Abstract
We report RNA-Seq results from skin of X. maculatus Jp 163 B after exposure to various doses of “cool white” fluorescent light (FL). We show that FL exposure incites a genetic transcriptional response in skin nearly as great as observed for UVB exposure; however, the gene sets modulated due to exposure to the two light sources are quite different. Known light responsive genes involved in maintaining circadian cycling (e.g., clock, cry2a, cry1b, per1b, per2, per3, arntl1a, etc.) exhibited expected shifts in transcriptional expression upon FL exposure. Exposure to FL also resulted in down-regulated transcription of many genes involved with cell cycle progression (e.g., cdc20, cdc45, cdca7b, plk1, cdk1, ccnb-3, cdca7a, etc.) and chromosome segregation (e.g., cenpe, cenpf, cenpi, cenpk, cenpo, cenpp, and cenpu; cep70; knstrm, kntc, mcm2, mcm5; smc2, etc.). In addition, several DNA replication and recombination repair genes (e.g., pola1, pole, rec52, rad54l, rpa1, parpbp, etc.) exhibit reduced expression in FL exposed X. maculatus skin. Some genes down modulated by FL are known to be associated with DNA repair and human diseases (e.g., atm2, brip1, fanc1, fancl, xrcc4, etc.). The overall suppression of genes involved with mitotic progression in the skin of adult fish is consistent with entry into the light phase of the circadian cycle. Current efforts are aimed at determining specific wavelengths that may lead to differential expression among the many genes affected by fluorescent light exposure.
Keywords: Fluorescent light, cool white light, RNA-Seq, Differential gene expression, Cell cycle progression, Circadian, DNA damage response
Introduction
Over the past decade physiological and psychological effects of artificial fluorescent lighting on humans have been shown to be significant and quantifiable (McColl and Veitch, 2001). Both the amount of light and composition of lighting are important parameters associated with human health. Differences in the in inflammatory response to specific light wavelengths have been shown between human males and females (Hurlbert and Ling, 2007; Vandewalle, et al, 2010). Other published reports suggest human physiological response to full spectrum vs. “cool white” lamps include differences in oxygen intake, heart rate, absorption of vitamins and minerals, etc (Wurtman, 1975a,b; Holick, 1996; McColl and Veitch, 2001). However, despite behavioral and physiological studies indicating artificial light sources are important to animal health, there exists a paucity of data regarding specific molecular genetic responses that occur in the intact animal upon exposure to varying types of artificial lighting.
Tropical fishes, such as Xiphophorus, may be expected to be both very sensitive and responsive to varying light conditions. In the wild, fishes must utilize light conditions for warmth, predation, predator avoidance, and to coordinate breeding cycles. Given the increasing set of genomic tools currently available, Xiphophorus represent a tractable vertebrate model to investigate the molecular genetic responses to light composition and varied light wavelengths.
Among the many documented Xiphophorus interspecies hybrid melanoma tumor models are a few known to exhibit melanoma induction only after exposure to ultraviolet light (UVB) (Setlow et al., 1989; Setlow et al., 1993; Nairn et al., 1996; Mitchell et al, 2010). Tumor induction studies using the UVB-inducible Xiphophorus hybrid tumor models have shown that post-UVB exposure to fluorescent light (FL) greatly diminishes or completely negates melanoma induction (Setlow et al, 1989, Setlow et al, 1993). This loss of UVB tumorigenic effect by exposure to FL is widely considered to be due to rapid repair of UVB-induced DNA damage by lesion specific DNA photolyases that are active in fish skin (Ahmed and Setlow, 1993; Meador et al, 2000; Mitchell et al, 2001). Studies from various fish species have shown fish photolyase gene expression is induced by exposure to visible light (Hart et al, 1977; Uchida et al, 1997; Armstrong et al, 2002). To assess this in Xiphophorus, we used quantitative real time PCR (qRT-PCR) to follow the expression levels of two photolyase genes (i.e., cyclobutane pyrimidine dimer and 6-4 photoproduct photolyases) in Xiphophorus skin upon exposure to FL (Walter et al, 2014). As expected, skin from parental fish (X. maculatus or X. couchianus) showed visible light induction of photolyase gene expression (≈2–3 fold). However, skin from interspecies hybrid fish (X. maculatus × X. couchianus F1 interspecies hybrids) appeared to have lost the ability to upregulate transcription of these two photorepair proteins upon FL exposure (Walter et al, 2014). Thus, it becomes difficult to explain loss of UVB-induced melanoma by post-UVB FL exposure solely based upon removal of CPD or 6-4 lesions in hybrid fish skin.
Here, we report RNA-Seq results from skin of X. maculatus after exposure to various doses of FL. We show that FL exposures incite a transcriptional response nearly as great in amplitude as that observed after UVB exposure (Yang et al, 2014) and is consistent with suppression of cell cycle progression as has been observed upon entry into the light phase of the circadian cycle. Although we identified a set of genes in fish skin that are similarly affected by both UVB and FL exposure, only FL serves to down-regulate expression of genes in critical pathways expected to curtail mitotic progression.
Materials and Methods
Fish Utilized and Fluorescent Light Exposure
Fish utilized were mature male X. maculatus Jp 163 B, 10 months of age, from the 101st generation (pedigree 101A and D) of sibling inbreeding were used. Skin from 4 individual fish treated as experimental fish but without FL exposure, were utilized for RNA isolation and Illumina sequencing (controls). For each FL time point (20, 40, and 60 min) three fish were individually exposed and RNA from the skin of 2 of these exposed fish was subjected to Illumina sequencing and utilized used for RNA-Seq analysis (Table 1). Power output of the FL light source was determined using a Newport 1918-R power meter (Newport Corporation, Irvine, CA, USA) with a 40 min. FL dose determined to be 10 kJ/m2. This is close to the UVB dose of 8kJ/m2 utilized in previous analyses of differential gene expression of X. maculatus Jp 163 B skin (Yang et al, 2014 and Figure 1). Prior to light exposure, each fish was placed into an individual 125 mL flask filled with 100 mL of filtered water from their home aquaria and kept in the dark 14 hrs. Fish from these individual flasks were randomly chosen for FL light exposure or to remain unexposed and serve as controls.
Table 1.
RNA-Seq and read mapping statistics.
| Exposure | Filtered Reads | Reads Mapped | % Mapped | Coverage |
|---|---|---|---|---|
| 0 Control A | 44,846,553 | 41,104,701 | 73.4 | 67.1X |
| 0 Control B | 44,699,687 | 40,949,014 | 73.4 | 66.8X |
| 0 Control C | 32,175,519 | 30,439,113 | 73.4 | 49.7X |
| 0 Control D | 24,988,902 | 24,178,684 | 73.4 | 39.5X |
| FL −20 min A | 43,612,238 | 39,827,303 | 73.6 | 65.0X |
| FL −20 min B | 47,343,728 | 42,550,103 | 75.7 | 69.5X |
| FL −40 min A | 48,980,752 | 43,639,847 | 76.8 | 71.2X |
| FL −40 min B | 56,608,706 | 45,536,298 | 74.8 | 74.3X |
| FL −60 min A | 57,118,191 | 52,357,400 | 73.8 | 85.5X |
| FL −60 min B | 48,035,072 | 43,179,638 | 78.2 | 70.5X |
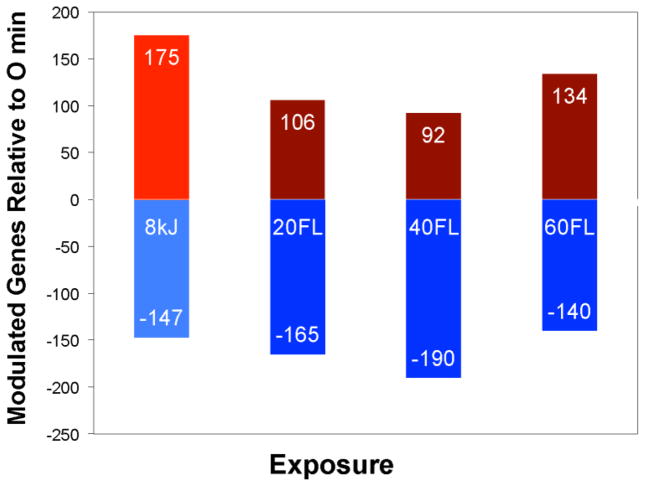
Figure 1.
The total number of up- and down-modulated genes in X. maculatus Jp 163 B skin due to UVB (8 kJ) or FL light exposures (20, 40, or 60 min). Within each bar are numbers of genes with increased transcription levels (top bar), or genes exhibiting depressed transcription levels (bottom bar) compared to the skin of unexposed sibling animals. The total number of genes showing modulated transcription (DE genes) are 322 for UVB, 271 for 20 min FL, 282 for 40 min FL, and 274 at 60 min FL.
