Abstract
Small conductance Ca2+-activated K+ (SK, KCa2) channels are unique in that they are gated solely by changes in intracellular Ca2+ and hence, function to integrate intracellular Ca2+ and membrane potentials on a beat-to-beat basis. Recent studies have provided evidence for the existence and functional significance of SK channels in the heart. Indeed, our knowledge of cardiac SK channels has been greatly expanded over the past decade. Interests in cardiac SK channels are further driven by recent studies suggesting the critical roles of SK channels in human atrial fibrillation, SK channel as a possible novel therapeutic target in atrial arrhythmias and up-regulation of SK channels in heart failure (HF) in animal models and human HF. However, there remain critical gaps in our knowledge. Specifically, blockade of SK channels in cardiac arrhythmias has been shown to be both anti-arrhythmic and proarrhythmic. This contemporary review will provide an overview of the literature on the role of cardiac SK channels in cardiac arrhythmias and to serve as a discussion platform for the current clinical perspectives. At the translational level, development of SK channel blockers as a new therapeutic target in the treatment of atrial fibrillation and the possible pro-arrhythmic effects merit further considerations and investigations.
Introduction
Cardiac action potentials (APs) are shaped by the intricate interplay of inward Na+, Ca2+ and outward K+ currents. Ca2+ influx through voltage-gated Ca2+ channels is critical not only for the initiation of cardiac excitation-contraction coupling, but also for the activation of multiple downstream molecules to couple the function of the proteins with changes in membrane potentials including Ca2+-activated ion channels.
The initial study of Ca2+-activated K+ channels in the heart dating back to 1983 did not support the functional role of the channels in the heart1. However, Giles and Imaizumi reported a few years later that Ca2+-activated K+ currents could be observed and were larger in atria than ventricles2. There were no additional reports on the functional roles of Ca2+-activated K+ channel in the heart until a decade ago when we reported the molecular identity and functional significance of small conductance Ca2+-activated K+ (SK) channels in human and mouse hearts3. Since then, our knowledge of cardiac SK channels has been greatly expanded over the past decade. Studies by our group and others have provided evidence to substantiate the important roles of SK channels in the heart4–15. Indeed, interests in cardiac SK channels are further fueled by recent studies suggesting the critical roles of SK channels in human atrial fibrillation (AF)16, 17, SK channel as a possible novel therapeutic target in atrial arrhythmias18–20 and up-regulation of SK channels in heart failure (HF) in animal models10,11 and human HF21 (see Figure 1). However, there remain major gaps in our knowledge. Conflicting studies have been reported regarding the existence of SK channels in the heart22. Moreover, blockade of SK channels in cardiac arrhythmias has been shown to be both anti-arrhythmic18–20 and proarrhythmic23–25 in various models (see Figure 1). This review attempts to provide an overview of the literature over the past decade on the role of cardiac SK channels in electrophysiology, molecular interactions, and cardiogenesis and to serve as a discussion platform for the current clinical perspectives.
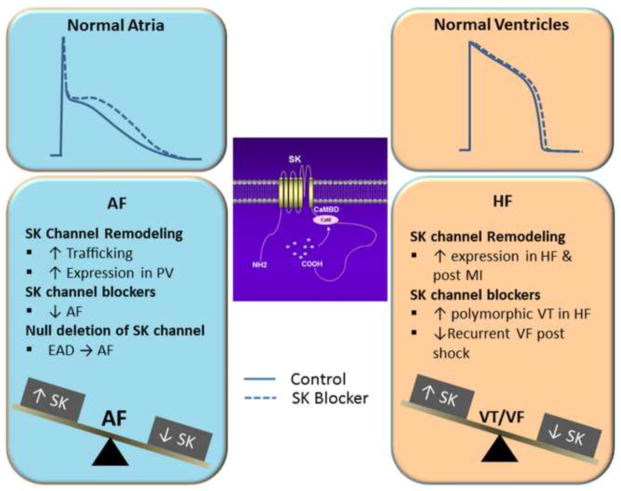
Figure 1. Functional roles of SK channels in normal and diseased hearts.
Distinct roles of SK channels in atria and ventricles are dipicted together with remodeling in AF, HF and post MI. EAD, early afterdepolarization; PV, pulmonary veins, AF, atrial fibrillation; VT/VF, ventricular tachycardia and fibrillation.
