Abstract
Objective:
This is a network meta-analysis of nonvenous drugs used in randomized controlled trials (RCTs) for treatment of acute convulsive seizures and convulsive status epilepticus.
Methods:
Literature was searched according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines for RCTs examining treatment of acute convulsive seizures or status epilepticus with at least one of the study arms being a nonvenous medication. After demographic and outcome data extraction, a Bayesian network meta-analysis was performed and efficacy results were summarized using treatment effects and their credible intervals (CrI). We also calculated the probability of each route–drug combination being the most clinically effective for a given outcome, and provided their Bayesian hierarchical ranking.
Results:
This meta-analysis of 16 studies found that intramuscular midazolam (IM-MDZ) is superior to other nonvenous medications regarding time to seizure termination after administration (2.145 minutes, 95% CrI 1.308–3.489), time to seizure cessation after arrival in the hospital (3.841 minutes, 95% CrI 2.697–5.416), and time to initiate treatment (0.779 minutes, 95% CrI 0.495–1.221). Additionally, intranasal midazolam (IN-MDZ) was adjudged most efficacious for seizure cessation within 10 minutes of administration (90.4% of participants, 95% CrI 79.4%–96.9%), and persistent seizure cessation for ≥1 hour (78.5% of participants, 95% CrI 59.5%–92.1%). Paucity of RCTs produced evidence gaps resulting in small networks, routes/drugs included in some networks but not others, and some trials not being connected to any network.
Conclusions:
Despite the evidence gaps, IM-MDZ and IN-MDZ exhibit the best efficacy data for the nonvenous treatment of acute convulsive seizures or status epilepticus.
IV administration of benzodiazepines including lorazepam (LOR) and diazepam (DZP) is considered the first-line treatment for control of acute convulsive seizures and status epilepticus.1,2 However, in many health care environments, establishing adequate venous access may be challenging, particularly in a convulsing patient. This difficulty may be augmented by lack of supplies, nonavailability of trained personnel at the point of care including out-of-hospital settings, collapsed peripheral veins, or malnutrition-related subcutaneous edema. Hence, nonvenous routes for administration of antiseizure medications to control acute convulsive seizures and convulsive status epilepticus have been explored, including sublingual (SL), rectal (PR), intranasal (IN), buccal (BC), and intramuscular (IM) routes. The present study is a systematic review of various nonvenous routes and drugs used in randomized controlled trials (RCTs) for treatment of acute convulsive seizures and convulsive status epilepticus along with a network meta-analysis (mixed or multiple treatment comparisons) of the data.3
METHODS
Literature search and data extraction.
Structured search was conducted for RCTs of treatment of acute convulsive seizures or convulsive status epilepticus where at least one of the study arms was a nonvenous medication (table e-1 on the Neurology® Web site at Neurology.org). All abstracts were screened by one of the authors (R.A.) and RCTs where the interventions seemed relevant were retained. Two authors (R.A. and H.K.) independently applied inclusion and exclusion criteria with disagreements being resolved by consensus. For eligible studies, one author (H.K.) extracted the data using a predesigned spreadsheet. Demographic and study methodology details were extracted for descriptive purposes. For each study arm, the number of participants, measured outcomes, and reported means (continuous variables) or proportions (dichotomous variables) were documented. Search results and study selection has been documented in a Preferred Reporting Items for Systematic reviews and Meta-Analyses diagram4 (figure e-1).
Outcome measures.
The dichotomous outcome measures included the proportion of participants with the following efficacy endpoints measured from the time of drug administration: seizure cessation within 10 minutes, seizure cessation within 5 minutes, and persistent seizure cessation for at least 1 hour. We included studies with only clinical and both clinical and EEG ascertainment of seizure termination for these outcomes. Since nonvenous routes find most utility in the out-of-hospital or resource-poor settings, clinical assessment of seizure activity was a representative endpoint. Further, the duration of continued seizure cessation after drug administration was reported differently by studies, with a minimum period of 1 hour. We have interpolated the reported proportions as representing participants who were seizure-free for at least 1 hour after receiving the study medication. Continuous outcomes included time from drug administration to termination of seizures, time to cessation of seizures after arrival to the health care facility, and time taken to initiate treatment. The time to initiate treatment has been reported differently in various studies, with respect to the initial timepoint (t = 0), and includes time to delivery of the drug after randomization, opening of the envelope, arrival in the hospital, time to prepare and administer the drug,5 or time from arrival of the nurse to medication administration.6 The paucity of and variable definitions used for adverse events data in the RCTs makes it impossible to rigorously address differential tolerability and safety of nonvenous medications.
Data synthesis and statistical methods.
