Abstract
Objective:
To estimate the ability of bedside information to risk stratify stroke in acute dizziness presentations.
Methods:
Surveillance methods were used to identify patients with acute dizziness and nystagmus or imbalance, excluding those with benign paroxysmal positional vertigo, medical causes, or moderate to severe neurologic deficits. Stroke was defined as acute infarction or intracerebral hemorrhage on a clinical or research MRI performed within 14 days of dizziness onset. Bedside information comprised history of stroke, the ABCD2 score (age, blood pressure, clinical features, duration, and diabetes), an ocular motor (OM)-based assessment (head impulse test, nystagmus pattern [central vs other], test of skew), and a general neurologic examination for other CNS features. Multivariable logistic regression was used to determine the association of the bedside information with stroke. Model calibration was assessed using low (<5%), intermediate (5% to <10%), and high (≥10%) predicted probability risk categories.
Results:
Acute stroke was identified in 29 of 272 patients (10.7%). Associations with stroke were as follows: ABCD2 score (continuous) (odds ratio [OR] 1.74; 95% confidence interval [CI] 1.20–2.51), any other CNS features (OR 2.54; 95% CI 1.06–6.08), OM assessment (OR 2.82; 95% CI 0.96–8.30), and prior stroke (OR 0.48; 95% CI 0.05–4.57). No stroke cases were in the model's low-risk probability category (0/86, 0%), whereas 9 were in the moderate-risk category (9/94, 9.6%) and 20 were in the high-risk category (20/92, 21.7%).
Conclusion:
In acute dizziness presentations, the combination of ABCD2 score, general neurologic examination, and a specialized OM examination has the capacity to risk-stratify acute stroke on MRI.
Patients with dizziness from stroke are challenging to identify because they often lack typical stroke warning signs or symptoms.1–6
Prior studies have been performed to assess bedside decision support tools that could help to discriminate stroke from other causes of acute dizziness.3,4,7 The ABCD2 score (age, blood pressure, clinical features, duration, and diabetes) may also be useful in discriminating vascular from nonvascular events.8–10 When assessed in a retrospective study of emergency department dizziness presentations, the visits with a low-risk ABCD2 score (ABCD2 < 4) had a stroke frequency of 1% (5/512 patients) compared with 8.1% (32/395) in the high-risk group (ABCD2 ≥ 4).3 Another tool that has been developed to identify cases of dizziness-stroke is the HINTS assessment (head impulse, nystagmus pattern, test of skew), which is based on a specialty bedside ocular motor (OM) examination.4 HINTS has shown promising results, superior to the ABCD2 score (sensitivity/specificity: ABCD2, 61%/62%; HINTS, 96.5%/84.4%) in a prospective study of acute dizziness.4
In this study, we expand on this prior work by evaluating the ability of the combination of bedside predictors of stroke—including both the ABCD2 score and the specialized OM examination—to stratify stroke risk using an MRI-based gold standard.
METHODS
Study design and setting.
This was a prospective, single-center, observational study that enrolled patients from November 21, 2009, to March 31, 2013. The main setting of recruitment was the level 1 trauma center emergency room with an annual volume of approximately 70,000 adult visits. A minority of patients were identified in the outpatient or inpatient settings.
Standard protocol approvals, registrations, and patient consents.
This study received approval from the University of Michigan institutional review board for the research using human subjects. Comprehensive written informed consent was obtained from all patients enrolled in the study.
Study population.
The target population was patients presenting for acute dizziness without an obvious cause who also had examination findings (i.e., nystagmus [spontaneous or gaze-evoked] or imbalance when walking) that could be attributable to neurologic dysfunction (table 1). To identify potentially eligible cases, we used active and passive surveillance methods (appendix e-1 on the Neurology® Web site at Neurology.org).
Table 1.
Inclusion and exclusion criteria
Baseline clinical measurements.
History of present illness information was obtained in a structured fashion by either a research assistant or investigator. The physical examination was performed in a structured fashion by a study investigator, either a neurologist fellowship trained in neuro-otology (K.A.K.) or vascular neurology (E.E.A.), or an emergency medicine physician fellowship trained in vascular neurology (W.J.M.). Study examinations were performed before the MRI whenever possible or blinded to the results of the MRI. The general neurologic examination included assessment of visual fields, cranial nerves, strength, sensation, coordination, and balance. Patients were given up to 2 attempts to walk 10 consecutive steps in tandem, and the maximum number of steps taken before a side step was recorded or a 0 was recorded if the patient declined the test. Hearing impairment was assessed using finger rub.
