Abstract
Background
Patent ductus arteriosus (PDA) is common in extremely premature infants and associated with increased morbidity and mortality. Medical management of PDA uses either indomethacin or ibuprofen. Despite numerous studies, uncertainty exists as to which drug is safer or more effective; we sought to fill this knowledge gap.
Methods
We identified infants <28 weeks gestational age discharged from neonatal intensive care units included in the Pediatrix Medical Group Clinical Data Warehouse between 2006 and 2012 who were treated with indomethacin or ibuprofen between postnatal day 2 and 14. Infants treated with both drugs or infants with a congenital malformation were excluded. We used multivariable logistic regression to determine the association of indomethacin versus ibuprofen on clinical outcomes.
Results
Of 6349 patients who met study criteria, 1177 (19%) received ibuprofen and 5172 (81%) received indomethacin. The median gestational age was 25 weeks (interquartile range 24–26), and 2894 (46%) infants were <750 g at birth. On unadjusted analysis, infants who received ibuprofen had significantly higher incidences of death prior to discharge, surgical ligation of the PDA prior to discharge, death or spontaneous intestinal perforation within 7 days of therapy, death or surgical ligation of the PDA prior to discharge, and an elevated creatinine within 7 days of treatment. However, on multivariable analysis, no significant differences in outcomes were observed (odds ratio for death/PDA ligation for ibuprofen vs. indomethacin = 1.12 [95% CI 0.91–1.39]).
Conclusions
We observed similar effectiveness and safety profiles for indomethacin and ibuprofen in the medical management of PDA in premature infants.
Keywords: premature infants, medical management, bronchopulmonary dysplasia, mortality
1. INTRODUCTION
The ductus arteriosus (DA) is a normal anatomic conduit in the fetus, allowing blood to bypass the pulmonary circuit [1]. The DA is kept open while in utero through a combination of prostaglandins, nitric oxide, and pressure differentials [2]. After birth, via contracture of the smooth muscle within its walls likely secondary to an increase in oxygen tension and a decrease in prostaglandins, the DA closes and becomes the ligamentum arteriosum [2]. Functional closure of the DA usually occurs within hours of birth in term infants; however, in premature infants, the DA frequently fails to close after birth or experiences substantial delay in closure [3]. This condition, known as patent ductus arteriosus (PDA) [4], has been associated with bronchopulmonary dysplasia (BPD), increased ventilation requirements, poor feeding tolerance, and increased mortality in premature infants [5,6].
Since the 1970s, indomethacin, an inhibitor of prostaglandin synthesis, has been used in the treatment of PDA [7–9]. However, indomethacin has been associated with adverse events, including increased risk of necrotizing enterocolitis and renal insufficiency [10–15]. To find a safer alternative to indomethacin, ibuprofen was approved for the treatment of PDA in 2006 [16]. However, ibuprofen has also recently been linked to adverse events including spontaneous intestinal perforation (SIP), leading to uncertainty over which drug has the better safety profile [17–19]. Further intensifying this debate, a voluntary recall by the supplier of ibuprofen in 2010 forced many clinicians to return to using indomethacin [20].
A number of studies have compared the safety and efficacy of ibuprofen and indomethacin [9,21–23]. These studies have consistently demonstrated similar effectiveness between the 2 drugs, but indomethacin tended to demonstrate a worse side effect profile [21]. Despite the available evidence, neonatologists continue to use both drugs for the treatment of PDA [24–26]. To better assess the risks and benefits of each drug, we performed a retrospective review to determine the safety and effectiveness of ibuprofen and indomethacin. We hypothesized that there would be no significant differences in effectiveness or safety between the 2 drugs.
2. METHODS
2.1. Data Source and Study Population
We obtained data for our study from the Pediatrix Medical Group Clinical Data Warehouse, a prospective clinical database that captures information from daily progress notes generated by clinicians on all infants discharged from 165 neonatal intensive care units managed by the Pediatrix Medical Group in the United States. We collected information on prenatal characteristics, demographics, timing and duration of exposure to ibuprofen or indomethacin, and clinical and laboratory diagnoses of interest, as well as clinical outcomes.
We included all inborn infants <28 weeks gestational age (GA) discharged between 2006 to 2012 who received either indomethacin or ibuprofen and were first exposed to either drug from postnatal day 2 to postnatal day 14. We excluded infants exposed to both drugs and infants diagnosed with a major congenital anomaly. We excluded infants treated on the first 2 days of life to eliminate infants receiving prophylaxis for intraventricular hemorrhage.
