SUMMARY
Deciphering the neural mechanisms of social behavior has propelled the growth of social neuroscience. The exact computations of the social brain, however, remain elusive. Here we investigated how the human brain tracks ongoing changes in social relationships using functional neuroimaging. Participants were lead characters in a role-playing game in which they were to find a new home and a job through interactions with virtual cartoon characters. We found that a two-dimensional geometric model of social relationships, a “social space” framed by power and affiliation, predicted hippocampal activity. Moreover, participants who reported better social skills showed stronger covariance between hippocampal activity and “movement” through “social space.” The results suggest that the hippocampus is crucial for social cognition, and imply that beyond framing physical locations, the hippocampus computes a more general, inclusive, abstract, and multidimensional cognitive map consistent with its role in episodic memory.
INTRODUCTION
Human social skills appear exceptional when compared to those of other animals, suggesting that the “social brain” evolved recently and is perhaps unique to humans. Neuroimaging has identified several structures specialized in processing social information (Adolphs, 2010; Ochsner and Lieberman, 2001). The functional anatomy of the social brain remains elusive, however, because social cognition does not simply map onto anatomically defined brain regions. Activity across many cortical regions, including parietal, temporal, prefrontal, and cingulate areas, varies with many social processes, including social perception, theory of mind, impression formation, and self-reflection. Yet the structural and functional definition of the social brain and the specific computations it performs are obscure (Stanley and Adolphs, 2013). Comparative neuroscience reveals that nonhuman primates modify future social interactions via the outcome of past encounters with individuals, showing that memory for past social events guides adaptive social behavior (Parr et al., 2000). Across species, the hippocampus is crucial for episodic memory: the personal, spatial, and temporal context of events (Eichenbaum and Cohen, 2014). Here we show that the hippocampus constructs an abstract geometric representation of social relationships during social interactions.
To investigate how the brain tracks social relationships, we developed a “choose-your-own-adventure” game in which participants played the lead role and interacted with six characters during functional neuroimaging (fMRI). The participants made choices throughout the game that reflected their social view of the characters along the two main factors that influence relationships: power (being submissive or authoritative) and affiliation (sharing private information or physical touch) (Fiske, 2012; Harris and Fiske, 2007; Todorov et al., 2005, 2008; Wiggins, 1979; Wiggins et al., 1989). Unlike previous social fMRI tasks, this task was designed to mimic real-life social interactions as a dynamic, rather than static, process. The design thereby helped to identify the neural computations that track ongoing social relationships over time. During these social interactions, the hippocampus represented relationships with other people as their location in a two-dimensional space centered on the self and framed by power and affiliation. Hippocampal activity varied with social distance defined by a vector from the participant to a character in an abstract space. This metric quantified social relationships between the participant and each character during each social interaction as a function of power and affiliation, and corresponded well with the participant’s subjective evaluations of the characters obtained after the social game. Moreover, the correlation between hippocampal activity and social locations was higher in participants who reported better social skills, as though “tracking” the outcome of social encounters with relatively high fidelity helps guide adaptive social behavior in real-world encounters. These findings suggest that the hippocampus constructs cognitive maps across domains that include, but are not limited to, two-dimensional Euclidean spaces (Tolman, 1948) (details in the Social Maps section below). Episodic memories encoded by the hippocampus within abstract cognitive maps may guide social navigation, and hippocampal dysfunction may contribute to maladaptive social behavior in previously unexpected ways.
Theoretical and Experimental Accounts of Social Relationships
Theories in social psychology and experimental evidence across species identify two main factors that define social relationships: power (competence, dominance, hierarchy, etc.) and affiliation (warmth, intimacy, trustworthiness, love, etc.). The interpersonal circle theory of personality (Wiggins et al., 1989) first proposed a representation of social relationships as vectors in a two-dimensional space. Empirical evidence for this geometric model quantified behavior with interpersonal adjectives scales: participants described themselves in relation to others using classifications like “arrogance” and “assurance”—the dominance dimension—and qualities like “agreeableness” or “gregariousness” —the love dimension (Wiggins, 1979). The stereotype content model (Fiske, 2012) used two similar axes to describe how we evaluate individuals in our social environment: an axis of warmth, our perception of the intentions of others, and an axis of competence, our perception of others’ ability to act on their intentions. The interaction between the two axes produces four quadrants and four possible stereotypes. Assigning people to one of the four stereotypes allows swift judgment of others (Harris and Fiske, 2007). The face evaluation model also proposed that two similar major axes, trustworthiness/valence and dominance/power, define face assessment (Todorov et al., 2005, 2008). This model proposes that people use facial features to evaluate others within this 2D space, and these evaluations predict the outcome of social behaviors as significant as election results.
Analogous models of social behavior apply to other species: non-human primates (Brent et al., 2013), birds (Fernald and Scharff, 2010), bees (Insel and Fernald, 2004), hyenas (Fernald, 2014), and fish (Fernald and Maruska, 2012). These studies describe social relationships using the same two dimensions of power (dominance, aggression) and affiliation (reproductive ties, parental bonding, kinship). Because rapid assessment of kin and status is fundamental for reproduction and survival of social animals, neural mechanisms for tracking power and affiliation during social interactions likely evolved early and were conserved in mammals.
Power and affiliation are forms of psychological distance. The construal level theory (Trope and Liberman, 2010) suggests that common cognitive mechanisms process different types of psychological distance, whether temporal, spatial, or social. Though social position, physical location, and temporal distance use distinct representations, the construal level theory proposes that each of these dimensions computes egocentric psychological distance. This view suggests that power and affiliation should be computed from an egocentric reference point. Several specific brain regions represent participants’ assessment of power (social status in the community) (Muscatell et al., 2012) or affiliation (familiarity of acquaintances) (Parkinson et al., 2014). Activity in these regions shows affiliation and power as independent factors with separate neural computations. Social theories (Fiske, 2012; Todorov et al., 2008; Wiggins et al., 1989), however, suggest that the interaction between power and affiliation, rather than each factor separately, is the major determinant of social perception. If such interactions are implemented directly in specific brain circuits, then their neural activity should co-vary with both power and affiliation, placing others in a two-dimensional social space at varying distance from ourselves.
Social Maps
The hippocampal system is crucial for remembering locations in physical space in many species (Bird and Burgess, 2008; Derdikman and Moser, 2010). O’Keefe and Nadel (1978) emphasized that the hippocampus represents locations within two-dimensional Euclidean space in rats, and extended the idea to more general cognitive mapping functions in humans. They defined “influence,” for example, as a non-Euclidean spatial dimension required to describe causal relationships in human language. The same neural systems used for computing two-dimensional Euclidian locations could also generalize to other abstract, higher-dimensional “spaces” and provide the same representational power. Relational memory theory emphasizes this view, proposing that hippocampal computations localize an individual in abstract “life” spaces (Eichenbaum and Cohen, 2014). Consistent with the “global amnesia” that follows hippocampal damage, these computations represent relationships among items that co-vary systematically (Eichenbaum and Cohen, 2014).
The same neural mechanisms that support episodic memory and spatial navigation may compute general conceptual spaces and provide a novel approach for investigating the social brain. Personal perspectives on the goal and outcome of social interactions frame social episodes, and remembering these events within appropriate social contexts is crucial for guiding adaptive responses. Representing one’s social positions with respect to others in terms of their power and affiliation exemplifies an abstract space, a social map constructed through episodic interactions. Just as “distance” and “direction” describe the similarity and independence of variables generally, they apply to social relationships that vary in power and affiliation. “Climbing the social ladder,” having a “tight social circle,” and “feeling close to someone” are metaphors that may reflect spatial computations that “place” individuals in an abstract space defined by the salient features and outcomes of social events.