The OM examination was performed including a nystagmus assessment, assessment of skew deviation, and the head impulse test (HIT) (appendix e-1). Patients were classified as having a central pattern of nystagmus if any of the following were observed: bidirectional gaze-evoked nystagmus, vertical nystagmus in any position, or isolated torsional nystagmus (i.e., nystagmus that was only in the torsional vector). Frenzel lenses were used as a complementary part of the nystagmus assessment in a convenience sample of 106 patients. Within individual patients, the categorization of nystagmus applied in this study (see categories below) did not change from the examination without Frenzel lenses to an examination with them, so further details are not presented. Skew deviation was classified as present when alternating vertical refixations were observed. The HIT vestibulo-ocular reflex to each side was scored by examiners as normal (0), inconsistent/slight corrective saccade (1), small-amplitude corrective saccade (2), medium-amplitude corrective saccade (3), or large-amplitude corrective saccade (4). The HIT vestibulo-ocular reflex was considered normal (finding absent) if the examiner scored 0 or 1 and abnormal (finding present) if the examiner scored 2, 3, or 4. A second examiner, when available, also performed an OM examination so that interobserver agreement could be estimated. So that examination reliability could be measured including all patients, the OM examination was also video-recorded.
From the medical record, we collected arrival date/time, first recorded blood pressure, demographic information, and administered medications. From the physician's note, we abstracted medical history and current medications.
Independent variables.
The ABCD2 score was calculated for each patient by assigning points as follows: age 60 years or older = 1; systolic blood pressure ≥140 or diastolic blood pressure ≥90 = 1; clinical features (new symptoms or examination findings of unilateral weakness = 2, speech disturbance without weakness = 1); duration of symptoms (<10 minutes = 0, 10–59 minutes = 1, ≥60 minutes = 2); and diabetes (either medical history or current use of an oral hypoglycemic medicine or insulin documented in the emergency department physician's note) = 1.8
The results of the OM examination were categorized into one of the following 3 categories for each patient: no nystagmus or skew, HINTS negative, or HINTS positive. Patients were assigned to the no-nystagmus or skew category when there was no nystagmus or skew deviation on examination. The HIT was not considered in this category because the value of the HIT in an acute presentation without nystagmus is uncertain as exemplified by the inclusion of patients without nystagmus in the first HINTS development study but not the second.4,11 Patients were assigned to the HINTS-negative category for the following findings: noncentral classification of nystagmus (i.e., direction-fixed horizontal nystagmus), plus an abnormal HIT (i.e., HIT score of ≥2, suggesting a peripheral lesion) to the side opposite the fast phase of the horizontal nystagmus, plus the absence of skew deviation. Patients were assigned to the HINTS-positive category for any of the following findings: a central pattern of nystagmus, or a normal HIT (i.e., HIT score of 0 or 1, suggesting a central lesion) to the side opposite the fast phase of direction-fixed horizontal nystagmus, or the presence of skew deviation.11 Using this scheme, a patient with nystagmus and bilaterally abnormal HITs would be included in the HINTS-negative category as long as central nystagmus and skew were not present.
A variable called “other CNS features” was used to indicate the presence of new CNS signs or symptoms not included in the ABCD2 or HINTS scales. This variable was dichotomized (0/1) with a score of 1 indicating the presence of any possible or mild bilateral weakness (note, the ABCD2 score specifies unilateral weakness) or sensory signs or symptoms, visual field deficit, or dysmetria on the finger-nose-finger test. As previously stated, patients with moderate to severe other CNS features were excluded from enrollment. The variable “prior stroke” (0/1) was scored as a 1 when the treating physician's note indicated that the patient had a medical history of stroke.
Outcome measure.
The primary outcome was an imaging-based definition of stroke, specifically any acute infarction or intracerebral hemorrhage (ICH) on MRI as determined by a neuroradiologist. All enrolled participants were offered a research MRI of the brain if a clinical MRI was not performed or was performed within 24 hours of symptom onset and was negative for acute infarction or ICH. Research MRIs were to be performed >24 hours from symptom onset because of the reduced sensitivity of MRI for acute infarction in the posterior fossa when performed within 24 hours of symptom onset.4,12 Diffusion-weighted MRIs with apparent diffusion coefficient maps were performed on either a 1.5T or 3T scanner (appendix e-1).