Due to a variety of medications and routes used in different combinations with lack of sufficient studies for every possible pairwise comparison, a network meta-analysis was considered an appropriate methodology to synthesize the available data, and to provide an unbiased effect estimate taking into account both direct and indirect comparisons.3,7 We performed network meta-analyses within a Bayesian framework and summarized the treatment effects and their credible intervals (CrI). For pairwise comparisons, effect size was captured with a posterior median odds ratio (OR), which is derived from the distribution resulting from use of an assumed prior, mathematical model, and observed data. This Bayesian posterior distribution is non-normal and the appropriate measure is a median. Further, for pairwise comparisons with at least 4 available studies, we illustrated the relative strength of treatment effects using a Forest plot, and explored heterogeneity with I2 and τ2 statistics. We also looked at publication bias using a funnel plot. The studies with missing data or disconnected from the respective outcome network were excluded from the meta-analysis. Instead, we have directly described the treatment effects from these studies.
We assumed a binomial distribution for dichotomous outcomes with use of logit-link function in the models, and log-normal distribution for continuous time-to-event outcomes. We calculated the probability of each route–drug combination being the most clinically effective for a given outcome, the second best, and so on, and provided their relative Bayesian hierarchical ranking using the methods described in Evidence Synthesis Technical Support Documents (Decision Support Unit, UK National Institute for Health and Clinical Excellence).8 For fixed effects model, the common relative treatment effects were assumed for all studies. For random effects model, we assumed trial-specific relative treatment effects to come from a normal distribution with a common random effects variance for all treatment comparisons. We selected the best model based on the deviance information criteria, which provided measures of model fit.9 The consistency between direct and indirect evidence was performed using node splitting methods.10 To estimate the absolute treatment effects, we modeled the treatment effects of the baseline treatment independently using a fixed-effect model.8 The posterior distributions of the absolute treatment effect of other treatments were obtained by synthesizing the posterior samples from both the baseline treatment model and the relative treatment effects model. The analyses were performed using Just Another Gibbs Sampler (JAGS 3.2.0), which simulates Bayesian hierarchical models using Markov chain Monte Carlo method.11 Noninformative priors were used for all model parameters: uniform distribution for random effects variance and normal distributions with a zero mean and very large variance for all other parameters.
