INTRODUCTION
Surgery on small mammals, reptiles and avian species poses unique challenges to the veterinary surgeon. The variety of tissue characteristics, blood volume, thermoregulatory properties and susceptibility to anesthesia and perioperative medications requires a tailored approach to each patient. Due to their large surface area-to-volume, most exotic species are at risk of rapid hypothermia and their relatively small blood volume can result in hypovolemia after seemingly minimal blood loss. Precise, efficient patient preparation, meticulous hemostasis, close patient monitoring, and brief operative periods are best to overcome these challenges.
PATIENT PREPARATION
Aseptic technique is paramount in all aspects of surgery and should be strictly followed in any procedure. Removal of fur and feathers decreases the bacterial burden and aids in visualization, but the process can be challenging and should be done with caution. Small mammals such as rabbits have particularly fragile skin that can be damaged during clipping, increasing the risk of blood loss and post-operative infection. Small and delicate clipper blades should be used and the skin stretched so that a flat taught surface is achieved to minimize the risk of iatrogenic laceration.
In avian species, smaller feathers can be plucked in groups of three to four. Gentle outward pressure is applied in the orientation of the follicle to avoid damage. These smaller feathers will be replaced quickly. Removal of larger flight feathers should be avoided. If there is soft tissue damage surrounding the follicle of large feathers, you may cut the feather, understanding that they will not regrow until the next molting cycle.1
Evaluation of the method and timing of hair removal has provided conflicting results in the literature. Currently, it is recommended to perform this process immediately pre-operatively (less than 2 hours before surgery) via electronic clippers (in patients with hair or fur). 1-3 The surgical field should be free from fur and feathers; however, extensive loss can lead to a decrease in insulation and subsequent hypothermia. Therefore, it is recommended that approximately 2-3 cm margins be achieved in small patients.1
Whether or not to wash or bathe the patient before surgery also remains controversial. The majority of existing studies in human and veterinary literature suggest only a transient decrease in bacterial numbers and potential damage to surrounding skin, which may actually predispose the patient to infection. Therefore, pre-operative bathing is not currently recommended in veterinary medicine.4
ANTISEPTICS
Following the removal of hair and fur, the skin is aseptically prepared using an antiseptic solution to reduce normal skin flora. The ideal antiseptic should be non-toxic, should not cause skin reaction, and should not interfere with the normal protective function of the skin. 3,5
Alcohols
Alcohols have strong bactericidal properties but are less effective at eliminating viral and fungal organisms. The bactericidal activity of alcohols is attributed to denaturation of proteins, alteration of metabolism, and direct cell lysis. Alcohols are most effective at concentrations greater than 60%.5 Undesirable effects of alcohols include skin irritation and decreased efficacy in the face of organic debris. For smaller patients, alcohols may also cause hypothermia so judicious use is advised.
Iodophors
The antiseptic activity of iodophors (e.g. povidone-iodine) is derived from the presence of molecular iodine and hypoiodic acid. Free iodine has broad-spectrum activity against bacteria, viruses and fungi. There are few undesirable effects associated with iodophor antiseptics but may include adverse skin reactions or systemic iodine toxicity when used in open wounds.5 Iodophors are minimally affected by organic debris when compared to other antiseptic solutions. Dilution to concentrations of 0.001 - 0.1 % may still possess antiseptic properties while decreasing the risk of complications.
Chlorhexidine
Chlorhexidine is a broad-spectrum antiseptic that is most effective against bacteria, with variable efficacy against most virus and fungal organisms. Concentration of chlorhexidine greatly influences its efficacy, with higher concentrations (2-4%) more bactericidal.6 At lower concentrations, the mechanism of action is attributed to disruption of the bacterial cell membrane, whereas at higher concentrations, chlorhexidine causes coagulation of cellular contents. Chlorhexidine is somewhat inhibited by the presence of organic debris; however, at least one study documented superior antiseptic properties in the face of blood when compared to povidone-iodine. Chlorhexidine binds to the tissue, leading to a long residual effect after application when compared to other antiseptic agents, making it an appealing alternative to other antiseptic agents.3 Chlorhexidine, particularly at doses of 0.05% or below, does not cause significant cytotoxicity; however, at higher concentrations it has been found to produce neurotoxic and ototoxic effects, making its use in areas surrounding the ear and eyes questionable.6 In exotic species, it is commonly recommended to use a warm, dilute 0.05% chlorhexidine solution for surgical preparation to avoid the hypothermic effects of alcohol-based or cold antiseptic solutions.
Comparison of Antiseptic Agents
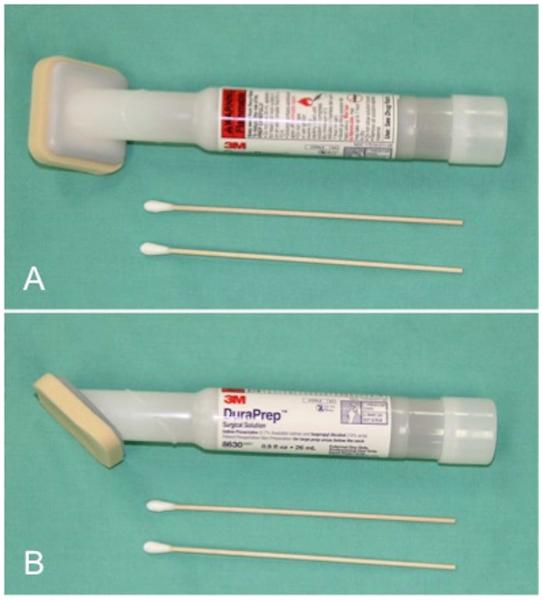
Aseptic techniques commonly used before surgery involve use of a chlorhexidine- or iodine-based scrub (Figure 1) in alternation with alcohol. Despite these recommendations, approximately 20% of the normal flora still remain, emphasizing the importance of intra-operative aseptic technique.3 Recent studies have found similar results when using a “one-step” process utilizing a combination of isopropyl alcohol with an iodophor (DuraPrep 3M, St. Paul, MN) (Figure 2)7-9 Direct comparisons between iodophor and chlorhexidine preparations have revealed similar efficacy in reducing bacterial burden, with a slightly higher rate of skin irritation when using povidone-iodine.3,10 Combinations of different antiseptic solutions have shown to be effective in certain circumstances at decreasing bacterial load.11 Impermeable adherent drapes impregnated with iodophors have been used following aseptic skin preparation to minimize contamination from neighboring tissue.6
Figure 1.