Statistical analysis.
Descriptive statistics were used to summarize clinical characteristics of the study participants. The volume of an acute infarction or ICH was measured using the ABC/2 method.13
Interrater agreement was calculated for both the 3-category OM classification scheme and a nystagmus-only scheme (i.e., central pattern of nystagmus vs any other pattern or no nystagmus) using the κ statistic. The 95% confidence intervals (CIs) were calculated using bootstrapping with 10,000 repetitions. The 3-category scheme did not include the presence or absence of skew deviation because the alternate cover test was not scored in second examinations. Agreement was calculated separately for each scheme between the first in-person assessor and the second assessor either in person or by video review. Methods to harmonize investigator examination scoring at the initiation of the study included discussion and review of the examination (after examination scores had been recorded) in 5 to 10 patients and 10 meetings to review videotaped OM examinations.
Patients who did not have an MRI within 14 days of the onset of continuous symptoms were excluded from further analysis. The proportion of patients with acute infarction or ICH on MRI was determined, along with the 95% CI calculated using the exact binomial method. Next, a multivariable logistic regression model was constructed to determine the association of the independent variables with acute infarction or ICH on MRI. The model's independent variables were ABCD2 score (continuous), the OM assessment, history of stroke, and other CNS features. The OM assessment used in the model was from the first in-person study examination. The likelihood ratio test was performed comparing the model without the OM assessment variable with the model that included it. In a separate model, the OM assessment was reduced to only the nystagmus assessment so that the discriminative ability of a simplified OM assessment could be measured. Model discrimination was measured with the C statistic including 95% CIs. Internal validation of model discrimination was assessed using bootstrapping with replacement (1,000 replications) as described previously.14 The ability of the models to stratify stroke risk was evaluated using model calibration that compared mean predicted probability to mean observed outcomes within prespecified risk categories defined as low (<5%), moderate (5% to <10%), and high (≥10%).
The following sensitivity analyses were performed: increasing the threshold for an abnormal HIT from a small to medium amplitude corrective saccade; reclassifying patients with possible incidental MRI infarction to the nonstroke category; expanding the HINTS-positive OM category to include patients with nystagmus who had a bilaterally abnormal HIT, imputation of the MRI result in enrolled cases who did not receive MRI; and limiting cases to those with the OM assessment conducted by K.A.K. Statistical analysis was performed using Stata 13.1 (StataCorp, College Station, TX). This report is in compliance with the STROBE statement recommendations (see supplementary materials).
RESULTS
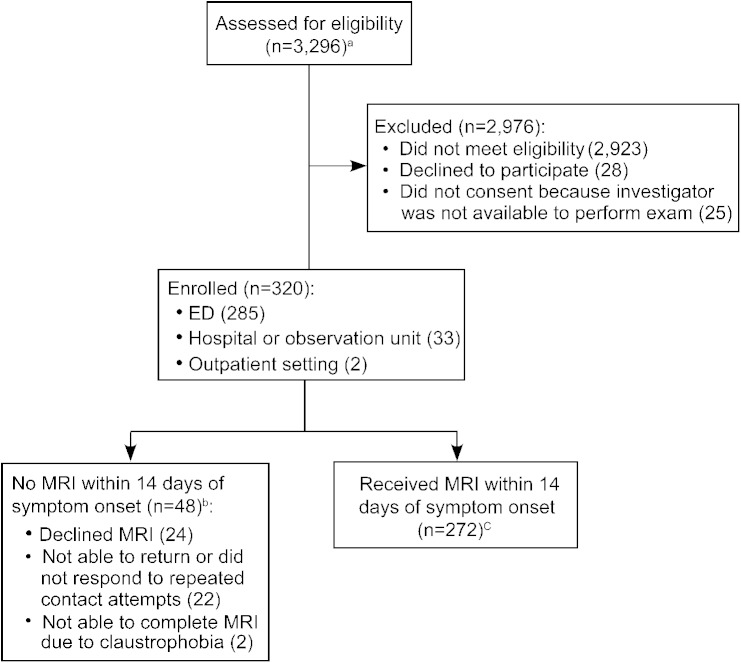
The study enrolled 320 patients and 272 (85%) received an MRI within 14 days of symptom onset (figure 1). Clinical characteristics of the study population are presented in table 2. Interrater agreement regarding the OM classification was fair for both the 3-category OM scheme and the nystagmus-only scheme (κ, 0.24–0.40) (tables e-1–e-3).