Light exposures were carried out essentially as previously detailed (Walter et al, 2014; Yang et al, 2014) except that in these studies flourescent lights (FL) were employed. Briefly, FL exposure occurred in a specially designed wooden box (77 cm in length, 41 cm in height, and 36 cm in depth), with a hinged wooden lid capable of sealing the interior of the box from external light. On the bottom of each of the two sides (41 cm × 36 cm) were 15.5 cm diameter high-speed fans that maintained interior temperatures of the closed box at less than 24°C. For FL exposures single fish were placed into UV transparent (UVT) plastic cuvettes (9 cm × 7.5 cm × 1.5 cm) in about 95 ml water and the cuvettes were suspended in a rack centered between and about 10 cm from the FL bulbs inside the exposure chamber. All animals were kept in the dark 12 hrs prior to exposure. A bank of “cool white” fluorescent lamps (Philips F20T-12/D, 4100K “cool white” lamps) provided FL exposures (20, 40 or 60 min).
After FL exposure, fish were maintained in the dark for 6 hrs to allow for gene expression changes prior to sacrifice and tissue dissection. At dissection fish were anesthetized in an ice bath and upon loss of gill movement were sacrificed by cranial resection. Skin were dissected directly into Tri-Reagent (Sigma Inc. St. Louis) placed in a dry ice-ethanol bath if the RNA was isolated at the time of dissection, or into RNALater (Ambion Inc. Austin, TX) and kept at −80°C for RNA isolation at a later time.
RNA isolation and RNA sequencing
Total RNA was isolated as previously detailed (Walter et al., 2014; Yang et al., 2014) using Tri-Reagent (Sigma Inc., St. Louis, MO, USA). Skin from each individual fish was homogenized in 600 μl Tri-Reagent followed by addition of 120 μl chloroform and the samples vigorously shaken and subjected to centrifugation at 12,000 g for 15min at 4°C. Total RNA was further purified using RNeasy mini RNA isolation kit (Qiagen, Valencia, CA, USA). Residual DNA was eliminated by DNase digestion on the column at 25°C for 15 min. RNA integrity was assessed by gel electrophoresis (2% agarose in TAE running buffer) and total RNA concentration was determined using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA quality was verified on a 2100 Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA) to confirm that RIN scores were above 8 prior to sequencing.
RNA sequencing was performed on libraries constructed using the Illumina TrueSeq library preparation system that employs a polyA selection. RNA libraries were sequenced as 75 bp paired-end fragments using an Illumina Hi-Seq 2000 system (Illumina, Inc., San Diego, CA, USA). 70–80 million raw reads were generated for each fish skin RNA sample. All raw reads were subsequently truncated by similarity to library adaptor sequences using a custom Perl script (Table 1). Short reads were then trimmed and filtered based on quality scores by using a custom filtration algorithm that removed low-scoring sections of each read and preserved the longest remaining fragment (Garcia et al., 2012). Our read depth was no less than 40x (O-D sample) for any sample and averaged about 58.5x (Table 1).
Computational Analyses
Filtered reads were mapped to the X. maculatus reference transcriptome (Ensembl v71) using Bowtie2 (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml), (Langmead et al, 2009; Langmead and Salzberg, 2013) and a custom Perl script to quantify gene expression. The percentage of reads mapped and unmapped as well as the nucleotide coverage was identified using SAMtools flagstat and depth respectively (Li et al., 2009). Differentially expressed (DE) genes were determined using the R-Bioconductor (www.bioconductor.org) package DESeq (Anders and Huber, 2010) with a fold-change cutoff of ±2 and a p-adj ≤ 0.05. Briefly, a gene expression count table containing raw counts, that represent how many short reads align to each gene was calculated by eXpress (Roberts, 2013) and used as the input file of DESeq. The raw count of each gene was then normalized by the size of each sample. The test for differential expression is based on a model using the negative binomial distribution. We defined differentially modulated genes as genes that have log2Fold Change (log2FC) ≤ −1 or ≥1, with a False Discovery Rate Adjusted p-value (p-adj) ≤ 0.05 between light-exposed skin samples and unexposed skin samples.
Determination of Basal Gene Expression Determination
To normalize gene expression levels on both transcript length and RNA-Seq library size, the eXpress generated gene expression raw count values were used to calculate Reads Per Kilobase per Million reads (RPKM) values, where RPKM = [(raw counts/total reads count per library) × 1million]/(transcript length/1000). We defined a gene as expressed if its RPKM value was ≥ 0.3 (Ramskold et al, 2009).
Biological Functional Analysis
Interacting genes may be expected to show nonrandom patterns in cell localization, molecular function, and/or biological process. Bioinformatics analysis of exposure data was performed using DAVID (http://david.abcc.ncifcrf.gov). DAVID allowed significant DE genes in each gene set to be clustered into functional gene ontologies. GeneMania (www.genemania.org, Warde-Farley et al., 2010) was used to visualize interactions and associations of gene networks. Nodes are differentially colored to designate between queried (black) and predicted (grey) genes.
NanoString
RNA isolated from X. maculatus skin was subjected to NanoString nCounter analysis (http://www.nanostring.com/applications/technology) to assess gene expression by direct counting (NanoString technologies, Seattle, WA). Hybridization protocols were strictly followed according to manufacturer’s instructions (Geiss et al., 2008) at the Baylor College of Medicine Microarray Core Facility (Houston, TX). Hybridized samples (500 ng RNA in 7 μL of solution) were incubated overnight at 65°C with a custom set of probes in the NanoString Prep Station and immediately evaluated with the NanoString nCounter based on color code signals. Data analysis was performed by lane normalization using a set of standard NanoString probes followed by sample normalization using a set of 10 housekeeping genes. Counts were generated by nCounter Digital Analyzer. Fold changes were calculated on normalized counts and plotted using Microsoft Excel. Two biological samples previously used in RNA-Seq analysis were selected for NanoString. Supplemental Table S1 presents the NanoString custom designed probe set for 18 test targets and 10 housekeeping controls.
Quantitative real time PCR
Quantitative real time PCR (qRT-PCR) was performed as previously described (Yang et al., 2014; Walter et al., 2014). Seven transcripts that exhibited differential modulation in response to FL by RNA-Seq analyses were confirmed using qRT-PCR. Briefly, cDNA synthesis was performed with 100 mM dNTPs and 10X RT random primers, RNase inhibitor, and Multiscribe Reverse Transcriptase mixed with 1.5 μg of RNA in a 20 μL reaction. Thermocycler conditions for cDNA synthesis were 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min.
Each cDNA reaction was diluted to a final volume of 500 μL and quantified using a Qubit 2.0 flourometer (Life Technologies, Grand Island, NY). Each sample was analyzed with qRT-PCR on Applied Biosystems 7500 Fast Real Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Primers used for qRT-PCR were designed using Geneious (Biomatters Ltd., Auckland, New Zealand) software version 6.1. Primer design included melting temperatures (Tm) between 60 and 62°C (50 mM Na, 3 mM K, and 0.8 mM dNTPs), lengths of 18–25 nt with 55–60% GC content, and not more than 1°C between Tms of each primer in a set. Target amplicons were limited to 100–150 bps in length, and were designed to amplify across at least one intron-exon junction. Primers specific for the Xiphophorus transcripts (Table S2) were tested for efficiency and only primers with efficiency values between 70 and 120% were utilized in downstream analyses. Each primer set was used to test the relative expression of each gene from cDNAs (15 ng) produced from X. maculatus skin in four qRT-PCR technical replicates. All plates were run using the SYBR Green protocol as previously described (Walter et al., 2014; Yang et al., 2014) wherein amplification for each target is normalized against the 18S rRNA transcript to determine ΔCT values. UV modulated fold changes are relative to 0 point control (ΔΔCT) values.
Results and Discussion
Genes up- and down-modulated in Xiphophorus skin by FL exposure
Data on the basal transcription activity of tissues from human and mouse studies have shown the number of genes expressed in most tissues varied from 11,000 to 13,000 applying a threshold of 0.3 RPKM. This corresponds to roughly 60–70% of RefSeq protein-coding genes (Ramskold et al, 2009). As many as 7,897 genes (42%) are observed to be ubiquitously expressed in all tissues and cell lines. Further, compared to the other tissues, testis was a clear outlier, expressing more than 15,000 different genes (84% of RefSeq genes) (Ramskold et al, 2009). Applying the same threshold constraints to RNA-Seq data generated for 10 individual skin samples derived from control X. maculatus Jp 163 B (i.e. dissected after 14hrs dark incubation) we observe 16,505 +/− 147 genes to be transcriptionally active in the fish skin. This is about 80.5% of the total gene models queried in the transcriptome (20,498 gene models; ftp.ensembl.org/pub/release-80/fasta/xiphophorus_maculatus/cdna/Xiphophorus_maculatus.Xipmac4.4.2.cdna.all.fa.gz). The large fraction of active genes observed, approaching the fraction observed in mammalian testes, highlights the dynamic nature and complexity of fish skin compared to the skin of mammals (Rakers et al, 2010; Xu et al, 2013).
Our conservative application of DEseq to the RNA-Seq data derived from FL exposed skin samples identified 522 genes to be differentially expressed (DE). Thus, we observe significant up- or down-transcriptional modulation for about 2.5% of the gene models, or about 3.0% of the number of genes expected to have been active in the fish skin before exposure to one of the three FL doses (20, 40 or 60 min.; Table S3).
The total number of DE genes (up or down modulated) in skin due to UVB (8 kJ/m2; 322) or FL light exposures of 20 min (271), 40 min (282), and 60 min (274) are presented in Figures 1 and 2. Overall, 522 differentially expressed (DE) genes from the transcriptome queried (20,218 gene models; ftp.ensembl.org/pub/release-80/fasta/xiphophorus_maculatus/cdna/Xiphophorus_maculatus.Xipmac4.4.2.cdna.all.fa.gz) showed significant up- or down-transcriptional modulation for at least one FL exposure (presented in Table S3). Of these genes, 11 DE genes are currently “uncharacterized” while 511 could be assigned gene names with presumed encoded protein functions.