4% chlorhexidine surgical scrub.
Figure 2.
Application device with 0.7% iodine and 74% isopropyl alcohol.
SURGICAL DRAPES
The concept of local barrier protection has led to development of a vast array of different techniques with the goal of preventing microbial spread from the patient into the surgical field. Ideal draping material should be impermeable to fluid, resistant to mechanical damage, and should remain in place during manipulation.5 The initial boundary should surround the planned incision site. An additional superficial layer extends to cover the body beyond the preparation site to minimize risk of contamination and allow surgical personnel to maneuver while abiding by aseptic technique. Historically this has been achieved with the use of woven and non-woven cloth drapes that can either be recycled or discarded after initial use.12
Draping poses a unique challenge in exotic species due to small body size and need for constant visual monitoring. Clear plastic drapes are commonly used in exotic surgery, allowing more precise patient monitoring and better thermoregulation (Figure 3). Clear adherent drapes are also available; however, recent evidence suggests these drapes may increase the risk of surgical site infections.13 Adhesive drapes can be challenging to maneuver due to the adhesive backing and can damage the delicate integument of smaller patients when removed. Therefore, adhesive drapes should be used with caution in exotic species. As mentioned previously, some adherent drapes are impregnated with antiseptics to further prevent bacterial contamination, though evidence that these drapes decrease the incidence of surgical site infection is lacking.14
Figure 3.
Application of a clear plastic drape with fenestration for laparotomy on a green iguana (Iguana iguana) (A). Example of pre-fenestrated drape with adhesive border (B).
The recommendation of the authors is to apply a primary layer of barrier drapes, covering the inner borders of the prepared surgical field, secured to the patient with towel clamps. A transparent drape with a fenestration made to match size of the proposed incision site can then be placed to cover the remainder of operative table. For smaller patients, a single fenestrated adherent plastic drape may be used to optimize patient monitoring and prevent iatrogenic trauma from towel clamps.
SURGICAL INSTRUMENTATION
Standard surgical instruments may be used for the majority exotic animal surgery, especially on larger patients. In smaller patients, however, small instruments are required for better surgical precision. The surgeon may consider using ophthalmic or microsurgery instruments. Microsurgery instruments are generally preferred over ophthalmic instruments. Microsurgery instruments are longer, reaching the surgical site within the patient while allowing the surgeon to rest the hands outside the body for stabilization. The microsurgery instruments should be comfortable in the surgeon’s hand to limit fatigue and allow fine control of instrument manipulation.1
Microsurgery instruments have a number of other attributes that make them an ideal choice for exotic animal surgery. They are counterbalanced for better hand control and more precise movement. The locking mechanisms, or lack thereof, tend to allow more precise control preventing sudden movements and forces that can bend and break smaller needles and suture. Microsurgery instruments are available with rounded handles allowing the instrument to be rolled between the thumb and index finger while holding the instrument with the preferred pencil grip. Microsurgery instruments most often have a satin finish for reduced glare.15 Many of the attributes that make microsurgery instruments excellent for small exotic animal surgery also require experience in using them. Therefore the surgeon should practice using them before attempting surgery in a live animal.
Basic surgery packs have been proposed for use in small mammals and exotics including the following instruments: Jones towel clamps (6), Adson dressing forceps (1), Adson tissue forceps (1), Bard-Parker scalpel blade #3 (1), blunt, curved tenotomy scissors (1), curved LeGrange scissors (1), Olsen-Hegar needle holder (1), Hartmann straight mosquito forceps (2), and Hartmann curved mosquito forceps (2). Jacobson or Packer mosquito forceps may replace the Hartmann forceps given their finer tips, which are advantageous for the extremely small vessels encountered with exotic animals.16,17
Small scalpel blades are preferred for small, exotic species, such as a #3 scalpel handle with a number 11 or 15 blade. Another option is a Beaver blade. These blades are smaller than 11 and 15 blades and come in a variety of configurations (Figure 4).
Figure 4.
Beaver blades have an advantage of being much smaller than standard 11 or 15 blades and come in a variety of configurations. Three different blade styles are shown above with a handle.
Small gauze pads may be included in the pack as well as cotton-tipped applicators. Cotton-tipped applicators may be used for gentle tissue manipulation as well as hemostasis. For tissue manipulation in very small animals, microbrushes may be adapted from dentistry use for very fine and delicate work (Figure 5).
Figure 5.
Microbrushes may be adapted form dentistry for gentle tissue manipulation. Two microbrushes are shown here next to a standard cotton-tipped applicator.
Retractors are an essential piece of equipment used during surgery. A surgical assistant may be of great value; however, an assistant’s hands may impede the surgeon’s visualization in very small surgical fields.16 Tissue retraction is necessary, but tension must be controlled to avoid morbidity associated with the retractor. Ring retractors are often the recommended retractor for exotic animal surgery. The most common is the Lone Star Retractor System (Jorgenson Laboratories). This system uses a plastic ring or frame that surrounds the operative field and has notches to accommodate individual stays. A hook is applied to retract the tissue as needed and the elastic band is then pulled to appropriate tension and inserted into one of the notches in the ring. Stays are added and adjusted as needed to achieve visualization (Figure 6).18
Figure 6.