Figure 1. Study flow diagram.
Flow diagram of patient screening, enrollment, and outcome completion. aIn an additional 73 visits, potential participants declined screening. bPatients not included in main analysis, but examination data were included in the interrater agreement analysis. cOf patients who received an MRI (n = 272), the initial MRI was performed for clinical purposes in 158 (58%) and for research purposes in 114 (42%). A research MRI (either first or second study) was performed in 177 (65%) of the patients. The first MRI was performed before the study bedside examination in 15% of patients (42/272). ED = emergency department.
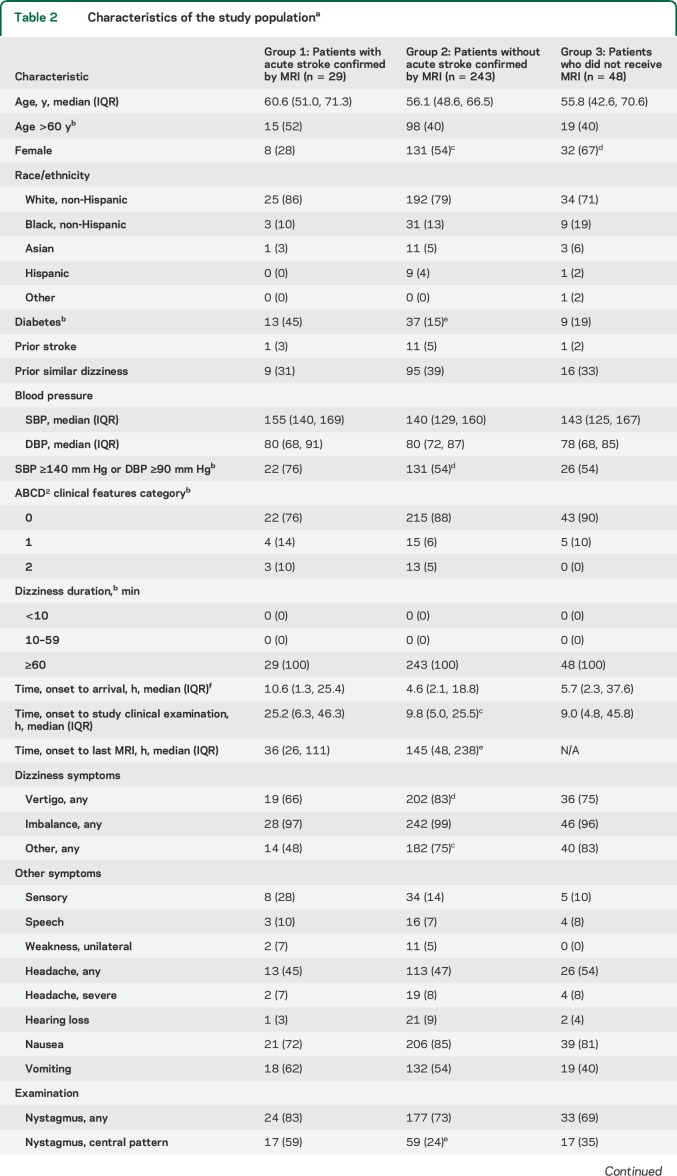
Table 2.
Characteristics of the study populationa
An acute infarction or ICH on MRI was identified in 29 of the 272 patients (10.7%; 95% CI 7.3%–15.0%) (26 infarctions, 3 ICH). Of patients without acute infarction or ICH on MRI, 91.4% (222/243) had the last MRI >24 hours after symptom onset. One stroke case had an initially negative MRI. In 3 cases with acute infarction, the finding was possibly incidental based on the lesion size and location (table e-4). Nonstroke cases with other acute CNS imaging findings were a cerebellar tumor (1), enhancing demyelinating plaque (1), presumed inflammatory brainstem lesion (nonenhancing) (1), and cerebellitis (1).