Taken together, these ideas suggest that a hippocampal social map could guide social navigation. Geometric models of space measure interactions between one or more dimensions. Vectors in two-dimensional spaces connect two points and define values on both dimensions. We theorized that during social interactions, the brain generates an egocentric representation of another’s position in a two-dimensional space framed by power and affiliation. Within this space, the brain represents others with respect to ourselves as vectors that signify the social relationship indicated by a given interaction. In this model, the orientation of the vector indicates the interaction between power and affiliation assigned to a particular individual relative to ourselves. The length of the vector represents the absolute “social distance”: lower affiliation and larger differences in power. By updating the values of the vector’s orientation and length during each episode, the brain tracks changes in power and affiliation indicated by the social interaction (see next section for details). This theoretical formulation predicts the following: social encounters should be represented in an abstract two-dimensional space from an egocentric point of view, representations of perceived social distances should engage the hippocampal system, and the extent to which social relationships are encoded in a spatial reference frame should predict adaptive social function.
RESULTS
Testing Geometric Modeling of Social Relationships
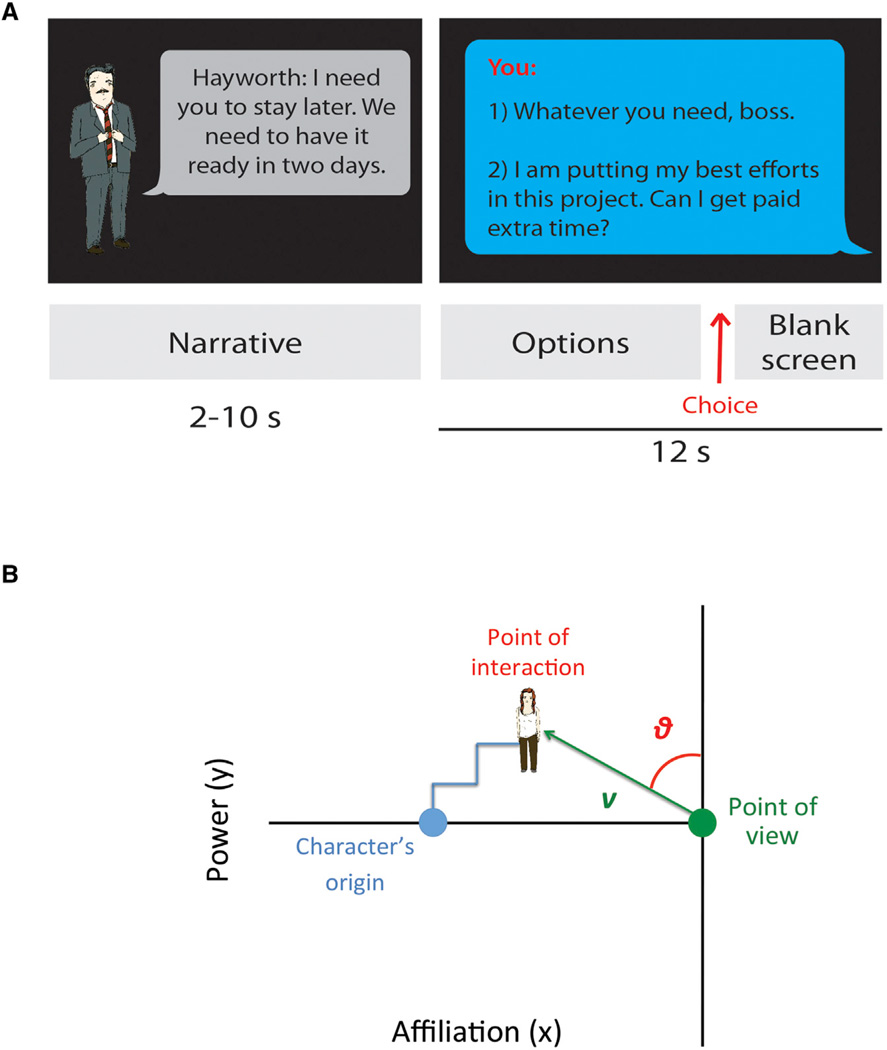
We tested these hypotheses using a role-playing game played by participants in the fMRI scanner (see Experimental Procedures). We told participants that they would be playing a social game in which they were moving to a new town, and that their goal was to find a job and a place to live by getting acquainted with the town’s people. During the game, the participants were shown slides of cartoon characters “speaking” through word bubbles. The slides included characters’ images and text and were devoid of visual indications of any spatial context. Each character had unique characteristics that indicated potential social position. One character, for instance, was an old friend from high school, while another owned a company where the participant might find a job. The participants responded to each social interaction slide by pressing one of two keys on a button box that selected one of two “replies” to the character. As in “choose-your-own-adventure” games, the participants followed the same story line, but their choices shaped the narrative of the story (Figure 1A; see Table S1 for examples of interactions).
Figure 1. Experimental Design.
(A) Schematic depiction of trial types and task structure. The example shows a power interaction (see Table S1 for examples of interactions; see Tables S2–S4 and Figure S4 for analysis of the Narrative and Options conditions).
(B) Schematic depiction of the geometrical representation of social coordinates. The example shows a character moving through the course of four social interactions (blue line trajectory to point of interaction from the character’s origin). To calculate social coordinates, we drew a vector between the theoretical point of view (maximum intimacy, neutral power) and the character’s position. We calculated the vector length (V) and the vector angle (θ) for each social interaction (see Figure S3 for analysis of a non-egocentric angle).
The outcome of each social interaction reflected changes in either the power or affiliation between the participant and the character. For example, the participant’s power over a character was signaled by deciding whether or not to comply with a character’s demand; affiliation with a character was signaled by engaging or not in personal conversation. The type of social interaction (power or affiliation) and the value of each choice (more or less power or affiliation) were validated separately (see Experimental Procedures). The participants’ choices were based on personal preference, representing a social dynamic for each character, a series of changes in power and affiliation depicted as a trajectory through a social space framed by power and affiliation (Figure 1B, blue line trajectory).
The final location of a character represented the outcome of all interactions, an individual “map” of each character’s “movement” through each participant’s unique social space (see Figure 2A for examples of individual trajectories in 3D, and Figure S1 for more examples of trajectories in 2D in different participants). The social map was represented as a theoretical game board. Because we measured relational distance, we ascribed a common origin for all presented characters: the starting point of each character in relation to the subject was distant from the participant’s point of view on the affiliation axis (reflecting a neutral distance and allowing the character to move toward or away from the participant across interactions), and at the same level on the power axis (reflecting neutral power from which the character could move above or below the participant) (Figure 1B). Though a character’s description could indicate a pre-assigned social role, only the participants’ choices during social interactions revealed their actual responses to this information. The accumulated responses defined the participant’s social view of a given character. Thus, all characters were assumed to acquire locations in a participant’s social space during the first interaction, and from then on change power and affiliation with respect to the participant.
