Abstract
Background:
Envenomation by Hemiscorpius lepturus is not painful and the clinical manifestations include bloody urine due to hemoglobinuria or hematuria, dermonecrotic reactions, cardiac arrhythmia and in minority of cases acute renal failure which may lead to death following disseminated intravascular coagulation in infants. Cardiac effects of envenomation by H. lepturus venom including inotropic, chronotropic and arrhythmogenic properties are not studied as now in rat hearts with Langendorff apparatus.
Methods:
Rat hearts were allowed to equilibrate in its buffer and cardiotropic plus arrhythmogenic effects induced by injection of different doses of H. lepturus venom were detected and recorded by computer acquisition based data in Langendorff apparatus. The neutralizing effects of Razi Institute antivenom and autonomic drugs were assayed in parallel studies.
Results:
Hemiscorpius lepturus venom (25 μg/100 l) treatment caused a negative inotropic (65.4 ± 3.2 versus 110.2 ± 3.4) and chronotropic effects (186.3 ± 4.2 versus 302 ± 6.3) in comparison to normal saline. Arrhythmogenic aspects including bradycardia, QRS widening and ST depression were induced by venom injection. Pre venom treatment (20 min) of Razi Institute antivenom (10 μl) neutralized cardiotropic effects but post venom injection (15 min later) had no therapeutic role. Pre (10 min before) and post (15 min after) injection of adrenaline (10 μl) neneutralized cardiotropic effects while pre venom injection (20 min) of propanolol (10 μl) had aggravating effects.
Conclusion:
Our study paves the way for further in vivo investigation of cardiovascular effects of this venom for finding suitable treatments instead of its ordinary antivenom medication in cardiogenic shock induced by the venom.
Keywords: Hemiscorpius lepturus, Contractility, Arrhythmogenicity, Envenomation
Introduction
Scorpion envenomation is common in tropical and subtropical areas of the world including southwestern part of Iran especially in Khuzestan province (Prendini 2000, Shahbazzadeh et al. 2009). Envenomation by H. lepturus belonging to family Hemiscorpiidae is not painful and the clinical manifestations in stung patients include bloody urine due to hemoglobinuria or hematuria, dermonecrotic reactions and in minority of cases acute renal failure which may lead to death following disseminated intravascular coagulation in infants (Radmanesh 1990, Jalali et al. 2010). This creature is one of the most dangerous scorpions, and responsible for 95 % of the scorpion-associated mortalities in Iran, that is probably due to the various pathological enzymes in its venom like Hemitoxin, Hemicalcin and Heminecrolysin (Shahbazzadeh et al. 2007, Srairi-Abid et al. 2008).
Generally there is only a few case reports on direct cardiotoxic effects of scorpion envenomation in human beings (Gueron et al. 1967, Bawaskar and Bawaskar 1998, Shapira et al. 1998, Cupo and Hering 2002). However previous studies on the envenomation by H. lepturus suggest that there are in vivo arrhythmogenic and negative inotropic effects in rabbits in spite of its small amount of accumulation of radiolabelled venom following subcutaneous injections in animal models during biodistribution study (Mirakabadi et al. 2011, Seyedian et al. 2012).
In this study, the direct effects of H. lepturus venom on rat hearts were investigated with Langendorff apparatus. First, inotropic and chronotrpic changes upon time were evaluated and then, neutralization properties of Iranian Razi Institute Antivenom and other drugs including epinephrine, propranolol and atropine were employed to analyze direct or indirect cardiotoxic effects of this venom thoroughly promoting further in vivo studies.
Materials and Methods
Drugs and chemicals
The lyophilized venom was acquired by direct electrical stimulation of H. lepturus telsons (15 mV) in Iran. The venom was centrifuged, lyophilized and stored at −20 °C until reconstitution by addition of Normal Saline in our laboratory. The Iranian pepsin digested polyvalent antivenom from horse plasma against 6 common endemic scorpions (Odontobuthus doriae, Mesobuthus eupeus, Androctonus crassicauda, Buthotus saulcyi, Buthotus sach and Hemiscorpius lepturus) (Latifi and Tabatabai 1979, Dehghani and Fathi 2012) were purchased from Razi Vaccine and Serum Research Institute. The neutralizing ability of the used batches was 26 LD50/ml. Adrenaline, atropine and propranolol were purchased from Sigma (St Loius, MO).