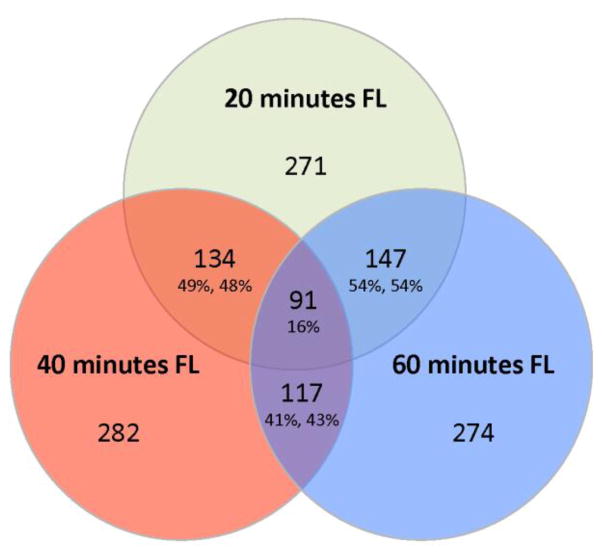
Figure 2.
Venn diagram comparison of DE genes from each FL exposure (20, 40, and 60 min). There are 134 genes in common between the 20 min (49%) and 40 min (48%) time points, 147 genes in common between the 20 min (54%) and 60 min (54%) time points, and 117 genes in common between the 40 min (41%) and 60 min (43%) time points. Among all three exposures, 91 DE genes are shared and expressed similarly (see Table S4).
The data show FL exposure resulted in a transcriptional response nearly as great as observed previously for exposure to 8 kJ/m2 UVB exposure (311 nm; see Yang et al, 2014). For example, by magnitude of the DE response, the 40 min FL exposure (282 genes) represents 87.5% of the UVB response (322 genes). However, UVB exposure results in a much higher fraction of up-regulated genes (54%) compared to FL exposure at 20 (38%), 40 (32%), or 60 min (48%) (Figure 1).
All genes that exhibited significant DE in two or more exposure time points are listed in Table S4 (216 genes). Of the 216 DE genes in Table S4, 6 were “uncharacterized” and could not be assigned a gene name after extensive homology comparisons to Ensembl and NCBI databases. Of the remaining 210 genes in this set, 115 (55%) were clustered into five overrepresented functional groups (discussed below) while the remaining 101 named genes did not segregate into clearly defined functional clusters, but appeared to represent a large and varied group of pathways.
Shared genes modulated by FL or UVB exposure
Comparing the sets of differentially expressed genes from each FL exposure (20, 40, and 60 min), we identified 91 genes that are DE similarly across all three FL doses (Figure 2). Additionally, nearly half of the responsive genes are common between subsequent time points with over 40% of the DE genes shared between the shortest and longest exposures (Figure 2).
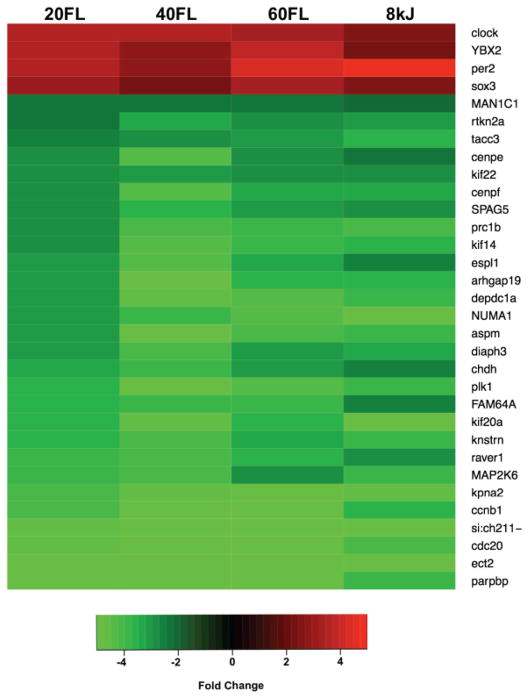
A set of 32 genes were identified as similarly affected by UVB or FL exposure (Figure 3). This set of 32 genes represents genes known to be responsive to light exposure, associated with mitosis, or involved in intercellular signaling. Interestingly, the light-responsive (ybx2, sox3) and circadian cycle genes (clock, per2) are up-regulated, while many of the down-regulated genes play a role in mitosis (aspm, cenpe, cenpf, FAM64a, kif14, kif20a, kif22, knstrn, kpna2, tacc3, etc.), cell cycle control (ccnb1, cdc20), or intercellular signaling (raver1, arhgap19, depdc1a). Outside of this small set of 32 genes, FL exposure modulates a fundamentally different set of genes in Xiphophorus skin then does UVB (Yang et al, 2014).
Figure 3.
Relative transcription levels of a set of genes identified as similarly affected by UV or FL exposure. This set of 32 genes include known light responsive genes (which are upregulated) and genes involved in mitosis and intercellular signaling (which are downregulated). For instance, light responsive (ybx2, sox3) and circadian cycle genes (clock, per2) are up regulated while many of down regulated genes play a role in mitosis (aspm, cenp-e, -f, fam64a, kif-14 and -20a, knstrn, kpna2, tacc3, etc.), cell cycling (ccnb1, cdc20), or intercellular signaling (raver1, arhgap19, depdc1a).
Clustering of the FL Results
DAVID gene ontology was employed to identify biological associations among the 210 named genes modulated in X. maculatus skin by exposure to FL and shared between two or more exposures (Table S4). Statistical significance was restricted for each category to p-values < 0.005. This analysis showed very strong clustering of 115 DE genes modulated in two or more exposures into five functional categories; Light Responsive (17 genes, p-value 6.60e-06), Oxidation/Reduction (14 genes, p-value 3.10e-04), Mitosis and Cell Cycle (38 genes, p-value 1.00e-39), Chromosome Structure (24 genes, p-value 1.10e-04), and DNA Replication and Repair (22 genes, p-value 4.60e-11).
Of the 210 named genes showing DE in two or more FL exposures, 69 (33%) were up-modulated. However, after segregation of the 115 DE genes into five functional clusters, we observe only 23 (20%) up-modulated while 92 (80%) exhibited suppressed transcription after FL exposure. Among these up-regulated genes, 19 of the 23 genes are disproportionately present in only 2 of the 5 functional classes; Light Responsive and Oxidation/Reduction. The 4 genes that exhibited up-modulation in the other 3 functional classes (ybx2, Chromosome structure; dusp1 and pim1, Mitosis and Cell Cycle; and nupriL, DNA Replication and Repair) each has shared functions that could have also resulted in their being assigned to the Light Responsive or Oxidation/Reduction classes. For example, ybx2 was up-regulated in both UVB and FL light exposures (Figure 3), while dusp1 is known to be responsive to oxidative stress and may also function within the Oxidation/Reduction functional class.
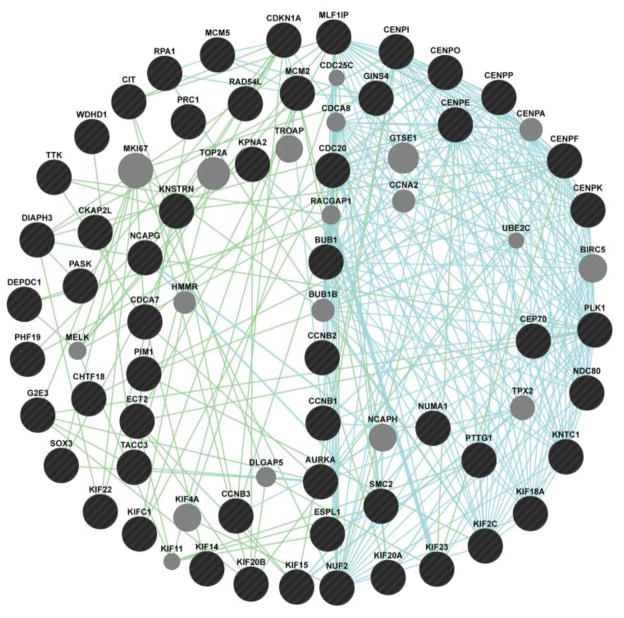
Inspection of the 115 genes DAVID segregated into the five functional classes (Table S4) shows many DE genes that could have been assigned to two or more classes (e.g., Mitosis/Cell Cycle and DNA Replication/Repair, or Mitosis/Cell Cycle and Chromosome Structure, etc.) as one might expect from these overlapping functional categories. Even so, the majority of genes within each functional category are clearly related by both functional and genetic interaction (Figure 4).
Figure 4.
Genetic associations of chromosome structure and mitosis genes differentially modulated in at least 2 time points (20, 40, and 60 min FL exposure) in X. maculatus JP 163 B skin. Biological associations are identified through genetic interaction (green lines) and pathways (blue lines). Grey circles are genes predicted through the GeneMania algorithm to be associated with query genes (black circles).