Identification and functional expression of SK channels in the heart
SK channels are gated solely by intracellular Ca2+ and hence, provide a critical link between changes in intracellular Ca2+ and membrane potentials. The discovery of SK channels started more than 70 years ago when convulsions in mice were observed following injection of bee venom26, 27. The active neurotoxin in bee venom is apamin, a remarkably specific blocker of SK channels28, 29. Indeed, the highly selective blockade by apamin is the signature of SK channels that enables the verification of the molecular identity of SK channels in mammalian brain30. The family of SK channels consists of three members with differential sensitivity to apamin: SK1 (or KCa2.1 encoded by KCNN1 gene) with the least sensitivity (EC50 for hSK1 ~10 nM), SK2 (or KCa2.2 encoded by KCNN2 gene) with the highest sensitivity (EC50 ~40 pM) and SK3 (or KCa2.3 encoded by KCNN3 gene) with intermediate sensitivity (EC50 ~1 nM)27. They have a relatively small single channel conductance (~10 pS in symmetrical K+) and are activated by submicromolar concentrations of intracellular Ca2+ ions (apparent Kd ~0.5 μM). They are highly conserved among mammalian species and are identified in many organisms including Drosophila27. Functional SK channels assemble to form homomeric30 or heteromeric5, 31 tetramers. An intermediate-conductance Ca2+-activated K+ channel (IK or SK4 encoded by KCNN4 gene) that is structurally and functionally similar to SK channels is classified to the same gene family27, 32.
SK channels were first identified in brain30, 32, and were later described in a variety of tissues including smooth muscle, endothelia, epithelia and blood cells32. SK4 expression is restricted to non-neuronal tissues such as muscle, epithelia and blood cells32, 33. Our laboratory demonstrated that all three isoforms of SK channels are expressed in mouse and human cardiomyocytes3, 4. Since then, expression of SK1 and SK3 in human heart tissues34 and SK2 and SK3 in rabbit pulmonary veins has also been reported7. The existence of SK currents in the heart was further supported by the findings of apamin-sensitive currents in rabbit pulmonary vein7, 9 and ventricular myocytes10, 11, human atrial myocytes12, 13, and rat ventricular myocytes14. Recently, presence of SK currents has also been demonstrated in canine pulmonary vein and left atrial myocytes using a SK-specific current blocker, NS859315. Moreover, SK channels have been identified in pacemaking cells including mouse atrioventricular nodal cells35 and rabbit sinoatrial nodal cells9. The SK currents show inward rectification profile which may result from the pore block by intracellular divalent cations at positive membrane potential or may be mediated by the intrinsic charged residues in the sixth transmembrane domain27, 42. Apamin-sensitive SK currents in atrial myocytes show the inward-rectified feature reminiscent of the hetero-expressed SK currents3, 30, 36.
Cardiac SK channel interactome
Ion channels do not exist and function in isolation, instead they form part of multi-protein complexes interacting with extracellular matrix and cytosolic proteins37–39. The composition of the ion channel complex significantly affects the localization, trafficking, activation and regulation of the channel function. Gating of SK channels depends on the interplay between the pore-forming α subunits and Ca2+-binding protein calmodulin (CaM)27, 36 through which the channels sense the intracellular Ca2+ leading to altered conformation. CaM binds to a highly conserved CaM-binding domain (CaMBD) residing within the C-terminus of the SK channels located immediately distal to the sixth transmembrane segment. Binding of Ca2+ to the EF hands of CaM results in changes in the conformation of the channels leading to channel activation. CaM is not only essential for Ca2+ sensitivity, but also critical to the trafficking of SK channels40. Specifically, previous studies have demonstrated that Ca2+-independent association between CaM and SK channels are necessary for cell surface expression. However, it is not known whether CaM help to anchor the SK channel or acts as a chaperone for channel trafficking.
A proteomics approach has identified protein kinase CK2 (casein kinase II) and protein phosphatase 2A (PP2A) as SK2 channel binding proteins41. CK2 and PP2A regulate SK channel’s sensitivity to intracellular Ca2+ by phosphorylating or dephosphorylating CaM27. However, CK2/PP2A modulation of SK channels has been demonstrated mainly in neurons and possible roles of CK2/PP2A in cardiac myocytes await further studies.