Animals
Female Wistar rats (body weight 180–230 g) were obtained from Jundishapur Medical University breeding center (Ahvaz, Iran) and placed in groups of three in PVC cages with free access to water and hard food pellets in animal house of Bushehr University of Medical Sciences. They were kept at 20 ± 2 °C and maintained at 12 hours light-dark cycle starting at 7 AM. The experimental protocol used in this study conforms to the guidelines of the National Institute of Health (NIH).
Langendorff perfused heart preparation
Rats were heparinized (300–400 IU) and anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally). After a bilateral thoracotomy, hearts were rapidly excised and after insertion of an aortic cannula perfused retrogradely at a constant perfusion pressure of 90 cm H2O with gassed (5% CO2, 95% O2) Krebs-Henseleit buffer (pH 7.4) containing NaCl (118.5 mM), NaHCO3 (25 mM), KCl (4.7 mM), KH2PO4 (1.2 mM), CaCl2. 2H2O (1.8 mM), MgSO47H2O (1.2 mM), D-glucose 11 mM) at 37 °C. An epicardial electrocardiogram (ECG) was continuously recorded using two fine stainless steel electrodes, one attached to the apex of the heart and the other placed on the right atrium and a metal clip attaching to the aortic cannula as the reference electrode. Left ventricular pressure was recorded throughout the experiment by a water filled latex balloon placed in the left ventricle and connected to a pressure transducer (MLT 844) through a fluid filled catheter. Volume of the balloon was adjusted to obtain end-diastolic pressure of 8–10 mm Hg at the end of stabilization period and unchanged for the remainder of the experiment. The electrical and hemodynamic functions of the heart were continuously monitored with a computer-based data acquisition system (PowerLab system with Chart 5 software, AD Instruments, Australia). Left ventricular developed pressure (LVDP), rate pressure product (RPP), Max and Min dp/dt and heart rate were calculated. Coronary flow (CF) was measured by timed collections of the coronary effluent.
Experimental protocol
Hearts were allowed to equilibrate for 20 min with its buffer. After the stabilization period, different doses of H. lepturus venom (25, 50 and 100 μg) dissolved in normal saline (100 μl) were infused directly acquiring cardiotropic, inotropic and arrhythmogenic changes with Langendorff apparatus. Razi Institute multivalent antivenom (10 μl) dissolved in Normal Saline (100 μl) was injected pre (20 minutes) and post venom (15 min) treatment. In another set of experiments incubated venom (25 μg) with Razi Institute antivenom (10 μl) was titrated to 100 μl with normal saline for 30 min at room temperature to investigate its neutralizing effect against cardiac aspects of H. lepturus venom. In order to evaluate the neutralizing effects of β adrenoceptors and cholinoceptors drugs, propranolol (10 μl) in addition to adrenaline (10 μl) and finally atropine (100 μl) were used pre and post venom injection. Coronary blood flow, left ventricular developed pressure, dp/dtmax and heart rate were recorded and outflow samples were collected every 1 min initially and every 10 min sequentially until the end of our experiments (60 min) to evaluate the coronary flow changes induced by the venom.
Statistical analysis
Results are expressed as mean ± SEM of three experiments. ANOVA plus post hoc test was carried out in cardiotropic experiments. Values of P< 0.05 were considered significant.
Results
Inotropic and chronotropic effects of Hemiscorpius lepturus venom
Cardiotropic effects of various doses (25, 50 and 100 μg) of H. lepturus venom reconstituted in Normal Saline (100 μl) are shown in Fig. 1–3 (n=3).
Fig. 1.
Heart rate changes by different doses of Hemiscorpius lepturus venom in rats in Langendorff apparatus
# Significantly different from normal saline at P< 0.05
Fig. 3.
Inotropic effects induced by injection of Hemiscorpius lepturus venom (25μg) in rat hearts with Langendorff apparatus
Fig. 2.
Contractility changes induced by Hemiscorpius lepturus venom in isolated rat hearts
# Significantly different from normal saline at P< 0.05
## Significantly different from normal saline at P< 0.01
Representative image of negative chronotropic effects of different doses from H. lepturus venom with the same volume (100 μl) on isolated rat heart in Langendorrf apparatus upon time versus normal saline injection was depicted in Fig 1. Results present mean ± SEM of 3 independent experiments.
Reducing effects on the contractile potency of the heart was induced shortly (two min later) following H. lepturus injection in langendorrf apparatus. Venom caused a statistically significant decrease in heart contractility (negative inotropic) and rate (negative chronotropism) for 40–60 min before ending the examination at 60 minutes. Coronary flow was significantly decreased (7.83 ± 0.21 ml to 2.81 ± 0.62 ml) post venom injection (25 μg) in our experiments.
