Abstract
Background:
Ticks are important vectors and reservoirs of Crimean Congo Hemorrhagic Fever (CCHF) virus. Human beings may be infected whenever the normal life cycle of the infected ticks on non-human vertebrate hosts is interrupted by the undesirable presence of humans in the cycle. A total of 26 species of Argasid and Ixodid ticks have been recorded in Iran; including nine Hyalomma, two Rhipicephalus, two Dermacentor, five Haemaphysalis, two Boophilus, one Ixodes and two Argas as well as three Ornithodoros species as blood sucking ectoparasites of livestock and poultries. The present paper reviews tick vectors of CCHF virus in Iran, focusing on the role of ticks in different provinces of Iran using reverse transcription polymerase chain reaction (RT-PCR) assay.
Methods:
During ten years study, 1054 tick specimens; including two species of Argasidae and 17 species of Ixodidae were examined for their infection to CCHF virus genome. The output of all studies as well as related publications were discussed in the current paper.
Results:
The results show that Rhipicephalus sanguineus, Hyalomma marginatum, H. anatolicum, H. asiaticum and H. dromedarii were known as the most frequent species which were positive for CCHF virus.
Conclusion:
The status of ticks which were positive for CCHF virus revealed that unlike the most common idea that Hyalomma species are the most important vectors of CCHF virus, other ticks including Rhipicephalus, Haemaphysalis and Dermacentor can be reservoir of this virus; thus, considering geographical distribution, type of host and environmental conditions, different tick control measurements should be carried out in areas with high incidence of CCHF disease.
Keywords: Vector tick, Domestic animals, CCHF, Iran, RT-PCR
A historical perspective of CCHF disease in Iran
The name CCHF derives from two regions in Asia and Africa where an often fatal human hemorrhagic fever disease occurred for the first time in the 1940s and 1950s (Nuttall 2001). Interestingly, a hemorrhagic disease similar to CCHF was reported by Dzhurzhoni, an Iranian physician, in 1110 A.D. (Hoogstraal 1979). The CCHFV is the type species of the genus Nairovirus, family Bunyaviridae. The disease is known with the local name “Kara-Mikh typhoid fever” in northwest of Iran (Chinikar 2007). Following publication of some reports about the occurrence of a hemorrhagic disease in human and domestic animals and confirming the presence of viral agent in collected ticks from Iran (Achrafi and Noriyan 1966, Chumakov et al. 1970, Asefi 1974, Saidi et al. 1975, Sureau et al. 1980, Ardoin and Karimi 1982), the disease are considered as a public health problem by Iranian health system in 1999 (Labbaf-Ghasemi 2006, Chinikar 2007).
The virus have been detected frequently in livestock (such as sheep, cow, goat and camel), human and tick vectors in many parts of Iran (Saidi et al. 1975, Moradi et al. 2008, Telmadarraiy et al. 2008a, Telmadarraiy et al. 2008b, Telmadarraiy et al. 2009, Chinikar et al. 2012, Mehravaran et al. 2013, Champour et al. 2014). So far, the disease has been reported from 23 out of 31 provinces of Iran that mostly centralized in southeast, due to a long border with two high risk countries, Afghanistan and Pakistan (Chinikar et al. 2010). Since 2000, the most CCHF positive cases have been reported from Sistan-o Baluchistan Province of Iran (Chinikar et al. 2010, Keshtkar-Jahromi et al. 2013).
Tick vectors
Ticks (Acari: Ixodidae) are the important vectors/reservoirs of Crimean Congo Hemorrhagic Fever virus (CCHFV). They play an important role in the survival of the virus in nature (Watts et al. 1988). The parasitic behavior of ticks during their all life stages, excessive blood feeding on hosts, wide range of vertebrate hosts, great reproductive potential, long term survival and adaptability to harsh and variable ecological conditions are among considerable characteristics of ticks as potential vectors of a large number of microbial agents (Linthicum and Bailey 1994). Humans may be infected via a tick bite whenever a normal cycle of virus-tick-nonhuman vertebrates is interrupted by the undesirable presence of human (such as herdsmen, tourists, veterinarian researchers) in the nature cycle. Domestic animals are known to be susceptible to CCHF virus; however, there is no evidence that any symptomatic disease develop (Eldridge et al. 2004). The disease occur in Palearctic, Oriental and some parts of sub-Saharan Africa wherever one or several Hyalomma to be distributed as widespread tick species (Linthicum and Bailey 1994).
A range of disease from mild to severe clinical illness occurs in human. In severe cases, petechial rash on the trunk and limbs, besides the appearance of hemorrhage from the body cavities are among the common clinical symptoms of the disease (Nuttall 2001). The approximate mortality rate of 22 % has been reported in CCHF cases infected by a tick bite, though the rate is variable in different areas (Hoogstraal 1979). The virus has been reported in two groups of ticks. The first group includes aggressive species, which search for human actively. For Instance, species such as Hyalomma marginatum and H. anatolicum are the main vectors in this group when epidemics is occurring (Hoogstraal 1979). Second group are less aggressive species seeking humans normally. For instance the genera Rhipicephalus, Boophilus, Haemaphysalis, Amblyomma, Dermacentor and even other Hyalomma are among species that chiefly maintain enzootic foci of CCHF virus among tick vectors and wild/domestic mammals (Hoogstraal 1979).