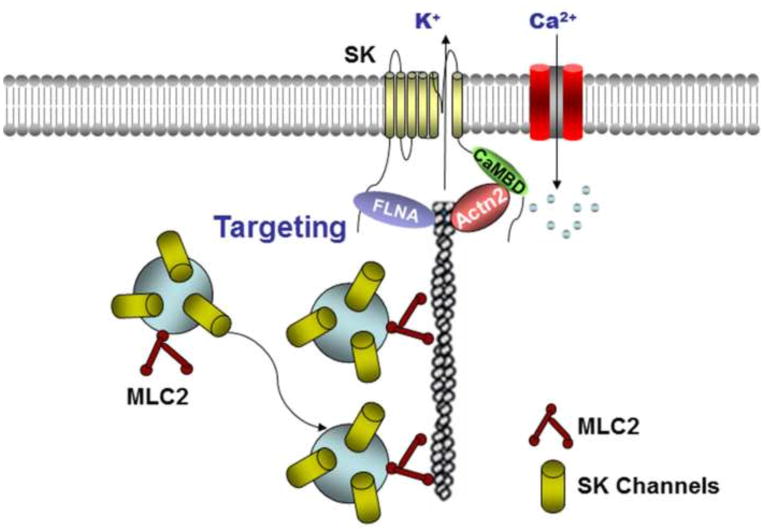
Using yeast two-hybrid screen against human heart library, we have identified several cytoskeletal proteins including α-actinin242,43, filamin A44, and myosin light chain 2 (MLC2)45 that directly interact with SK2 channels (Figure 2). Specifically, α-actinin2 and MLC2 interact via the C-termini and filamin A via the N-termini of the SK2 channel, respectively. Moreover, cardiac SK2 channels coupled with L-type calcium channels, Cav1.3 and Cav1.2, through a physical bridge, α-actinin2. SK2 channels do not physically interact with Ca2+ channels, instead the two channels co-localize via their interaction with α-actinin2 along the Z-line in atrial myocytes (Figure 2). The co-localization of SK and Ca2+ channels suggests the possibility that local subsarcolemmal Ca2+ resulting from opening of Ca2+ channels is sufficient to activate SK channels as was demonstrated for hippocampal neurons46. However, two recent studies suggested that sarcoplasmic reticulum (SR) Ca2+ release is required for the activation of cardiac SK channels47,48. One of these studies utilized over-expressed SK channels and the regulation of the over-expressed SK channels maybe distinct compared to native cardiomyocytes. Nonetheless, it is possible that in cardiomyocytes, both Ca2+ entry and Ca2+ release from SR are required to activate SK channels.
Figure 2. Localization, trafficking and molecular parters of cardiac SK channels.
SK channels interactome includes α-actinin2 (Actin2), filaminA (FLNA), and myosin light chain 2 (MLC2). Cardiac SK channels have been shown to couple to L-type calcium channels through a physical bridge, α-actinin2. SK2 channels do not physically interact with the Ca2+ channels, instead the two channels co-localize via their interaction with α-actinin2 along the Z-line in atrial myocytes. An increase in intracellular Ca2+, as evident during rapid AF or atrial tachycardia, is predicted to increase SK2 channel expression leading to shortening of the atrial APs and maintenance of arrhythmias.
In addition, trafficking of SK2 channels is critically dependent on the direct protein-protein interactions of the channels with α-actinin2, MLC2, and filamin A43–45 (see Figure 2). Knockdown of α-actinin2 or filamin A results in a decrease in SK2 channel expression on the membrane and localization of SK2 channels in endosome suggesting a reduction in recycling of SK2 channels from endosome44–45. Finally, SK2 channel trafficking is Ca2+-dependent in the presence of α-actinin2. A decrease in intracellular Ca2+ results in a significant reduction of SK2 channel membrane localization44. An increase in intracellular Ca2+, as evident during rapid AF or atrial tachycardia, is predicted to increase SK2 channel expression leading to shortening of the atrial APs and maintenance of arrhythmias. One previous study using rapid pacing in isolated rabbit atria demonstrated a significant increase in SK2 immunostaining from a perinuclear pattern to the plasma membrane at the pulmonary vein after burst pacing suggesting an increase in forward trafficking of SK2 channels7.
Overall, SK channel complexes in cardiomyocytes consist at least of homomeric or heteromeric SK channel α subunits, CaM, α-actinin2, filamin A and MLC2. The interaction amongst the multi-proteins in the SK channel complexes is essential for the gating, regulation, and membrane trafficking of the channels in cardiomyocytes. With the aid of the advanced proteomics techniques, more interacting proteins may be identified as new candidates that participate in the SK channel complexes.
Roles of SK channels in cardiac repolarization
Repolarization of cardiac action potential (AP) relies on the orchestrated activity of multiple K+ channels and transporters. One critical question following the identification of cardiac SK channels is whether the channels contribute to cardiac repolarization. The initial study reported that inhibition of SK currents by apamin prolonged AP duration (APD) in mouse and human atrial myocytes, however, the effects were less prominent in ventricular myocytes suggesting the unique role of the channels in atrial repolarization3. Consistently, subsequent experiments using global SK2 knockout mice demonstrated that ablation of SK2 channel resulted in a significant prolongation of APD prominently in the late phase of the repolarization in atrial myocytes6. Importantly, the null mutant mice showed an increased susceptibility to atrial fibrillation (AF). On the other hand, there was no significant alteration in the APD in ventricular myocytes and no ventricular arrhythmias were induced in the null mutant animals6. In contrast, a mouse model of SK3 channel overexpression showed a significant shortening of APD in atrial myocytes8. These gene-targeted mouse models were not restricted to only cardiac tissues. Indeed, SK channels are abundantly expressed in a number of non-cardiomyocyte cells in the heart. Nonetheless, single isolated cardiomyocytes were used in these studies in addition to in vivo studies to circumvent possible contributions from the effects of SK2 knockout or SK3 overexprssion in other cell types including neuronal cells.