Ring retractor used in a macaw undergoing ventral midline coeliotomy. The ring retractor uses hooks on adjustable stays attaching to the ring frame.
Other options for retraction include small self-retaining retractors. Commonly recommended retractors are the Alm and Heiss retractors. These are small adjustable retractors that tension can be adjusted to the size of the wound. The Alm retractor uses a thumbscrew adjustment, while the Heiss retractor uses a ratchet mechanism (Figure 7). For extremely small patients or wounds, one may consider retractors such as the Agricola or the Trigger Finger Self-Retaining Retractor (buxtonbio.com). These retractors are lighter and less than 4 mm in total length and have a thumbscrew to adjust tension. Eyelid retractors have been proposed for use in exotic animals surgery; however, these are not recommended as they are maintained in an open position by a spring mechanism, and tension is not adjustable.18
Figure 7.
The Heiss retractor is a small self-retaining retractor that uses a ratchet mechanism to maintain retraction. This can be adjusted to appropriate tension for the tissue it is retracting.
MAGNIFICATION
Magnification is strongly recommended for proper visualization of the surgical field in small mammals and exotic species. Various forms of magnification exist, including surgical loupes or operating microscopes. Surgical loupes offer magnification ranging from 2.5 – 5 X from many manufacturers, while operating microscopes offer magnification from 5 – 40 X. For most patients, surgical loupes will be sufficient; however, an operating microscope may be worthwhile for very small patients or delicate procedures. Magnification decreases the field of vision, depth perception, and fine motor movements, and thus training is required to become proficient in the use of magnification during surgery.
Loupes may range from inexpensive hobby loupes to more costly high-resolution loupes with or without an attached focal light source. Newer loupes have either Galilean or prismatic (Keplerian) lenses mounted on a pair of glasses (Figure 8). Surgical loupes differ in the degree of magnification and size of the telescope. Prismatic telescopes in general have higher magnification but more substantial telescope size and weight. Galilean telescopes, though lower in magnification, are sufficient for most applications while providing decreased weight and user fatigue. The telescope may be mounted on or within the lens (through-the-lens technology) or front-mounted on the bridge of the glasses. Surgical loupes are custom made for the user to fit interpupillary distance and focal working length. They should fit comfortably such that the surgeon does not have to bend over or strain to work. In general, the field width decreases with an increase in magnification. This does not hold true between telescope types, however, as a prismatic loupe would have a larger field width than a Galilean telescope of the same magnification.
Figure 8.
2.5x through-the-lens surgical loupes with an attached headlight. Surgical loops provide from 2.5X to 6X magnification, and the headlight provides bright focused unobstructed lighting on the surgical field.
Surgical microscopy is an attractive option for magnification in surgery of small patients. Surgical microscopes have become more economically feasible and have been described in use outside of the teaching hospital setting.19 Studies in human medicine including neurosurgery and dentistry have shown that operating microscopes provide better outcomes for patients as they offer better visualization and comfort for the surgeon (Figure 9).20,21
Figure 9.
Surgical microscope. Surgical microscopy provides greater magnification than surgical loops. Better patient outcome has been demonstrated with microscopy while providing better visualization and surgeon comfort.
FOCAL LIGHT SOURCE
As visualization of the surgical field is improved through tissue retraction and magnification, appropriate lighting should also be available. Often overhead lighting cannot be aimed or focused into the patient. A focal light source allows the surgeon to direct the light into the patient and the surgical field reducing shadows, glare, and artifact. This is accomplished best when the light source is attached to the magnification used as in surgical loupes (Figure 8). With the advent of light-emitting diode (LED) technology, newer light sources offer improved brightness and decreased heat and weight over earlier light sources.
HEMOSTASIS
Electrosurgery
Electrosurgery has been refined over the past 100 years, yet the fundamental principles remain the same - heat from a developed electrical circuit causes collagen denaturation and tissue shrinkage.5 Electrosurgical technology transfers energy from electrons in the instrument to electrolyte-rich living tissues. Electrosurgery should not be confused with electrocautery, which coagulates or cuts local tissues by using heat from an electrical current.
Electrosurgery can be applied using either a monopolar or bipolar instrument. Monopolar electrosurgery requires the use of an inactive electrode or “grounding pad” that creates an electrical circuit. Radiofrequency generated within the circuit causes cells to dehydrate and vessels to coagulate. Due to the larger circuit, higher settings are required in comparison to bipolar cautery. The frequency and amplitude of the waveforms can generate different outcomes. Continuous, low-amplitude current results in a tissue cutting, whereas an interrupted, high-amplitude current results in coagulation of tissues. The field must be dry and the electrode kept free from debris to function properly. The instrument may be applied directly to the tissue, or indirectly by touching the electrode tip to an instrument holding tissue. Indirect application results in greater precision and less collateral tissue damage.22,5
Bipolar cautery consists of a single instrument that has dispersive and active electrodes at the tip, which allows for more isolated energy transfer (Figure 10). The circuit is contained within the tips of the instrument, negating the need of a grounding pad. Additional benefits include efficacy in the face of moisture and less peripheral tissue damage due to the confines of the current application. Thermal dissipation into surrounding tissues can lead to collateral damage to neurovascular structures warranting caution in certain areas. However, direct comparisons between skin incisions made by cold scalpel and electrosurgery have found no difference in post-surgical infection or cosmesis in human patients.23
Figure 10.