The false-negative frequency (i.e., frequency of stroke in the lowest-risk categories) was as follows: ABCD2 < 4, 5.1% (8/157); OM assessment, 5.9% (9/152) (4.9% [4/82], for HINTS peripheral findings); other CNS features, 7.8% (17/219); and prior stroke, 10.8% (28/260). The OM assessment was positive for a central lesion in 20 of the 29 stroke patients (69%). Of the 9 stroke patients who did not have the central OM findings, 7 were in the no-nystagmus category (5) and/or had an acute infarction that was possibly incidental (3).
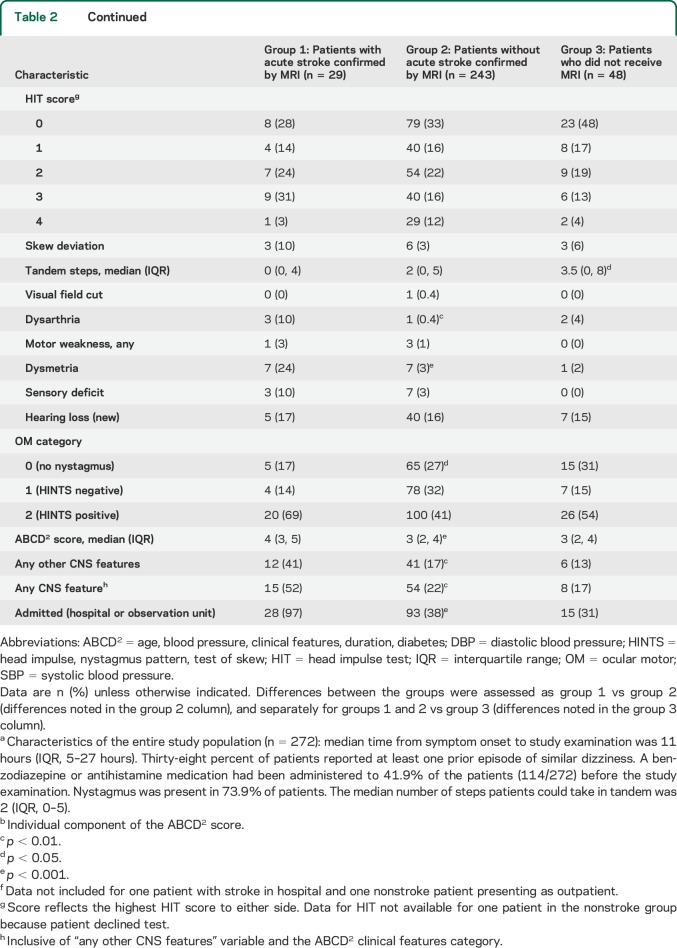
The results of the multivariable logistic regression models are presented in table 3. The model 1 (including full HINTS battery) C statistic was 0.77. Associations with acute stroke were significant for ABCD2 score (p < 0.01) and other CNS features (p < 0.05), borderline significant for the OM assessment (p = 0.06), but not significant for prior stroke (p = 0.54). The results were similar in the 4 sensitivity analyses (table e-5). The assessment of model 1 calibration (i.e., ability of the model to stratify stroke risk) revealed that no stroke cases occurred in the predicted low-risk category (0/86, 0%), whereas 9 occurred in the predicted moderate-risk category (9/94, 9.6%) and 20 in the predicted high-risk category (20/92, 21.7%) (figure 2). In model 2, which reduced the OM assessment to only the nystagmus component, the central pattern of nystagmus was associated with acute stroke (p < 0.01). However, model 2 calibration revealed that one patient in the low-risk category had a stroke (1/109, 0.9%), which was an acute cerebellar infarction (volume 0.08 cm3).
Table 3.
Results of multivariable logistic regression models with dependent variable of acute infarction or intracerebral hemorrhage on MRI
Figure 2. Model calibrations.