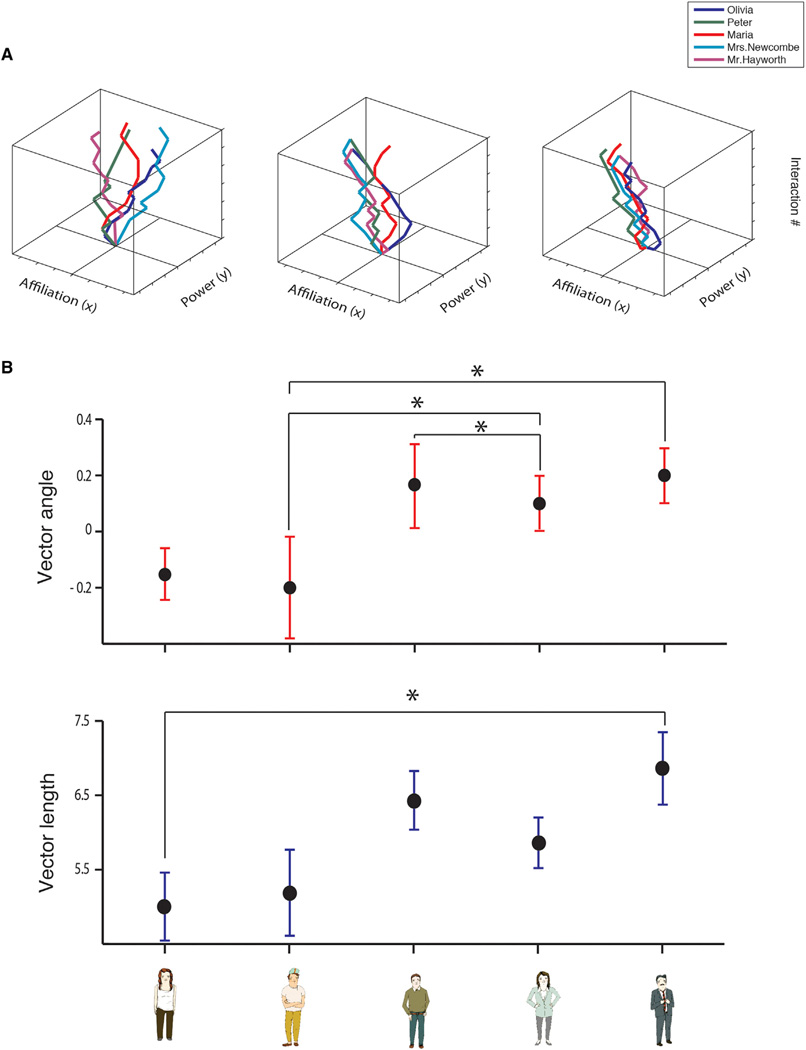
Figure 2. Participants’ Behavior in the Task.
(A) Examples of characters’ trajectories from three participants in a 3D view. The x axis represents affiliation, the y axis represents power, and the z axis represents the 12 total social interactions with each character (see Figure S1 for examples of 2D trajectories).
(B) Mean and SD values of the vector angle (cosine θ) and length (V, arbitrary units) at the final time point for each of the five main characters (the sixth character served as control and did not change position; see Experimental Procedures). One-way ANOVA with a main factor of character type yielded a significant main effect for the vector angle and vector length (F = 5.46, F = 2.99, respectively; p < 0.05). Asterisks represent significant difference between two corresponding characters (post hoc t tests, p < 0.05).
To calculate a geometric proxy of social relationships over time we determined the characters’ location in the participants’ social space during each interaction. Locations in a two-dimensional space can be represented interchangeably as either Cartesian (x and y axis values) or polar coordinates (angle and length of a vector drawn from a given reference point). A Cartesian model represents power and affiliation as independent dimensions with locations measured from a neutral origin. Because social theories advocate the interdependency between power and affiliation, and that both dimensions are “seen” from an egocentric point of view, we modeled social relationships using a polar coordinate system. This representation emphasizes the combined contribution of both dimensions and allows the participant’s point of view to serve as the reference point (the two models are compared later).
The characters moved in discrete fixed steps either up or down along the power axis (above or below the participant’s point of view) or back and forth on the affiliation axis (closer to or away from the participant’s point of view), and we calculated the angle (θ) and the length (V) of the vector in each interaction throughout the game. The vector angle (orientation) represents the normalized function of power modulated by affiliation, and the vector length represents the absolute “social distance,” i.e., lower affiliation and larger differences in power (low abscissa values indicate “proximity,” locations near the origin of a social space centered on the participant). Because equal increments in power (ordinate) produce larger changes in angle as the abscissa approaches 0, the effect of power is magnified by affiliation (and reduced by “social distance”). Together, the vector angle and length described the specific location of each character in the participant’s theoretical social space, and were used to predict neural activity during each interaction throughout the social navigation game.
Neural Tracking of Social Coordinates
To identify neural signatures tracking the vector angle and length during social interactions, we compared two types of trials: narrative (slides in which the storyline develops) and options (slides that prompted participants to choose one of two possible options of interacting with the characters). After making a choice, the participants saw a blank screen for the remaining time, and these inter-trial intervals were treated as baseline activity (Figure 1A). We hypothesized that the neural tracking of social coordinates occurs at the time of choice, where the participants “move” the position of the character according to perceived social space. We therefore calculated a parametric weight for the options condition, based on the coordinate values of each character as determined by the participants’ choice at each options trial. The value for the parametric modulator indicated the updated relationship status (see below tests of alternative hypotheses and model validation analyses).
A total of 18 participants completed the experiment (mean age = 29.7 ± 3.4 years, age range = 24–34 years, 10 males). The mean final values of the vector angle and length, corresponding to the final power and affiliation assigned to each character across participants, are depicted in Figure 2B. The variance in each character’s final location captures the individual differences among participants. The differences between characters within each participant reflect the characters’ perceived social role. Thus, some characters tended to gain relatively more power and/or more affiliation compared to other characters depending on their particular role in the storyline (see Experimental Procedures for plot summary), but these positions varied across participants.
To identify the neural correlates of the characters’ location in social space, we conducted a whole-brain analysis using a general linear model (GLM) consisting of separate regressors for the narrative and options conditions, as well as a parametric regressor for the social coordinates, consisting of either the vector angle or length values throughout the task during the options condition. All analyses were corrected for multiple comparisons using cluster size threshold ensuring FWE rate of p < 0.05 (see Experimental Procedures for full details).
Hippocampal Activity Tracks Power Modulated by Affiliation
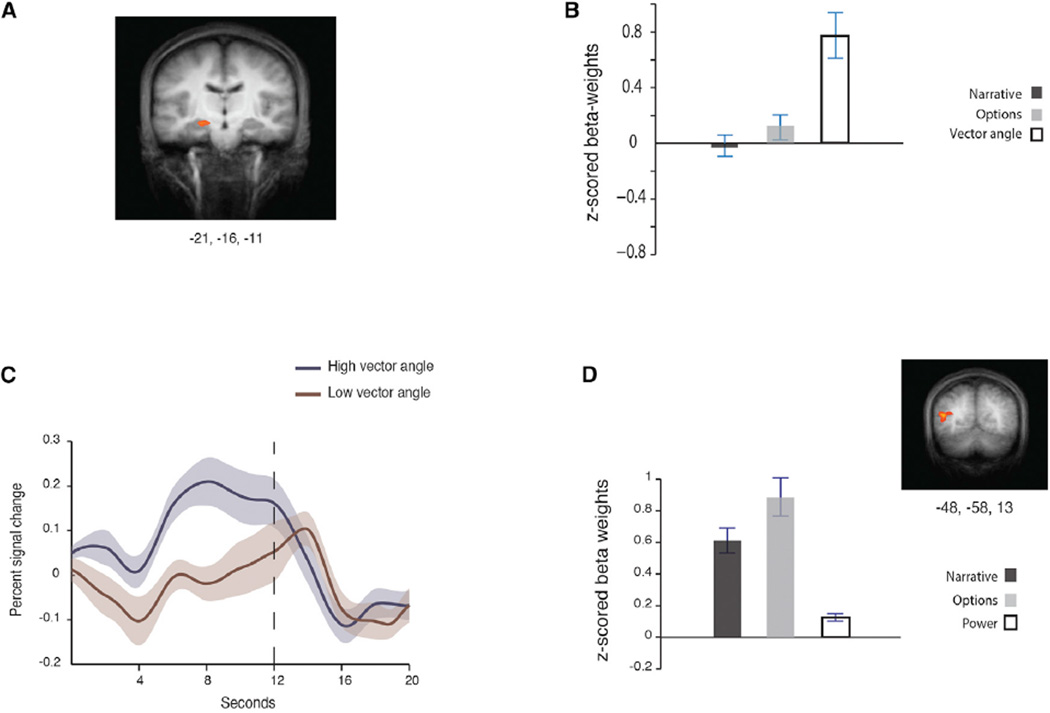
To test our main hypothesis, we contrasted the parametric angle regressor against baseline (see below analyses of the other regressors). Consistent with our prediction, we found that blood oxygen level-dependent (BOLD) signal in the left hippocampus correlated with the vector angle, signifying the characters’ location in social space as the interaction between their power and affiliation relative to the participant (Figure 3A). In contrast, the hippocampal BOLD response did not correlate with the narrative or options regressors (Figure 3B; see below additional analyses testing alternative hypotheses). To examine the direction of the parametric modulation in this region, we inspected the BOLD response divided by low and high angle values (Figure 3C). The left hippocampus mean percent signal change was higher when the character was perceived as having more power modulated by affiliation (cosine θ closer to 1).