Consistent with these studies, a recent report using optical mapping in isolated canine left atria demonstrated that inhibition of SK channels by either apamin or UCL168449 prolonged APD23. In addition, SK channel inhibitors, NS8593 and ICAGEN50, prolonged APD in isolated human atrial myocytes13. The above studies supported the critical role of SK channels in the repolarization not only in mouse and canine but also human atrial myocytes51.
In atrioventricular nodal cells, SK2 channel overexpression results in shortening of spontaneous APs and an increase in the firing frequency, while ablation of SK2 channels results in the opposite effects35. Recently, Chen et al studied the apamin modulation of pulmonary vein and SAN cells from rabbit heart9. They found that SAN cells have larger SK currents than pulmonary vein cardiomyocytes. Apamin treatment decreases the firing rate and prolongs APD50 and APD75 in SAN cells and pulmonary vein cardiomyocytes.
SK channels and atrial fibrillation (AF)
Significance of cardiac SK channels lies in the fact that the channels are preferentially expressed in atria compared to the ventricles3. The differential expression of SK channels in the heart may offer a unique therapeutic strategy to target atria without interfering with ventricular function. Moreover, the possible role of SK channels in human AF was recently reported using genome-wide association analysis (GWAS) revealing an association between an intronic single-nucleotide polymorphism (SNP) in KCNN3 gene with lone AF16, 17. In addition, Olesen et al reported one known exonic synonymous SNP in KCNN3 that was also associated with lone AF52. To further test the role of SK3 channels in atrial repolarization and AF, Zhang et al took advantage of a SK3 transgenic mouse model to demonstrate that overexpression of SK3 results in significant shortening of APD in atrial myocytes, an abbreviation of atrial effective refractory period (AERP) and an increased susceptibility to AF8. A separate study using the same mouse model showed that overexpression of SK3 channels can increase the risk of sudden cardiac death associated with bradyarrhythmias, heart block and an increased susceptibility to atrial arrhythmias53.
SK channel remodeling in AF
Evidence is accumulating to support SK channel remodeling in AF. The first evidence showing the AF-induced remodeling of SK channel came from a study to test the effect of a rare stimulation on the susceptibility of atrial arrhythmias54. Apamin prevented the progressive shortening of APD near burst pacing electrode in coronary sinus and pulmonary vein (PV) regions suggesting the involvement of SK channels. Ozgen et al further studied the mechanism underlying the phenomenon7. They found that the burst pacing-induced APD shortening in pulmonary vein-atria interface in a rabbit model resulted from SK2 channel trafficking to the cell membrane, leading to increased apamin-sensitive outward currents7. Similarly, Qi et al reported that atrial-tachypacing in a canine model enhanced SK currents in PV and left atrial myocytes15. Moreover, inhibition of SK channels by a known SK channel blocker (NS8593)55 significantly reduced AF inducibility. Of interest is the fact that SK2 expression was found to be more abundant in pulmonary vein than left atrial myocytes. Mechanistically, our recent study has provided evidence to suggest that an increase in intracellular Ca2+ as seen during rapid atrial tachycardia results in an enhanced trafficking of SK channels to the membrane44.
Contrary to the above studies, a recent report found that SK1 and SK2 channel expression as well as apamin-sensitive currents were significantly reduced in right atrial appendages recovered from chronic AF patients12. Similarly, a recent study showed that SK3 expression was down-regulated in patients with permanent AF, which was attributed to the up-regulation of microRNA-49956. Taken together, it is possible that AF may result in the up-regulation of SK channels as the initial response. With progression of the disease, there may be a down-regulation of the channels.
Anti-arrhythmic and proarrhythmic effects of SK channel inhibition
Recently, Diness et al utilized three different SK channel inhibitors including UCL1684, N-(pyridin-2-yl)-4-(pyridin-2-yl)thiazol-2-amine (ICA)50 and NS8593 in ex vivo and in vivo models of AF in rat, guinea pig, and rabbit18. Inhibition of SK channels resulted in prolongation of AERP and termination of AF. Similar anti-arrhythmic effects of NS8593 and UCL1684 were observed for paroxysmal AF in a hypertensive rat model19. In an acute pacing-induced AF model in rats, it was found that intravenous application of NS8593 reduced AF duration in a dose-dependent manner and the antiarrhythmic effect is associated with increased AERP20. Similar results were also obtained using UCL1684 and apamin in the same study. The results from these studies suggest that SK channels may represent a potential therapeutic target for the treatment of atrial arrhythmias.