Bipolar electrosurgical forceps.
There are a wide variety of tips and instrument types for use in exotic species but the most commonly utilized include fine-tip bipolar cautery units such as the Harrison forceps with one bent tip. This is primarily used as a method of coagulation in mammalian species but can also be used for skin incisions in birds.1
Laser
The term laser is an acronym standing for Light Amplification by Stimulated Emission of Radiation. All lasers work by delivering energy in the form of light. A laser beam is created from a substance called an active medium, which when stimulated by light or electricity produces photons of a specific wavelength. The light produced by a laser is both monochromatic (of one wavelength) and coherent (all waves are in phase with one another in both time and space). Medical lasers emit light anywhere from ultraviolet light, to visible light, to the infrared portions of the electromagnetic spectrum. They can deliver a large amount of energy to a focal source without actually touching the tissue. The tissue effect of lasers results from a complex interaction of the light emitted from the laser with the tissue, resulting in photochemical, photothermal, and photomechanical effects. Most surgical lasers have a photothermal effect on tissues, resulting in coagulation, cutting, cauterization, vaporization, or welding of tissues.
There are many types of surgical lasers, typically named by the type of lasing material employed. These include gas lasers (CO2, helium, or argon-ion), excimer lasers (e.g. argon-fluoride or xenon-monochloride), solid-state lasers (neodymium:yttrium-aluminum garnet “Nd:YAG” or holmium:YAG “Ho:YAG”) lasers, or semiconductor lasers (diode lasers), each producing a different wavelength of light. CO2 laser technology is the most commonly utilized laser in small animal surgery and has gained popularity in exotic animal surgery (Figure 11). It produces its effect through instantaneous heating of intracellular water resulting in cellular vaporization and ablation that can seal vessels measuring less than 0.6 mm in diameter.17 Other reported benefits include simultaneous sealing of nerve endings and lymphatics resulting in less discomfort and swelling post-operatively.22
Figure 11.
CO2 laser unit (A) and pen hand unit (B).
More recently, the use of a diode laser has been evaluated for use in exotic species and offers unique benefits including the ability to function in fluid environments, improved hemostasis, and capability of sealing vessels as large as 2 mm.24 The diode lasers are in the near infrared spectrum, typically with a wavelength of 810 nm. They can be used in direct contact with tissue (contact mode) or at a distance from tissue (non-contact). Penetration into tissue can be up to 4 mm in non-contact mode and 0.3 mm in contact mode with greater efficacy in muscle and non-keratinized tissues.24 Another intriguing feature of the diode laser is that it has the ability to be used in both endoscopic and open procedures. The contact mode is particularly useful to aid in hemostasis during endoscopic procedures, a technique gaining popularity in exotic animal surgery. It’s use has been explored in reptiles, birds, small mammals, amphibians an even fish due its the efficacy in aqueous environments. Diode lasers may lead to delayed primary intention healing.
The potential for retinal damage requires all surgical personnel to wear appropriate protective eye wear when using any medical lasers in surgery.
Electrothermal Bipolar Vessel Sealing Devices
Bipolar vessel sealing devices provide hemostasis by denaturing collagen and elastin of the vessel wall and surrounding connective tissue, without reliance on thrombosis. These units have been used extensively in human and veterinary medicine, offering a rapid and safe alternative to suture or clip ligation of vessels. Other applications include the sealing and removal of hollow organ structures <9mm in diameter in general urologic, thoracic, plastic and reconstructive procedures.25
The most commonly used bipolar vessel sealing device in veterinary surgery is the LigaSure™ (ValleyLab, Boulder, CO – a division of Covidien), which includes a bipolar electrosurgery generator and a dedicated bipolar electrosurgery instrument (Figure 12).26 The LigaSure™ is able seal vessels up to 7 mm in diameter. The more recently developed SurgRx™ EnSeal™ device (Ethicon Endo-Surgery, Inc, Cincinnati, OH – a division of Johnson & Johnson) is also able to seal vessels up to 7 mm, but also limits thermal spread to 1mm, thereby preventing damage to neighboring tissues.4, 27
Figure 12.
Impact (A) and small jaw (B) instruments.
Bipolar vessel sealing devices are often used in a minimally invasive manner during endoscopic procedures of people and animals; however, they can also be used in open abdominal or thoracic surgery. The size of instrumentation may limit its utility in many small exotic species. Use in procedures such as castration and hysterectomy in a variety of species, including lizards and chinchillas, documents the versatility and potential application in exotic species.28
Ultrasonic Energy – The Harmonic® System
The Harmonic® (Ethicon Endo-Surgery) device system delivers ultrasonic waves to tissue, and can be used in both open and laparoscopic procedures. High-frequency vibrations cause an oscillating saw effect as well as vibration-induced heat and coagulation. The unit consists of a current generator, a handpiece that houses the ultrasonic transducer, an instrument with an end effector used to cut the tissue, a foot pedal, and a hand-switching adaptor. The Harmonic® device is able to seal vessels up to 5 mm in diameter. The primary advantage of the Harmonic® system is that it is able to cut and coagulate tissue by using lower temperatures than those used by electrosurgery, resulting in potentially less lateral thermal spread and tissue damage.22,26
Stapling devices
Staplers have been used widely for 50 years and offer a rapid sealing of vessels and organ parenchyma. Their use is limited by the size of the device and finite sizes of clip cartridges. Guidelines for the use of stapling equipment have been previously outlined. 29
Do not staple tissues that are inflamed, edematous, or lack a vascular supply.
Every staple must penetrate all tissue layers.
Staple size should be accurate; tissues should not be too thick to be penetrated or too thin to support the staple.