Figure 3. Neural Correlates of Power Modulated by Affiliation, Measured by the Vector Angle.
The regressor tracking the angle between the participant’s point of view and the character (cosine θ), which corresponds to a character’s interaction between power and affiliation at each social interaction throughout the task, was contrasted with baseline in a whole-brain analysis.
(A) The resulting statistical map overlaid on the groups’ average anatomical image shows the hippocampus (p < 0.001, FWE rate of p < 0.05; K = 33). Talairach coordinates (x, y, z) of the peak voxel are indicated below the maps (see Figure S2 for three other regions).
(B) The Z-scored beta weights extracted from that peak voxel are shown for each condition.
(C) Options trials were separated into two types: high cosine θ and low cosine θ. Event-related averaging of the hippocampal mean percent signal change (from the entire cluster) shows higher BOLD responses for options trials where characters are perceived as having more power relative to the participant, modulated by affiliation (cosine θ closer to 1).
(D) The parametric regressor tracking the character’s power as a Cartesian coordinate at each social interaction throughout the task correlated with the BOLD signal in the left middle temporal gyrus (BA 37). The right panel shows the resulting statistical map overlaid on the groups’ average anatomical image (p < 0.001, FWE rate of p < 0.05; K = 32). Talairach coordinates (x, y, z) of the peak voxel are indicated below the maps. The left panel shows the Z-scored beta weights extracted from that peak voxel for each condition. Error bars indicate SEM.
Three other brain regions showed BOLD correlations with the vector angle (Figure S2). The left inferior parietal lobule (BA 39) has been linked with spatial-visual, language, and arithmetic skills (Dehaene, 2009; Krause et al., 2014; Muscatell et al., 2012). Notably, this region was specifically involved in associating numbers with a spatial representation (Dehaene, 2009; Krause et al., 2014), and computed a common representation of distance from oneself in either spatial, temporal, or social familiarity domains (Muscatell et al., 2012). The left dorsolateral prefrontal cortex (dlPFC; BA 9) may contribute to executive control, spatial working memory, and goal-directed behavior, especially in a social context (Courtney et al., 1997; du Boisgueheneuc et al., 2006; Gariépy et al., 2014; Haxby et al., 2000; Miller and Cohen, 2001). The pre-supplementary motor area (pre-SMA; BA 6), extending to the dorsal anterior cingulate cortex (dACC; BA 32), has been specifically linked to movement planning, motor decision making, attention, and orientation (Shenhav et al., 2013). The BOLD signal in this region did not correlate uniquely with the angle regressor, but also correlated with the non-parametric options regressor (Figure S2C), possibly reflecting the decision-making process and the motor response during the options trials. None of these regions was activated during the narrative trials.
Tracking Social Coordinates in the Hippocampus Correlates with Social Skills
If hippocampal activation during experimental social interactions is relevant to actual social navigation, then it should correlate with social skills. To test this hypothesis, the participants filled out questionnaires assessing social anxiety, social effectiveness, and personality traits (see Experimental Procedures) after the fMRI task. We examined the correlation between the participants’ behavioral scores and the beta-weights of their angle regressor (Figure 4). Hippocampal parametric activity associated with the angle values (power modulated by affiliation) correlated negatively with social avoidance (Pearson’s r = −0.52, p = 0.03) and neuroticism (r = −0.69, p = 0.002), and correlated positively with conscientiousness (r = 0.53, p = 0.03; this correlation did not survive correction for multiple comparisons). These correlations were unique to the hippocampus. The three other brain regions that tracked the vector angle did not correlate with the questionnaire scores.
Figure 4. Hippocampal BOLD Correlations with Social Skills and Personality Scores.
Significant linear correlations (Pearson’s r, p < 0.05) between the hippocampal beta weight values of the vector angle regressor, corresponding to the interaction between power and affiliation, and social avoidance (Liebowitz Social Anxiety Scale, avoidance dimension), neuroticism (Neo Personality Inventory), and conscientiousness (Neo Personality Inventory).
Together, these findings suggest that hippocampal BOLD signal during social navigation correlates with social skills and personality traits. Participants exhibiting stronger hippocampal tracking of the characters’ relative movement in power and affiliation reported being less socially avoidant and neurotic, and more conscientious. Hippocampal function during social navigation, therefore, appears to be linked to social and psychological wellbeing.
The Posterior Cingulate Cortex Tracks Social Distance
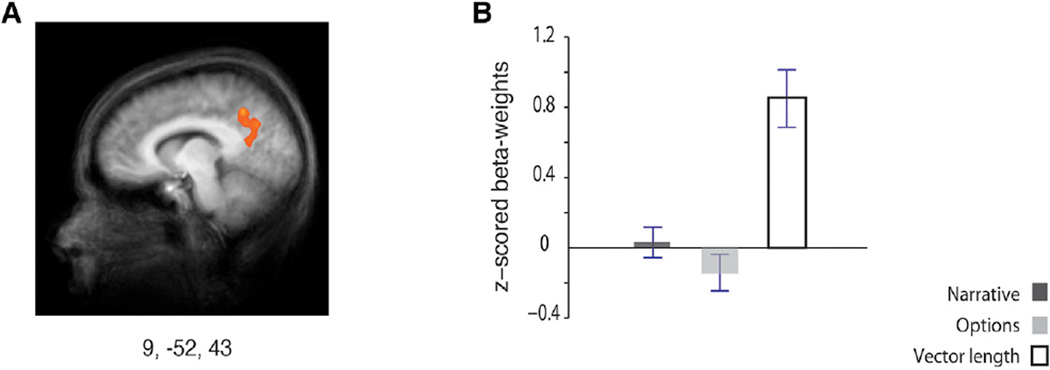
Though hippocampal activity tracked the relative interaction between power and affiliation assigned to each character (the vector angle), it did not predict the absolute social distance between the character and the participant (the vector length). Analyzing brain activity that tracked the vector length investigated how different brain regions represented social distance, reflecting changes in affiliation and differences in power (higher values indicate larger distance influenced by both power and affiliation). A whole-brain analysis found one region that tracked vector length: the precuneus/posterior cingulate cortex (PCC; BA 31; Figure 5A). The PCC has been linked with first impressions of other people (Schiller et al., 2009) and dynamic updating of these impressions (Mende-Siedlecki et al., 2013). The BOLD response in the PCC/precuneus did not correlate with the narrative or options regressors (Figure 5B), nor did it correlate with social skills assessed by the questionnaire scores. By comparison, if hippocampal activity guides social navigation by locating people within a personal context framed by power and affiliation, then PCC/precuneus activity signals only the social distance between others and ourselves.
Figure 5. Neural Correlates of the Magnitude of Social Distance, Measured by the Vector Length.