The neutralizing capacity of Razi Institiute antivenom against cardiogenic effects of Hemiscorpius lepturus
Razi Institute antivenom (10 μl) was used as pre (20 min) and post (15 min) venom (25 μg) injection and its neutralizing effect against the venom were depicted in Fig. 4 A, B.
Fig. 4.
Inotropic changes following pre and post venom (25 μg) injection of Razi Institute antivenom (10 μl)
Razi Institute antivenom pretreatment (20 min before venom injection) or incubation with venom for 30 min at room temperature significantly neutralized negative inotropic effects while post venom injection had no effect.
In another study incubated venom and antivenom (25 μg in 10 μl) for 30 min at room temperature was titrated with normal saline (100 μl) and injected to evaluate the neutralizing effects of this remedy on rat hearts (Fig. 4C). There was no bradycardia and negative inotropic effects following H. lepturus venom (25 μg) injection pre incubated with the antivenom and the post treatment of antivenom failed to counteract the cardiotoxic effects of venom injection.
Arrhythmogenic effects of Hemiscorpius lepturus venom
Venom perfusion prominently induced bradycardia, with widened QRS complexes and ST depression after passing 50 min in all the envenomed hearts as shown in Fig. 5A.
Fig. 5.
Arrhythmogenic affects of Hemiscorpius lepturus venom injection (25 μg) upon time (5A) versus normal saline (5B). Bradycardia, QRS widening and ST depression were induced with venom injection in Langendorff apparatus
Normal heart rate and ECG parameter were induced by normal saline (100 μl) infusion to serve as control (not shown). Antivenom administration (10 μl), 20 min before venom injection had neutralized QRS widening and bradycardia induced by H. lepturus venom (Fig. 5B).
Injection of adrenaline and atropine in our study counteracted the late arrhythmogenic effects induced by H. lepturus venom (data not shown).
Protection and aggrevating experiments using adrenaline, atropine and propranolol
Pre and post injection of adrenaline (10 μl) following using of H. lepturus venom are represented in Fig. 6A, B.
Fig. 6.
Changes in inotropic effects upon the pre and post treatment of epineprine (10 μl) added before and after venom (25 μg) injection
Adrenaline (10 μl) treatment 10 min before and 15 min after venom injection neutralized negative inotropic effects upon time.
According to these diagrams negative inotropic effects were significantly neutralized by this drug since there was no decreasing of LVDP (left ventricular diastolic pressure) following venom injection upon time. In another experiment, pretreatment of propranolol (10 μl) resulted in additive negative effects on LVDP in isolated rat hearts (Fig. 7) and in our final examinations incubation of atropine (100 μl) as an anticholinergic drug with our venom (25 μg) prior to injection neutralized its inotropic effects showing the probability of its indirect mechanism (Fig. 8).
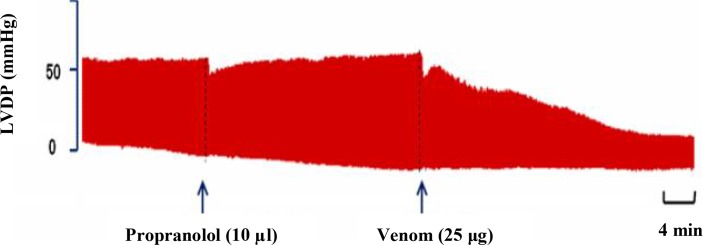
Fig. 7.
LVDP changes following pretreatment with propranolol (10 μl), added 20 min before venom (25 μg) injection
Fig. 8.
LVDP chnages by the venom (25 μg) preincubated with atropine (100 μl) for 30 min
Pretreatment of isolated rat hearts with propranolol, 20 min before venom injection had no neutralizing effect on negative inotropic aspects as represented in this figure.
Incubation of venom and atropine for 30 min had no negative inotropic effects in isolated rat hearts upon time.
Discussion
In this study, in vitro negative inotropic, chronotropic and late arrhythmogenic effects of H. lepturus envenomation in rats were shown with Langendorff apparatus.
The most interesting findings in envenomed patients by H. lepturus are hemoglobinuria, hematuria, cutaneous necrotic ulcers and in minority of cases disseminated intravascular coagulation leading to death (Radmanesh 1990, Shayesteh et al. 2011) as seen in Loxosceles envenomation (Bay et al. 1997, Tavares et al. 2004). Envenomation with H. lepturus which is not painful is commonly seen in South West Provinces of Iran and treated with intramuscular injection of Razi Institute poly valent antivenom and followed by close monitoring.