Tissues should be inspected thoroughly before stapler application to ensure proper alignment and no capturing of inadvertent tissues.
Stapling devices should be removed carefully to avoid disrupting the staples.
Tissues should be grasped gently before removal of the stapler to check for hemorrhage, leakage, or loose staples. The thoracoabdominal (TA) stapler comes in 30 mm, 45 mm, 60 mm or 90 mm cartridge sizes that apply staggered rows of metal staples into tissues (Figure 13). Several studies have documented the effectiveness of TA staplers by decreasing operative time, hemorrhage and necrosis of tissue. There is concern for incomplete ligation of vascular tissues that may require reinforcement by oversewing the exposed pedicle to prevent leakage. The use of surgical staples has been described in a multitude of organ systems.
Figure 13.
TA 30 (A), TA 55 (B) and TA 90 (C) staplers with cartridges.
The GIA or, gastrointestinal and intestinal linear anastomosis stapler can provide a linear communication between neighboring tissues by using a cutting function (Figure 14). Examples of described uses include gastropexy, lobectomies and end-to-end anastomosis.
Figure 14.
Linear stapler with cartridge.
The LDS or ligate-divide stapler has the ability to severe tissue while providing 2 titanium staples, one on either side of the cut ends, and is useful in procedures that require the transection of multiple vessels.
The size of all of the surgical stapling devices makes their use potentially challenging in smaller exotic species.
Hemostatic clips
In an effort to expedite the ligation of smaller vessels, the use of vascular clips can be used. This advantage, however, is coupled with a reduction in strength and security when compared to traditional suture or electrosurgery techniques. They can be comprised of metal in the traditional chevron design (Figure 15) (Hemoclip®; Weck, Triangle Park, NC USA) or polymer composition (Absolak extra clips; Ethicon Endo-Surgery, Cincinnati, OH) which lock in place after deployment and can withstand a greater weight (> 950 g) and intravascular pressure (> 300mm Hg) when compared to single and double Hemoclip® placement.30 Polymer designs also have the advantage of preventing interference during higher diagnostic imaging such as CT or MRI and are absorbable over time. 30 To optimize security, the vessels should be carefully dissected from neighboring tissue and the size should not be greater than two-thirds and no less than one-third than the size of the clip used.31 Arteries and veins should be separated and clipped individually before application, allowing several millimeters between placement of the clip and the cut end of the vessel. 5 The application of vascular clips in endoscopic surgery has been well documented and offers an appealing alternative to other traditional techniques.
Figure 15.
Metal chevron design hemoclip with applicator.
Hemostatic agents
A number of techniques can be used to ameliorate hemorrhage before, during and after surgery. Decreasing blood flow to the region of interest can be achieved through the applications of direct pressure or tamponade, decreasing perfusion via hypothermia or hypotension, or the use of tourniquets to control distant blood flow. Similarly, the use of systemic medications such as serine protease inhibitors, lysine analogues, desmopressin, and ethamsylate can aid in preventing blood loss by decreasing fibrinolysis, providing synthetic coagulation factors and increasing platelet adhesiveness and aggregation. This section, however, will concentrate on the use of topical hemostatic agents in the clinical setting.
The advent of hemostatic agents has gained popularity in the veterinary community in the effort to minimize blood loss in patients with small volumes. It is important to note that the majority of agents used rely heavily on the patients own hemostatic potential, and factors deterring this process will make them less efficacious. Products can be classified as mechanical agents, active agents or hemostatic sealants. The appropriate selection of agent should be based on a number of factors but availability and cost are often times the limiting factor. None of these agents are without the potential for complication and may include local swelling, exothermic reactions, immunogenic reactions, foreign body reactions, and delayed healing.
Mechanical agents
Mechanical agents are absorbable materials that promote blood stasis and absorption at the site of hemorrhage and provide a matrix for clot formation and stabilization. They are widely available and can be combined with procoagulants to optimize hemostasis as is the case of thrombin added to porcine gelatin. Gelatin products are commonly utilized (Gelfoam®; Pharmacia and Upjohn Co, Kalamazoo MI USA) and are available in a foam-based medium (Figure 16) in addition to a powder based form. The main disadvantage is the occurrence of swelling, foreign body reactions, and inhibition of healing. They are not involved in active platelet aggregation and are absorbed over several weeks by granulomatous inflammation.32
Figure 16.
Absorbable compressed sponge.
Bovine collagen is another agent that not only acts as a mechanical activator but also enhances platelet aggregation resulting in a superior hemostatic response.32,33 It is available in sheets or a powder form and has similar complications risks when compared to gelatin but the cost-prohibitive nature of the product makes it less commonly used in veterinary medicine.
Another type of topical hemostatic agent includes oxidized regenerated cellulose (Surgicel®; Ethicon, Inc., Johnson and Johnson, Somerville, NJ USA). Not only can this cause clot formation independent of the coagulation pathway it also has antibacterial properties. The mechanism of action is not well understood but it is generally considered to be a weaker hemostatic agent due to the inactivation of thrombin.33
Bone wax is a variant of beeswax that can be used to decrease hemorrhage on osseous surfaces. It has been associated with a higher risk of infection and delayed bone healing according to certain reports. A newer formulation of alkylene oxide copolymer, called Ostene, is available and does not possess the same risk of infection and delayed healing and may also adhere better to moist surfaces. 34
Active Agents
Active agents can be used solely or in combination with other mechanical agents as described. Thrombin is the most active common agent, which works by converting fibrinogen to fibrin. Thrombin is available in bovine, human and recombinant forms. Its use in humans has led to coagulopathic reactions following repeated administration and so frequent use is not recommended.