In contrast to these studies, genetic ablation of SK2 channels in a mouse model prolonged atrial APD, increased occurrences of early after depolarization (EAD) and inducible AF6. In addition, a recent report found that apamin and UCL1684 promoted arrhythmia in isolated canine left atrium23. The prolongation of APD by SK channel inhibition was accompanied by increased APD heterogeneity, occurrences of electrical alternans, and wave breaks. In addition to the previously suggested increased occurrence of EAD by ablation of SK2 channels, inhibition of SK channels may lead to more heterogeneous APD and altered dispersion of repolarization, which can promote development of reentrant arrhythmia24. Moreover, SK2 channels expression in left atrial appendages was higher at the base compared to the apex. Taken together, key questions remain regarding the role of SK channels in AF, i.e., whether inhibition of the channels is proarrhythmic or antiarrhythmic.
Thus, both reduced and enhanced activity of SK channels may predispose atria to AF, likely from different mechanisms depending on the heterogeneous expression, heart rate and AF-induced electrical remodeling. The discrepancy between the above studies may be related to the different AF models, varied species, different experimental techniques and conditions. Indeed, the mechanisms of AF in patients are likely to be far more complex. Further studies are necessary to address the functional roles of SK channels in AF.
Remodeling of SK channels in heart failure (HF)
Contrary to the atria, apamin does not significant affect the APD of dog, rat, rabbit, and human ventricles under normal physiological condition10, 22. However, in a rabbit model with HF and spontaneous ventricular fibrillation, apamin prolonged the APD and eliminated the recurrent spontaneous ventricular fibrillation10. Failing ventricular myocytes showed a significant increase in SK currents compared to the normal ventricular myocytes possibly as a result of the increased sensitivity of SK channels to intracellular Ca2+ in failing heart. More importantly, there is a transmural gradient of SK currents with higher density in epicardial than in midmyocardial and endocardial layers in the failing heart. Similar up-regulation of SK currents was demonstrated in a post myocardial infarction rabbit model11 and failing human ventricles21. It was further demonstrated that a commonly used antiarrhythmic agent, amiodarone, inhibited SK2 channels and prevented post-shock APD shortening in rabbit failing heart suggesting the significance of SK channel inhibition in HF57. A more recent study demonstrated that apamin prolonged ventricular repolarization and EAD in dog and human with end-stage HF58. In a volume-overload HF model in rat, it was found that there was a significant increase of SK1 and SK3 expression and increased apamin-sensitive currents in the HF ventricle compared to sham control. Treatment with a β-blocker, bisoprolol, reduced the SK1 and SK3 expression, apamin-sensitive currents and their sensitivity to intracellular Ca2+ 14. Similarly, in an in vivo acute myocardial infarction model in rat, pretreatment with apamin or UCL1684 significantly increased APDs in the infarcted area of the left ventricle and inhibited spontaneous ventricular tachycardia and ventricular fibrillation59.
The potential mechanisms underlying SK channel remodeling in HF remain unclear. Abnormal Ca2+-handling in HF has been well described and may represent a significant contributor. In addition, remodeling in failing heart involves complex changes in gene expression, phosphorylation of targeted proteins and modulation of Ca2+ signaling pathways. One previous study has documented an increase in SK2 protein expression in failing human ventricles using total homogenates from ventricular tissues21. Therefore, alteration of SK channel expression, Ca2+ sensitivity in HF or possibly increased trafficking may be critical factors in SK channel remodeling in HF and contribute towards recurrent ventricular fibrillation and electrical storm. Additional studies are necessary to gain further mechanistic insight into the regulation of SK channels in HF. Finally, these findings provide a cautionary view for the use of SK channel blockers in the treatment of AF. AF is commonly seen associated with HF and up-regulation of SK channels in heart failure may represent an adaptive response to prevent excessive AP prolongation in the ventricles58. Indeed, the use of SK channel blockers has been shown in an animal model to result in occurrence of torsades de pointes ventricular arrhythmias60. The therapeutic potential for AF needs further assessment in patients with HF.
SK channels in stem cell cardiogenesis
Recent studies have demonstrated that SK channels play an important role in directed stem cell differentiation. Functional SK1, 2, 3, and 4 channels have been shown to be present in pluripotent stem cells (PSCs)61, 62. Activation of SK channels by small molecule 1-ethyl-2-benzimidazolinone (EBIO) improves the differentiation of pacemaking cardiomyocytes from mouse PSCs at two independent differentiation stages, by increasing the induction of the mesoderm lineage and the pacemaking cardiomyocytes subtype specification61. Transcriptional data demonstrated that SK4 is the dominant isoform in murine PSCs and responsible for the facilitated differentiation. The most immediate consequence of SK4 activation was membrane potential hyperpolarization. Similar to murine PSCs, SK channel activation facilitated induction of pacemaking cardiomyocytes from human PSCs, as assessed by expression of hyperpolarization-activated cyclic nucleotide-modulated (HCN)4 channel62. However, electrophysiological studies were not performed. Therefore, it remains to be determined if activated SK channel-induction of pacemaking cardiomyocytes is as robust in human PSCs as that in murine cells. Moreover, contrary to their murine counterpart, human PSCs have virtually no SK4 expression but SK2 as the dominant isoform with low SK1 and minimal SK3 transcripts.