2.2. Definitions
We identified infants as exposed to indomethacin or ibuprofen and analyzed the first course of therapy for each infant. We defined small for gestational age (SGA) as <10th percentile for age [27]. As surrogates for severity of illness, we identified exposure to any inotropic medication (dopamine, dobutamine, epinephrine, milrinone, and phenylephrine) or systemic hydrocortisone, as well as any mechanical ventilator support or need for supplemental oxygen (FiO2) on the first day of therapy. We defined renal insufficiency at the onset of therapy as any serum creatinine >1 mg/dL on the first day of therapy or up to 2 days prior. We identified several outcomes of interest: death prior to discharge, death or PDA requiring surgical ligation prior to discharge, SIP within 7 days of start of therapy, death or SIP within 7 days of start of therapy, death or BPD prior to discharge, serum creatinine >1 mg/dL within 7 days of the start of therapy, and the combined outcome of any one of these events. BPD was defined as patients who received continuous supplemental oxygen or respiratory support from a corrected GA of 36 0/7–36 6/7 weeks.
2.3. Statistical Analysis
The unit of observation for this study was the infant. We used standard summary statistics, including medians (interquartile ranges) and counts (percentages), to describe continuous and categorical study variables. We described the use of indomethacin and ibuprofen by year over the course of the study, as well as variation in the use of the 2 drugs by site. We compared the distribution of predictor variables and the outcomes of interest between infants exposed to indomethacin versus ibuprofen using Wilcoxon rank sum and chi-square tests of association. We used multivariable mixed logistic regression with fixed effects for site to evaluate the association between indomethacin and ibuprofen exposure and each outcome of interest. We conducted standard model assumptions diagnostics and evaluation for collinearity [28]. We first evaluated a model including all plausible predictor variables available, including their interactions as covariates. Initial predictors included GA, SGA, inotrope use, hydrocortisone use on first day of therapy, creatinine >1 mg/dL, mechanical ventilation, supplemental oxygen on first day of therapy, prenatal steroid exposure, discharge year, and postnatal age on first day of therapy. We then performed likelihood ratio tests to compare the full model to a priori determined reduced models and reported the most parsimonious model that fit the data well. A priori reduced models were produced by systematically dropping variables that were less likely to be related to the outcome. The final multivariable logistic regression model used site as a fixed effect and included the following covariates: GA, SGA status, prenatal steroid exposure, discharge year, postnatal age on the first day of therapy and the need for inotropic support, hydrocortisone use, mechanical ventilation, supplemental oxygen on the first day of therapy, and baseline creatinine >1 mg/dL.
We further analyzed trends in the use of indomethacin and ibuprofen over the study period, as well as variation by center in the use of each drug following the removal of any site with <10 patients during the study period. All statistical analyses were conducted using Stata12 (College Station, TX). A p<0.05 was defined as statistically significant. This study was approved by the institutional review board at the corresponding author’s university.
3. RESULTS
3.1. Study Population
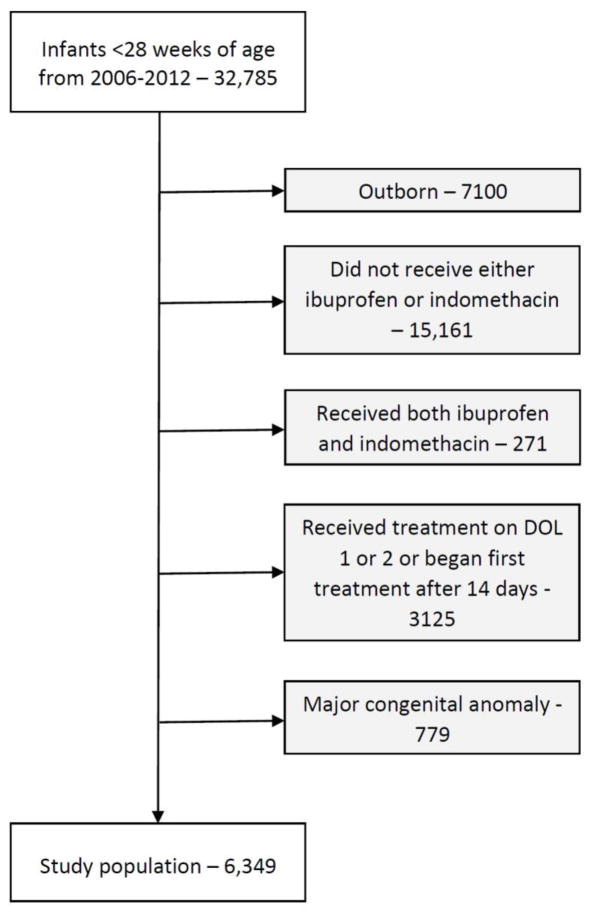
We identified 6349 infants from 165 sites who met our study criteria (Fig. 1); 1177 (19%) received ibuprofen and 5172 (81%) received indomethacin. Median GA was 25 weeks (interquartile range 24–26, Table 1). Birth weight, SGA status, race/ethnicity, the use of antenatal steroids, baseline creatinine >1 mg/dL, requirement of supplemental oxygen, and use of hydrocortisone on the first day of therapy were significantly different between the ibuprofen and indomethacin groups; however, the absolute differences between groups were small.