RESULTS
Characteristics of the included studies.
Twenty studies, published between 1997 and 2012, were eligible for the qualitative synthesis (figure e-1).5,6,12−29 Study participants ranged from newborns to 102 years of age, medications included LOR, DZP, midazolam (MDZ), and paraldehyde (PLD), and most studies were single-center (15/20). Four studies were excluded due to the following: not connected to the networks for any of the outcomes (n = 2)16,22 (figure 1), did not specify the number of participants allocated to each arm (n = 1),21 and did not report any of the meta-analysis outcomes (n = 1).13 Thus, 16 open-label studies were included in the quantitative synthesis, and 15/16 comprised only pediatric (≤18 years) participants. The methods used for randomization were either suboptimal (5/16) or not reported (2/16) in some studies (table e-2).
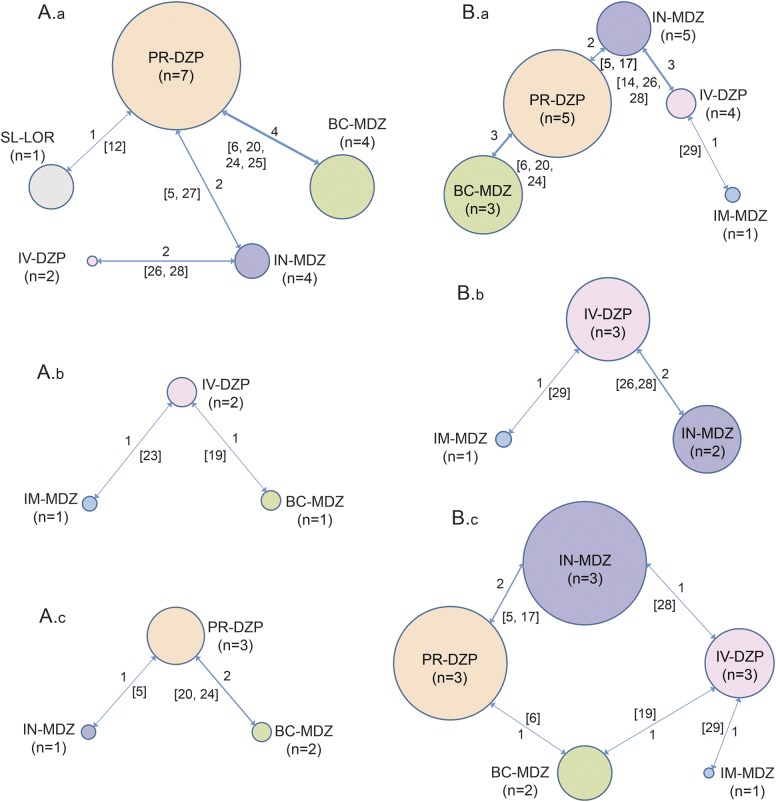
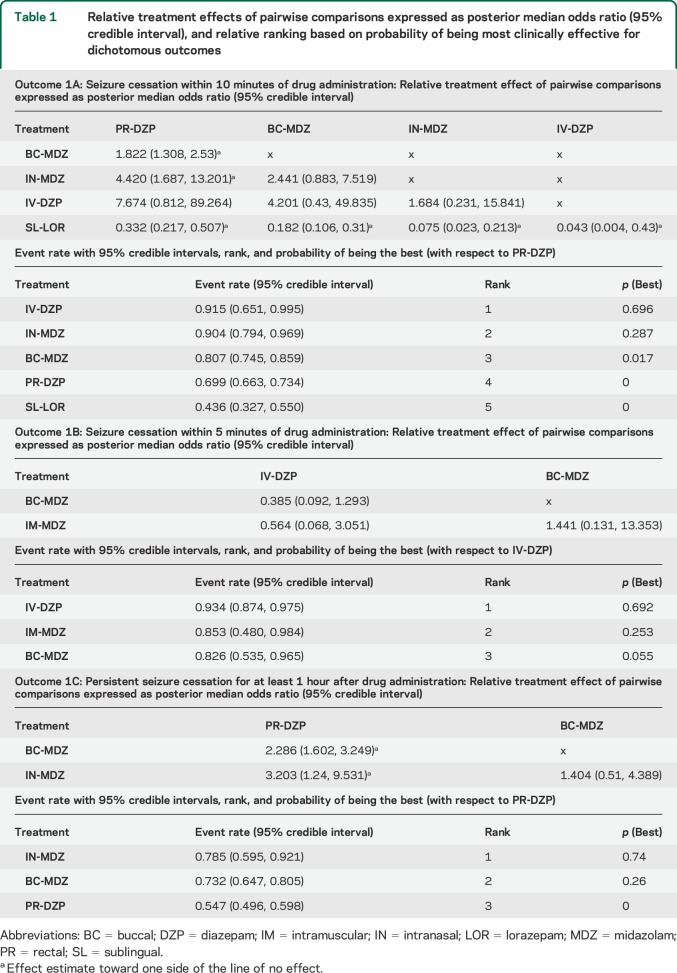
Figure 1. Bayesian network representations for individual outcomes.
The size of the bubble represents the total number of participants randomized to receive a particular route–drug combination. The number by the side of a line joining 2 bubbles indicates the number of trials for that particular comparison (A.a: seizure cessation within 10 minutes of drug administration, A.b: seizure cessation within 5 minutes of drug administration, A.c: persistent seizure cessation for at least 1 hour after drug administration, B.a: time from drug administration to termination of seizure [minutes], B.b: time to cessation of seizures after arrival in hospital [minutes], B.c: time to initiate treatment [minutes]) (reference numbers in square brackets). BC = buccal; DZP = diazepam; IM = intramuscular; IN = intranasal; LOR = lorazepam; MDZ = midazolam; PR = rectal; SL = sublingual.
Seizure cessation within 10 minutes of drug administration.
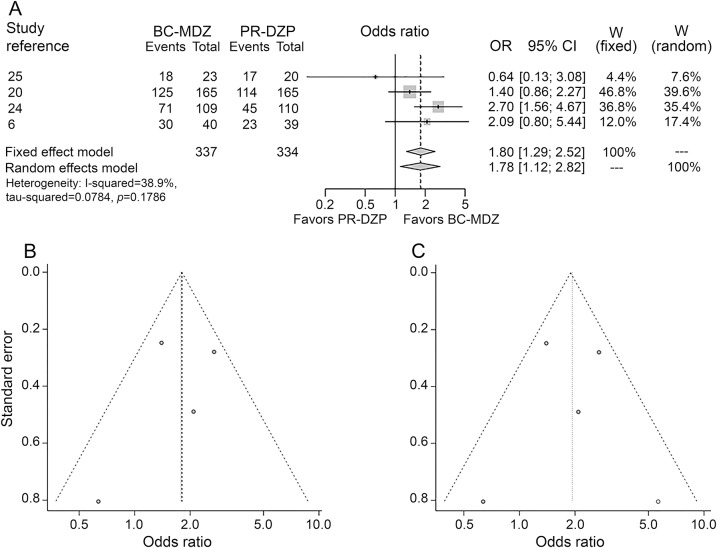
Eleven studies reported this outcome with 1 being disconnected from the network (figure 1A.a).16,22 The route–medication combinations reported by these studies included SL-LOR, IN-LOR, BC-MDZ, IN-MDZ, PR-DZP, IV-LOR, IM-PLD, and IV-DZP (table e-3). Out of the nonvenous route–drug combinations, IN-MDZ was the best (weighted event rate [WER] 90.4%) followed by BC-MDZ (80.7%) and PR-DZP (69.9%). On pairwise comparisons, IN-MDZ was superior to PR-DZP (OR 4.420, 95% CrI 1.687–13.201). Also, BC-MDZ was superior to PR-DZP (OR 1.822, 95% CrI 1.308–2.53), whereas SL-LOR was inferior to all other medications (table 1). Studies for IM-MDZ and IV-LOR were disconnected from the network, rendering this outcome uninformative for these medications. Although IN-LOR was disconnected from the network, the proportion of patients meeting the above endpoint was 78.8% (pooled event rate).