The regressor tracking the length of the vector drawn between the participant’s point of view and the character, which corresponds to the magnitude of a character’s social distance to the participant at each social interaction throughout the task, was contrasted against baseline in a whole-brain analysis. The resulting statistical map overlaid on the groups’ average anatomical image (left panel) revealed a single region (p < 0.005, FWE rate of p < 0.05; K = 82), the PCC/precuneus. Talairach coordinates (x, y, z) of the cluster’s peak voxel are indicated below the map. The bar graph of the mean Z-scored beta weights extracted from that peak voxel is shown in the right panel. Error bars indicate SEM.
Testing Alternative Hypotheses
Independent Representations of Power and Affiliation
Though social psychology theories suggest otherwise, the brain may yet track the power and affiliation of a character as two independent components (x and y axis values in a Cartesian coordinate system), rather than the interaction between them relative to the participant (vector angle and length in a polar coordinate system).To test this alternative hypothesis, we created two separate parametric regressors corresponding to the location of the characters on the affiliation and power axes at each interaction throughout the game and examined their neural correlates. The affiliation axis regressor did not predict significant BOLD activation even at a liberal statistical threshold (p < 0.05, FWE corrected). The power axis regressor revealed one active region in the left middle temporal gyrus (Figure 3D). Previous studies reported language-related activation (Ashtari et al., 2004) in this region, and indeed its activity did not distinguish between narrative and options trials, indicating that it did not specifically track power (Figure 3D). The interaction between power and affiliation signaled by the correlation between the vector angle and hippocampal activity may provide a more precise representation of social coordinates that helps guide specific social interactions.
The Conjunction and Simple Interaction Models
Even if power and affiliation as separate regressors are relatively poor predictors of how the brain tracks the social location of characters during the game, their combined values may still correlate with the BOLD signal. We therefore performed two analyses: a conjunction analysis between power and affiliation as two parametric regressors in the same GLM, and a simple interaction analysis using an alternative GLM with a single parametric predictor calculated from the a priori multiplication of the power and affiliation values. The conjunction analysis found no BOLD signal correlates even at a liberal threshold (p < 0.05, FWE corrected). The simple interaction analysis yielded no significant correlates at the threshold used for the main model analysis (p < 0.001, FWE corrected). This model predicted activation in the prefrontal cortex and ventral striatum at a liberal threshold (p < 0.05, FWE corrected), but activity in these regions was better predicted by the non-parametric regressors of the narrative and options trials, suggesting a more general role in the task. These results indicate that a polar coordinate system, which reflects relative locations as a vector between two points, provides a better model for how the brain may be tracking social relationships.
The Non-egocentric Model
Consistent with the construal level theory (Trope and Liberman, 2010), we hypothesized that tracking a person’s location in social space should be egocentric, i.e., represented from the participant’s point of view. Alternatively, the brain could track the movement of others in a global space independent of our own position. In other words, we might not represent ourselves in the center of the social world, but rather represent the location and movement of others on the axes of power and affiliation as viewed “from above.” To test this hypothesis, we calculated the vector angle and length using the starting point of the characters, rather than the participant’s point of view, as the origin of the vector, which reflected the trajectory of the character alone (Figure S3A). The neural correlates of this angle (p < 0.001, FWE corrected; Figure S3B) revealed a pre-SMA/dACC region (BA 32, overlapping with the region found for the vector angle from the point of view), as well as the insula (BA 13) and left inferior parietal lobule (BA 40), but activity in these regions was strongly correlated with the non-parametric options condition (Figure S3C). This activity pattern suggests that these regions are engaged during social interactions perhaps by contributing to choice selection or action. The more precise correlations of hippocampal activity with specific social choices support the hypothesis that the hippocampus tracks social relationships from an egocentric point of view.
Tracking Social Coordinates during Passive Reception of Social Narrative
As mentioned above, we examined the neural correlates of social coordinates during the time of choice, when the participants “move” the position of characters according to their perceived social coordinates. If the tracking does not depend on active social choices, but rather on the passive reception of social narratives, then brain activity should correlate better during the narrative trial and before the participant’s choice. To test this possibility we measured the correlation between the vector angle defined by the choice and the BOLD activity recorded at the end of the narrative, before the option trials. Placing the coordinates at the end of the narrative did not predict BOLD activation even at a liberal statistical threshold (p < 0.05, FWE corrected), confirming that neural tracking of social coordinates occurs at the time of choice.
Validation of the Results
Ecological Validity
To test if the social coordinates inferred from the participants’ choices in the game reflected the participants’ subjective perception of power and affiliation, we asked participants to place dots with each character’s name on a graph with power and affiliation axes (the first time the participants were exposed to this representation of “social space”). We then measured the difference between the subjective locations and the final locations of the characters in the game quantified by the iterative algorithm that tracked social location during the game. The differences between the subjective and algorithmic locations were compared to differences calculated between algorithmic and randomly generated locations. Across participants, the subjective and game-computed locations were significantly closer to one another than the distance between the game and randomly assigned locations (paired two-tailed t test, p < 0.05). Thus, the locations computed from the participants’ choices during the game reflected their subjective perception of power and affiliation attributed to each character.
Scrambling Analysis
To further verify that the recorded pattern of BOLD results reflected participants’ subjective choices, we scrambled the choice data by assigning each participant social coordinates (parametric regressors of angle and vector length) generated by another participant. No significant BOLD correlates emerged for the scrambled parametric regressors even at a liberal statistical threshold (p < 0.05, FWE corrected).
Narrative and Options Trials
BOLD responses analyzed during narrative or options trials relative to baseline showed the expected engagement of visual and language areas (Tables S1 and S2). The options trials also engaged the left motor cortex, as expected due to participants’ button pressing when making a choice (Tables S2 and S3). The caudate showed enhanced BOLD responses bilaterally during the options trials (Figure S4), consistent with the well-documented role of this region in economic and social decision-making, and motor response (Brosch and Sander, 2013; Fareri and Delgado, 2014; Guitart-Masip et al., 2014; Lim et al., 2011).
Reaction Time
Time to respond during the options trials did not correlate with the vector angle or the vector length regressors in any participant (angle: mean r = −0.075, SD = 0.13, mean p value = 0.48; vector length: mean r = 0.051, SD = 0.10, mean p value = 0.51), precluding reaction time as an intervening factor.
Memory Test
To assess the extent to which memory processing interacted with hippocampal function during the task, we tested each participant’s memory for the characteristics and events related to the different characters (see Experimental Procedures). The participants’ mean memory accuracy overall was 88.9%. Memory performance for all characters was significantly above chance, indicating that memory demands and performance were relatively constant throughout the task, and could not explain variations in the BOLD signal.
DISCUSSION
Hippocampal BOLD activity predicted changes in subjective affiliation and power between people and fictional characters in a virtual role-playing game. The results were best characterized as movement through a social space framed by power and affiliation that depended on active social choices, rather than the passive reception of social narratives. This outcome suggests that beyond metaphorical description, the concept of social “space” may reflect how the brain represents our position in the social world. Spatial descriptions of social location such as “she is above him,” or “he is closer to me,” might reveal a mechanism of social cognition that locates others in a two-dimensional space of power and affiliation. “Finding our place” in a given social environment may be the outcome of navigating through a geometric representation of social relationships.
Previous studies have identified unique brain regions involved in processing different kinds of psychological distance (Addis and Schacter, 2008; Brosch and Sander, 2013; Maglio et al., 2013; Mason and Just, 2011; Muscatell et al., 2012; Parkinson et al., 2014; Tamir and Mitchell, 2011; Weiler et al., 2010). Going beyond a one-dimensional representation of psychological distance, we tested predictions based on two-dimensional space as a reference frame for social interactions, where neural computations of relationships reflect the ongoing interaction between the dimensions of power and affiliation. The results showed that the hippocampus tracks how we represent others in terms of the interaction between power and affiliation, whereas the PCC/precuneus tracks a broader measure of social distance.