In previous in vivo studies, bradycardia (decreasing from 227 beats/min to 94 beats/min) and ST elevation in lead II was induced with intravenous injection of extreme doses (50 μg/kg) of venom in rabbits with no significant changes of lactate dehydrogenase and Creatinine phosphokinase one hour after envenomation showing no myocardial necrosis (Mirakabadi et al. 2010). In this paper, a Langendorff model was assembled to analyze the direct and indirect chronotropic and inotropic effects of H. lepturus venom on rat hearts.
Our venom induced a persistant negative dose response inotropic and chronotropic effects in rat hearts until up to 60 min, similar to loxosceles envenomation (Shahbazzadeh et al. 2007, Chatzaki et al. 2012). This study prudently approved that Hemiscorpius lepturus cardiotoxicity was highly dependent on dose and time of its administration. Coronary blood flow was significantly decreased (10.4 ± 2.21 to 3.16 ± 2.58) in envenomed hearts with no changes in LDH and CPK before and 60 min after envenomation (Data not shown) ruling out the myocardial necrosis. According to the nature of poisonous animals, cardiotoxic effects of scorpion venoms could be explained with prevention or releasing of neurotransmitters including acetylcholine and epinephrine from nerve terminals inducing changes in heart rate and contractility (Tarasuik et al. 1994, Sauviat et al. 1995).
Our data suggest that H. lepturus venom could be suppressing, at least partially on the adrenoceptors mimicking agent presenting in venom or from varicosities in the heart like other poisonous creatutres (Gomes et al. 2010) in addition to direct binding to its myocardial receptors that their shape and origin will be clarified in further studies. In our experiment, pre and post injection of epinephrine (10 μl) counteracted negative inotropic and chronotropic effects induced with H. lepturus venom. It should be noted that coronary blood flow was not decreased by pre and post venom injection of adrenaline (data not shown) indicating H. lepturus indirect effect via supression of β receptors as one of the most autonomic determinants of cardiac contractility and coronary perfusion (Gordon et al. 1998). In a parallel study, preinjection of propranolol (10 μl) as a sympathetic antagonist had an additive role on cardiotropic effects of H. lepturus venom supporting our hypothesis (Fig. 7).
Based on our study pre venom injection of atropine or incubated mixture of venom with this drug, had significant neutralizing effect in contractility following envenomation clearly showing that negative inotropic effect of this venom possibly was also dependent on cholinergic system by direct stimulation or activation of muscarinic receptors in atria with releasing of acetylcholine from nerve terminals like Tityus cerrulatus scorpion venom (Teixeira et al. 2001). Post venom injection of Atropine had no neutralizing effect on cardiotropic aspects possibly due to binding of the venom to its ion channel receptors strongly. There is a great debate about the efficacy of scorpion antivenom in neutralization of the detrimental effects induced by envenomation (Gueron et al. 1967, Abroug et al. 1999, Ismail 2003, Chatzaki et al. 2012) since clinical efficacy of treatment for envenomed patients is affected deeply with the time between scorpion sting and administration of antivenom. Generally H. lepturus venom like other poisonous animals have low molecular weight diffusing rapidly to target organs including kidney and heart while the heavy chain molecules of antivenom have not this ability making it unsuitable for treating envenomed patients (Ismail et al. 1983, Seyedian et al. 2010, Seyedian et al. 2012).
In our experiment, prevenom injection of antivenom in addition to incubation of venom and antivenom completely antagonized all deterimental effects including negative inotropic, chronotropy, decreasing coronary blood flow and even late arrhythmogenic effects (bradycardia, QRS prolongation and ST depression after 60 min) in isolated rat hearts while post venom injection had no effect. It seems that antivenom could bind firmly to venom and prevents its direct and indirect effects while injected before envenomation but due to its high molecular weight structure and different pharmacokinetic and biodistribution profile versus H. lepturus venom had no treating effects in envenomed hearts even when injected 5 min after envenomation (data not shown). Scorpion venoms generally act mainly on Na+ and to lesser degrees on calcium and potassium channels provoking electrocardiographic changes (Gordon et al. 1998). Arrhythmogenic changes in our envenomed hearts could be induced by late ischemia or prolongation of repolarization phase with some purified toxins from H. lepturus as Hemicalcins and Heminecrolysin (Shahbazzadeh et al. 2007, Borchani et al. 2011) acting on ion channels. Those effects were neutralized by prevenom injection of Razi Institute multivalent antivenom. Arrythmogenic changes with venom including QRS prolongation and ST depression were not observed during treatments with other drugs (atropine, propranolol and adrenaline) and antivenom as control.