Table 1.
Relative treatment effects of pairwise comparisons expressed as posterior median odds ratio (95% credible interval), and relative ranking based on probability of being most clinically effective for dichotomous outcomes
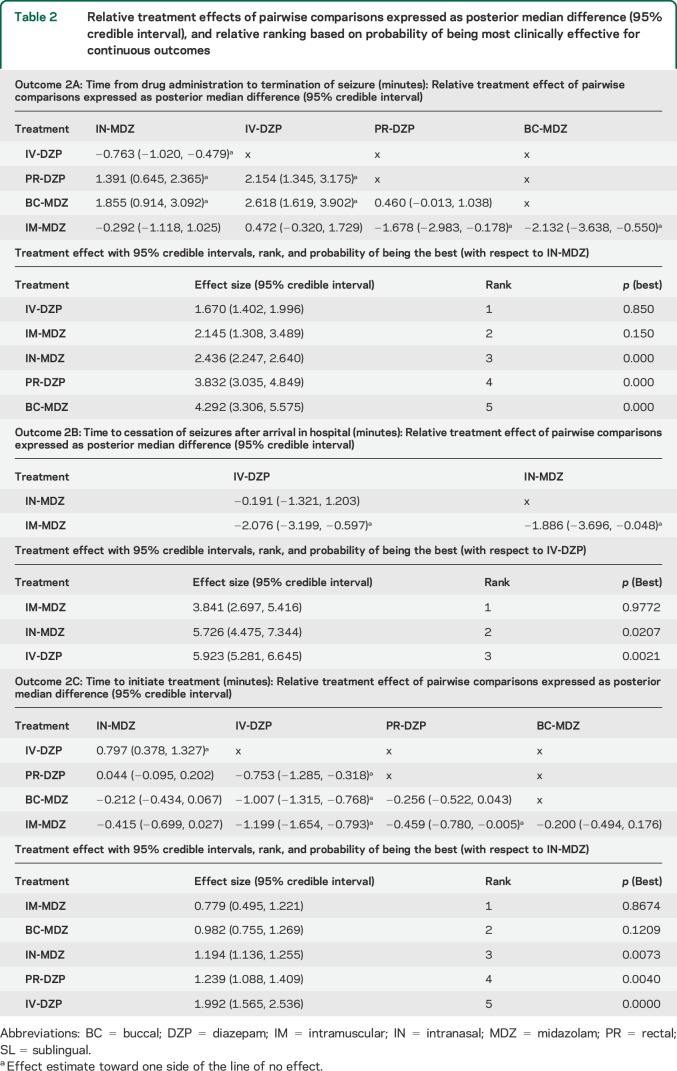
Since 4 studies compared BC-MDZ with PR-MDZ for this outcome, we performed a nested head-to-head meta-analysis. BC-MDZ was superior to PR-DZP (random effects OR 1.78, 95% confidence interval [CI] 1.12–2.82) with trivial heterogeneity in the evidence (I2 = 38.9%, figure 2). The funnel plot did not reveal any significant impact on the conclusion about relative treatment effect, even when a “pseudostudy” was added using trim-and-fill method to adjust for funnel plot asymmetry30 (figure 2).
Figure 2. Forest plot and funnel diagrams for BC-MDZ and PR-DZP head-to-head comparison.
Forest plot (A) for conventional head-to-head comparison between buccal (BC) midazolam (MDZ) and rectal (PR) diazepam (DZP) for seizure cessation within 10 minutes of drug administration shows minimal heterogeneity and similar relative effect compared to network meta-analysis. Funnel plots without (B) and with (C) a pseudostudy (open circle, created using trim-and-fill method to adjust for funnel plot asymmetry) shows no relevant influence on relative treatment effect of the publication bias. CI = confidence interval; OR = odds ratio.
Seizure cessation within 5 minutes of drug administration.