Alginate is a seaweed-derived protein that is most often used as a wound dressing but it also has hemostatic properties relating to the release of calcium enhancing activation of the coagulation cascade and platelet activation.35 It should not be used in open body cavities and must be removed before closure to prevent foreign body reactions.
Hemostatic Sealants
Hemostatic sealants act as glue on tissues creating a barrier for additional hemorrhage and therefore do not rely on normal hemostatic factors. Fibrin sealants provide both thrombin and fibrinogen to the site and can be derived from pooled plasma sources or can be obtained from the patient’s own plasma by mixing with collagen and thrombin.5,36 They are not widely available in veterinary medicine. Synthetic sealants such as Coseal (Baxter) and Duraseal (Covidien) consist of polyethylene polymers that form hydrogels to seal tissue. The major concern is swelling at the application site. More and more products are becoming available for use, but the lack of prospective analysis, particularly in veterinary medicine, warrants caution for the surgeon when debating their use in the clinical setting.
Suture Materials
As with other species, wounds and surgical incisions of exotic species will often be closed with suture. The ideal suture material should have high tensile strength to resist fragmentation and provide sufficient time to allow tissue healing, have good knot security, resist infection, and cause minimal inflammatory reaction. Although suture material used is the same as other species often ranging from 3-0 to 8-0, the technique may vary.17 Rabbits and rodents tend to traumatize incisions and intradermal patterns with or without the additional use of tissue adhesive, or skin staples may be used.37 In reptiles, the skin is the holding layer and it is recommended to close with an everting pattern (Figure 17). An Aberdeen knot may be used instead of a square knot to minimize suture material while achieving a higher breaking.38 A significant variety of suture materials are available and selection should be based upon tissue characteristics and expected healing times (Table 1).5,1
Figure 17.
Everting horizontal mattress suture pattern for closure of a prefemoral skin incision in red-eared slider turtle (Trachemys scripta elegans).
Table 1.
Suture characteristics for commonly used materials in veterinary medicine.
| Suture trade name | Composition | Configuration | Reduction in tensile Strength |
Complete absorption |
Relative knot security |
Tissue reactivity |
|---|---|---|---|---|---|---|
| Surgical gut (chromic) | Intestinal serosa/ submucosa |
twisted monofilament |
33% at 7 days | 60 days | − | +++ |
| Vicryl® | Polyglactin 910 | braided | 25% at 14 days | 56-70 days | ++ | + |
| Vicryl Rapide™ | Polyglactin 910 | braided | 50% at 5 days | 42 days | ++ | + |
| Dexon™ | Polyglycolic acid | 35% at 14 days | 60-90 days | ++ | + | |
| Polysorb™ | Glycolide/lactide | 20% at 14 days 70% at 21 days |
60 days | +++ | − | |
| Perma-Hand® | Silk | monofilament | 30% at 14 days 50% at 1 year |
>2 years | ||
| Caprosyn™ | Polyglytone 6211 | monofilament | 40-50% at 5 days | 56 days | +++ | + |
| Monocryl® | Poliglecaprone 25 | monofilament | 40-50% at 7days | 90-120 days | ++ | + |
| Biosyn™ | Glycomer 631 | monofilament | 25% at 14 days 60% at 21 days |
90-110 days | ++ | + |
| PDS® II | Polydioxanone | monofilament | 14% at 14 days 31% at 42 days |
180 days | ++ | + |
| Maxon™ | Polyglyconate | monofilament | 30% at 14 days 45% at 21 days |
180 days | ++ | + |
| Prolene® | Polypropylene | monofilament | Non-absorbable | +++ | − | |
| Ethilon® | Polyamide (Nylon) | monofilament | 30% at 2 years | Non-absorbable | + | − |
Relative knot security: (−) = poor (<60%); (+) = fair (60%-70%); (++) = good (70% to 85%); (+++) = excellent (>85%). Tissue reactivity: (−) = minimal to none; (+) = mild; (++) = moderate; (+++) = severe.
Data from Bennett RA. Preparation and equipment useful for surgery in small exotic pets. Vet Clin North Am Exot Anim Pract. 2000;
3: 563-585 and Tobias KM, Johnston SA. Veterinary Surgery: Small Animal (ed 1). St. Louis, MO: Elsevier Saunders, 2012.
In closing wounds in reptiles, cyanoacrylate glue should be considered as an alternative to sutures. It should be noted that while the overall process of wound healing in reptiles is similar to that in mammals, it is slower, and certain aspects of wound healing are unique that should be taken into consideration. In particular, it is thought that the responding inflammatory cells and proteolytic enzymes involved in wound healing in reptiles may be different than that of mammals, leading to altered breakdown of suture materials. A recent study evaluated the histologic reaction to commonly used suture materials, including cyanoacrylate tissue adhesive, in the musculature and skin of ball pythons. The cyanoacrylate glue did not cause a significant inflammatory response compared to the negative control; however all suture materials let to a significant inflammatory response. None of the sutures were absorbed by then end of the 90-day study period, and several sutures appeared to be in the process of extrusion. The authors concluded that cyanoacrylate glue should be considered to close small superficial wounds in snakes with minimal inflammatory response associated. Due to slower absorption, the authors suggest that shorter acting suture materials may be more appropriate for use in reptiles; however, it is important to note that healing time and complications of surgery were not analyzed in this study.39
SUMMARY
The general principles of surgical technique, patient preparation, and instrumentation are similar between exotic species and mammals. However, due to small patient size, small mammals, reptiles and avian species are prone to rapid hypothermia, life threatening blood loss, and challenges in adequate tissue visualization. Further, unique wound healing processes necessitate the use of different suture materials and patterns to optimize wound healing. Recent availability of high power magnification with focal lighting sources, novel methods of hemostasis, and small, precise surgical instruments allow for safe completion of advanced surgical procedures in exotic species. These advances have been highlighted in this article, focusing on recent literature that assesses performance in exotic animal species. Each exotic patient poses a unique challenge to the veterinary surgeon, and a tailored approach to each patient is required.