Consistent with the general role of hippocampus in relational processing, we suggest that hippocampal activity represents memories for social interactions as vector angles describing the power and affiliation relationships between ourselves and others in an abstract, multidimensional, egocentric memory space. The neuronal activity correlated with vector length represents the absolute psychological distance between ourselves and others. Representations of social events likely include other episodic or contextual information, including place and time as well as outcome expectancies, and social rules. Memories for social events in turn help inform sense of empowerment, obedience, and other aspects of social cognition that are likely computed by other brain regions.
The construal level theory predicts that subjective psychological distance is modified by changes in perceived distance across any number of physical and social dimensions (Maglio et al., 2013; Tamir and Mitchell, 2011). The temporal interval between events, for example, might influence our estimate of their spatial proximity. By the same token, individuals in higher power positions tend to feel more social distance between themselves and others than lower-power individuals (Magee and Smith, 2013; Maglio et al., 2013; Tamir and Mitchell, 2011). These observations, together with the present results, suggest that the brain represents social relationships in a multidimensional space and that the neural basis of social cognition can be described in terms of specific social computations within an abstract geometric framework.
Two related theories of hippocampal function emphasize different computations to account for its role in learning and memory across species. The cognitive map theory posits that the hippocampus supports memory by computing an allocentric or world-centered spatial framework that links items to locations in an environment (O’Keefe and Nadel, 1978). Research guided by this theory focuses largely on spatial tasks solved by computing two-dimensional locations based on the distance and direction between a subject and an environmental context. The relational memory theory suggests that the hippocampus computes a more general framework that links items and events in a “memory space” that includes temporal and personal as well as spatial context (Eichenbaum, 2004; Eichenbaum et al., 1999). Research guided by the memory space theory focuses on how hippocampal function contributes to remembering events distinguished by time, internal states, or stimulus properties that generalize across locations.
Our results complement the previous studies by varying social interactions in a constant spatial environment and testing the extent to which changes in social relationships altered hippocampal activation. In other words, experiences varied within a personal, not a spatial, reference frame. By quantifying the dynamic response patterns of people making decisions based on social interactions, we found that the hippocampus tracks relationships within an egocentric, two-dimensional personal space framed by affiliation and power. Our findings support a broader role for the hippocampus in relational memory, which represents the relationships between items and events that vary in many dimensions, including space, time, motivation, and abstract concepts (Eichenbaum and Cohen, 2014). Though our methods did not explicitly vary mnemonic demands, the results provide new insights into how abstract spatial computations may contribute to episodic memory and language (Maguire and Mullally, 2013; Ryan et al., 2010).
The cognitive map theory argued that the hippocampus encodes allocentric representations, i.e., signals locations of the individual relative to an environmental reference frame (O’Keefe and Nadel, 1978). More recent work suggests that the hippocampus constructs spatially coherent scenes that are representative egocentrically (Maguire and Mullally, 2013). Indeed, neither place nor grid fields are purely allocentric in rats, but are modulated by heading direction, so changes in position in particular directions strongly influence activity (Muller et al., 1994). The present study did not compare egocentric and allocentric tasks and cannot distinguish these claims directly. The present task did not vary physical or virtual space, but varied social interactions that occurred in one place, and measured perceived social “distance” in a personal, egocentric model. Nonetheless, the results fit better to an egocentric than an allocentric model. Changes in locations and distances between characters that could be detected as movement through an allocentric social space (the “non-egocentric model” analysis above) did not predict brain activity, whereas movement of characters with respect to the participant’s egocentric view did, indicating that social processing in the hippocampus is likely framed egocentrically. Consistent with this view, a previous fMRI study that manipulated spatial and relational variables (Kumaran and Maguire, 2005) reported higher correlation of hippocampal activity to memory for where people interacted (whether or not they lived physically closer) than to their social relationships (whether or not they knew one another), suggesting that the hippocampus does not encode an allocentric representation of social relationships. Future studies could use this model to investigate the interaction between the narrative and participants’ predispositions, especially in clinical populations (e.g., modeling social expectancies or prejudice, etc.).
Navigating through social space may be relevant to the many psychiatric disorders that impair social cognition, such as sociopathy, borderline personality disorder, schizophrenia, depression, and autism. Many of these disorders involve hippocampal dysfunction (Amaral et al., 2008; Nunes et al., 2009; Sheline, 2011; Sigurdsson et al., 2010). Some social cognition deficits may be a consequence of hippocampal dysfunction and impaired social navigation. Poor memory for the outcome of social interactions or relative insensitivity to affiliation or power cues could impair tracking others’ social coordinates and impoverish the “social map.” Consistent with this possibility, the present results show that the fidelity of social tracking measured by hippocampal activity correlated with social and personality traits within the normal range of social function. The results predict that an impaired geometric representation of social space in the hippocampus may accompany social dysfunction across psychiatric populations.
EXPERIMENTAL PROCEDURES
Participants
A total of 21 people participated in the study. One participant was excluded following psychiatric evaluation (PDSQ, Psychiatric Diagnostic Screening Questionnaire). Two other participants were excluded due to exaggerated head motion in the fMRI scanner (> 2 mm). The final analysis included 18 medically and psychiatrically healthy adults (mean age = 29.7 years, age range = 24–34 years, 10 males). The Institutional Review Board of the Icahn School of Medicine at Mount Sinai approved the experimental protocol. All participants provided written informed consent and were compensated for their participation.
Task
To create a naturalistic paradigm of social encounters, we wrote a storyline (see below) using the principles of role-playing games and resembling a “choose-your-own-adventure” game. The storyline took each participant through several social interactions. The participants were the protagonists in the narrative and chose how to interact with six characters. Twelve interaction opportunities with each of the five main characters (the sixth character was neutral) were divided into six power and six affiliation interactions. The categorization was validated before the study by eight volunteers asked to make social choices, report their level of engagement with the storyline, and classify each interaction as power or affiliation. We used only interactions that were classified consistently (> 75% agreement between judges, or at least six out of eight judges). Power interactions were defined as giving or receiving orders/instructions/demands (i.e., imperative sentences), and affiliation interactions were defined as engaging or not in personal conversation or accepting/ initiating physical touch (see examples in Table S1). The story also included a sixth character as a control who appeared throughout the story 12 times, with whom the participants had three neutral interactions (e.g., talking about the weather).
After the initial validation we divided the story into scenes, arranged the text as a slide presentation, and hired an illustrator to create cartoons for each character. Narration was presented as simple text on slides. Characters would “talk” among themselves and with the participant using gray word bubbles, and options slides were distinguished by displaying a single blue word bubble with two options to choose from and the indication “YOU:” in red. We estimated an average time for reading each slide, added about an excess second for each, which resulted in each “narrative” slide presented between 2 and 10 s. For options slides, we defined a maximum of 12 s, which was ample time to read, and that would allow creating an intermediate blank screen after the response to be used as a variable inter-trial interval. The Cogent Toolbox (http://www.vislab.ucl.ac.uk/cogent.php) in Matlab (MathWorks) scripted these parameters and presented the task in the scanner. Participants’ choices and reaction times were recorded during presentation. The task was gender balanced by alternating randomly between four versions of the story, in which the genders of the characters were switched, maintaining an equal number of characters of each gender and assigned to equal numbers of male and female participants.