This outcome was reported by only 3 studies, 1 being disconnected from the network (figure 1A.b).12 The route–drug combinations analyzed were SL-LOR, BC-MDZ, IM-MDZ, PR-DZP, and IV-DZP (table e-3). For the nonvenous medications, IM-MDZ had a higher WER of 85.3% compared to BC-MDZ (82.6%), though none of the pairwise comparisons was statistically significant (table 1).
Persistent seizure cessation for at least 1 hour after drug administration.
Five studies reported this outcome, 2 being disconnected from the network (figure 1A.c).16,22 The route–drug combinations analyzed were IN-LOR, BC-MDZ, IN-MDZ, IM-MDZ, IV-LOR, PR-DZP, and IV-DZP (table e-3). Among the nonvenous route–drug combinations, IN-MDZ had the highest WER of 78.5% followed by BC-MDZ (73.2%). In pairwise comparisons, IN-MDZ was significantly better than PR-DZP (OR 3.203, 95% CrI 1.24–9.531) but not BC-MDZ (OR 1.404, 95% CrI 0.51–4.389, table 1). BC-MDZ was superior to PR-DZP (OR 2.286, 95% CrI 1.602–3.249). Though comparative analysis was not possible for IM-MDZ and IN-LOR, the pooled event rates were found to be 69.2% and 61.9%, respectively, for these medications.
Time from drug administration to termination of seizure.
A majority of studies reported this outcome (n = 14). However, 2 studies were disconnected from the network (figure 1B.a),16,22 3 others had missing data points,15,21,23 and 1 study had censored observations at 10 minutes.20 The route–drug combinations analyzed were IN-MDZ, BC-MDZ, IN-LOR, IM-MDZ, IV-DZP, PR-DZP, IV-LOR, and IM-PLD (table e-3). Among the nonvenous medications, IM-MDZ was most effective, with a weighted effect size (WES) of 2.145 minutes, followed respectively by IN-MDZ (2.436 minutes), PR-DZP (3.832 minutes), and BC-MDZ (4.292 minutes). In pairwise comparisons, IM-MDZ was superior to PR-DZP (weighted posterior median difference [WMD] −1.678 minutes, 95% CrI −2.983 to −0.178) and BC-MDZ (WMD −2.132 minutes, 95% CrI −3.638 to −0.550), but not to IN-MDZ and IV-DZP (table 2). Also, both PR-DZP (WMD 1.391 minutes, 95% CrI 0.645–2.365) and BC-MDZ (WMD 1.855 minutes, 95% CrI 0.914–3.092) were inferior to IN-MDZ (table 2). Although the 2 studies including IN-LOR were disconnected from the network, the (variance-weighted) mean time to seizure termination for IN-LOR was 6.80 minutes.
Table 2.
Relative treatment effects of pairwise comparisons expressed as posterior median difference (95% credible interval), and relative ranking based on probability of being most clinically effective for continuous outcomes
Time to termination of seizure after arrival at the health care facility.
Four studies reported this outcome, all of which were included in the network, though 1 study had missing data (figure 1B.b).21 The route–drug combinations analyzed included IN-MDZ, IM-MDZ, and IV-DZP (table e-3). IM-MDZ was the best nonvenous medication (WES 3.841 minutes), and was superior to both IV-DZP (WMD −2.076 minutes, 95% CrI −3.199 to −0.597) and IN-MDZ (WMD −1.886 minutes, 95% CrI −3.696 to −0.048) on pairwise comparisons (table 2).
Time taken to initiate treatment.
Seven studies reported this outcome, with only 1 being disconnected (figure 1B.c).22 The route–drug combinations analyzed were IN-MDZ, BC-MDZ, IN-LOR, IM-MDZ, PR-DZP, IV-DZP, and IM-PLD (table e-3). The best nonvenous medication was IM-MDZ (WES 0.779 minutes), followed by BC-MDZ (0.982 minutes), IN-MDZ (1.194 minutes), and PR-DZP (1.239 minutes). On pairwise comparisons, IM-MDZ was significantly superior (allowed faster administration) compared to IV-DZP (WMD −1.199 minutes, 95% CrI −1.654 to −0.793) and PR-DZP (WMD −0.459 minutes, 95% CrI −0.780 to −0.005), but not to IN-MDZ and BC-MDZ (table 2). Additionally, both PR-DZP (WMD −0.753 minutes, 95% CrI −1.285 to −0.318) and BC-MDZ (WMD −1.007 minutes, 95% CrI −1.315 to −0.768) were superior to IV-DZP (table 2).
DISCUSSION
This network meta-analysis showed that IM-MDZ is the most efficacious nonvenous medication regarding rapidity of administration and action for treatment of acute convulsive seizures and convulsive status epilepticus. IM-MDZ was the fastest nonvenous medication to terminate seizures after administration (WES 2.145 minutes, 95% CrI 1.308–3.489), and can be administered most rapidly among nonvenous drugs (WES 0.779 minutes, 95% CrI 0.495–1.221). Also, IM-MDZ was fastest to terminate seizures when measured from arrival to the health care facility (WES 3.841 minutes, 95% CrI 2.697–5.416, table 2).