Conclusion
It seems that cardiotropic changes including negative inotropism and chronotropism of H. lepturus venom in addition to late arrhythmogenic manifestations has a detrimental effect in heart failure induced by envenomation in human beings. We believe that indirect and direct mechanisms are involved in this phenomenon since injection of adrenaline and atropine according to their high and low presence in the rat heart changed our results.
Pre venom injection of Razi Institute antivenom as the customary treatment could neutralize its cardiotropic effects but unfortunately it had no effect even 5 min after envenomations making its beneficial role on detrimental cardiac consequences questionable.
Acknowledgments
The authors would like to thank the authorities of Bushehr University of Medical Sciences for providing facilities.
References
- Abroug F, Elatrous S, Nouria S, Haguiga H, Touzi N, Bouchoucha S. ( 1999) Serotherapy in scorpion Envenomation: a randomized controlled trial. Lancet. 354: 906– 909. [DOI] [PubMed] [Google Scholar]
- Bawaskar H, Bawaskar P. ( 1998) Indian red scorpion envenoming. Ind J Pediatr. 65: 383– 391. [DOI] [PubMed] [Google Scholar]
- Bey TA, Walter FG, Lober W, Schmidt J, Spark R, Schlievert PM. ( 1997) Loxosceles arizonica Bite Associated with Shock. Ann Emerg Med. 30: 701– 703. [DOI] [PubMed] [Google Scholar]
- Borchani L, Sassi A, Shahbazzadeh D, Strub JM, Tounsi-Guetteti H, Boubaker MS, Akbari A, Van Dorsselaer A, El ayeb M. ( 2011) Heminecrolysin, the first hemolytic dermonecrotic toxin purified from scorpion venom. Toxicon. 58: 130– 139. [DOI] [PubMed] [Google Scholar]
- Chatzaki M, Horta C, Almeida M, Pereira N, Mendes T, Dias-lopes C, Guimarães G, Moro L, Chávez-olórtegui C, Horta M. ( 2012) Cutaneous loxoscelism caused by Loxosceles similis venom and neutralization capacity of its specific antivenom. Toxicon. 60: 21– 30. [DOI] [PubMed] [Google Scholar]
- Cupo P, Hering SE. ( 2002) Cardiac troponin I release after severe scorpion envenoming by Tityus serrulatus. Toxicon. 40: 823– 830. [DOI] [PubMed] [Google Scholar]
- Dehghani R, Fathi B. ( 2012) Scorpion sting in Iran: A review. Toxicon. 60: 919– 933. [DOI] [PubMed] [Google Scholar]
- Gomes HL, Andrich F, Mauad H, Sampaio KN, De lima ME, Figueiredo SG, Moyses MR. ( 2010) Cardiovascular effects of scorpionfish (Scorpaena plumieri) venom. Toxicon. 55: 580– 589. [DOI] [PubMed] [Google Scholar]
- Gordon D, Savarin P, Gurevitz M, Zinnjustin S. ( 1998) Functional anatomy of scorpion toxins affecting sodium channels. Toxin Rev. 17: 131– 159. [Google Scholar]
- Gueron M, Stern J, Cohen W. ( 1967) Severe myocardial damage and heart failure in scorpion sting: Report of five cases. Am J Card. 19: 719– 726. [DOI] [PubMed] [Google Scholar]
- Ismail M. ( 2003) Treatment of the scorpion envenoming syndrome: 12-years experience with serotherapy. Int J Antimicrob Agents. 21: 170– 174. [DOI] [PubMed] [Google Scholar]
- Ismail M, Shibl A, Morad A, Abdullah M. ( 1983) Pharmacokinetics of 125 I-labelled antivenin to the venom from the scorpion Androctonus amoreuxi. Toxicon. 21: 47– 56. [DOI] [PubMed] [Google Scholar]
- Jalali A, Pipelzadeh MH, Sayedian R, Rowan E. ( 2010) A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpius lepturus (Hemiscorpiidae) in Iran. Toxicon. 55: 173– 179. [DOI] [PubMed] [Google Scholar]
- Latifi M, Tabatabai M. ( 1979) Immunological studies on Iranian scorpion venom and antiserum. Toxicon. 17: 617– 620. [DOI] [PubMed] [Google Scholar]