Light responsive genes modulated by FL exposure
Seventeen genes are known to be light responsive and are largely up-regulated after FL exposure (Table 2). Many members of this cluster are circadian associated genes (arntl1a, bhlhe40, clock, cry2a, npas2, per1b, per2, per3, timeless) and are well-documented to exhibit light-affected transcriptional regulation (for reviews see Somade, 2014; Borgs, et al, 2009). These genes behaved exactly as one might predict from previously published studies. In addition, other genes previously shown to exhibit light dependent regulation and associated with DNA repair (cipc, il12b, junb, nfil3-2) or genes we have observed in many independent experiments to be light inducible (dnah7, fggy, klhl38b, opn1) (Caballero, 2015; Chang et al, 2015) appear in this category.
Table 2.
Light responsive genes showing DE upon FL exposure (20, 40 and 60 min). Shaded boxes are up-regulated while down-regulated are unshaded. np = not significant for that exposure.
| Ensembl I.D | Gene | Fold Change | Description | ||
|---|---|---|---|---|---|
| 20 | 40 | 60 | |||
| ENSXMAT00000015691 | arntl1a | 2.75 | 2.33 | 2.35 | aryl hydrocarbon receptor nuclear translocator-like 1a |
| ENSXMAT00000002257 | bhlhe40 | −3.74 | −2.41 | −2.44 | basic helix-loop-helix family, member e40 |
| ENSXMAT00000017010 | clock | 3.62 | 3.51 | 3.45 | clock |
| ENSXMAT00000008440 | cipc | −2.07 | np | −2.07 | clock interacting pacemaker |
| ENSXMAT00000009327 | cry2a | 3.32 | np | 3.13 | cryptochrome 2a |
| ENSXMAT00000007381 | dnah7 | 3.72 | 2.74 | 3.62 | dynein, axonemal, heavy chain 7 |
| ENSXMAT00000018361 | fggy | −3.34 | −3.47 | −3.47 | FGGY carbohydrate kinase domain containing |
| ENSXMAT00000000472 | il12bb | 6.61 | 3.83 | 3.58 | interleukin 12B, with IL23 activates Jak/Stat pathway |
| ENSXMAT00000019606 | junbb | 2.50 | np | 3.55 | jun B proto-oncogene b |
| ENSXMAT00000008690 | klhl38b | 14.81 | 19.37 | 17.14 | kelch-like 38b (Drosophila) |
| ENSXMAT00000019605 | nfil3-2 | 2.54 | 2.56 | 2.34 | nuclear factor, interleukin 3 regulated, member 2 |
| ENSXMAT00000012307 | npas2 | 3.47 | np | 3.71 | neuronal PAS domain protein 2, SAD associated |
| ENSXMAT00000013132 | opn1sw1 | −2.45 | np | −4.04 | opsin 1 (cone pigments), short-wave-sensitive 1 |
| ENSXMAT00000015376 | per1b | −2.49 | −2.92 | −2.45 | period homolog 1b (Drosophila) |
| ENSXMAT00000016248 | per2 | 3.54 | 2.84 | 4.42 | period homolog 2 (Drosophila) |
| ENSXMAT00000004015 | per3 | −3.11 | −2.63 | −2.01 | period homolog 3, p53 degradation |
| ENSXMAT00000003313 | timeless | −2.00 | −2.29 | np | timeless homolog (Drosophila), stress response, pole |
Overall the behavior of these known light responsive genes, showing similar values in all three exposures (11 of 17), with the overwhelming up-regulation (10 of the 17 genes) and the down regulation of specific circadian genes (i.e., bhlhe40, per1b, per 3, and timeless) upon FL light exposure is in agreement with previous published reports (Somade, 2014; Johnson, 2010).
Oxidation/reduction
Fourteen genes associated with Oxidation/Reduction states are observed modulated in skin upon exposure to FL light (Table 3). Of these, 9 are up-regulated. The spectrum of the “cool white” FL light (Supplemental Figure 1) used for these exposures shows some emission in the lower blue-violet wavelengths with two peaks of emission between 400–450 nm. This region may be expected to result in absorption by many different cellular photosensitizers (i.e. melanin, flavins, heme, etc.) and photoactive proteins (Horner and Weber. 2012). Proteins that require light in this region often use flavin or modified rhodopsin as a photoreceptor to become active (e.g., photolyases and flavin, Li et al., 1991; Mitani et al., 1996). For example, the cryptochrome family proteins, involved in circadian phasing, have many members that undergo photoactivation (Bouly et al., 2007). It seems likely the intense FL light encountered by the fish skin in these exposures led to excited electron states in cellular photosensitizers that may have resulted in production of reactive oxygen species (ROS). In addition, the regulation of heavy metals within the cell becomes important in the presence of light energy. Consistent with this, several genes up-regulated in the Oxidation/Reduction category are expected to encode proteins that bind metals (e.g., cyp24a1, ferritin, smox, etc.). Others in this cluster are involved with peroxide metabolism (gpx6, ehhadh), aromatic amino acid or sterol metabolism (akr1d1, hgd, pah, tm7sf2), associated with the control of mitochondria-derived reactive oxygen species (ucp2, chdh), or involved with general metabolic activities (grhph, hpdb). Overall, these 14 genes exhibit a very strong cluster p-value (3.10e-04) as an Oxidation/Reduction functional class. However, as with most proteins, overlapping functions of the encoded proteins in several cellular functional classes is very likely.
Table 3.
Oxidation/reduction responsive genes showing DE upon FL exposure (20, 40, and 60 min). Shaded boxes are up-regulated while down-regulated are unshaded. np = not significant for that exposure.
| Ensembl I.D | Gene | Fold Change | Description | ||
|---|---|---|---|---|---|
| 20 | 40 | 60 | |||
| ENSXMAT00000004428 | akr1d1 | 2.27 | 2.76 | 2.21 | aldo-keto reductase family 1, member D1 |
| ENSXMAT00000019354 | chdh | −3.39 | −3.80 | −2.96 | choline dehydrogenase |
| ENSXMAT00000010260 | cyp24a1 | 4.49 | np | 3.27 | cytochrome P450, family 24, subfamily A, polypeptide 1 |
| ENSXMAT00000015599 | ehhadh | −2.94 | −3.46 | np | Enoyl-CoA, hydratase/3-hydroxyacyl Co A dehydrogenase |
| ENSXMAT00000008621 | LOC102238129 | 2.46 | np | 2.53 | ferritin, middle subunit-like; 100% identity |
| GPX6.CDS | gpx6 | −2.66 | np | −4.42 | Glutathione Peroxidase 6 (Olfactory) |
| ENSXMAT00000008475 | grhpr | −2.53 | np | −2.60 | glyoxylate reductase/hydroxypyruvate reductase a |
| ENSXMAT00000019083 | hgd | 2.58 | 3.72 | 2.69 | homogentisate 1,2-dioxygenase |
| ENSXMAT00000015288 | hpdb | 2.27 | 2.75 | 2.24 | 4-hydroxyphenylpyruvate dioxygenase b |
| ENSXMAT00000017348 | pah | 2.28 | 3.08 | 2.50 | phenylalanine hydroxylase |
| ENSXMAT00000005538 | smox | 3.12 | 2.22 | np | spermine oxidase |
| ENSXMAT00000014172 | snca | 4.18 | 4.02 | np | synuclein, alpha (non A4 component of amyloid precursor) |
| ENSXMAT00000005176 | tm7sf2 | −5.94 | −3.91 | −5.22 | transmembrane 7 superfamily member 2 |
| ENSXMAT00000011982 | ucp2 | 2.59 | 2.76 | 2.61 | uncoupling protein 2, mitochondrial oxidation/reduction |
Mitosis, cell cycle and chromosome structure
One of the most striking co-regulatory shifts in the transcription of skin RNA after FL exposure is a generalized suppression of 62 genes (i.e., 38 in the Mitosis and Cell Cycle, Table 4; and 24 in Chromosome Structure, Table 5) that are collectively associated with cell cycle progression and mitosis. Genes encoding protein components of the centromeres, kinetochore, and involved with spindle attachment (cenpe, cenpf, cenpi, cenpk, cenpo, cenpp, cenpu, cep70, haus5, kif 14, kif 15, kif20a, kif20b, kif22, kif18a, kif23, kifc, kifc1, knstm, kntc12, ndc80, nuf2, ttk), chromosome condensation and chromatin structure (aspm, ckap2l, chtf18, esp1, ncapg, smc2, pik1), as well as cell cycle regulators and kinases (aurka, bub1, ccb1, ccb2, ccb3, cdc7a, cdc20, cdkn1d, cit, ect2, pask, pim1, prc1a, prc1b, ybx2) all appear suppressed in Xiphophorus skin after exposure to FL light. Other cell cycle associated genes are similarly affected and many in this group also share function, not only between Mitosis/Cell Cycle and Chromosome Structure categories, but also with the DNA Replication and Repair cluster of genes (gins4, mcm2, cmc5, pttg1, wdhd1, etc.). Overall, this list of suppressed genes suggests cessation of mitotic progression in cells that prior to exposure were actively dividing or preparing to do so. GeneMania analysis of genes in these functional classes show genetic association and strong pathway association among the encoded gene products (Figure 4). This level of interaction may be expected in a coordinated response to light as has been reported for cells entering the light phase of the circadian cycle (Borgs et al., 2009; Johnson, 2012; Somade, 2014). Circadian control of the cell cycle has been demonstrated in most organisms including mammalian cultured cells, rodent models (Reppert and Weaver, 2002; Destici et al, 2011) and circadian-entrained mitotic activity has been shown active in human skin (Bjarnason and Jordan, 2002). Zebrafish has been shown to possess light regulated cell cycling in larvae and evidence of peripheral circadian clock oscillators by direct light absorption has been suggested (Whitmore et. al, 1998; Whitmore et. al, 2000; Dekens et al, 2003; Peyric et al, 2013). The results shown here extend this work into live-bearing fish and imply the cell cycle stalls in the skin of adult male Xiphophorus upon FL exposure. Given the time of exposure and dissection it is likely this occurs via direct light absorption by the skin and thus implicates peripheral circadian oscillators that gate cell cycle progression upon FL light exposure.