Procedure
The participants were told they would be playing a virtual social game in the scanner, and choosing how to interact with a set of characters by using a button box. While lying down in the scanner, they were shown a first set of two slides where they could read the game’s instructions. The first slide welcomed them to play a “virtual social reality game.” They were asked not to overthink their choices and behave as in real life. They were told characters would speak in gray word bubbles, and they would be prompted to make choices on how to interact with characters in the blue word bubble slides by picking choice 1 or 2 by pressing keys 1 (index finger) or 2 (middle finger) in the button box. The assignment of keys to choice direction was counterbalanced (for example, in some trials key 1 indicated choice of more power, whereas on other trials key 1 indicated the opposite direction). The participants acclimated to the setting before the story started by viewing the characters’ cartoons (6 s each) at the beginning and the end of the task (trials excluded from main design). The participants were instructed to press the button box keys for testing. The storyline was introduced in a slide telling the participants that they just moved to a new town, “Greenville,” and had to find a job and a place to live while getting acquainted with the town’s people. After the participants read the instructions, we asked (by intercom) if everything was clear and if they were ready to start. The functional scan started, the story presentation began, and the participants followed along and made choices throughout the 26 minutes of the task.
Summary of the Storyline
The story starts with participants being told they are on a street in the town Greenville as the first character, Olivia (Peter on the gender-counterbalanced version), approaches them. Olivia tells the participant that she knows him/her back from high school, and from then on she keeps acting as a possible friend. The second character is Peter (Olivia in the opposite version), and when he shows up Olivia mysteriously disappears. Peter behaves as another potential peer. However, the participants later learn that Peter broke up with Olivia, and are put in a position where they have to navigate a potentially tricky social setting where they have to choose sides. There are a number of interactions with these first two characters, over coffee or lunch at the local spot, the “Flying Biscuit.” An older and potentially more powerful character, Mrs. Newcomb (or Mr.), is then introduced by showing up at the Flying Biscuit and interacting with Olivia and Peter. Newcomb can help the participant get a job, while she also mentions knowing the participant’s parents and creates an affiliation potential. Newcomb then hosts a dinner where the participant is invited along with Olivia, Peter, and the control character, Kayce (Anthony on opposite gender). The fifth character to be introduced is the one that might directly hire the participant, Mr. (or Mrs.) Hayworth. He is well known and admired in the town, and the participant is sent for an interview with him. There he meets his assistant, Anthony (Kayce on counterbalanced version). The participant gets the job after a series of interactions (including a dinner and a golf course visit) with Hayworth and his assistant. This ends the game “Level 1.” On “Level 2,” the participant now knows all characters and keeps interacting with all of them. The participant is working with Hayworth and Anthony and looking for a place to live with the potential help of all characters. The game ends after the last interaction, when the participant has to chose whether to rent an in-law unit from Newcomb or share an apartment with Anthony/Kayce.
Post-Task Questionnaires
After the scanning task, the participants were given a battery of questionnaires. The first was a memory questionnaire: 30 multiple choice questions about facts in the storyline such as, “who did you have coffee with on your first day in town”? Each question had five answer options, where each was a different character name and only one was correct. This was followed by an implicit power and affiliation assessment of characters: participants saw a set of seven houses of increasing size and attributed the houses to each one of the characters and to themselves, as they also spread them in a square space. Participants then saw an explicit graph with power and affiliation axes and dots labeled with the names of the characters; we asked the participants to distribute the dots according to their subjective evaluation of the characters’ position relative to themselves. Finally, participants completed a set of standardized self-report questionnaires: the Liebowitz social anxiety scale (Fresco et al., 2001) (which yields two scores: social avoidance and social fear), the general self-efficacy scale (Luszczynska et al., 2005) (which yields two scores: general efficacy and social efficacy), the NEO personality inventory revised (Costa and McCrae, 2000) (which yields five scores: extraversion, agreeableness, conscientiousness, neuroticism, and openness to experience), and the MacArthur Scale of Subjective Social Status (which asks participants to place themselves on a picture format social ladder) (Cundiff et al., 2013).
fMRI Acquisition
Functional data were acquired on a Siemens Allegra 3.0 Tesla scanner in one run, approximately 26 min in length, using a single-shot gradient echo T2*-weighted echo-planar imaging sequence (flip angle = 90°, echo time = 35 ms, repetition time = 2,000 ms) and 36 contiguous transversal interleaved slices with a voxel size of 3 × 3 × 3 mm3 (field of view = 192 cm). AT1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) protocol (176 sagittal slices, 256 × 256 matrix) recorded high-resolution (1 × 1 × 1 mm3) anatomical images.
fMRI Data Preprocessing
fMRI data were processed and analyzed offline using Brain Voyager QX version 2.10 (Brain Innovation, Maastricht), Matlab (MathWorks), and software implemented in the NeuroElf toolbox (http://neuroelf.net). Images were corrected for slice timing (using sinc interpolation), head movements, and linear drifts, and low frequencies (below three cycles per time course) were filtered out from the data. Images were spatially smoothed using a 6-mm full-width at half-maximum (FWHM) Gaussian kernel. The anatomical and functional data of each participant were spatially normalized by extrapolation into a 3D volume in Talairach space and re-sliced into iso-voxel dimensions of 3 mm3.
Social Coordinates and Parametric Regressor Calculation
Each participant had six power and six affiliation interactions with each one of five main characters (and three neutral interactions with the control character). Each interaction had two possible directions, which were recorded as +1 or −1 in the respective axis (x = affiliation, y = power), and choices accumulated as “social coordinates” for that given character.
Polar coordinates (vector length and angle) were calculated from the Cartesian values of the power and affiliation axes using the length of a vector between the theoretical point of view of the participants (6,0) and the Cartesian coordinates of the character at that time (the neutral character remained at 0,0 throughout the game). To control for number of prior interactions with each character at each step, we “elevated” the point-of-view to point-of-interaction vector on a hypothetical z axis representing number of interactions (0–12), and calculated the directional angle between the vectors point-of-view to point-of-interaction and point-of-view to top right corner of the theoretical gameboard (6,6). The resulting angle was normalized into a parametric predictor using a cosine function. To obtain the vector’s length, we calculated the norm of point-of-view to point-of-interaction vector.
fMRI Data Analysis
The recorded time series indicated the temporal position of the two types of trials, narrative and options, for each participant, and these were used to construct individual design matrixes for imaging data analysis. Additionally, the social coordinates values calculated at each options trial (polar coordinates or other controls referred to throughout the text) were applied as a parametric weight for the options trials. Data from all participants were probed with random effects GLM. The predictors were convolved with a standard canonical hemodynamic response function. Structural and functional data of each participant were transformed to standard Talairach stereotaxic space (Talairach and Tournoux, 1998). In a whole-brain analysis, the various regressors were contrasted to baseline. All analyses were corrected for multiple comparisons using cluster size threshold ensuring FWE rate of p < 0.05. Regions nomenclature was as per Neurosynth database atlas (http://neurosynth.org) and Neuroelf toolbox and cross-referenced on Brede database (http://neuro.imm.dtu.dk/cgi-bin/brede_loc_query.pl). Beta weights values were extracted from the regions that survived statistical correction. Correlations between these statistical outputs and the participants’ scores in the relevant psychological questionnaires were tested using Matlab Statistical toolbox.
Supplementary Material
Highlights.
Power and affiliation guide social interactions in many species
Participants interacted with characters in a role-playing game during fMRI
Hippocampal activity located each character in a 2D power-affiliation “map”
Participants’ social skills correlated with more distinct hippocampal paths
ACKNOWLEDGMENTS
The authors thank Mark Baxter, Meehan Crist, Harold Koenigsberg, Ifat Levy, Ushma Neill, and Galit Yovel for helpful comments and discussions; Scott Berger and Caroline Overacker for assistance with behavioral data analysis; Jochen Weber for technical advice on Neuroelf; and Taylor Williams for the original illustrations of the game characters. D.S. is supported by the Klingenstein-Simons Foundation Award in the Neurosciences.