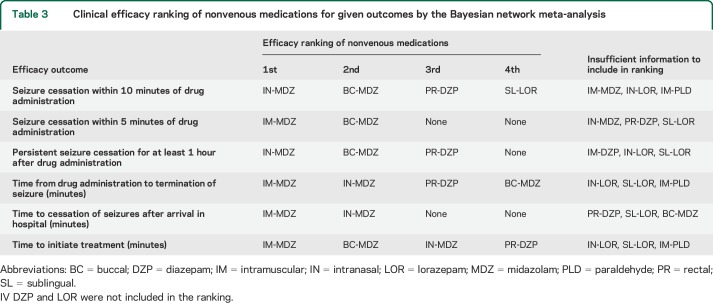
IN-MDZ was the most efficacious nonvenous medication for seizure cessation within 10 minutes of drug administration, and persistent seizure cessation for at least 1 hour (table 3). More than 90% (WER 90.4%, 95% CrI 79.4%–96.9%) of patients attained seizure termination within 10 minutes of administration of IN-MDZ and nearly 80% (WER 78.5%, 95% CrI 59.5%–92.1%) sustained seizure remission for at least 1 hour (table 1). Of the 7 studies including IN-MDZ, none reported using atomizers: 3 studies used syringes (not reported whether atomizers were attached),21,26,28 1 used nasal dropper,5 and the others did not mention their method of administration.14,17,27 Hence, these results for IN-MDZ should be interpreted carefully, since there is some evidence for altered absorption kinetics when an atomizer is used for nasal delivery.31
Table 3.
Clinical efficacy ranking of nonvenous medications for given outcomes by the Bayesian network meta-analysis
The Bayesian hierarchical analysis also showed BC-MDZ to be second most efficacious nonvenous medication for some of these outcomes (table 3). Notably, whereas the time to initiate treatment was not significantly different for IM-MDZ, BC-MDZ, and IN-MDZ, these drugs differed regarding time to seizure termination such that IM-MDZ and IN-MDZ were not statistically different, but both were superior to BC-MDZ in pairwise comparisons (table 2). These are important clinical considerations when prescribing a rescue medication for out-of-hospital use.
Guidelines for management of acute convulsive seizures or convulsive status epilepticus recommend IV-LOR as the first drug.32–34 The safety and efficacy of IV-LOR has been demonstrated in multiple settings.1,16,35 In the Veterans Affairs study,1 IV-LOR successfully stopped all clinical and electrical seizure activity within 20 minutes of administration with no recurrence for 60 minutes in 67% of patients (n = 100) with overt status epilepticus.1 Recently, among children aged 3 months to 18 years, IV-LOR (97/133, 72.9%) was not found to be superior to IV-DZP (101/140, 72.1%) for cessation of convulsive status epilepticus for 10 minutes without recurrence within 30 minutes (absolute difference 0.8%, 95% CI −11.4% to 9.8%).2
While these IV medications are suitable for well-equipped emergency rooms adept at rapid vascular access and well-trained emergency response teams, there is a lack of standard recommendations regarding nonvenous route–drug combinations.36,37
In the United States, PR-DZP is perhaps the most commonly prescribed nonvenous medication for use as a caregiver-administered rescue treatment of acute convulsive seizures. However, our analyses showed that it achieves seizure cessation within 10 minutes in only ∼70% of patients, with sustained seizure cessation for ≥1 hour in only ∼55% of patients, being inferior to IN-MDZ and BC-MDZ (table 1). PR-DZP was also inferior to other nonvenous medications regarding time for seizure termination and time to initiate treatment (table 2). In the community, particularly in adolescents, rectal medication may be socially embarrassing and potentially stigmatizing.
The safety and efficacy of IM-MDZ is also attested by Class I evidence from the Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) study.13 After prehospital treatment of status epilepticus by paramedics, seizures were absent without the rescue medication on arrival to the emergency department in 329/448 (73.4%) of subjects in the IM-MDZ group compared to 282/445 (63.4%) in the IV-LOR group (absolute difference 10%, 95% CI 4.0–16.1, p < 0.001). While the median time to administration was significantly shorter for IM-MDZ compared to IV-LOR (1.2 vs 4.8 minutes), the termination of seizures took longer with the former (3.3 vs 1.6 minutes).13 For IM-MDZ, these outcomes are similar to the pooled estimates obtained in the present study (0.8 and 2.2 minutes, respectively, table 2). Adverse event rates were similar in both groups. These results together with our meta-analyses argue strongly to consider IM-MDZ as the nonvenous drug of choice for treatment of acute convulsive seizures and convulsive status epilepticus, with IN-MDZ as an alternative where IM injection is not feasible.