Unresolved issues and future perspectives
A decade of study on cardiac SK channels has opened new and exciting fields in cardiac electrophysiology and arrhythmias. The cumulative findings so far have greatly enhanced the current understanding of the critical crosstalk between Ca2+ signaling and K+ channels in the regulation of cardiac excitability. However, challenges and important knowledge gaps remain.
First, controversies exist regarding the roles of SK channels in cardiac repolarization. Nagy et al recorded APs from rat, dog and human atrial and ventricular tissue preparations and found that no effects of apamin on APDs22. Diness et al also found that apamin does not cause significant APD prolongation in atrial myocytes from guinea pigs18. Interspecies differences and different experimental conditions may contribute to the observed discrepancies. Moreover, the heterogeneous expression of SK channels in the heart is not well defined even though a number of studies suggest that there is a transmural gradient in the expression of SK channels in failing ventricles10, and the expression of SK channels appears to be variable in the different regions of the atria24. Detailed information on the region-specific expression of SK channels in the heart awaits further studies. Functional roles of SK channels in pacemaking cells and PSCs are only beginning to be recognized and need to be further explored. Finally, the role of SK channels in AF and VF is not completely defined. On the translational level, the use of SK channel blockers in the treatment of AF and the possible pro-arrhythmic effects of SK channel blockers in patients with HF require further considerations and investigations.
Acknowledgments
This work was supported by the National Institutes of Health Grants (R01 HL075274 and R01 HL085844 to NC), the Department of Veteran Affairs Merit Review Grant (I01 BX000576 to NC), American Heart Association Beginning Grant-in-Aid (14BGIA18870087 to XDZ), and the California Institute of Regenerative Medicine (RB4-05764 to DL).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisner DA, Vaughan-Jones RD. Do calcium-activated potassium channels exist in the heart? Cell Calcium. 1983 Dec;4:371–386. doi: 10.1016/0143-4160(83)90015-5. [DOI] [PubMed] [Google Scholar]
- 2.Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003 Dec 5;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 4.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA, Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005 Dec;289:H2714–2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 5.Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA, Chiamvimonvat N. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010 Oct 1;107:851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Timofeyev V, Tuteja D, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009 Mar 1;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, Boyden PA, Rosen MR. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res. 2007 Sep 1;75:758–769. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang XD, Timofeyev V, Li N, Myers RE, Zhang DM, Singapuri A, Lau VC, Bond CT, Adelman J, Lieu DK, Chiamvimonvat N. Critical roles of a small conductance Ca2+-activated K+ channel (SK3) in the repolarization process of atrial myocytes. Cardiovasc Res. 2014 Feb 1;101:317–325. doi: 10.1093/cvr/cvt262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WT, Chen YC, Lu YY, Kao YH, Huang JH, Lin YK, Chen SA, Chen YJ. Apamin modulates electrophysiological characteristics of the pulmonary vein and the Sinoatrial Node. European journal of clinical investigation. 2013 Sep;43:957–963. doi: 10.1111/eci.12125. [DOI] [PubMed] [Google Scholar]
- 10.Chua SK, Chang PC, Maruyama M, et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011 Apr 15;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YS, Chang PC, Hsueh CH, Maruyama M, Park HW, Rhee KS, Hsieh YC, Shen C, Weiss JN, Chen Z, Lin SF, Chen PS. Apamin-sensitive calcium-activated potassium currents in rabbit ventricles with chronic myocardial infarction. J Cardiovasc Electrophysiol. 2013 Oct;24:1144–1153. doi: 10.1111/jce.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu T, Deng C, Wu R, Guo H, Zheng S, Yu X, Shan Z, Kuang S, Lin Q. Decreased expression of small-conductance Ca2+-activated K+ channels SK1 and SK2 in human chronic atrial fibrillation. Life Sci. 2012 Jan 30;90:219–227. doi: 10.1016/j.lfs.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Skibsbye L, Poulet C, Diness JG, Bentzen BH, Yuan L, Kappert U, Matschke K, Wettwer E, Ravens U, Grunnet M, Christ T, Jespersen T. Small-conductance calcium-activated potassium (SK) channels contribute to action potential repolarization in human atria. Cardiovasc Res. 2014 Jul 1;103:156–167. doi: 10.1093/cvr/cvu121. [DOI] [PubMed] [Google Scholar]