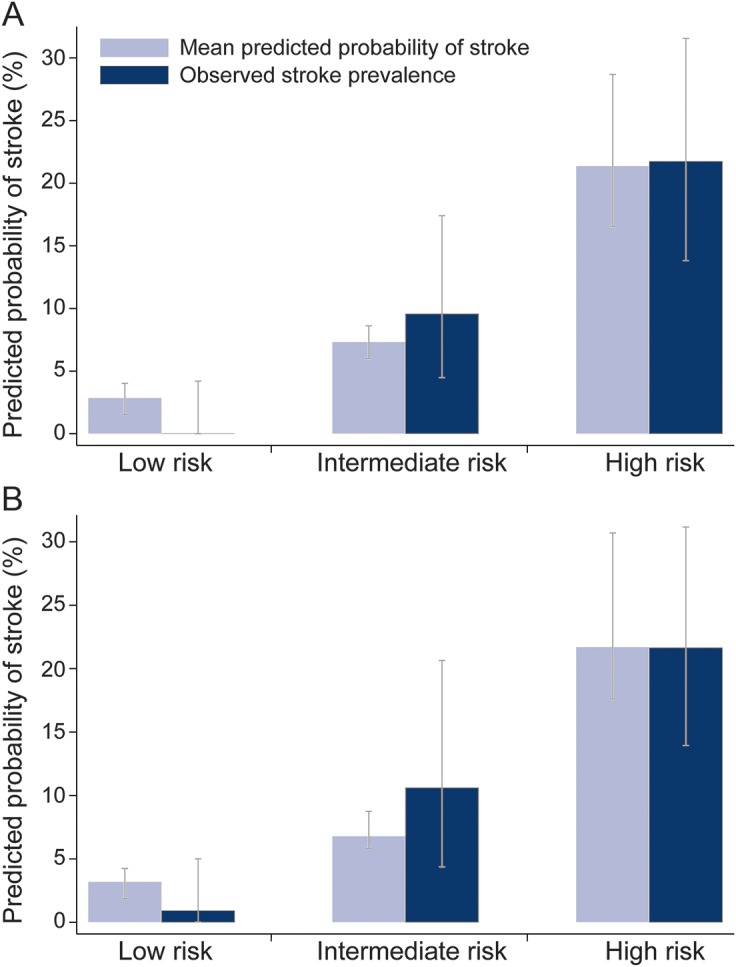
Calibration for model 1 (A) (including ocular motor 3-category scheme variable) and model 2 (B) (limiting the ocular motor examination to only the nystagmus component). Calibration was assessed using low, intermediate, and high predicted probability risk categories. Light blue bars represent the mean predicted probability of acute infarction or intracerebral hemorrhage (ICH) on MRI within each category. Dark blue bars represent the frequency of observed acute infarction or ICH on MRI within each category. Model 1 distribution of patients (stroke patients/total patients) within each category: low-risk category, 0/86; intermediate-risk category, 9/94; and high-risk category, 20/92. Model 2 distribution of patients (stroke patients/total patients) within each category: low-risk category, 1/109; intermediate-risk category, 7/66; and high-risk category, 21/97. Error bars represent 95% confidence intervals within each category calculated using the exact binomial method for observed stroke and 1,000 bootstrap samples for the predicted probability.
Details regarding the cases with acute infarction or ICH on MRI are presented in tables e-4 and e-6.
DISCUSSION
In this prospective study that used rigorous case capture methods, a systematic clinical patient evaluation, and objective imaging-based outcome, we found that the combination of readily obtained clinical information and a specialized OM assessment has the capacity to meaningfully stratify stroke risk in a population of patients presenting with acute dizziness and nystagmus or imbalance.
This study was unique because we used active and passive surveillance methods to prospectively capture acute dizziness cases and provided research MRIs to study participants when a clinical MRI was not performed. These methods enable a more generalizable estimate of stroke prevalence—11% of the population had acute infarction or ICH—than any prior study of patients with acute dizziness and nystagmus or imbalance.
Our findings indicate that the ABCD2 score and the specialized OM evaluation both meaningfully influence the probability of stroke at the individual patient level in this acute dizziness cohort, even adjusting for all model predictors. Prior studies have only analyzed the ABCD2 score and OM assessment as singular diagnostic tests in acute dizziness presentations.3,4 Our results do not support the prior studies' conclusions that the ABCD2 or OM assessment alone can identify a sufficiently low-risk group because we found that the stroke frequency in the low-risk group of each of these variables was >5% (more than 1 in 20). The difference between our findings and those of the prior ABCD2-based study likely relate to the study populations. The population in the prior retrospective ABCD2-based study was all dizziness symptom presentations including patients with obvious medical causes, transient or chronic symptoms, and a normal examination.3 This differed from our study, which required new-onset and constant symptoms in addition to examination abnormalities.