Approximately, 26 soft (Argasidae) and hard (Ixodidae) tick species have been recorded from Iran so far. They include nine Hyalomma, two Rhipicephalus, two Dermacentor, five Haemaphysalis, two Boophilus, one Ixodes, two Argas and three Ornithodoros species as ectoparasites of livestock and poultries (Maghami 1968, Mazlum 1971, Rahbari et al. 2007). Studies on tick vectors of CCHFV in Iran have begun since 1980s, when Sureau et al. (1980) detected the virus in nymphs of Ornithodoros lahorensis collected from goat, however, the antibody of CCHFV (CCHF virus) was detected in the serum of the sheep sent from Iran to Russia, for the first time (Chumakov et al. 1970). Few years later, Saidi et al. (1975) reported the presence of CCHFV antibody in the sera of man, sheep, goats, cattle and some small mammals in Iran.
Iran has different climatic conditions and appropriate landscapes suitable for the occurrence of many species of bloodsucking ticks (up to the present 50 species) as external parasites on mammals, birds and other animals (Kamali et al. 2001). The circulation of CCHF virus in ticks and livestock plays an important role in the maintenance of virus in Iran (Chinikar et al. 2010). Considering to the importance of ticks in the survival of the virus, a series of studies have been conducted by our group on ticks suspected to transmit the CCHFV between domestic animals and human.
The present paper reviews the tick vectors of CCHFV in Iran and summarizes the results of ten years study conducted in 14 provinces of Iran. Similar studies associated with tick vectors of CCHFV have also been discussed in the present study.
Argasidae
The Argasid or soft ticks are the most common ectoparasites found on the poultries and domestic animals in Iran (Rafyi and Maghami 1965). To date, five human associated soft ticks are known in Iran, all of them play important role in transmission of arthropod borne diseases (Djanbakhsh 1956, Rafyi and Rak 1985, Barmaki et al. 2010, Oshaghi et al. 2011, Rafinejad et al. 2011).
Argas
Two Argas species are widely found in Iran namely A. persicus, a parasite of poultries, and A. reflexus, a parasite of pigeon and doves. Recently, a third species is also found on Pipistrellus pipistrellus which can parasitize bats (Hosseini-Chegeni and Tavakoli 2013).
Ornithodoros
Five Ornithodoros species have been recorded in Iran, including O. erraticus and O. tartakovskyi (parasites of rodents), O. lahorensis and O. canestrinii (parasites of livestock) and O. tholozani (parasites of mammals, birds and even human) (Djanbakhsh 1956, Rafyi and Maghami 1965, Rafyi and Rak 1985).
Ixodidae
Totally, 21 Ixodid or hard tick species have been reported in Iran. All of the species parasitize domestic animals, including sheep, goat, cattle, camel, horse, and donkey, moreover, humans might be infested accidentally (Chinikar 2007).
Dermacentor
The genus Dermacentor is a most attractive tick having an ornate scutal pattern. The polymorphic D. marginatus and D. niveus (= D. daghestanicus) are two well-known species in Iran (Rahbari et al. 2007) as well as a Less known species D. raskemensis; however, their species status need to be revised.
Haemaphysalis
Six species of Haemaphysalis includes H. concinna, H. erinacei, H. inermis, H. parva, H. punctata and H. sulcata were found in Iran. The latter species (H. sulcata) is the most widespread species in Iran (Rahbari et al. 2007, Hosseini-Chegeni et al. 2014).
Hyalomma
The genus of Hyalomma is the most important tick species associates with livestock. The most frequent species of this genus found in Iran are as follows: H. anatolicum, H. asiaticum, H. detritum, H. dromedarii, H. excavatum, H. marginatum, H. rufipes and H. schulzei (Hosseini-Chegeni et al. 2013).
Ixodes
The species I. ricinus belonging to Ixodes genus has been reported on livestock in Iran. The prevalence of this species is confined to Hyrcanian zone in north part of Iran. This multi-host species parasitize over three hundred different hosts; including domestic and wild animals (Sonenshine et al. 2002). Two other species of Ixodes genus have been reported on wild animals (Filippova et al. 1976).
Rhipicephalus
R. sanguineus is the most common species of the genus Rhipicephalus throughout Iran. As well as a rare species, R. bursa, it may also be found on domestic and wild animals in Iran (Rahbari et al. 2007).
Vectors of CCHFV in Iran, Past to Present
Since 2004, we examined different tick species of two families Argasidae (two genera, two species) and Ixodidae (five genera, 17 species) for detection of CCHF virus genome. The infection rate of tick species that were positive for CCHF virus has been shown in the Table 1. The collection sites of tick in different geographical zones of Iran have also been shown in Fig. 1. Our results showed that Rhipicephalus sanguineus, Hyalomma marginatum, H. anatolicum, H. asiaticum and H. dromedarii were the most frequent tick species which were positive for CCHFV whereas other species were less infected (Fig. 2, Fig. 3).