The limitations of this study result from evidence gaps including paucity of high-quality RCTs, variability in study populations and drug dosages, and small networks with variably disconnected studies. The meta-analysis can accommodate but cannot reconcile the scant source data. Also, all studies included in the quantitative synthesis were open-label. Certain nonvenous routes used in acute care settings, like intraosseous, are not represented due to lack of RCTs. Hence, this meta-analysis' conclusions will have the same or slightly higher level of evidence than these source RCTs. Further, in network analysis, indirect comparisons may be biased if the factors that differ across trials are effect modifiers of the response. Some studies were limited to convulsive status epilepticus while others also included relatively brief seizures. Individual studies excluded patients with prior administration of benzodiazepines,12,17 any antiseizure medications,14,16,26 signs of upper respiratory infection,14 prior IV access,18,28 or certain etiologies of acute symptomatic seizures.19 Although the doses of PR-DZP (0.3–0.5 mg/kg) and BC-MDZ (0.2–0.5 mg/kg, fixed 10 mg) varied across studies,6,19,20 the doses of IM-MDZ (0.2 mg/kg) and IN-MDZ (0.2 mg/kg) were fairly consistent.19,20,23,29 Also, studies have measured the time to initiate treatment differently in terms of initial reference timepoint, which can potentially bias the results. For example, the outcome can be overestimated if measured from the time of arrival in the hospital compared to if measured from time of randomization. Finally, these results only incorporated efficacy and not safety of the analyzed medications, due to variability in defining and reporting adverse events across studies. Hence, the present results should be used in conjunction with factors specific to the individual patient and the health care system.
In many health care environments, nonvenous treatment of acute convulsive seizures is highly desirable. This meta-analysis together with the RAMPART study13 should encourage health care providers to consider IM-MDZ as an acceptable first choice nonvenous medication, when the IM route is feasible. IM administration may be most appropriate when used by paramedics in the field or in the hospital before IV access is established. Future availability of inexpensive, easy-to-use autoinjectors may facilitate IM administration even by nonmedical personnel in out-of-hospital settings. When the IM route is not feasible, IN-MDZ is an easily administered alternative, particularly for out-of-hospital use by nonmedical personnel. Wider use of atomizers may further improve and promote IN administration. Nonvenous formulations for treatment of seizure emergencies currently being developed are further expected to provide effective and user-friendly alternatives.38
Among the various route–drug combinations used in RCTs, IM-MDZ was the most efficacious nonvenous medication for time to seizure termination after administration, and time to initiate treatment. IN-MDZ was the most efficacious nonvenous medication regarding seizure cessation within 10 minutes of administration and sustained seizure cessation for at least 1 hour. The results of this study support a practice change towards wider use of IM-MDZ and IN-MDZ in appropriate settings.
Supplementary Material
GLOSSARY
- BC
buccal
- CI
confidence interval
- CrI
credible intervals
- DZP
diazepam
- IM
intramuscular
- IN
intranasal
- LOR
lorazepam
- MDZ
midazolam
- OR
odds ratio
- PLD
paraldehyde
- PR
rectal
- RAMPART
Rapid Anticonvulsant Medication Prior to Arrival Trial
- RCT
randomized controlled trial
- SL
sublingual
- WER
weighted event rate
- WES
weighted effect size
- WMD
weighted posterior median difference
Footnotes
Supplemental data at Neurology.org
Editorial, page 1830
AUTHOR CONTRIBUTIONS
R.A.: concept, design, literature search, data analysis, and drafting the manuscript. H.K.: literature search, data extraction. Z.Z.: data analysis. B.H.: data analysis, critical review of the manuscript. P.S.H.: data analysis, critical review of the manuscript. T.A.G.: data analysis, critical review of the manuscript. All authors approve the final version.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
R. Arya receives research support from the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award (pSERG). H. Kothari reports no disclosures relevant to the manuscript. Z. Zhang currently works at Eli Lilly and Company. This work was done outside his scope of employment with Eli Lilly and Company. B. Han reports no disclosures relevant to the manuscript. This work was done outside his scope of employment with Biogen. P. Horn receives royalties for his book (Horn PS, Pesce AJ. Reference Intervals: A User's Guide. Washington, DC: AACC Press, 2005); consulting fees from InventivHealth, and Cincinnati Consulting Inc.; and research support from NIH/NINDS (R21 NS081420-01A1, coinvestigator), and The John Merck Fund (coinvestigator). T. Glauser is funded by NIH grants 2U01-NS045911, U10-NS077311, R01-NS053998, R01-NS062756, R01-NS043209, R01-LM011124, and R01-NS065840. He has received consulting fees from Supernus, Sunovion, Eisai, UCB, Lundbeck, and Questcor. He also serves as an expert consultant for the US Department of Justice and has received compensation for work as an expert on medico-legal cases. He receives royalties from a patent license from AssureRx Health. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus: Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–798. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain JM, Okada P, Holsti M, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA 2014;311:1652–1660. [DOI] [PubMed] [Google Scholar]
- 3.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 2008;11:956–964. [DOI] [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol 2006;34:355–359. [DOI] [PubMed] [Google Scholar]
- 6.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet 1999;353:623–626. [DOI] [PubMed] [Google Scholar]
- 7.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health 2011;14:429–437. [DOI] [PubMed] [Google Scholar]
- 8.NICE Decision Support Unit. Evidence Synthesis TSD Series. Available at: http://www.nicedsu.org.uk/. Accessed May 15, 2014.