Our population was similar to the prior studies concluding that the OM assessment could be used to identify a sufficiently low-risk group.4,11 These study populations were restricted to the acute dizziness segment that has been labeled the “acute vestibular syndrome,” which requires new-onset constant symptoms and nystagmus or gait abnormalities. An important difference between our study and the HINTS development studies might be that the HINTS population comprised more severe dizziness cases since that study used referral-based recruitment (resulting in the capture of only 193 patients over 14 years), nearly all patients underwent clinical MRIs, and the population had a very high stroke prevalence (60%).4 High disease prevalence and increased disease severity are both factors known to bias assessments of tests toward higher accuracy.15,16 Factors that could have reduced the accuracy of our OM assessments were the inclusion of patients without nystagmus (patients without nystagmus were included in some prior HINTS analyses but not others4,11), potentially incidental infarctions, or inferior examinations compared with HINTS development studies, which reported an area under the curve of 0.995 (95% CI 0.985–1.000) for detecting central causes.4
Another unique aspect of the current study was the measurement of the interobserver agreement of the OM assessment. We found the agreement to be in the fair range, which is not uncommon with neurologic examination components.17 Agreement in the current study could reflect test characteristics or a difference in examiner characteristics (skills/techniques). Examiners in this study were from different backgrounds and only one was a neuro-otologist. The fair agreement thus approximates what might be expected if this classification scheme was used by a variety of physicians of differing backgrounds after a small number of training/harmonization sessions. If the reliability of the OM examination is lower in practice than when performed by a neuro-otologist, the performance of the overall strategy would decline. This might require that the OM assessment strategy use different training/harmonization methods, stipulate the level of examiner specialty training, or use an automated measurement device.18
Future work should be performed to define acceptable risk tolerance (i.e., frequency of false-negative results) for stroke diagnosis. This information is necessary for designating decision cutpoints that are based on the probability of stroke. Acceptance of higher risk (e.g., 5%) is likely to enable a decision tool with fewer components than if a lower risk is required (e.g., <1%).
There were limitations to this study. This was a single-center study at a tertiary medical center. The generalizability of our examination (both for patient selection [e.g., excluding patients with posterior canal benign paroxysmal positional vertigo] and model item components) to providers outside this study is not known. The predictiveness of the model for other central disorders was not evaluated. We prespecified the MRI-based stroke outcome because stroke is the most common dangerous central cause of dizziness and because ABCD2 and HINTS were exclusively or primarily developed as stroke identification tools. The focus on acute infarction/ICH may increase the importance of the ABCD2 score relative to the OM assessment. Prior reports indicate that MRI can miss acute infarction.4 Our methods to obtain MRI >24 hours after onset should have mitigated this occurrence. A composite stroke determinate measure (incorporating clinician judgment) was not applied since the clinicians would have used the index test components to classify the outcome (incorporation bias).19 Additional validation work is necessary before these approaches are endorsed for use in routine practice.
Supplementary Material
GLOSSARY
- ABCD2
age, blood pressure, clinical features, duration, diabetes
- CI
confidence interval
- HINTS
head impulse, nystagmus pattern, test of skew
- HIT
head impulse test
- ICH
intracerebral hemorrhage
- OM
ocular motor
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.A.K., D.L.B., W.J.M., A.T., L.B.M., T.P.H., J.F.B., A.M.F., and E.G.H. made substantial contributions to the concept or design of the study. K.A.K., W.J.M., E.E.A., and E.G.H. acquired the data. K.A.K., J.F.B., A.T., D.L.B., L.B.M., W.J.M., and T.P.H. planned or performed the statistical analysis. K.A.K. drafted the manuscript. All authors contributed to planning, interpretation, and writing of the manuscript and critically revised the manuscript for intellectual content. All authors provided final approval of the submitted manuscript. K.A.K. is guarantor.
