobile-a smartphone-based intervention to reduce sedentary time in overweight/obese individuals: A within-subjects experimental trial. PloS one. 2014a;9(6):e100821. doi: 10.1371/journal.pone.0100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DS, Thomas JG, Raynor HA, Vithiananthan S, Ryder BA, Trautvetter J, Wing RR. B-MOBILE: A smartphone-based intervention to reduce sedentary time in postoperative bariatric surgery patients. Abstract retrieved April. 2014b;21:2015. from https://guidebook.com/guide/23140/poi/2326290/?pcat=73014. [Google Scholar]
- Bond DS, Unick JL, Jakicic JM, Vithiananthan S, Pohl D, Roye GD, Wing RR. Objective assessment of time spent being sedentary in bariatric surgery candidates. Obesity Surgery. 2011;21:811–814. doi: 10.1007/s11695-010-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond DS, Vithiananthan S, Thomas JG, Trautvetter J, Unick JL, Jakicic JM, Wing RR. Bari-Active: a randomized controlled trial of a preoperative intervention to increase physical activity in bariatric surgery patients. Surgery for Obesity and Related Diseases. 2015b;11:169–177. doi: 10.1016/j.soard.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Blair SN, Haskell W. Why study physical activity and health? In: Bouchard C, Blair SN, Haskell WL, editors. Physical activity and health. Champaign, IL: Human Kinetics; 2007. pp. 4–19. [Google Scholar]
- Butte NF, Ekelund U, Westerterp KR. Assessing physical activity using wearable monitors: measures of physical activity. Medicine and Science in Sports and Exercise. 2012;44(1 Suppl 1):S5–12. doi: 10.1249/MSS.0b013e3182399c03. [DOI] [PubMed] [Google Scholar]
- Carson V, Wong SL, Winkler E, Healy GN, Colley RC, Tremblay MS. Patterns of sedentary time and cardiometabolic risk among Canadian adults. Preventive Medicine. 2014;65:23–27. doi: 10.1016/j.ypmed.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Cell phone and smartphone ownership demographics. 2013 Dec 17; Retrieved from http://www.pewinternet.org/data-trend/mobile/cell-phone-and-smartphone-ownership-demographics/
- Chapman N, Hill K, Taylor S, Hassanali M, Straker L, Hamdorf J. Patterns of physical activity and sedentary behavior after bariatric surgery: An observational study. Surgery for Obesity and Related Diseases. 2014;10:524–530. doi: 10.1016/j.soard.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, Goodpaster BH. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. The Journal of Clinical Investigation. 2015;125:248–257. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey PC, Owen N, Biddle SJ, Dunstan DW. Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Current Diabetes Reports. 2014;14:522. doi: 10.1007/s11892-014-0522-0. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports and Exercise. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- Gorin AA, Stone AA. Recall biases and cognitive errors in retrospective self-reports: A call for momentary assessments. In: Baum A, Revenson T, Singer J, editors. Handbook of health psychology. Mahwah, NJ: Lawrence Erlbaum; 2001. pp. 405–413. [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Herman KM, Carver TE, Christou NV, Andersen RE. Keeping the weight off: physical activity, sitting time, and weight loss maintenance in bariatric surgery patients 2 to 16 years postsurgery. Obesity Surgery. 2014;24:1064–1072. doi: 10.1007/s11695-014-1212-3. [DOI] [PubMed] [Google Scholar]
- Inside wearables: How the science of human behavior change offers the secret to long-term engagement. 2014 Jan; Retrieved from http://endeavourpartners.net/assets/Endeavour-Partners-Wearables-and-the-Science-of-Human-Behavior-Change-Part-1-January-20141.pdf.