Fig. 1.
Study cohort. DOL, day of life.
Table 1.
Demographics and baseline indicators of severity of illness of infants <28 weeks gestational age, by treatment group.
| Ibuprofen (N = 1,177) | Indomethacin (N = 5,172) | p | |
|---|---|---|---|
| Gestational age (weeks, continuous) | 25 (24, 26) | 25 (24, 26) | 0.08 |
| Gestational age (weeks, categorical) | 0.42 | ||
| <25 | 346 (29%) | 1,422 (27%) | |
| 25–26 | 560 (48%) | 2,530 (49%) | |
| 27–28 | 271 (19%) | 1,220 (24%) | |
| Birth weight (g) | 750 (640, 888) | 770 (651, 910) | <0.01 |
| Birth weight <750 g | 575 (49%) | 2,319 (45%) | 0.01 |
| Small for gestational age | 175 (15%) | 653 (13%) | 0.04 |
| Male Sex | 629 (53%) | 2,631 (51%) | 0.11 |
| Race/ethnicity | <0.01 | ||
| White | 458 (40%) | 2,343 (47%) | |
| Black | 444 (39%) | 1,387 (28%) | |
| Hispanic | 192 (17%) | 957 (19%) | |
| Other | 48 (4.2%) | 317 (6.3%) | |
| Antenatal steroids | 916 (78%) | 4,258 (82%) | <0.01 |
| Age on day 1 of therapy (days) | 4 (3, 7) | 4 (3, 7) | 0.11 |
| >4 days of age on day 1 of therapy | 694 (59%) | 2,964 (57%) | 0.30 |
| Baseline creatinine on day 1 of therapy (mg/dL) | 1.0 (0.8, 1.1) | 0.9 (0.8, 1.1) | <0.01 |
| Baseline creatinine >1.0 mg/dL on day 1 of therapy | 258 (22%) | 892 (17%) | <0.01 |
| Mechanical ventilation on day 1 of therapy | 866 (74%) | 3,743 (73%) | 0.22 |
| FiO2 >21% on day 1 of therapy | 694 (62%) | 3,240 (66%) | 0.03 |
| Inotrope support on day 1 of therapy | 211 (18%) | 857 (17%) | 0.26 |
| Hydrocortisone therapy on day 1 of therapy | 75 (6.4%) | 130 (2.5%) | <0.01 |
Continuous variables listed as median (interquartile range). Categorical variables listed as frequency (percentage).
3.2. Outcomes
The incidences of death prior to discharge (17% vs. 13%, p<0.01), PDA ligation prior to discharge (27% vs. 24%, p=0.04), death or PDA ligation prior to discharge (40% vs. 34%, p<0.01), death or SIP within 7 days of therapy (18% vs. 14%, p<0.01), and creatinine >1.0 mg/dL within 7 days of treatment (36% vs. 32%, p<0.01) were significantly higher in the ibuprofen group compared with the indomethacin group (Table 2). Following adjustment, no statistically significant difference in outcomes remained.
Table 2.
Morbidity and mortality associated with each treatment group.
| Ibuprofen (N = 1177) | Indomethacin (N = 5172) | Unadjusted ORa (95% CI) | Adjusted ORb (95% CI) | |
|---|---|---|---|---|
| Death prior to discharge | 186 (17%) | 603 (13%) | 1.37 (1.14, 1.64) | 1.10 (0.82, 1.47) |
| PDA requiring surgical ligation prior to discharge | 318 (27%) | 1,249 (24%) | 1.16 (1.01, 1.34) | 1.10 (0.87, 1.37) |
| Death/PDA requiring surgical ligation prior to discharge | 473 (40%) | 1,768 (34%) | 1.29 (1.14, 1.47) | 1.12 (0.91, 1.39) |
| SIP within 7 days of therapy | 37 (3.1%) | 161 (3.1%) | 1.01 (0.70, 1.45) | 0.87 (0.52, 1.51) |
| Death/SIP within 7 days of therapy | 213 (18%) | 707 (14%) | 1.40 (1.18, 1.65) | 1.11 (0.85, 1.45) |
| Death/BPD prior to discharge | 703 (60%) | 3,016 (58%) | 1.06 (0.93, 1.21) | 1.03 (0.84, 1.27) |
| Creatinine >1.0 mg/dL within 7 days of therapy | 422 (36%) | 1,651 (32%) | 1.19 (1.04, 1.36) | 1.16 (0.87, 1.54) |
| Any adverse outcome | 802 (68%) | 3,395 (66%) | 1.12 (0.98, 1.28) | 1.14 (0.92, 1.42) |
OR, odds ratio; CI, confidence interval.