Table 1.
Infection rates (%) of Iranian Tick Species with CCHF Virus in Different Localities of Iran
|
Tick species |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Province• | T/P | D. marginatus | H. anatolicum | H. asiaticum | H. detritum | H. dromedarii | H. marginatum | H. schulzei | H. sp | Ha. inermis | Ha. punctata | O. lahorensis | R. bursa | R. sanguineus |
| Ardebil* | 130/35 | - | - | - | - | 0.8 | 0.8 | 4.6 | 6.9 | 13.8 | ||||
| Azarbayjan-e Sharqi** | 56/15 | 7 | - | 3.7 | 5.4 | - | 11 | - | ||||||
| Golestan | 130/7 | 2.3 | - | - | 1.6 | 0.7 | - | 0.7 | ||||||
| Hamadan*** | 88/10 | 1.2 | - | 2.3 | 3.4 | 1.2 | - | 1.2 | 2.3 | |||||
| Ilam | 74/3 | 1.35 | 1.35 | - | 1.35 | |||||||||
| Khorasan-e Joonobi | 115/7 | - | 1.8 | 1.8 | - | - | 0.9 | - | - | 0.9 | 0.9 | |||
| Khorasan-e Shomali | 67/5 | - | 4.5 | - | 3 | |||||||||
| MazandaranI | 58/1 | 1.7 | ||||||||||||
| MazandaranII | 42/4 | - | - | 2.4 | 2.4 | 2.4 | 2.4 | |||||||
| Qom | 14/1 | - | - | 7.1 | - | |||||||||
| Sistan-o Baluchistan**** | 140/6 | - | - | - | 8.3 | - | ||||||||
| Tehran | 50/2 | - | 2,E | - | - | - | - | 2 | ||||||
| Yazd***** | 140/8 | - | 0.7 | 0.7 | 1.4 | 2.1 | 0.7 | - | ||||||
| Total | 1112/108 | 7 | 11.85 | 9.55 | 1.4 | 23.9 | 14.95 | 0.8 | 17.1 | 10.7 | 3.6 | 17.9 | 18.3 | 38 |
References related to other studies including papers authored/co-authored by senior author (ZT) including;
D: Dermacentor, Ha. Haemaphysalis, H: Hyalomma, R: Rhipicephalus, O: Ornithodoros
Numbers arranged according to collection sites in the map (Fig. 2)
T/P: total tested/positive tick specimen, I. Pushtekooh district, II. Ghaemshahr county, E: egg mass, Blank places: not done, -: negative
Fig. 1.
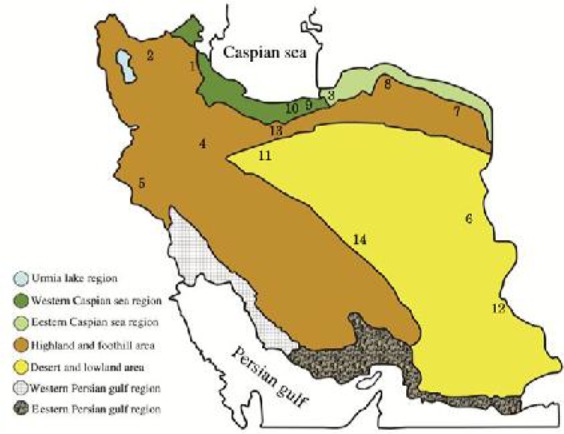
The seven geographical zones of Iran; numbers 1–14 represented different localities of tick collection, 1. Ardabil, 2. Azarbayjan-e Sharqi, 3. Golestan, 4. Hamadan, 5. Ilam (Abdanan township), 6. Khorasan-e Joonobi, 7. Khorasan-e Razavi, 8. Khorasan-e Shomali, 9. Mazandaran (Pushtekooh district), 10. Mazandaran (Ghaemshahr County), 11. Qom, 12. Sistan-o-Baluchistan, 13. Tehran, 14. Yazd
Fig. 2.

The male of most frequent Iranian Ixodid ticks infected to CCHFV, I) Rhipicephalus sanguineus, II) Hyalomma marginatum, III) Hyalomma anatolicum, IV) Hyalomma asiaticum, V–VI) Hyalomma dromedarii dorsal and ventral view respectively. (Black and white arrows indicated the most taxonomic characters of certain species)
Fig. 3.

The male of less frequent Iranian Ixodid and Argasid ticks infected to CCHFV, I) Hyalomma schulzei, II) Hyalomma detritum, III) Dermacentor marginatus, IV) Rhipicephalus bursa, V–VI) Haemaphysalis sulcata ventral and dorsal view respectively, VII–VIII) Ornithodoros lahorensis dorsal and ventral view. (Black and white arrows indicated the most taxonomic characters of certain species)
Based on the results obtained from reverse transcription polymerase chain reaction (RT-PCR), Argasid ticks, including Argas persicus, Ixodid ticks, Boophilus annulatus, Haemaphysalis sulcata, Ha. concina, and Ha. erinacei as well as Rhipicephalus turanicus were negative for CCHF virus.