Table 4.
Mitosis and cell cycle progression genes showing DE upon FL exposure. Shaded boxes are up-regulated while down-regulated are unshaded. np = not significant for that exposure.
| Ensembl I.D | Gene | Fold Change | Description | ||
|---|---|---|---|---|---|
| 20 | 40 | 60 | |||
| ENSXMAT00000000723 | aspm | −3.15 | −5.80 | −4.14 | asp (abnormal spindle)-like, coordination of mitotic processes. |
| ENSXMAT00000004556 | bub1 | −3.41 | −3.47 | np | BUB1 mitotic checkpoint ser/threo kinase, Mitotic complex activating spindle checkpoint |
| ENSXMAT00000008618 | ccnb1 | −4.09 | −4.78 | −5.01 | cyclin B1 |
| ENSXMAT00000014334 | ccnb2 | −4.92 | −4.78 | −5.71 | cyclin B2 role in the regulation of cytokinesis. |
| ENSXMAT00000015109 | ccnb3 | −3.47 | −3.68 | −3.27 | cyclin B3 |
| ENSXMAT00000016054 | cdc20 | −4.71 | −4.84 | −6.77 | cell division cycle 20 homolog |
| ENSXMAT00000013526 | cenpi | −2.08 | −2.25 | np | centromere protein I, Mitotic porgression, Kinetochore assembly |
| ENSXMAT00000000137 | cenpk | −3.25 | np | −2.93 | centromere protein K |
| ENSXMAT00000015198 | cenpo | −2.39 | −2.86 | −2.61 | centromere protein O, Centromere, spindle assembly |
| ENSXMAT00000010514 | cenpp | −2.14 | np | −2.18 | centromere protein P, Centromere-kinetochore |
| ENSXMAT00000004074 | cenpu | −2.63 | −2.78 | −2.36 | centromere protein U, Kinetochore assembly, replaces histone H3 |
| ENSXMAT00000018911 | cep70 | −3.05 | −3.25 | np | centrosomal protein |
| ENSXMAT00000018396 | cit | −2.40 | −4.71 | −2.40 | citron rho-interacting ser/threo kinase |
| ENSXMAT00000016865 | ckap2l | −2.47 | −2.67 | −2.30 | cytoskeleton associated protein 2-like, Mitotic spindle formation and cell cycle progression |
| ENSXMAT00000005117 | diaph3 | −3.18 | −4.03 | −3.18 | diaphanous homolog 3 (Drosophila), Required for cytokinesis |
| ENSXMAT00000009693 | dusp1 | 2.25 | np | 2.69 | dual specificity phosphatase 1, Skin fibroblast oxidative stress, inactivates MSAP kinase |
| ENSXMAT00000014150 | ect2 | −4.97 | −5.23 | −4.88 | epithelial cell transforming sequence 2 oncogene |
| ENSXMAT00000004776 | g2e3 | np | −5.03 | −4.85 | G2/M-phase specific E3 ubiquitin ligase |
| ENSXMAT00000001883 | gins4 | −2.35 | −2.48 | np | GINS complex sub4 (Sld5 homolog), DNA replication progression. S phase |
| ENSXMAT00000006058 | kif14 | np | −4.44 | −3.79 | kinesin family member 14, Important role in cytokinesis, spindle |
| ENSXMAT00000013566 | kif15 | −2.16 | −3.61 | np | kinesin family member 15, Spindle assembly |
| ENSXMAT00000012995 | kif20a | −3.64 | −4.65 | −3.71 | kinesin family member 20A, Mitotic segregation |
| ENSXMAT00000004879 | kif20b | −2.71 | −4.29 | −2.40 | kinesin family member 20Ba, Chromosome movement, G2 phase, Cytokinesis |
| ENSXMAT00000018645 | kif22 | −2.75 | −2.98 | −2.81 | kinesin family member 22 |
| ENSXMAT00000015773 | kif2c | np | −3.92 | −3.96 | kinesin family member 2C, Important role in cytokinesis, spindle |
| ENSXMAT00000007687 | knstrn | −3.64 | −4.01 | −3.42 | kinetochore-localized astrin/SPAG5 binding protein, Mitotic segregation |
| ENSXMAT00000017710 | kntc1 | −2.87 | −3.72 | −2.43 | kinetochore associated 1 |
| ENSXMAT00000008371 | kpan2 | −3.99 | −4.52 | −4.88 | karyopherin alpha 2 (RAG cohort 1, importin alpha 1), Essential for cell cycle control G2/M |
| ENSXMAT00000008384 | mastl | np | −3.68 | −2.48 | microtubule associated serine/threonine kinase-like, entry into M phase |
| ENSXMAT00000000306 | mcm2 | −2.29 | −3.26 | −2.08 | MCM2 minichromosome maintenance deficient 2, MCM complex, S phase entry |
| ENSXMAT00000004575 | mcm5 | −2.03 | −2.42 | np | MCM5 minichromosome maintenance deficient 5, MCM complex. Go-G1/S phase replication |
| ENSXMAT00000011043 | MTBP | −3.13 | −3.65 | −2.24 | MDM2 binding protein |
| ENSXMAT00000006891 | nuf2 | −2.06 | −2.32 | np | NUF2, NDC80 kinetochore complex component, Centromere Prophase |
| ENSXMAT00000011193 | numa1 | −3.09 | −3.97 | −4.43 | nuclear mitotic apparatus protein 1, Circadian clock gene |
| ENSXMAT00000008117 | phf19 | −2.69 | −2.96 | −2.23 | PHD finger protein 19, binds histone H3-traHC3, transition from active to repressed state. |
| ENSXMAT00000016450 | pim1 | 2.21 | np | 2.66 | pim-1 oncogene, Cell cycle progression, regulates MYC |
| ENSXMAT00000012882 | plk1 | −3.47 | −4.78 | −4.40 | polo-like kinase 1, Master regulator of M phase, centrosome/spindle many other functions |
| ENSXMAT00000009875 | wdhd1 | −2.14 | −2.91 | np | WD repeat and HMG-box DNA binding protein, Replication initiation |
Table 5.
Chromosome structure genes showing DE upon FL exposure. Shaded boxes are up-regulated while down-regulated are unshaded. np = not significant for that exposure.