KEY POINTS.
Surgery on exotic species requires a tailored approach with challenges relating to hemostasis, thermoregulation and small body size.
Preparation for surgery should focus on decreasing bacterial burden while minimizing time under anesthesia and permitting adequate patient monitoring.
Unique instruments and appropriate magnification and lighting should be considered to optimize visualization, tissue handling, and dissection.
Advances in electrosurgery and laser technology have improved hemostasis during surgery and offers diversity to the surgeon.
SYNOPSIS.
The diversity implicit in exotic animal surgery requires a tailored approach to optimize successful outcomes. Outlined is information on patient preparation, instrumentation, hemostatic techniques and magnification as it pertains to the exotic animal. Application of topical antiseptic solutions and judicious removal of pelage and feathers will decrease bacterial load during patient preparation. The use of specific barrier protection ensures proper aseptic technique and enables optimal patient monitoring. Magnification combined with a focal light source enhances visual acuity allowing for better use of delicate instrumentation and identification of anatomical structures. The advent of electrosurgery, laser technology and hemostatic agents provides improved hemostasis and tissue healing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors have nothing to disclose.
REFERENCES
- 1.Bennett RA. Preparation and equipment useful for surgery in small exotic pets. Vet Clin North Am Exot Anim Pract. 2000;3:563–585. doi: 10.1016/s1094-9194(17)30063-4. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JW, Fischer JE, Boyajian M, Palmquiest J, Morris MJ. The influence of hair-removal methods on wound infections. Arch Surg. 1983;118:347–352. doi: 10.1001/archsurg.1983.01390030079013. [DOI] [PubMed] [Google Scholar]
- 3.Bhavan KP, Warren DK. Surgical preparation solutions and preoperative skin disinfection. J Hand Surg Am. 2009;34:940–941. doi: 10.1016/j.jhsa.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Ayliffe G. The effect of antibacterial agents on the flora of the skin. J Hosp Infect. 1980;1:111–124. doi: 10.1016/0195-6701(80)90043-2. [DOI] [PubMed] [Google Scholar]
- 5.Tobias KM, Johnston SA. Veterinary Surgery: Small Animal. 1 Elsevier Saunders; St. Louis, MO: 2012. [Google Scholar]
- 6.DeBaun B. Evaluation of the antimicrobial properties of an alcohol-free 2% chlorhexidine gluconate solution. AORN J. 2008;87:925–933. doi: 10.1016/j.aorn.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Gibson KL, Donald AW, Hariharan H, McCarville C. Comparison of two pre-surgical skin preparation techniques. Can J Vet Res. 1997;61:154–156. [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson C, Osmon DR, Hanssen A, Trousdale RT, Pagnano MW, Pyrek J, Berbari E, Naessens J. Prevention of wound contamination using DuraPrep solution plus Ioban 2 drapes. Clin Orthop Relat Res. 2005;439:32–37. doi: 10.1097/01.blo.0000182245.29830.bc. [DOI] [PubMed] [Google Scholar]
- 9.Moen MD, Noone MB, Kirson I. Povidone-iodine spray technique versus traditional scrub-paint technique for preoperative abdominal wall preparation. Am J Obstet Gynecol. 2002;187:1434–1436. doi: 10.1067/mob.2002.129922. [DOI] [PubMed] [Google Scholar]
- 10.Osuna DJ, DeYoung DJ, Walker RL. Comparison of three skin preparation techniques in the dog. Part 1: Experimental trial. Vet Surg. 1990;19:14–19. doi: 10.1111/j.1532-950x.1990.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 11.Guzel A, Ozekinci T, Ozkan U, Celik Y, Ceviz A, Belen D. Evaluation of the skin flora after chlorhexidine and povidone-iodine preparation in neurosurgical practice. Surg Neurol. 2009;72:207–210. doi: 10.1016/j.surneu.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Garibaldi RA, Maglio S, Lerer T, Becker D, Lyons R. Comparison of nonwoven and woven gown and drape fabric to prevent intraoperative wound contamination and postoperative infection. Am J Surg. 1986;152:505–509. doi: 10.1016/0002-9610(86)90216-3. [DOI] [PubMed] [Google Scholar]
- 13.Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2015;4:CD006353. doi: 10.1002/14651858.CD006353.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewan PA, Van Rij AM, Robinson RG, Skeggs GB, Fergus M. The use of an iodophor-impregnated plastic incise drape in abdominal surgery—a controlled clinical trial. Aust N Z J Surg. 1987;57:859–863. doi: 10.1111/j.1445-2197.1987.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 15.Boothe HW. Instrumentation. In: Tobias KM, Johnston SA, editors. Veterinary Surgery: Small Animal. 1 Elsevier Saunders; St. Louis, MO: 2012. pp. 152–163. [Google Scholar]
- 16.Capello V. Common surgical procedures in pet rodents. J Exotic Pet Med. 2011;20:294–307. [Google Scholar]