After Dengue virus, CCHFV is the second most widespread arboviruse type disease throughout the world (Appannanavar and Mishra 2011). The biological role of ticks is also important, not only as virus vectors, but also as reservoirs of the virus in nature (Whitehouse 2004). The long term survival of the virus occurs in live dormant ticks at unfavorable climatic conditions (Chumakov 1965). Hoogstraal (1979) reviewed the epidemiology of tick borne Crimean Congo Hemorrhagic Fever virus. He introduced the trans-stadial and trans-ovarial (vertical) transmission of the virus in ticks (Hoogstraal 1979).
Another route that CCHF virus could be transmitted among tick vector is an interesting mechanism called co-feeding behavior in which virus transferred between feeding ticks on the host (Gordon et al. 1993).
Except tick bite, the other route for transmissions of virus to human being is the direct contact with blood or tissue of the infected livestock and nosocomial infection. The biting of the infected tick or contact of human to the infected tick hemolymph through naked hands or other parts of the body and also other tick borne transmission routes are considered non-significant in the epidemiology of CCHF virus in Iran (Shirzadi 2003). Based on Chinikar et al. (2007) the direct infection of humans by tick bite is rare in Iran and has been observed only in few cases (Chinikar 2007). CCHFV propagates in tick vectors and within the natural host vertebrate including domestic animals. From biological point of view, the virus is transmitted from tick to the host via tick saliva during blood feeding. The virus is transmitted from tick to tick through co-feeding phenomenon (Nuttall 2001). Thus, in Iran, the essential role of ticks in the epidemiology of CCHF virus may be debatable and never considered merely according to patient’s statements and health worker reports (Izadi et al. 2003).
Ornithodoros lahorensis was reported as the most frequent soft tick infected to CCHFV in northwest (Ardebil and Azerbaijan-e Sharqi) northeast and central parts of Iran (Sureau et al. 1980, Shirani et al. 2004). Shirani et al. could detect the virus in the body of living Ornithodoros lahorensis maintained in the laboratory conditions for three months. The authors also concluded that Argasid ticks might be considered as a potential reservoir of CCHFV (Shirani et al. 2004). However, the few other studies showed that Argasid ticks cannot be infected in the laboratory condition (Linthicum and Bailey 1994, Shirani et al. 2004). Three Argas ticks reported from Iran including A. persicus, A. vespertilionis and A. refelexus, all have been considered as the main vectors of animal diseases (Hosseini-Chegeni and Tavakoli 2013). The infection of the latter species to CCHFV was confimed in Hamedan Province, Iran (Tahmasebi et al. 2010). An expected reason of the minor implication of soft ticks in the transmission of CCHFV is their quick feeding behavior (excluding some exceptions e g, larvae of O. lahorensis, O. canestrinii and Argas persicus) (Hoogstraal 1985). In many literatures Hyalomma ticks were introduced as the main vectors of CCHFV thus the correct identification of species is an essential work since it may be a complicated and controversial issue in Iran (Hosseini et al. 2011, Hosseini and Tavakoli 2012, Tavakoli et al. 2012). In spite of the only report on low infection rate of Hyalomma detritum (Salim-Abadi et al. 2011), Tahmasebi et al. (2010) reported 48 positive ticks out of 294 of H. detritum tested for CCHFV which is somewhat doubtful (Tahmasebi et al. 2010). The other less frequent and patchy distributed tick is Rhipicephalus bursa that is the most frequent tick infected to CCHFV in Fereydoonshahr of Isfahan Province (Chinikar et al. 2012) and northwest of Iran (Telmadarraiy et al. 2009). Many literatures, refer Hyalomma tick as main vector of CCHFV in nature; however, in our study wherever Hyalomma was absent or not considered as the predominant vector (in Ghaemshahr County, Mazandaran Province), CCHFV was detected from other ticks such as Rhipicephalus and Haemaphysalis. It seems that these species are key species in the survival of the virus in the absence of Hyalomma. On the other hand, sometimes the infection rate of Hyalomma to CCHFV was in low level in comparison with Rhipicephalus (i.e, totally 5 positive out of 8 Rhipicephalus versus only a few positive Hyalomma out of 97 specimens in Ghaen county, Khorasan-e Joonobi Province).
There are little information on genetic structure and molecular phylogenetic analysis on CCHF viruses isolated from different tick vectors in Iran. However, the phylogeny studies on the Iranian CCHFV isolates in comparison with a representative set of CCHFV sequences from other regions of the world based on S-segment sequences revealed that the Iranian isolates are mostly related to Asian 1 group particularly to the Pakistani strains (Chinikar et al. 2012) although a few isolates were associated with Iraq (Chinikar et al. 2012) or Europe 1 strains (Morovvati et al. 2014).