- Mirakabadi A, Khatoonabadi S, Teimoorzadeh S. ( 2011) Antivenom injection time related effects of Hemiscorpius lepturus scorpion envenomation in rabbits. Arch Razi Inst. 66: 139– 145. [Google Scholar]
- Mirakabadi A, Khatoonabadi S, Teimourzadeh S, Sabiri GH. ( 2010) Serum enzymes studies in scorpion (Hemiscorpius lepturus) dose related envenomation in rabbits. Arch Razi Inst. 65: 83– 89. [Google Scholar]
- Prendini L. ( 2000) Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): an exemplar approach. Cladistics. 16: 1– 78. [DOI] [PubMed] [Google Scholar]
- Radmanesh M. ( 1990) Clinical study of Hemiscorpion lepturus in Iran. J Trop Med Hyg. 93: 327– 332. [PubMed] [Google Scholar]
- Sauviat MP, Garnier P, Goudey-perriere F, Perriere C. ( 1995) Does crude venom of the stonefish (Synanceia verrucosa) activate β-adrenoceptors in the frog heart muscle? Toxicon. 33: 1207– 1213. [DOI] [PubMed] [Google Scholar]
- Seyedian R, Jalali A, Babaee M, Pipelzadeh M, Rezaee S. ( 2012) A biodistribution study of Hemiscorpius lepturus scorpion venom and available polyclonal antivenom in rats. J Ven Anim Toxins Trop Dis. 18: 375– 383. [Google Scholar]
- Seyedian R, Pipelzadeh MH, Jalali A, Kim E, Lee H, Kang C, Cha M, Sohn ET, Jung ES, Rahmani AH , Mirakabady AZ. ( 2010) Enzymatic analysis of Hemiscorpius lepturus scorpion venom using zymography and venom-specific antivenin. Toxicon. 56: 521– 525. [DOI] [PubMed] [Google Scholar]
- Shahbazzadeh D, Amirkhani A, Djadid ND, Bigdeli S, Akbari A, Ahari H, Amini H, Dehghani R. ( 2009) Epidemiological and clinical survey of scorpionism in Khuzestan province, Iran (2003). Toxicon. 53: 454– 459. [DOI] [PubMed] [Google Scholar]
- Shahbazzadeh D, Srairi-abid N, Feng W, Ram N, Borchani L, Ronjat M, Akbari A, Pessah IN, De waard M, El ayeb M. ( 2007) Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem J. 404: 89– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira MY, Haviv YS, Sviri S. ( 1998) Second degree atrio-ventricular block and cardiotoxicity secondary to envenomation by the scorpion Leiurus quinquestriatus (Yellow Scorpion')-an indication for serotherapy? Hum Exp Toxicol. 17: 541– 543. [DOI] [PubMed] [Google Scholar]
- Shayesteh AA, Zamiri N, Peymani P, Zargani FJ, Lankarani KB. ( 2011) A novel management method for disseminated intravascular coagulation like syndrome after a sting of Hemiscorpius lepturus: a case series. Trop Biomed. 28: 518– 523. [PubMed] [Google Scholar]
- Srairi-abid N, Shahbazzadeh D, Chatti I, Mlayah-bellalouna S, Mejdoub H, Borchani L, Benkhalifa R, Akbari A, El ayeb M. ( 2008) Hemitoxin, the first potassium channel toxin from the venom of the Iranian scorpion Hemiscorpius lepturus. FEBS J. 275: 4641– 4650. [DOI] [PubMed] [Google Scholar]
- Tarasiuk A, Sofer S, Huberfeld SI, Scharf SM. ( 1994) Hemodynamic effects following injection of venom from the scorpion Leiurus quinquestriatus. J Crit Care. 9: 134– 140. [DOI] [PubMed] [Google Scholar]
- Tavares FL, Sousa-e-silva MCC, Santoro ML, Barbaro KC, Rebecchi IMM, Sanomartins IS. ( 2004) Changes in hematological, hemostatic and biochemical parameters induced experimentally in rabbits by Loxosceles gaucho spider venom. Hum Exp Toxicol. 23: 477– 486. [DOI] [PubMed] [Google Scholar]
- Teixeira A, Fontoura B, Freire-maia L, Machado C, Camargos E, Teixeira M. ( 2001) Evidence for a direct action of Tityus serrulatus scorpion venom on the cardiac muscle. Toxicon. 39: 703– 709. [DOI] [PubMed] [Google Scholar]