- 17.Lennox AM. Equipment for exotic mammal and reptile diagnostics and surgery. J Exotic Pet Med. 2006;2:98–105. doi: 10.1053/j.jepm.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett RA, Mullen HS. Soft Tissue Surgery. In: Quesenberry KE, Carpenter JW, editors. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery. 2nd Elsevier Saunders; St. Louis, MO: 2004. pp. 316–328. [Google Scholar]
- 19.Ford S. Surgical microscopy and fluoroscopy in avian practice. J Exotic Pet Med. 2006;15:91–97. [Google Scholar]
- 20.Kumar SS, Mourkus H, Farrar G, Yellu S, Bommireddy R. Magnifying loupes versus microscope for microdisectomy and microdecompression. J Spinal Disord Tech. 2012;25:235–239. doi: 10.1097/BSD.0b013e31825010ae. [DOI] [PubMed] [Google Scholar]
- 21.Mamoun J. Use of high-magnification loupes or surgical operating microscope when performing dental extractions. N Y State Dent J. 2013;79:28–33. [PubMed] [Google Scholar]
- 22.Diamantis T, Kontos M, Arvelakis A, Syroukis S, Koronarchis D, Papalois A, Agapitos E, Bastounis E, Lazaris AC. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, ultracision, and ligasure. Surg Today. 2006;36:908–913. doi: 10.1007/s00595-006-3254-1. [DOI] [PubMed] [Google Scholar]
- 23.Aird LN, Brown CJ. Systematic review and meta-analysis of electrocautery versus scalpel for surgical skin incisions. Am J Surg. 2012;204:216–221. doi: 10.1016/j.amjsurg.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Divers SJ. Diode laser surgery: Principles and application in exotic animals. Semin Avian Exot Pet Med. 2002;11:208–220. [Google Scholar]
- 25.Mison MB, Steficek B, Lavagnino M, Teunissen BD, Hauptman JG, Walshaw R. Comparison of the effects of the CO2 surgical laser and conventional surgical techniques on healing and wound tensile strength of skin flaps in the dog. Vet Surg. 2003;32:153–160. doi: 10.1053/jvet.2003.50003. [DOI] [PubMed] [Google Scholar]
- 26.Barrera JS, Monnet E. Effectiveness of a bipolar vessel sealant device for sealing uterine horns and bodies from dogs. Am J Vet Res. 2007;73 doi: 10.2460/ajvr.73.2.302. 2, 302-305. [DOI] [PubMed] [Google Scholar]
- 27.Landman J, Kerbel K, Rehman J, Andreoni C, Humphrey PA, Collyer W, Olweny E, Sundaram C, Clayman RV. Evaluation of a vessel sealing system, bipolar electrosurgery, harmonic scalpel, titanium clips, endoscopic gastrointestinal anastomosis vascular staples and sutures for arterial and venous ligation in a porcine model. J Urol. 2003;169:697–700. doi: 10.1097/01.ju.0000045160.87700.32. [DOI] [PubMed] [Google Scholar]
- 28.Hruby GW, Marruffo FC, Durak E, Collins SM, Pierorazio P, Humphrey PA, Mansukhani MM, Landman J. Evaluation of surgical energy devices for vessel sealing and peripheral energy spread in a porcine model. J Urol. 2007;178:2689–2693. doi: 10.1016/j.juro.2007.07.121. [DOI] [PubMed] [Google Scholar]
- 29.Lipscomb V. Surgical staplers: toy or tool? Practice. 2012;34:472–479. [Google Scholar]
- 30.Hsu TC. Comparison of holding power of metal and absorbable hemostatic clips. Am J Surg. 2006;191:68–71. doi: 10.1016/j.amjsurg.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Toombs JP. Basic operative techniques. In: Clark KM, Slatter D, editors. Textbook of small animal surgery. 3 Vol. 1. Elsevier Saunders; Philadelphia, PA: 2003. p. 199. [Google Scholar]
- 32.Solheim E, Anfinsen OG, Holmsen H, Sudmann E. Effect of local hemostatics on platelet aggregation. Eur Surg Res. 1991;23:45–50. doi: 10.1159/000129135. [DOI] [PubMed] [Google Scholar]
- 33.Wagner WR, Pachence JM, Ristich J, Johnson PC. Comparative in vitro analysis of topical hemostatic agents. J Surg Res. 1996;66:100–108. doi: 10.1006/jsre.1996.0379. [DOI] [PubMed] [Google Scholar]
- 34.Magyar CE, Aghaloo TL, Atti E, Tetradis S. Ostene, a new alkylene oxide copolymer bone hemostatic material, does not inhibit bone healing. Neurosurgery. 2008;63:373–378. doi: 10.1227/01.NEU.0000316859.03788.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal HC, Hunt BJ, Gilding K. The effects of alginate and non-alginate wound dressings on blood coagulation and platelet activation. J Biomater Appl. 1998;12:249–257. doi: 10.1177/088532829801200305. [DOI] [PubMed] [Google Scholar]
- 36.Crow SS, Sullivan VV, Ayosola AE, Key NS, Harker-Murray P, Foker JE, Steiner ME. Postoperative coagulopathy in a pediatric patient after exposure to bovine topical thrombin. Ann Thoracic Surg. 2007;83:1547–1549. doi: 10.1016/j.athoracsur.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins JR. Soft Tissue Surgery. In: Quesenberry KE, Carpenter JW, editors. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery. 3rd Elsevier Saunders; St. Louis, MO: 2012. pp. 269–278. [Google Scholar]
- 38.Schaaf O, Glyde M, Day RE. In vitro comparison of secure Aberdeen and square knots with plasma- and fat-coated polydioxanone. Vet Surg. 2010;39:553–560. doi: 10.1111/j.1532-950X.2009.00640.x. [DOI] [PubMed] [Google Scholar]
- 39.McFadden MS, Bennett RA, Kinsel MJ, et al. Evaluation of the histologic reactions to commonly used suture materials in the skin and musculature of ball pythons (Python regius) Am J Vet Res. 2011;72:1397–1406. doi: 10.2460/ajvr.72.10.1397. [DOI] [PubMed] [Google Scholar]