Further molecular epidemiology studies are necessary to find out the origin and the phylogenetic relationship of Iranian CCHFV to improve the surveillance and control measurements of the disease in the country.
Control of ticks
Based on CCHF vector, different control measurements should be performed in high risk areas where disease presents (Hoogstraal 1979). For instance, in Ghaemshahr County of Mazandaran Province, and Azarbayjan-e Gharbi Province, Haemaphysalis punctata and Ha. inermis are the most prevalent species. In Azerbaijan-e Sharqi Province, D. marginatus is considered as target of control whereas in Hamadan, Yazd and Kurdistan Provinces, Hyalomma ticks (e g, H. marginatum) are the most important vectors, tick control measures may be focused on domestic mammals and animal housings especially with reference to behaviorally unusual ridiculous ticks, H. detritum and H. anatolicum (Telmadarraiy et al. 2004, Salari-Lak et al. 2008, Nasiri et al. 2010). Hyalomma and other ticks can parasitize small wild rodents (Rattus norvegicus, Rhombomys opimus, Tatera indica, Meriones libycus, Meriones hurrianae and the lesser mouse eared bat, Myotis blythii) (Kia et al. 2009, Tajedin et al. 2009, Vatandoost et al. 2010, Nateghpour et al. 2013). The control measures of livestock ticks recommended by the Iranian Veterinary Organization including the application of Acaricides, at animal housing, as anti tick bathroom and pure on or spot on domestic animals (Rezaii 2014). Infusing Ivermectin is currently being used as a relative chief anti tick method in the Iranian ruminants, but concerning its harmful residues on animals products (milk and meat), it is not advised by the Iranian Veterinary Organization. Flumethrin is also reported as a pesticide suitable for the control of hard and soft ticks especially Hyalomma tick parasitizing livestock whenever use as pour-on host (Vatandoost et al. 2012).
Conclusion
The high frequency of tick species which are infected to CCHF virus reveals that unlike a most common idea which believes that Hyalomma species are the main vectors of CCHF virus, other ticks including Rhipicephalus, Haemaphysalis and Dermacentor can be reservoir of this virus; thus, considering geographical distribution, type of host and environmental conditions, different tick control measurements should be acquired in areas with high incidence of CCHF disease.
Acknowledgments
We would like to appreciate staffs of the Laboratory of Arboviruses and Viral Hemorrhagic Fevers, Institute of Pasteur of Iran as national Reference Laboratory, for their technical support. We would also like to thank students of Department of Medical Entomology and Vector Control of School of Public Health, Tehran University of Medical Sciences for their efforts in collecting and identifying tick specimens. Our sincere thanks go to Engs. Tigi and Zarei, Dr Hosseini, Dr Shakeri, and Ms Shirzaiiyan-Zaroni for their kind helps in this research. This study was financially supported by the grant offered by Tehran University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- Achrafi A, Noriyan A. (1966) The report first observation of hemorrhagic fever in one region located in vills of Azarbaijan-e-Sharqi. J Fac Med Univ Tabriz. 6: 182– 188. [Google Scholar]
- Appannanavar SB, Mishra B. (2011) An update on Crimean Congo hemorrhagic fever. J Global Infect Dis. 3: 285– 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardoin A, Karimi Y. (1982) Foyer de purpura thrombocytopenique en Iran dans l’Azerbaidjan de l’est. Med Trop. 42: 319– 326. [PubMed] [Google Scholar]
- Asefi V. (1974) Etude clinique de 60 patients atteints d’un syndrome infectieux et hemorragique en Azerbaidjan l’est (Iran). Iran J Public Health. 3: 140– 146 [In French]. [Google Scholar]
- Barmaki A, Rafinejad J, Vatandoost H, Telmadarraiy Z, Mohtarami F, Leghaei SH, Oshaghi MA. (2010) Study on presence of Borrelia persica in soft ticks in western Iran. J Arthropod Borne Dis. 4: 19– 25. [PMC free article] [PubMed] [Google Scholar]