| Ensembl I.D | Gene | Fold Change | Description | ||
|---|---|---|---|---|---|
| 20 | 40 | 60 | |||
| ENSXMAT00000006097 | aurka | np | −3.56 | −3.87 | aurora kinase A, ser/thr kinase important for cell cycle progression and p53 |
| ENSXMAT00000000581 | cdca7b | −2.77 | −2.42 | −2.04 | cell division cycle associated 7b |
| ENSXMAT00000004312 | cdkn1d | 2.23 | 2.18 | 2.14 | cyclin-dependent kinase inhibitor 1D, Circadian regulator |
| ENSXMAT00000010065 | cenpe | −2.71 | −4.45 | −2.87 | centromere protein E, Kinetochore, chromosome alignment and movement |
| ENSXMAT00000015178 | cenpf | −2.76 | −4.41 | −3.27 | centromere protein F, (mitosin), Centromere at G2, localizes to spindle in anaphase & telophase |
| ENSXMAT00000007642 | chtf18 | −2.09 | −2.55 | np | CTF18, chromosome transmission fidelity factor 1, Chromosome cohesion, S phase |
| ENSXMAT00000000722 | depdc1a | −3.04 | −4.58 | −4.26 | DEP domain containing 1a, represses A20 leading to transport of NF-Kβ |
| ENSXMAT00000002159 | espl1 | −3.00 | −4.42 | −3.27 | extra spindle poles like 1, Mitotic spindle function |
| ENSXMAT00000018919 | haus5 | −2.92 | −3.16 | np | HAUS augmin-like complex, subunit 5, Involved in mitotic spindle, vital to spindle assembly |
| ENSXMAT00000003800 | kif18a | −2.48 | −3.05 | np | kinesin family member 18A, Kinetochore function, regulates CENPE |
| ENSXMAT00000003706 | kif23 | −2.58 | −3.70 | −2.71 | kinesin family member 23, Chromosome movement, spindle, cytokinesis |
| ENSXMAT00000016886 | kifc1 | np | −3.96 | −3.26 | kinesin family member 2C, Important role in cytokinesis, spindle |
| ENSXMAT00000009865 | ncapg | np | −3.54 | −3.11 | non-SMC condensin II complex, subunit D3, condensation of chromosomes during mitosis |
| ENSXMAT00000017114 | ndc80 | −2.29 | −2.40 | −2.10 | NDC80 homolog, kinetochore complex component, Kinetochore, chromosome segregation |
| ENSXMAT00000015146 | pask | −2.53 | −2.78 | −2.21 | PAS domain ser/threo kinase, Regulates changing environmental conditions, energy homeostasis |
| ENSXMAT00000006913 | prc1a | np | −3.44 | −3.49 | protein regulator of cytokinesis 1a |
| ENSXMAT00000018132 | prc1b | −2.90 | −4.04 | −3.91 | protein regulator of cytokinesis 1b |
| ENSXMAT00000013737 | pttg1 | −2.20 | np | −2.34 | pituitary tumor-transforming 1, Chromosome stability, DNA repair, negatively regulates p53 |
| ENSXMAT00000004597 | smc2 | −2.58 | −3.11 | −2.38 | structural maintenance of chromosomes 2, Critical for mitotic condensation and DNA repair |
| ENSXMAT00000019592 | sox3 | 2.96 | 2.33 | 3.38 | SRY-box containing gene 3 |
| ENSXMAT00000010119 | spag5 | −2.81 | −3.57 | −3.20 | sperm associated antigen 5, Functions at spindle, chrom. segregation, anaphase progression |
| ENSXMAT00000013574 | tacc3 | −2.51 | −2.92 | −3.02 | transforming, acidic coiled-coil containing protein 3, Stabilization of the mitotic spindle |
| ENSXMAT00000007097 | ttk | np | −3.34 | −2.98 | ttk protein kinase, centrosome alignment, mitotic segregation |
| ENSXMAT00000015815 | ybx2 | 3.59 | 2.91 | 3.81 | Y box binding protein 2, transcription factor and RNA binding |
DNA replication and repair
A large number of FL DE genes (20) segregated into the functional class of DNA Replication and Repair (Table 6). Several of these genes are involved with replication as one may expect during S phase of the cell cycle (i.e., cdc45, orc1, rpa1, rnaseh2a) and these share functional similarity with several genes that are segregated into both the Mitosis and Cell Cycle, and Chromosome Structure classes (gins4, mcm2, chtf18, cmc5, pttg1, and wdhd). This repression of transcription in genes encoding products that function in S phase may suggest that cells actively in mitosis, at the time of FL exposure, cease their mitotic progression. This would be in addition to the stalling of cells at the G1-S transition by reduction in many mitotic and cell cycle regulators (see above).
Table 6.
DNA replication and repair genes showing DE upon FL exposure. Shaded boxes are up-regulated while down-regulated are unshaded. np = not significant for that exposure.
| Ensembl I.D | Gene | Fold Change | Description | ||
|---|---|---|---|---|---|
| 20 | 40 | 60 | |||
| ENSXMAT00000017007 | atm-2 | −6.54 | −12.28 | −3.53 | ATM serine/threonine kinase, DNA repair, ATM (Ataxia telangiectasia), recombination |
| ENSXMAT00000005761 | brip1 | −2.26 | −2.87 | −2.15 | BRCA1 interacting protein C-terminal helicase 1, BRCA1 Complex, also called FANCJ |
| ENSXMAT00000014828 | cdc45 | −2.36 | −2.29 | np | CDC45 cell division cycle 45 homolog, Required for Initiation of DNA replication |
| ENSXMAT00000004764 | cpd-phr | np | 2.03 | 2.35 | deoxyribopyrimidine photolyase like |
| ENSXMAT00000000980 | fanci | −2.44 | −3.43 | np | Fanconi anemia, compl. group I, DS break DNA repair, FANC comples and BRCA1 |
| ENSXMAT00000007264 | fancl | −3.55 | −3.43 | np | Fanconi anemia, complementation group L |
| ENSXMAT00000010557 | hmmr | −3.00 | −4.08 | −2.59 | hyaluronan-mediated motility receptor. complex with BRCA1, BRCA2, DNA repair |
| ENSXMAT00000016798 | map2K6 | −3.86 | −4.09 | −2.71 | mitogen-activated protein kinase 6 |
| ENSXMAT00000011013 | mdc1 | −2.24 | −3.08 | np | mediator of DNA-damage checkpoint1, G2-M phase checkpoint control, BRCA1 |
| ENSXMAT00000004105 | mms22l | −2.31 | −2.62 | np | MMS22-like, DNA repair protein, Genome integrity during replcation |
| ENSXMAT00000002242 | neil3 | −2.42 | −3.32 | np | nei endonuclease VIII-like 3 (E. coli), Oxidative damage N-glycosylase |
| ENSXMAT00000020089 | nupr1l | 2.22 | 3.76 | 2.46 | nuclear protein, transcriptional regulator, 1-like |
| ENSXMAT00000013066 | orc1 | −2.34 | −3.22 | np | origin recognition complex, subunit 1, Part of complex for replication initiation, S phase |
| ENSXMAT00000017412 | parpbp | −5.24 | −7.44 | −9.15 | PARP1 binding protein |
| ENSXMAT00000003968 | pif1 | np | −5.89 | −7.52 | PIF1 5′-to-3′ DNA helicase homolog (S. cerevisiae) replication and repair |
| ENSXMAT00000019149 | pola1 | −2.25 | −3.08 | np | polymerase (DNA directed), alpha 1, Initiation of Replication, S phase |
| ENSXMAT00000007844 | pole | −2.45 | −3.69 | np | polymerase (DNA directed), epsilon, DNA repair and replication |
| ENSXMAT00000009167 | rad52 | −2.58 | −2.61 | np | RAD52 homolog (S. cerevisiae), Double strand break repair and recombination |
| ENSXMAT00000000633 | rad54l | −2.21 | −2.88 | np | RAD54-like (S. cerevisiae), Interaction with BRCA1 complex |
| ENSXMAT00000004146 | rpa1 | −2.41 | −3.10 | np | replication protein A1, Replication and DNA repair, xeroderma pigmentosum |
| ENSXMAT00000006984 | rnaseh2a | −2.51 | −2.56 | −2.45 | ribonuclease H2, subunit A, reeplication, removal of priming RNA |
| ENSXMAT00000006766 | xrcc4 | −2.47 | np | −2.78 | X-ray repair complementing repair in Chinese hamster 4, XPD, DS break repair |
Quite a few of the DNA repair genes suppressed in light-exposed skin are thought to be involved with recombination (rad52, rad54l) and are noteworthy in being associated with human disorders when mutated (i.e., atm, ataxia telegentasia; brip1 and hmmr, BRCA1 complex; fanci and fancl, Fanconi’s anemia; xrcc4 and rpa1, xeroderma pigmentosum class D). Other genes that become suppressed upon FL exposure are also associated with DNA repair processes (cpd-phr, cit, neil3, pif1) as well as DNA repair polymerases (pola1 and pole).
The observed suppression on DNA repair gene transcription is consistent with dramatically reduced cell division in the skin, and is likely due to circadian control upon light exposure.
NanoString and qRT-PCR Results
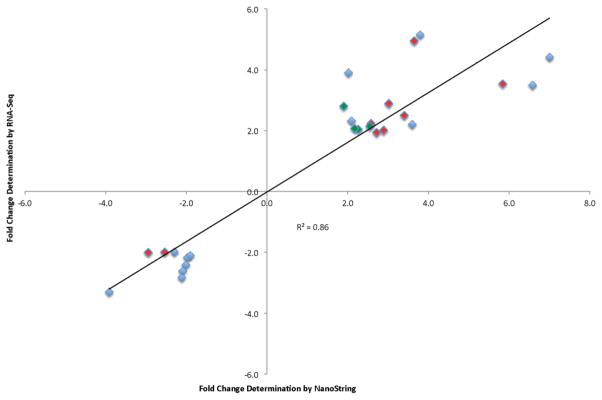
Validation of a subset of RNA-Seq gene expression values was performed by analysis of 18 individual transcripts using a custom NanoString nCounter panel. Each exposure (20, 40 and 60 min FL) was subject to nCounter analysis; ten transcripts were differentially modulated following 20 min FL, four transcripts were differentially modulated following 40 min FL and 13 transcripts were differentially modulated following 60 min FL. Generally, the direction and magnitude of gene expression change was confirmed for all 18 genes, with an R2 value of 0.86 (Figure 5 and Table 7).
Figure 5.
NanoString analysis confirmed the RNA-Seq data for 18 genes in both direction and magnitude (R2 = 0.86) following 20 (red), 40 (green) and 60 (blue) min FL exposure. Fold changes are represented as the average change of 2 biological replicates determined independently for each target plotted and at each exposure.
Table 7.