- Josbeno DA, Kalarchian M, Sparto PJ, Otto AD, Jakicic JM. Physical activity and physical function in individuals post-bariatric surgery. Obesity Surgery. 2011;21:1243–1249. doi: 10.1007/s11695-010-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Belle SH, Eid GM, Dakin GF, Inabnet WB, Mitchell JE, Wolfe BM. Physical activity levels of patients undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery study. Surgery for Obesity and Related Diseases. 2008;4:721–728. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, Wolfe BM. Pre-to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2) Surgery for Obesity and Related Diseases. 2012;8:522–532. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Chen J, Bond DS, Belle SH, Courcoulas AP, Patterson EJ, Wolfe BM. Objective assessment of changes in physical activity and sedentary behavior in bariatric surgery patients: pre- through 3-years post-surgery. Obesity (Silver Spring) 2015;23:1143–1150. doi: 10.1002/oby.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. American Journal of Epidemiology. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Smith SN, Tewari A, Witkiewitz K, Collins LM, Spring B, Murphy S. Just in time adaptive interventions (JITAIs): An organizing framework for ongoing health behavior support. Methodology Center technical report. 2014:14–126. doi: 10.1007/s12160-016-9830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Cornier MA, Mazzone T, Stiles S, Cummings S, Klein S, Franklin BA. Bariatric surgery and cardiovascular risk factors a Scientific Statement from the American Heart Association. Circulation. 2011;123:1683–1701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
- Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. The International Journal of Behavioral Nutrition and Physical Activity. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Marrero FA, Miles J, Joyner MJ, Curry TB. Self-reported and objective physical activity in postgastric bypass surgery, obese and lean adults: association with body composition and cardiorespiratory fitness. Journal of Physical Activity and Health. 2014;11:145–151. doi: 10.1123/jpah.2012-0048. [DOI] [PubMed] [Google Scholar]
- Reid RE, Carver TE, Andersen KM, Andersen RE. Physical Activity and Sedentary Behavior in Bariatric Patients Long-Term Post-Surgery. Obesity Surgery. 2015 doi: 10.1007/s11695-015-1624-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rutten GM, Savelberg HH, Biddle SJ, Kremers SP. Interrupting long periods of sitting: good STUFF. International Journal of Behavioral Nutrition and Physical Activity. 2013;10:1. doi: 10.1186/1479-5868-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquée, Nutrition, et Metabolisme. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- Shah M, Snell PG, Rao S, Adams-Huet B, Quittner C, Livingston EH, Garg A. High-Volume Exercise Program in Obese Bariatric Surgery Patients: A Randomized, Controlled Trial. Obesity (Silver Spring) 2011;19:1826–1834. doi: 10.1038/oby.2011. [DOI] [PubMed] [Google Scholar]
- Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. The Journal of Cardiovascular Nursing. 2013;28:320–329. doi: 10.1097/JCN.0b013e318250a3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Atienza AA, Nebeling L. Historical roots and rationale of ecological momentary assessment (EMA) In: Stone AA, Shiffman S, Atienza AA, Nebeling L, editors. The science of real-time data capture: Self-reports in health research. New York: Oxford University Press; 2007. pp. 3–10. [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Controlled Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Thomas JG, Bond DS. Review of innovations in digital health technology to promote weight control. Current Diabetes Reports. 2014;14:485. doi: 10.1007/s11892-014-0485-1. [DOI] [PubMed] [Google Scholar]
- Thomas JG, Bond DS, Sarwer DB, Wing RR. Technology for behavioral assessment and intervention in bariatric surgery. Surgery for Obesity and Related Diseases. 2011;7:548–557. doi: 10.1016/j.soard.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick JL, Bond DS, Jakicic JM, Vithiananthan S, Ryder BA, Roye GD, Wing RR. Comparison of two objective monitors for assessing physical activity and sedentary behaviors in bariatric surgery patients. Obesity Surgery. 2012;22:347–352. doi: 10.1007/s11695-011-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatier C, Henegar C, Ciangura C, Poitou-Bernert C, Bouillot JL, Basdevant A, Oppert JM. Dynamic relations between sedentary behavior, physical activity, and body composition after bariatric surgery. Obesity Surgery. 2012;22:1251–1256. doi: 10.1007/s11695-012-0619-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.