- Champour M, Mohammadi GR, Chinikar S, Razmi GR, Shah-Hosseini N, Khakifirouz S, Mostafavi E, Jalali T. (2014) Seroepidemiology of Crimean-Congo hemorrhagic fever virus in one-humped camels (Camelus dromedarius) population in northeast of Iran. J Vector Borne Dis. 51: 62– 65. [PubMed] [Google Scholar]
- Chinikar S. (2007) Crimean-Congo hemorrhagic fever infection in Iran. In: Ergonul O, Whitehouse CA. (Eds.): Crimean-Congo hemorrhagic fever: A global perspective. Springer, Dordrecht Netherlands. [Google Scholar]
- Chinikar S, Ghiasi SM, Moradi M, Goya MM, Shirzadi MR, Zeinali M, Meshkat M, Bouloy M. (2010) Geographical distribution and surveillance of Crimean-Congo hemorrhagic fever in Iran. Vector Borne Zoonotic Dis. 10: 705– 708. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Ghiasi SM, Naddaf S, Piazak N, Moradi M, Razavi MR, Afzali N, Haeri A, Mostafavizadeh K, Ataei B, Khalilifard-Brojeni M, Husseini SM, Bouloy M. (2012) Serological evaluation of Crimean-Congo hemorrhagic fever in humans with high-risk professions living in enzootic regions of Isfahan Province of Iran and genetic analysis of circulating strains. Vector Borne Zoonotic Dis. 12: 733– 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov MP. (1965) A short review of investigation of the virus of Crimean hemorrhagic fever. In: Chumakov MP. (Ed.) Endemic viral infections (hemorrhagic fever with renal syndrome, Crimean hemorrhagic fever, Omsk hemorrhagic fever, and Astrakhan virus from Hyalomma pl. plumbeum tick). Sbor Trudy Inst Polio Virus Entsef Akad Med Nauk SSSR, Moscow, USSR. [Google Scholar]
- Chumakov MP, Ismailova ST, Rubin SG, Smirnova SE, Zgurskaya GN, Khankishiev AS, Berezin VV, Solovei EA. (1970) Detection of Crimean hemorrhagic fever foci in Azerbaijan SSR from results from serological investigations of domestic animals. Trudy Inst Polio Virus Entsef Akad Med Nauk SSSR. 18: 120– 122. [Google Scholar]
- Eldridge BF, Scott TV, Day JF, Tabachnick WJ. (2004) Arbovirus diseases. In: Eldridge BF, Edman JD. (Eds.): Medical entomology a textbook on public health and veterinary problems caused by arthropods. Kluwer Academic Publishers, Netherlands. [Google Scholar]
- Filippova NA, Neronov VM, Farhang-Azad A. (1976) Data on ixodid tick fauna (Acarina, Ixodidae) of small mammals in Iran. Entomol Obozreniye. 55: 467– 479. [Google Scholar]
- Hoogstraal H. (1979) The epidemiology of tick-borne Crimean Congo haemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 15: 307– 417. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H. (1985) Argasid and Nuttalliellid ticks as parasites and vectors. Adv Parasitol. 24: 135– 238. [DOI] [PubMed] [Google Scholar]
- Hosseini-Chegeni A, Hosseine R, Tavakoli M, Telmadarraiy Z, Abdigoudarzi M. (2013) The Iranian Hyalomma (Acari: Ixodidae) with a key to the identification of male species. Persian J Acarol. 2: 503– 529. [Google Scholar]
- Hosseini-Chegeni A, Tavakoli M. (2013) Argas vespertilionis (Ixodida: Argasidae): A parasite of Pipistrel bat in Western Iran. Persian J Acarol. 2: 321– 330. [Google Scholar]
- Hosseini-Chegeni A, Telmadarraiy Z, Salimi M, Arzamani K, Banafshi O. (2014) A record of Haemaphysalis erinacei (Acari: Ixodidae) collected from Hedgehog and an identification key for the species of Haemaphysalis occurring in Iran. Persian J Acarol. 3: 203– 215. [Google Scholar]
- Hosseini A, Dalimi A, Abdigoudarzi M. (2011) Morphometric study on male specimens of Hyalomma anatolicum (Acari: Ixodidae) in west of Iran. J Arthropod-Borne Dis. 5: 23– 31. [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Tavakoli M. (2012) A taxonomic study on hard ticks Hyalomma marginatum species group (Acari: Ixodidae) in Iran. Proceeding of 1st International and 8th National Congress of Parasitology and Parasitic Disease in Iran, October 16–18, Kerman, Iran. [Google Scholar]
- Izadi S, Holakouie-Naieni K, Majdzadeh SR, Rakhshani F, Chinikar S, Nadim A, Hooshmand B. (2003) Crimean-Congo haemorrhagic fever in Sistan and Baluchesatan Province of Iran, a case-control study about epidemiological characteristics. J Vet Fac Univ Tehran. 57: 27– 32. [Google Scholar]
- Kamali K, Ostovan H, Atamehr A. (2001) A catalog of mites and ticks (Acari) of Iran. Islamic Azad University Scientific Publication Center, Tehran, Iran. [Google Scholar]