NanoString targets (18) tested confirmed RNA-Seq determined fold changes following 20, 40 and 60 min of FL exposure.
| Exposure | Ensembl ID | Gene Name | NanoString | RNA-Seq |
|---|---|---|---|---|
|
| ||||
| 20 min FL | ENSXMAT00000002257 | bhlhe40 | −2.9 | −2.0 |
| ENSXMAT00000002359 | nr1d4b | 3.4 | 2.5 | |
| ENSXMAT00000015815 | ybx2 | 2.6 | 2.2 | |
| ENSXMAT00000017010 | clock | 2.7 | 1.9 | |
| ENSXMAT00000000471 | adrb2b | 3.0 | 2.9 | |
| ENSXMAT00000008690 | klhl38b | 3.6 | 4.9 | |
| ENSXMAT00000009163 | tgm8* | 11.8 | 3.4 | |
| ENSXMAT00000014172 | snca | 2.9 | 2.0 | |
| ENSXMAT00000016248 | per2 | 5.8 | 3.5 | |
| ENSXMAT00000016798 | map2k6 | −2.5 | −2.0 | |
|
| ||||
| 40 min FL | ENSXMAT00000009327 | cry2a | 2.3 | 2.0 |
| ENSXMAT00000015815 | ybx2 | 2.2 | 2.1 | |
| ENSXMAT00000017010 | clock | 2.5 | 2.1 | |
| ENSXMAT00000016248 | per2 | 1.9 | 2.8 | |
|
| ||||
| 60 min FL | ENSXMAT00000004757 | ccnf | −2.0 | −2.2 |
| ENSXMAT00000014334 | ccnb2 | −2.1 | −2.8 | |
| ENSXMAT00000008371 | kpna2 | −2.1 | −2.6 | |
| ENSXMAT00000011193 | numa1 | −2.0 | −2.4 | |
| ENSXMAT00000014517 | arhgap19 | −1.9 | −2.1 | |
| ENSXMAT00000015815 | ybx2 | 2.1 | 2.3 | |
| ENSXMAT00000017010 | clock | 2.0 | 3.9 | |
| ENSXMAT00000003968 | pif1 | −3.9 | −3.3 | |
| ENSXMAT00000008690 | klhl38b | 3.8 | 5.2 | |
| ENSXMAT00000009163 | tgm8 | 6.6 | 3.5 | |
| ENSXMAT00000016248 | per2 | 7.0 | 4.4 | |
| ENSXMAT00000016798 | map2k6 | −2.3 | −2.0 | |
| ENSXMAT00000019435 | xpc | 3.6 | 2.2 | |
tgm8 confirmed the RNA-Seq determined fold change in direction but did not correlate in magnitude and was therefore not included Figure 5.
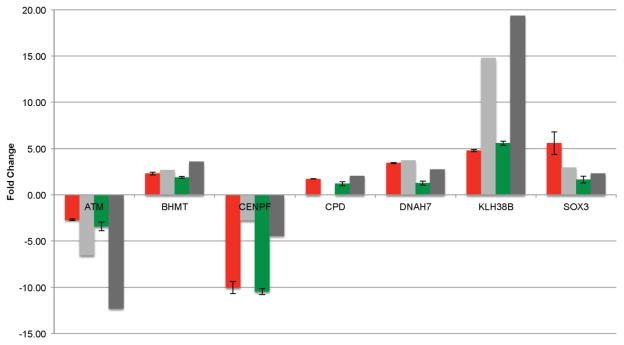
In addition to the NanoString nCounter analysis, an additional confirmation of 7 DE gene targets was performed using qRT-PCR. Like the nCounter analysis, all reported fold changes from the RNA-Seq analysis agreed with the qRT-PCR in direction (Figure 6).
Figure 6.
RNA-Seq validation (20 min FL- light grey; 40 min FL- dark grey) was performed on seven targets using qRT-PCR following 20 (red) and 40 (green) min FL exposure. Mean fold change relative to an unexposed sample was calculated using the ΔΔCT method with an 18S rRNA internal standard for 3 technical replicates.
Conclusions
Fluorescent light exposure, from commonly utilized “cool white” lights, induced a robust genetic response (≥ 87% of the response induced by UVB) in the skin of male X. maculatus across all examined FL exposures. The FL response is significantly different than that observed from UVB exposure (Figure 2). The FL genetic response appears to saturate at the earliest exposure (20 min.) since the genes modulated remain relatively constant in number, type, and direction of effect though 60 min.
One-third of the genes that demonstrated significant DE after FL which includes over half of the genes modulated in at least two of the three FL doses, cluster into 5 functional classes (Table S4): (1) Light Responsive, (2) Oxidation/Reduction. (3) Mitosis and Cell Cycle, (4) Chromosome Structure, and (4) DNA Replication and Repair (Tables 2–6).
Exposure to FL results in depression of transcription levels for of a large set of genes involved in mitosis and cell cycle progression, as well as genes involved in the repair (recombination) and replication of DNA. Repression of this gene set in the Mitosis/Cell Cycle and Chromosome Structure categories shows genetic interaction and encoded gene products that are functionally related (Figure 4).
Tumor induction studies using the UVB inducible Xiphophorus interspecies hybrid models aimed at determination of the wavelengths responsible for melanomagenesis have been controversial. While it is established that UVB exposure can induce melanoma in susceptible Xiphophorus hybrids (Nairn et al, 1996; Patton et al, 2010; Mitchell et al, 2010) there are also reports showing UVA may play a major role in tumor induction (Setlow et al, 1993; Wood et al, 2006). We have shown that exposure to as little as 20 min of fluorescent light may substantially decrease the transcription levels of genes involved in cell cycle regulation, mitosis, chromosome structure, and DNA repair. While fluorescent light does not induce melanoma the molecular genetic effects of FL exposure in fish skin appear to be substantial. Thus, the light conditions prior to a UV tumor induction experiment have the potential to play a role in the outcome. It remains to be seen if Xiphophorus hybrid skin behaves genetically as the parental X. maculatus Jp 163 B utilized herein. In previous studies of DNA repair, Xiphophorus hybrid fish have been shown to lose light induced regulation or have reduced DNA repair capability compared to their parental lines (Mitchell et al, 2004; David et al, 2004; Walter et al, 2014).
The rather rapid and sustained (20 to 60 min) reduction on transcription of genes involved in cell cycle progression suggests peripheral circadian oscillators may exist in Xiphophorus skin capable of gating the cell cycle. It remains to be determined how different light sources, with substantially varied spectral emission, may affect such mechanisms. Having evolved in a full spectrum environment, the genes of tropical fishes such as Xiphophorus, are likely to be fine tuned to a full spectrum of wavelengths that fluorescent lighting does not mimic. As a result, one cannot say the genetic effect shown herein is “normal”, but only that it is observed upon “cool white” fluorescent light exposure. At the minimum, given the cost associated with maintaining aquatic research animals (Axolotl, Xiphophorus, zebrafish, Fundulus, medaka, Aplysia, sea urchins, etc.) more investigations are warranted to ensure lighting conditions utilized are most appropriate. Given the amount of fluorescent light that humans and animals are subjected to on a daily basis, further investigation of these effects would be prudent.
Supplementary Material
NanoString custom designed probe set for 18 test targets and 10 housekeeping controls. Asterisked genes represent housekeeping genes.
Spectral distribution of the FL source. The spectral output of the FL lamps was determined using an Ocean Optics STS 350–800 nm Microspectrometer (Ocean Optics Inc., Dundedin, FL, USA) and OceanView software v1.5 (http://oceanoptics.com/product/oceanview/). The Microspectrometer was calibrated to a known standard using Ocean Optics Halogen Calibrated Light Source HL-3P-CAL (Ocean Optics Inc., Dundedin, FL, USA).
Custom designed primers set for 7 genes tested by qRT-PCR for consistency with RNA-Seq and the 18S rRNA control sequence.
522 DE genes from the transcriptome (20,218 gene models queried) that showed significant up- or down-transcriptional modulation for at least one FL exposure.
216 genes that exhibited significant DE in two or more exposure time points. Also shown are the five functional clusters identified with DAVID comprised of 115 DE genes within the set of DE genes for two or more exposures.
Acknowledgments
The authors would like to thank the staff of the Xiphophorus Genetic Stock Center, Texas State University, for maintaining the pedigreed fish lines and caring for the animals used in this study. The authors would also like to thank Drs. Yingjia Shen and Tzuni Garcia for setting up the bioinformatic pipelines used in some of the data analysis presented herein. Thanks also to Drs. Chris Walter and Karen Lewis for critical reading of this manuscript. This work was supported by the National Institutes of Health, Division of Comparative Medicine, R24 OD-011120, R24 OD-011199 and R24 OD-018555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dylan J. Walter, Email: djw3@txstate.edu.
William T. Boswell, Email: wb1016@txstate.edu.
Kaela L. Caballero, Email: klc140@txstate.edu.
Mikki Boswell, Email: mboswell@txstate.edu.
Yuan Lu, Email: y_l54@txstate.edu.
Jordan Chang, Email: changj@txstate.edu.
Markita G. Savage, Email: markita@txstate.edu.