STUDY FUNDING
This project was supported by grant R18HS017690 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
DISCLOSURE
K. Kerber has received research support from NIH/NCRR K23 RR024009, AHRQ R18 HS017690, NIH/NIDCD R01DC012760, AHRQ R18HS022258, and NIH/NIDCD U01DC013778, received speaker or author honoraria from the American Academy of Neurology (AAN), was an expert witness for National Medical Consultants, and received publishing royalties from Oxford University Press. W. Meurer has received research support from AHRQ (R18HS017690) and NIH. D. Brown serves as an editorial board member of Neurology® and Stroke, is funded by NIH grants R01 NS062675, R01 NS070941, U10NS086526, and R01 DC012760, and received research support from the Blue Cross Blue Shield of Michigan Foundation, Michigan Department of Community Health, and the University of Michigan for stroke-related research. J. Burke has received an honorarium from AAN Continuum for writing an article. Dr. Burke is supported by National Institute of Neurological Disorders and Stroke (NINDS) grant K08-NS082597 and NIMHD grant R01-MD008879. Dr. Burke has received modest fees for reviewing medical malpractice defense documents. T. Hofer has received research support from NIH (P30 DK092926, P01 CA163233), AHRQ (R18HS017690. R01 HS018334), Veterans Affairs (VA DIB 98-001, CIN 13-408, IIR 13-079, SDR 10-180, EPID-011-11S, IIR 12-116, IIR 11-088), and Robert Wood Johnson Foundation. A. Tsodikov has received research support from AHRQ (R18HS017690) and NIH. E. Hoeffner has received research support from AHRQ (R18HS017690). A. Fendrick has received research support from AHRQ (R18HS017690) and serves as a coeditor for the American Journal of Medical Care. E. Adelman has received research support from AHRQ (R18 HS017690) and currently receives research support from NINDS (U01 NS062835) and Medtronic. L. Morgenstern has received research funding from NIH and AHRQ, St. Jude Medical Corporation, and has served as an expert witness. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med 2007;14:63–68. [DOI] [PubMed] [Google Scholar]
- 2.Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology 2006;67:1178–1183. [DOI] [PubMed] [Google Scholar]
- 3.Navi BB, Kamel H, Shah MP, et al. Application of the ABCD2 score to identify cerebrovascular causes of dizziness in the emergency department. Stroke 2012;43:1484–1489. [DOI] [PubMed] [Google Scholar]
- 4.Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med 2013;20:986–996. [DOI] [PubMed] [Google Scholar]
- 5.Kuruvilla A, Bhattacharya P, Rajamani K, Chaturvedi S. Factors associated with misdiagnosis of acute stroke in young adults. J Stroke Cerebrovasc Dis 2011;20:523–527. [DOI] [PubMed] [Google Scholar]
- 6.Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011;183:E571–E592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase M, Goldstein JN, Selim MH, et al. A prospective pilot study of predictors of acute stroke in emergency department patients with dizziness. Mayo Clin Proc 2014;89:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–292. [DOI] [PubMed] [Google Scholar]
- 9.Josephson SA, Sidney S, Pham TN, Bernstein AL, Johnston SC. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke 2008;39:3096–3098. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan OC, Merwick A, Kelly LA, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke 2009;40:3449–3454. [DOI] [PubMed] [Google Scholar]
- 11.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol 2000;21:1434–1440. [PMC free article] [PubMed] [Google Scholar]
- 13.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009;72:2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol 2014;180:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med 2004;140:189–202. [DOI] [PubMed] [Google Scholar]
- 16.Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ 2002;324:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein LB, Simel DL. Is this patient having a stroke? JAMA 2005;293:2391–2402. [DOI] [PubMed] [Google Scholar]
- 18.Newman-Toker DE, Saber Tehrani AS, Mantokoudis G, et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke 2013;44:1158–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geijer B, Lindgren A, Brockstedt S, Stahlberg F, Holtas S. Persistent high signal on diffusion-weighted MRI in the late stages of small cortical and lacunar ischaemic lesions. Neuroradiology 2001;43:115–122. [DOI] [PubMed] [Google Scholar]
- 21.Lansberg MG, Thijs VN, O'Brien MW, et al. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol 2001;22:637–644. [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Abnormalities on diffusion weighted magnetic resonance imaging performed several weeks after a minor stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 2003;74:734–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke 2004;35:2459–2465. [DOI] [PubMed] [Google Scholar]
- 24.Burdette JH, Ricci PE, Petitti N, Elster AD. Cerebral infarction: time course of signal intensity changes on diffusion-weighted MR images. AJR Am J Roentgenol 1998;171:791–795. [DOI] [PubMed] [Google Scholar]
- 25.Johkura K. Central paroxysmal positional vertigo: isolated dizziness caused by small cerebellar hemorrhage. Stroke 2007;38:e26–e27. [DOI] [PubMed] [Google Scholar]
- 26.Kim HA, Yi HA, Lee H. Apogeotropic central positional nystagmus as a sole sign of nodular infarction. Neurol Sci 2012;33:1189–1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.