- Keshtkar-Jahromi M, Sajadi MM, Ansari H, Mardani M, Holakouie-Naieni K. (2013) Crimean-Congo hemorrhagic fever in Iran. Antiviral Res. 100: 20– 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia EB, Moghddas-Sani H, Hassanpoor H, Vatandoost H, Zahabiun F, Akhavan AA, Hanafi-Bojd AA, Telmadarraiy Z. (2009) Ectoparasites of rodents captured in Bandar Abbas, southern Iran. J Arthropod-Borne Dis. 3: 44– 49. [PMC free article] [PubMed] [Google Scholar]
- Labbaf-Ghasemi R. (2006) The most important Iranian programs for control of communicable diseases. In: Hatami H, Razavi SM, Eftekhar AH, Majlesi F, Sayed Nozadi M, Parizadeh SMJ. (Eds.): The textbook of public health. Ministry of Health and Medical Education (Iran), Tehran Iran: [In Persian]. [Google Scholar]
- Linthicum KJ, Bailey CL. (1994) Ecology of Crimean-Congo Hemorrhagic Fever. In: Sonenshine DE, Mather TN. (Eds.): Ecological dynamics of tick-borne zoonoses. Oxford University press, Oxford, UK. [Google Scholar]
- Maghami G. (1968) External parasites of livestocks in Iran. Arch Inst Razi Iran. 20: 81– 83. [Google Scholar]
- Mazlum Z. (1971) Ticks of domestic animals in Iran: geographic distribution, host relation, and seasonal activity. J Vet Fac Univ Tehn Iran. 27: 1– 32 [In Persian]. [Google Scholar]
- Mehravaran A, Moradi M, Telmadarraiy Z, Mostafavi E, Moradi AR, Khakifirouz S, Shah-Hosseini N, Sadat-Rasi-Varaie F, Jalali T, Hekmat S, Ghiasi SM, Chinikar S. (2013) Molecular detection of Crimean-Congo haemorrhagic fever (CCHF) virus in ticks from southeastern Iran. Ticks Tick-borne Dis. 4: 35– 38. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Chinikar S, Oshaghi MA, Vatandoost H, Houlakoui-Naeini K, Zahirnia AH, Telmadarraiy Z. (2008) Molecular detection of Crimean-Congo hemorrhagic fever (CCHF) virus in ticks (Ixodidae, Argasidae) of Hamedan Province, Iran. Biochem Cell Arch. 8: 119– 123. [Google Scholar]
- Morovvati A, Ghalyanchi-Langeroudi A, Soleimani M, Mousavi-Nasab SD, Majidzadeh AK. (2014) Emergence of a new genotype of Crimean-Congo hemorrhagic fever virus in Iran. Iranian J Virol. 6: 24– 29. [Google Scholar]
- Nasiri A, Telmadarraiy Z, Vatandoost H, Chinikar S, Moradi A, Oshaghi MA, Salim Abadi Y, Sheikh Z. (2010) Tick infestation rate of sheep and their distribution in Abdanan county, Ilam Province, Iran, 2007–2008. J Arthropod-Borne Dis. 4: 56– 60. [PMC free article] [PubMed] [Google Scholar]
- Nateghpour M, Akhavan AA, Hanafi-Bojd AA, Telmadarraiy Z, Ayazian Mavi S, Hosseine Vasoukolaei N, Motevalli-Haghi A, Akbarzadeh K. (2013) Wild rodents and their ectoparasites in Baluchistan area, southeast of Iran. Trop Biomed. 30: 72– 77. [PubMed] [Google Scholar]
- Nuttall PA. (2001) Crimean–Congo haemorrhagic fever. In: Service MW. (Ed.) Encyclopedia of arthropod-transmitted infections of man and domesticated animals. CABI Publishing, New York, USA. [Google Scholar]
- Oshaghi MA, Rafinejad J, Choubdar N, Piazak N, Vatandoost H, Telmadarraiy Z, Mohtarami F, Ravasan NM. (2011) Discrimination of relapsing fever Borrelia persica and Borrelia microtti by diagnostic species-specific primers and polymerase chain reaction–restriction fragment length polymorphism. Vector Borne Zoonotic Dis. 11: 201– 207. [DOI] [PubMed] [Google Scholar]
- Rafinejad J, Choubdar N, Oshaghi MA, Piazak N, Satvat T, Mohtarami F, Barmaki A. (2011) Detection of Borrelia persica infection in Ornithodoros tholozani using PCR targeting rrs gene and xenodiagnosis. J Arthropod-Borne Dis. 40: 138– 145. [PMC free article] [PubMed] [Google Scholar]
- Rafyi A, Maghami G. (1965) Etat actuel de nos connaissances les Argasidae de l’Iran. Arch Inst Razi Iran. 17: 1– 16. [Google Scholar]
- Rafyi A, Rak H. (1985) Parasitology of arthropods (entomology). Tehran University Perss, Tehran Iran: [In Persian]. [Google Scholar]
- Rahbari S, Nabian S, Shayan P. (2007) Primary report on distribution of tick fauna in Iran. Parasitol Res. 101: S175– S177. [DOI] [PubMed] [Google Scholar]
- Rezaii AA. (2014) Executive instructions of office of health and management of animal diseases. Iranian Veterinary Organization, Tehran Iran: [In Persian]. [Google Scholar]