- 14.Ni Y, Wang T, Zhuo X, Song B, Zhang J, Wei F, Bai H, Wang X, Yang D, Gao L, Ma A. Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Mol Cell Biochem. 2013 Dec;384:95–103. doi: 10.1007/s11010-013-1785-5. [DOI] [PubMed] [Google Scholar]
- 15.Qi XY, Diness JG, Brundel BJ, Zhou XB, Naud P, Wu CT, Huang H, Harada M, Aflaki M, Dobrev D, Grunnet M, Nattel S. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014 Jan 28;129:430–440. doi: 10.1161/CIRCULATIONAHA.113.003019. [DOI] [PubMed] [Google Scholar]
- 16.Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010 Mar;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012 Jun;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Aug;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- 19.Diness JG, Skibsbye L, Jespersen T, Bartels ED, Sorensen US, Hansen RS, Grunnet M. Effects on atrial fibrillation in aged hypertensive rats by Ca2+-activated K+ channel inhibition. Hypertension. 2011 Jun;57:1129–1135. doi: 10.1161/HYPERTENSIONAHA.111.170613. [DOI] [PubMed] [Google Scholar]
- 20.Skibsbye L, Diness JG, Sorensen US, Hansen RS, Grunnet M. The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca2+-activated K+ channels. J Cardiovasc Pharmacol. 2011 Jun;57:672–681. doi: 10.1097/FJC.0b013e318217943d. [DOI] [PubMed] [Google Scholar]
- 21.Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, Tao W, Rubart M, Chen PS, Chen Z, Ai T. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. J Am Heart Assoc. 2013 Feb;2:e004713. doi: 10.1161/JAHA.112.004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy N, Szuts V, Horvath Z, et al. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol. 2009 Nov;47:656–663. doi: 10.1016/j.yjmcc.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm. 2013 Jun;10:891–898. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner S, Maier LS. Small conductance Ca-activated K channel: small but powerful proarrhythmogenic? Heart Rhythm. 2013 Jun;10:899–900. doi: 10.1016/j.hrthm.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Mahida S. Expanding role of SK channels in cardiac electrophysiology. Heart Rhythm. 2014 Jul;11:1233–1238. doi: 10.1016/j.hrthm.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Habermann E. Apamin. Pharmacology & therapeutics. 1984;25:255–270. doi: 10.1016/0163-7258(84)90046-9. [DOI] [PubMed] [Google Scholar]
- 27.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 28.Hugues M, Schmid H, Lazdunski M. Identification of a protein component of the Ca2+-dependent K+ channel by affinity labelling with apamin. Biochem Biophys Res Commun. 1982 Aug 31;107:1577–1582. doi: 10.1016/s0006-291x(82)80180-0. [DOI] [PubMed] [Google Scholar]
- 29.Blatz AL, Magleby KL. Single apamin-blocked Ca2+-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23–29;323:718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- 30.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996 Sep 20;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- 31.Strassmaier T, Bond CT, Sailer CA, Knaus HG, Maylie J, Adelman JP. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J Biol Chem. 2005 Jun 3;280:21231–21236. doi: 10.1074/jbc.M413125200. [DOI] [PubMed] [Google Scholar]
- 32.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nature reviews Neuroscience. 2004 Oct;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 33.Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev. 2010 Oct;90:1437–1459. doi: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]
- 34.Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004 Jun;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Timofeyev V, Lu L, Li N, Singapuri A, Long MK, Bond CT, Adelman JP, Chiamvimonvat N. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008 Feb 29;102:465–471. doi: 10.1161/CIRCRESAHA.107.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998 Oct 1;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 37.Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005 Aug 15;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Catterall WA. Signaling complexes of voltage-gated sodium and calcium channels. Neuroscience letters. 2010 Dec 10;486:107–116. doi: 10.1016/j.neulet.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adsit GS, Vaidyanathan R, Galler CM, Kyle JW, Makielski JC. Channelopathies from mutations in the cardiac sodium channel protein complex. J Mol Cell Cardiol. 2013 Aug;61:34–43. doi: 10.1016/j.yjmcc.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004 Jan 15;554:255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007 Feb 28;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007 Jan 5;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 43.Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR, Chiamvimonvat N. Alpha-actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel) Proc Natl Acad Sci U S A. 2009 Oct 27;106:18402–18407. doi: 10.1073/pnas.0908207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rafizadeh S, Zhang Z, Woltz RL, et al. Functional interaction with filamin A and intracellular Ca2+ enhance the surface membrane expression of a small-conductance Ca2+-activated K+ (SK2) channel. Proc Natl Acad Sci U S A. 2014 Jul 8;111:9989–9994. doi: 10.1073/pnas.1323541111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, Sirish P, Zhang Z, Woltz RL, Li N, Timofeyev V, Knowlton AA, Zhang X, Yamoah EN, Chiamvimonvat N. Regulation of Gene Transcription by Voltage-Gated L-Type Calcium Channel, Cav1.3. J Biol Chem. 2015 Dec 23; doi: 10.1074/jbc.M114.586883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998 Oct 29;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 47.Terentyev D, Rochira JA, Terentyeva R, Roder K, Koren G, Li W. Sarcoplasmic reticulum Ca2+ release is both necessary and sufficient for SK channel activation in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2014 Mar 1;306:H738–746. doi: 10.1152/ajpheart.00621.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mu YH, Zhao WC, Duan P, Chen Y, Zhao WD, Wang Q, Tu HY, Zhang Q. RyR2 modulates a Ca2+-activated K+ current in mouse cardiac myocytes. PLoS One. 2014;9:e94905. doi: 10.1371/journal.pone.0094905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosa JC, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. Bis-quinolinium cyclophanes: 6,10-diaza-3(1,3),8(1,4)-dibenzena-1,5(1,4)-diquinolinacyclodecaphane (UCL 1684), the first nanomolar, non-peptidic blocker of the apamin-sensitive Ca2+-activated K+ channel. J Med Chem. 1998 Jan 1;41:2–5. doi: 10.1021/jm970571a. [DOI] [PubMed] [Google Scholar]
- 50.Gentles RG, Grant-Young K, Hu S, Huang Y, Poss MA, Andres C, Fiedler T, Knox R, Lodge N, Weaver CD, Harden DG. Initial SAR studies on apamin-displacing 2-aminothiazole blockers of calcium-activated small conductance potassium channels. Bioorganic & medicinal chemistry letters. 2008 Oct 1;18:5316–5319. doi: 10.1016/j.bmcl.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 51.Nattel S, Qi XY. Calcium-dependent potassium channels in the heart: clarity and confusion. Cardiovasc Res. 2014 Feb 1;101:185–186. doi: 10.1093/cvr/cvt340. [DOI] [PubMed] [Google Scholar]
- 52.Olesen MS, Jabbari J, Holst AG, Nielsen JB, Steinbruchel DA, Jespersen T, Haunso S, Svendsen JH. Screening of KCNN3 in patients with early-onset lone atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011 Jul;13:963–967. doi: 10.1093/europace/eur007. [DOI] [PubMed] [Google Scholar]
- 53.Mahida S, Mills RW, Tucker NR, Simonson B, Macri V, Lemoine MD, Das S, Milan DJ, Ellinor PT. Overexpression of KCNN3 results in sudden cardiac death. Cardiovasc Res. 2014 Feb 1;101:326–334. doi: 10.1093/cvr/cvt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sosunov EA, Anyukhovsky EP, Hefer D, Rosen TS, Danilo P, Jr, Janse MJ, Rosen MR. Region-specific, pacing-induced changes in repolarization in rabbit atrium: an example of sensitivity to the rare. Cardiovasc Res. 2005 Aug 1;67:274–282. doi: 10.1016/j.cardiores.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Strobaek D, Hougaard C, Johansen TH, Sorensen US, Nielsen EO, Nielsen KS, Taylor RD, Pedarzani P, Christophersen P. Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol. 2006 Nov;70:1771–1782. doi: 10.1124/mol.106.027110. [DOI] [PubMed] [Google Scholar]
- 56.Ling TY, Wang XL, Chai Q, et al. Regulation of the SK3 channel by microRNA-499--potential role in atrial fibrillation. Heart Rhythm. 2013 Jul;10:1001–1009. doi: 10.1016/j.hrthm.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turker I, Yu CC, Chang PC, Chen Z, Sohma Y, Lin SF, Chen PS, Ai T. Amiodarone inhibits apamin-sensitive potassium currents. PLoS One. 2013;8:e70450. doi: 10.1371/journal.pone.0070450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonilla IM, Long VP, 3rd, Vargas-Pinto P, et al. Calcium-activated potassium current modulates ventricular repolarization in chronic heart failure. PLoS One. 2014;9:e108824. doi: 10.1371/journal.pone.0108824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gui L, Bao Z, Jia Y, Qin X, Cheng ZJ, Zhu J, Chen QH. Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K+ channels. Am J Physiol Heart Circ Physiol. 2013 Jan 1;304:H118–130. doi: 10.1152/ajpheart.00820.2011. [DOI] [PubMed] [Google Scholar]
- 60.Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF, Chen PS. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm. 2013 Oct;10:1516–1524. doi: 10.1016/j.hrthm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleger A, Seufferlein T, Malan D, et al. Modulation of calcium-activated potassium channels induces cardiogenesis of pluripotent stem cells and enrichment of pacemaker-like cells. Circulation. 2010 Nov 2;122:1823–1836. doi: 10.1161/CIRCULATIONAHA.110.971721. [DOI] [PubMed] [Google Scholar]
- 62.Muller M, Stockmann M, Malan D, et al. Ca2+ activated K+ channels-new tools to induce cardiac commitment from pluripotent stem cells in mice and men. Stem Cell Rev. 2012 Sep;8:720–740. doi: 10.1007/s12015-011-9324-9. [DOI] [PubMed] [Google Scholar]