- 9.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J Roy Stat Soc B 2002;64:583–616. [Google Scholar]
- 10.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–944. [DOI] [PubMed] [Google Scholar]
- 11.Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In: Hornik K, Leisch F, Zeileis A, eds. Proceedings of the 3rd International Workshop on Distributed Statistical Computing; Vienna; 2003. [Google Scholar]
- 12.Malu CK, Kahamba DM, Walker TD, et al. Efficacy of sublingual lorazepam versus Intrarectal diazepam for prolonged convulsions in Sub-Saharan Africa. J Child Neurol 2013;29:895–902. [DOI] [PubMed] [Google Scholar]
- 13.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr 2012;22:1–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Nakken KO, Lossius MI. Buccal midazolam or rectal diazepam for treatment of residential adult patients with serial seizures or status epilepticus. Acta Neurol Scand 2011;124:99–103. [DOI] [PubMed] [Google Scholar]
- 16.Arya R, Gulati S, Kabra M, Sahu JK, Kalra V. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open-label study. Epilepsia 2011;52:788–793. [DOI] [PubMed] [Google Scholar]
- 17.Holsti M, Dudley N, Schunk J, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med 2010;164:747–753. [DOI] [PubMed] [Google Scholar]
- 18.Ashrafi MR, Khosroshahi N, Karimi P, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol 2010;14:434–438. [DOI] [PubMed] [Google Scholar]
- 19.Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev 2009;31:744–749. [DOI] [PubMed] [Google Scholar]
- 20.Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics 2008;121:e58–e64. [DOI] [PubMed] [Google Scholar]
- 21.Mittal P, Manohar R, Rawat AK. Comparative study of intranasal midazolam and intravenous diazepam sedation for procedures and seizures. Indian J Pediatr 2006;73:975–978. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet 2006;367:1591–1597. [DOI] [PubMed] [Google Scholar]
- 23.Shah I, Deshmukh CT. Intramuscular midazolam vs intravenous diazepam for acute seizures. Indian J Pediatr 2005;72:667–670. [DOI] [PubMed] [Google Scholar]
- 24.McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet 2005;366:205–210. [DOI] [PubMed] [Google Scholar]
- 25.Baysun S, Aydin OF, Atmaca E, Gurer YK. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr 2005;44:771–776. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav 2004;5:253–255. [DOI] [PubMed] [Google Scholar]
- 27.Fisgin T, Gurer Y, Tezic T, et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol 2002;17:123–126. [DOI] [PubMed] [Google Scholar]
- 28.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ 2000;321:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care 1997;13:92–94. [DOI] [PubMed] [Google Scholar]
- 30.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 31.Wermeling DP. Intranasal delivery of antiepileptic medications for treatment of seizures. Neurotherapeutics 2009;6:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowenstein DH. Seizures, and epilepsy. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine, 18th ed New York: NY McGraw-Hill; 2011:3251–3269. [Google Scholar]
- 33.Capovilla G, Beccaria F, Beghi E, Minicucci F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus in childhood: recommendations of the Italian league against epilepsy. Epilepsia 2013;54(suppl 7):23–34. [DOI] [PubMed] [Google Scholar]
- 34.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 35.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631–637. [DOI] [PubMed] [Google Scholar]
- 36.Wait S, Lagae L, Arzimanoglou A, et al. The administration of rescue medication to children with prolonged acute convulsive seizures in the community: what happens in practice? Eur J Paediatr Neurol 2013;17:14–23. [DOI] [PubMed] [Google Scholar]
- 37.Lagae L. Clinical practice: the treatment of acute convulsive seizures in children. Eur J Pediatr 2011;170:413–418. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal SK, Cloyd JC. Development of benzodiazepines for out-of-hospital management of seizure emergencies. Neurol Clin Pract 2015;5:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.