- Saidi S, Casals J, Faghih MA. (1975) Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man and domestic and small mammals in Iran. Am J Trop Med Hyg. 24: 353– 357. [DOI] [PubMed] [Google Scholar]
- Salari-Lak S, Vatandoost H, Telmadarraiy Z, Entezar-Mahdi R, Kia EB. (2008) Seasonal activity of ticks and their importance in tick-borne infectious diseases in west Azerbaijan, Iran. J Arthropod-Borne Dis. 2: 28– 34. [Google Scholar]
- Salim-Abadi Y, Chinikar S, Telmadarraiy Z, Vatandoost H, Moradi M, Oshaghi MA, Ghiasi SM. (2011) Crimean-Congo hemorrhagic fever: a molecular survey on hard ticks (Ixodidae) in Yazd Province, Iran. Asian Pac J Trop Med. 4: 61– 63. [DOI] [PubMed] [Google Scholar]
- Shirani M, Asmar M, Chinikar S, Mirahmadi R, Piazak N, Mazaheri V. (2004) Detection of CCHF virus in soft ticks (Argasidae) by RT-PCR method. J Infect Dis Trop Med. 9: 11– 15. [Google Scholar]
- Shirzadi MR. (2003) Crimean-Congo hemorrhagic fever and other viral hemorrhagic fevers. Seda publication, Tehran Iran: [In Persian]. [Google Scholar]
- Sonenshine DE, Lane RS, Nicholson WL. (2002) Ticks (Ixodida). In: Mullen GR, Durden LA. (Eds.): Medical and veterinary entomology. Academic Press, San Diego, USA. [Google Scholar]
- Sureau P, Klein JM. (1980) Arbovirus enIran. Méd Trop. 40: 549– 554. [PubMed] [Google Scholar]
- Sureau P, Klein JM, Casals J, Digoutte JP, Salaun JJ, Piazak N, Calvo MA. (1980) Isolement des virus Thogoto, Wad Medani, Wanowrie et de la fievre hemorragique de Crimée-Congo en Iran à partir de tiques d’animaux domestiques. Annu Virol (Institue Pasteur). 131: 185– 200. [Google Scholar]
- Tahmasebi F, Ghiasi SM, Mostafavi E, Moradi M, Piazak N, Mozafari A, Haeri A, Fooks AR, Chinikar S. (2010) Molecular epidemiology of Crimean-Congo hemorrhagic fever virus genome isolated from ticks of Hamadan Province of Iran. J Vector Borne Dis. 47: 211– 216. [PubMed] [Google Scholar]
- Tajedin L, Rassi Y, Oshaghi MA, Telmadarraiy Z, Akhavan AA, Abai MR, Arandian MH. (2009) Study on ectoparasites of Rhombomys opimus, the main reservoir of zoonotic cutaneous leishmaniasis in endemic foci in Iran. J Arthropod-Borne Dis. 3: 41– 45. [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Hosseini-Chegeni A, Mehdifar D. (2012) Occurrence of morphologic variability in tick Hyalomma anatolicum anatolicum (Acari: Ixodidae). Iranian J Vet Med. 6: 177– 185. [Google Scholar]
- Telmadarraiy Z, Bahrami A, Vatandoost H. (2004) A survey on fauna of ticks in west Azerbaijan Province, Iran. Iran J Public Health. 33: 65– 69. [Google Scholar]
- Telmadarraiy Z, Ghiasi SM, Moradi M, Vatandoost H, Eshraghian MR, Faghihi F, Zarei Z, Haeri A, Chinikar S. (2009) A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand J Infect Dis. 2: 37– 41. [DOI] [PubMed] [Google Scholar]
- Telmadarraiy Z, Moradi AR, Vatandoost H, Mostafavi E, Oshaghi MA, Zahirnia AH, Haeri A, Chinikar S. (2008a) Crimean-Congo hemorrhagic fever: a seroepidemiological and molecular survey in Bahar, Hamadan Province of Iran. Asian J Anim Vet Adv. 3: 321– 327. [Google Scholar]
- Telmadarraiy Z, Tighi S, Chinikar S, Vatandoost H, Oshaghi MA, Rassi Y, Mirahmadi R, Zarei Z, Ahmadnezhad P, Rafinejad J, Jedari M, Aboulhasani M, Mohtarami M, Faghihi F, Mashrabi M. (2008b) Immunological and serological study on the infectivity of host animals and ticks (Ixodidae, Argasidae) to CCHF virus in east Azerbaijan, Iran. 17th International Congress for Tropical Medicine and Malaria, Jeju Korea. [Google Scholar]
- Vatandoost H, Moradi Asl E, Telmadarraiy Z, Mohebali M, Masoumi Asl H, Abai MR, Zarei Z. (2012) Field efficacy of flumethrin pour-on against livestock ticks in Iran. Int J Acarol. 38: 457– 464. [Google Scholar]
- Vatandoost H, Telmadarraiy Z, Sharifi M, Moradi A, Kamali M, Taran M. (2010) Ectoparasites of lesser mouse eared bat, Myotis blythii from Kermanshah Iran. Asian Pac J Trop Med. 3: 371– 373. [Google Scholar]
- Watts DM, Ksiazek TG, Linthicum KJ, Hoogstraal H. (1988) Crimean-Congo haemorrhagic fever. In: Monath TP. (Ed.) The Arboviruses: epidemiology and ecology. CRC Press, Florida USA. [Google Scholar]
- Whitehouse CA. (2004) Crimean-Congo hemorrhagic fever. Antiviral Res. 64: 145– 160. [DOI] [PubMed] [Google Scholar]