Footnotes
Supplemental Information includes four tables and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.06.011.
AUTHOR CONTRIBUTIONS
R.M.T. and D.S. conceived and designed the experiments. R.M.T. collected and analyzed the data and wrote the first draft of the manuscript. A.M., Y.G., and C.H.W. contributed to data analysis. All authors contributed to data interpretation and manuscript write-up.
REFERENCES
- Addis DR, Schacter DL. Constructive episodic simulation: temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Lencz T, Zuffante P, Bilder R, Clarke T, Diamond A, Kane J, Szeszko P. Left middle temporal gyrus activation during a phonemic discrimination task. Neuroreport. 2004;15:389–393. doi: 10.1097/00001756-200403010-00001. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Skene JHP, Platt ML. Genetic origins of social networks in rhesus macaques. Sci. Rep. 2013;3:1042. doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch T, Sander D. Neurocognitive mechanisms underlying value-based decision-making: from core values to economic value. Front. Hum. Neurosci. 2013;7:398. doi: 10.3389/fnhum.2013.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Overview: innovations in assessment using the revised NEO personality inventory. Assessment. 2000;7:325–327. doi: 10.1177/107319110000700402. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Cundiff JM, Smith TW, Uchino BN, Berg CA. Subjective social status: construct validity and associations with psychosocial vulnerability and self-rated health. Int. J. Behav. Med. 2013;20:148–158. doi: 10.1007/s12529-011-9206-1. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Origins of mathematical intuitions: the case of arithmetic. Ann. N Y Acad. Sci. 2009;1156:232–259. doi: 10.1111/j.1749-6632.2009.04469.x. [DOI] [PubMed] [Google Scholar]
- Derdikman D, Moser EI. A manifold of spatial maps in the brain. Trends Cogn. Sci. 2010;14:561–569. doi: 10.1016/j.tics.2010.09.004. [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83:764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Delgado MR. The importance of social rewards and social networks in the human brain. Neuroscientist. 2014;20:387–402. doi: 10.1177/1073858414521869. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Communication about social status. Curr. Opin. Neurobiol. 2014;28:1–4. doi: 10.1016/j.conb.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD, Scharff C. Neurobiology of behavior. Curr. Opin. Neurobiol. 2010;20:746–747. doi: 10.1016/j.conb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Fernald RD, Maruska KP. Social information changes the brain. Proc. Natl. Acad. Sci. USA. 2012;109(Suppl 2):17194–17199. doi: 10.1073/pnas.1202552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske ST. Warmth and competence: stereotype content issues for clinicians and researchers. Can. Psychol. 2012;53:14–20. doi: 10.1037/a0026054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol. Med. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Gariépy J-F, Watson KK, Du E, Xie DL, Erb J, Amasino D, Platt ML. Social learning in humans and other animals. Front. Neurosci. 2014;8:58. doi: 10.3389/fnins.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart-Masip M, Duzel E, Dolan R, Dayan P. Action versus valence in decision making. Trends Cogn. Sci. 2014;18:194–202. doi: 10.1016/j.tics.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LT, Fiske ST. Social groups that elicit disgust are differentially processed in mPFC. Soc. Cogn. Affect. Neurosci. 2007;2:45–51. doi: 10.1093/scan/nsl037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380–391. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu. Rev. Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Krause F, Lindemann O, Toni I, Bekkering H. Different brains process numbers differently: structural bases of individual differences in spatial and nonspatial number representations. J. Cogn. Neurosci. 2014;26:768–776. doi: 10.1162/jocn_a_00518. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The human hippocampus: cognitive maps or relational memory? J. Neurosci. 2005;25:7254–7259. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-L, O’Doherty JP, Rangel A. The decision value computations in the vmPFC and striatum use a relative value code that is guided by visual attention. J. Neurosci. 2011;31:13214–13223. doi: 10.1523/JNEUROSCI.1246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczynska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J. Psychol. 2005;139:439–457. doi: 10.3200/JRLP.139.5.439-457. [DOI] [PubMed] [Google Scholar]
- Magee JC, Smith PK. The social distance theory of power. Pers. Soc. Psychol. Rev. 2013;17:158–186. doi: 10.1177/1088868312472732. [DOI] [PubMed] [Google Scholar]
- Maglio SJ, Trope Y, Liberman N. Distance from a distance: psychological distance reduces sensitivity to any further psychological distance. J. Exp. Psychol. Gen. 2013;142:644–657. doi: 10.1037/a0030258. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL. The hippocampus: a manifesto for change. J. Exp. Psychol. Gen. 2013;142:1180–1189. doi: 10.1037/a0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Just MA. Differentiable cortical networks for inferences concerning people’s intentions versus physical causality. Hum. Brain Mapp. 2011;32:313–329. doi: 10.1002/hbm.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Cai Y, Todorov A. The neural dynamics of updating person impressions. Soc. Cogn. Affect. Neurosci. 2013;8:623–631. doi: 10.1093/scan/nss040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Muller RU, Bostock E, Taube JS, Kubie JL. On the directional firing properties of hippocampal place cells. J. Neurosci. 1994;14:7235–7251. doi: 10.1523/JNEUROSCI.14-12-07235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, Lieberman MD, Dapretto M, Eisenberger NI. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60:1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes PM, Wenzel A, Borges KT, Porto CR, Caminha RM, de Oliveira IR. Volumes of the hippocampus and amygdala in patients with borderline personality disorder: a meta-analysis. J. Pers. Disord. 2009;23:333–345. doi: 10.1521/pedi.2009.23.4.333. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel J. The Hippocampus as a Cognitive Map. Oxford Clarendon Press; 1978. [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. Am. Psychol. 2001;56:717–734. [PubMed] [Google Scholar]
- Parkinson C, Liu S, Wheatley T. A common cortical metric for spatial, temporal, and social distance. J. Neurosci. 2014;34:1979–1987. doi: 10.1523/JNEUROSCI.2159-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Winslow JT, Hopkins WD, de Waal FB. Recognizing facial cues: individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) J. Comp. Psychol. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Lin C-Y, Ketcham K, Nadel L. The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus. 2010;20:11–18. doi: 10.1002/hipo.20607. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat. Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Depression and the hippocampus: cause or effect? Biol. Psychiatry. 2011;70:308–309. doi: 10.1016/j.biopsych.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DA, Adolphs R. Toward a neural basis for social behavior. Neuron. 2013;80:816–826. doi: 10.1016/j.neuron.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; 1998. [Google Scholar]
- Tamir DI, Mitchell JP. The default network distinguishes construals of proximal versus distal events. J. Cogn. Neurosci. 2011;23:2945–2955. doi: 10.1162/jocn_a_00009. [DOI] [PubMed] [Google Scholar]
- Todorov A, Mandisodza AN, Goren A, Hall CC. Inferences of competence from faces predict election outcomes. Science. 2005;308:1623–1626. doi: 10.1126/science.1110589. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said CP, Engell AD, Oosterhof NN. Understanding evaluation of faces on social dimensions. Trends Cogn. Sci. 2008;12:455–460. doi: 10.1016/j.tics.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychol. Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Construal-level theory of psychological distance. Psychol. Rev. 2010;117:440–463. doi: 10.1037/a0018963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I. When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behav. Brain Res. 2010;212:196–203. doi: 10.1016/j.bbr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Wiggins J. A psychological taxonomy of trait-descriptive terms: The interpersonal domain. J. Pers. Soc. Psychol. 1979;37:395–412. [Google Scholar]
- Wiggins J, Phillips N, Trapnell P. Circular reasoning about interpersonal behavior: Evidence concerning some untested assumptions underlying diagnostic classification. J. Pers. Soc. Psychol. 1989;56:296–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.