References
- Ahmed FE, Setlow RB. Ultraviolet radiation-induced DNA damage and its photorepair in the skin of the platyfish Xiphophorus. Cancer Res. 1993;15(53):2249–55. [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TN, Reimschuessel R, Bradley BP. DNA damage, histological changes and DNA repair in larval Japanese medaka (Oryzias latipes) exposed to ultraviolet-B radiation. Aquat Toxicol. 2002;58 (1–2):1–14. doi: 10.1016/s0166-445x(01)00212-0. [DOI] [PubMed] [Google Scholar]
- Bjarnason G, Jordan R. Circadian variation of cell proliferation and cell cycle protein expression in man: Clinical implications. Prog Cell Cycle Res. 2000;4:193–206. doi: 10.1007/978-1-4615-4253-7_17. [DOI] [PubMed] [Google Scholar]
- Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Malgrange B. Cell “Circadian” Cycle. Cell Cycle. 2009;8 (6):8332–837. doi: 10.4161/cc.8.6.7869. [DOI] [PubMed] [Google Scholar]
- Bouly JP, Schleicher E, Dionisio-Sese M, Vandenbussche F, Van Der Straeten D, Bakrim N, Maier S, Batschauer A, Galland P, Bittl R, Ahmad M. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem. 2007;282(13):9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- Caballero KL. MS Thesis in Biochemistry. Texas State University; San Marcos, Texas: 2015. Differential gene expression in the Skin of Xiphophorus maculatus Jp 163 B in response to full spectrum (10,000K) fluorescent light. [Google Scholar]
- Chang J, Boswell WT, Boswell M, Caballero KL, Lu Y, Walter RB. Molecular Genetic Response in Xiphophorus maculatus Skin to Varying Wavelengths of Light. 2015 submitted. [Google Scholar]
- David WM, Mitchell DL, Walter RB. DNA repair in hybrid fish of the genus Xiphophorus. Comp Biochem Physiol Part C. 2004;138 (3):300–310. doi: 10.1016/j.cca.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Destici E, Oklejewicz M, Saito S, Van Der Horst GTJ. Mammalian cryptochromes impinge on cell cycle progression in a circadian clock-independent manner. Cell Cycle. 2011;10 (21):3788–3797. doi: 10.4161/cc.10.21.17974. [DOI] [PubMed] [Google Scholar]
- Denkins MPS, Santoriello C, Vallone D, Grassi G, Whitmore D, Foulkes NS. Light regulates the cell cycle in zebrafish. Current Biol. 2003;13:2051–2057. doi: 10.1016/j.cub.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Garcia TI, Shen Y, Crawford D, Oleksiak MF, Whitehead A, Walter RB. RNA-Seq reveals complex genetic response to Deepwater Horizon oil release in Fundulus grandis. BMC Genomics. 2012;13:474–483. doi: 10.1186/1471-2164-13-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, Fell HP, Ferree S, George RD, Grogan T, James JJ, Maysuria M, Mitton JD, Oliveri P, Osborn JL, Peng T, Ratcliffe AL, Webster PJ, Davidson EH, Hood L, Dimitrov K. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nature Biotech. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- Hart RW, Setlow RB, Woodhead AD. Evidence that pyrimidine dimers in DNA can give rise to tumors. Proc Natl Acad Sci USA. 1977;74 (12):5574–5578. doi: 10.1073/pnas.74.12.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D and bone health. J Nutrition. 1996;126:1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- Honer M, Weber W. Molecular switches in animals cells. FEBS Lett. 2012;586:2084–2096. doi: 10.1016/j.febslet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Hurlbert AC, Ling Y. Biological components of sex differences in color preference. Curr Biology. 2007;17 (16):623–625. doi: 10.1016/j.cub.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Johnson CH. Circadian clocks and cell division. Cell Cycle. 2010;9 (19):3864–3873. doi: 10.4161/cc.9.19.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Meth. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Filipski E, Iurisci I, Li Innominato XM. Cross-talk between circadian timing system and cell division cycle determine cancer biology and therapeutics. Cold Spring Symp Quant Biol. 2007;72:465–475. doi: 10.1101/sqb.2007.72.030. [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. J Gerontol. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SL, Veitch JA. Full-spectrum fluorescent lighting: a review of its effects on physiology and health. Psychol Med. 2001;31 (6):949–964. doi: 10.1017/s0033291701004251. [DOI] [PubMed] [Google Scholar]
- Meador JA, Walter RB, Mitchell DL. Induction, distribution and repair of UV photodamage in the platyfish, Xiphophorus signum. Photochem Photobiol. 2000;72 (2):260–266. doi: 10.1562/0031-8655(2000)072<0260:idarou>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitani H, Uchida N, Shima A. Induction of cyclobutane pyrimidine dimer photolyase in cultured fish cells by UVA and blue light. Photochem Photobiol. 1996;64 (6):943–948. doi: 10.1111/j.1751-1097.1996.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Meador JA, Byrom M, Walter RB. Resolution of UV-induced DNA damage in Xiphophorus fishes. Mar Biotechnol. 2001;3:S61–S71. doi: 10.1007/s101260000000. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Nairn RS, Johnston DA, Byrom M, Kazianis S, Walter RB. Decreased levels of DNA excision repair in hybrid fish of the genus Xiphophorus. Photochem Photobiol. 2004;79:447–452. doi: 10.1562/ca-03-14.1. [DOI] [PubMed] [Google Scholar]
- Mitchell DL, Fernandez AA, Nairn RS, Garcia R, Paniker L, Trono D, Thames HD, Gimenez-Conti I. Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model. Proc Natl Acad Sci USA. 2010;107 (2):9329–9334. doi: 10.1073/pnas.1000324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn RS, Kazianis S, McEntire BB, Coletta LD, Walter RB, Morizot DC. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish-swordtail hybrids. Proc Natl Acad Sci USA. 1996;93:13042–13047. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pig Cell Mel Res. 2010;23:314–337. doi: 10.1111/j.1755-148X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyric E, Moore HA, Whitmore D. Circadian clock regulation of the cell cycle in the zebrafish intestine. PLOS One. 2013;8 (8):e73209. doi: 10.1371/journal.pone.0073209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakers S, Gebert M, Uppalapati S, Meyer W, Maderson P, Sell AF, Kruse C, Paus R. Fish matters’: the relevance of sh skin biology to investigative dermatology. Exp Dermatology. 2010;19:313–324. doi: 10.1111/j.1600-0625.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Ramsko D, Wang ET, Burge CB, Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol. 2009;5(12):e1000598. doi: 10.1371/journal.pcbi.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Pachter L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nature methods. 2013;10:71–73. doi: 10.1038/nmeth.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: Platyfish-swordtail hybrid. Proc Natl Acad Sci USA. 1989;86:8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somade OT. Role of circadian clock genes in the regulation of cerll cycle processes. Rom J Biochem. 2014;512 (2):151–178. [Google Scholar]
- Uchida N, Mitani H, Todo T, Ikenaga M, Shima A. Photoreactivating enzyme for (6-4) photoproducts in cultured goldfish cells. Photochem PhotobioL. 1997;65(6):964–968. doi: 10.1111/j.1751-1097.1997.tb07955.x. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Schwartz S, Grandjean D, Wuillaume C, Balteau E, Degueldre C, Schabus M, Phillips C, Luxen A, Dijk DJ, Maquet P. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci USA. 2010;107 (45):19549–19554. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter DJ, Boswell M, Volk de Garcia SM, Walter SM, Breitenfeldt EW, Boswell W, Walter RB. Characterization and differential expression of CPD and 6-4 DNA photolyases in Xiphophorus species and interspecies hybrids. Comp Biochem Physiol Part C. 2014;163:77–85. doi: 10.1016/j.cbpc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucl Acids Res. 2010;38:214–220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Strahle U, Sassone-Corsi P. Zebrafish clock rhythmic expression revels independent peripheral circadian oscillators. Nat Neurosci. 1998;1:701–707. doi: 10.1038/3703. [DOI] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells n culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- Wood SR, Berwick M, Lev RD, Walter RB, Setlow RB, Timmins GS. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci USA. 2006;103(11):4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ. The effects of light on the human body. Scien Amer. 1975a;233:68–77. [PubMed] [Google Scholar]
- Wurtman RJ. The effects of light on man and other mammals. Ann Review Physiol. 1975b;37:467–483. doi: 10.1146/annurev.ph.37.030175.002343. [DOI] [PubMed] [Google Scholar]
- Xu Z, Parra D, Gómez D, Salinas I, Zhang YA, von Gersdorff Jørgensen L, Heinecke RD, Buchmann K, LaPatra Sunyer JO. Teleost skin, an ancient mucosal surface that elicits gut-like mucosal immune responses. Proc Natl Acad Sci USA. 2013;110:13097–13102. doi: 10.1073/pnas.1304319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Boswell M, Walter DJ, Downs KD, Gaston-Pravia K, Garcia T, Shen Y, Mitchell DL, Walter RB. UVB-induced gene expression in the skin of Xiphophorus maculatus Jp 163 B. Comp. Biochem Physiol Part C. 2014;163:86–94. doi: 10.1016/j.cbpc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NanoString custom designed probe set for 18 test targets and 10 housekeeping controls. Asterisked genes represent housekeeping genes.
Spectral distribution of the FL source. The spectral output of the FL lamps was determined using an Ocean Optics STS 350–800 nm Microspectrometer (Ocean Optics Inc., Dundedin, FL, USA) and OceanView software v1.5 (http://oceanoptics.com/product/oceanview/). The Microspectrometer was calibrated to a known standard using Ocean Optics Halogen Calibrated Light Source HL-3P-CAL (Ocean Optics Inc., Dundedin, FL, USA).
Custom designed primers set for 7 genes tested by qRT-PCR for consistency with RNA-Seq and the 18S rRNA control sequence.
522 DE genes from the transcriptome (20,218 gene models queried) that showed significant up- or down-transcriptional modulation for at least one FL exposure.
216 genes that exhibited significant DE in two or more exposure time points. Also shown are the five functional clusters identified with DAVID comprised of 115 DE genes within the set of DE genes for two or more exposures.