Indomethacin as reference.
Indomethacin as reference, adjusted for GA, SGA status, prenatal steroid exposure, discharge year, postnatal age in days on the first day of therapy, the receipt of inotropic support, hydrocortisone use on day 1 of therapy, mechanical ventilation, and supplemental oxygen on the first day of therapy.
3.3. Temporal Trends and Site-Based Differences
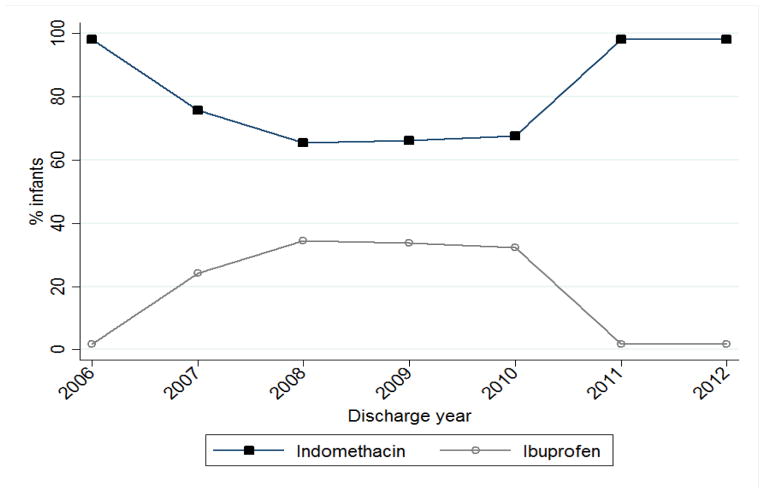
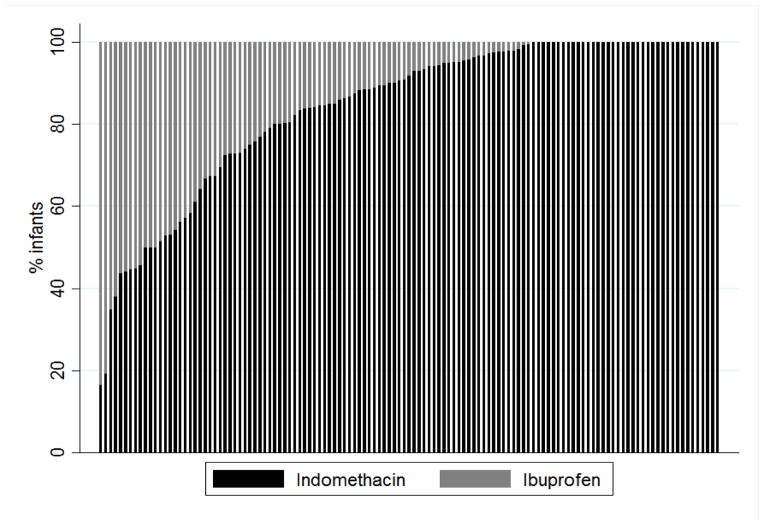
The use of indomethacin decreased from 98% of treated infants in 2006 to 66% of cases in 2008 before rebounding back to 98% in 2011 (Fig. 2). Following removal of any site with <10 cases during the study period, we determined how ibuprofen and indomethacin use varied by site (Fig. 3). Among sites with ≥10 infants treated with either ibuprofen or indomethacin during the study period, indomethacin use varied between 17% and 100%, with 38/125 sites (30%) using only indomethacin.
Fig. 2.
Ibuprofen and indomethacin use between 2006 and 2012.
Fig. 3.
Variability in the use of ibuprofen and indomethacin among the 125 sites that treated 10 or more infants over the study period.
4. DISCUSSION
We found that exposure to indomethacin or ibuprofen resulted in no significant difference in hospital mortality, surgical ligation, SIP, BPD, or renal insufficiency between groups after adjusting for baseline severity of illness. Although, historically, indomethacin was the treatment of choice for medical closure of PDA, concerns over side effects, especially those affecting the gastrointestinal (particularly when used with hydrocortisone) and renal systems, led to the increasing use of ibuprofen for this indication [10,12,16,21]. Our findings are consistent with previous studies that also found similar outcomes with regard to mortality, surgical ligation, SIP, and BPD [21–23,29].
Our findings, however, did not demonstrate a higher risk of elevated creatinine associated with indomethacin as observed in prior studies [21–23,30]. For instance, Van Overmeire et al. randomized 148 infants to treatment with indomethacin or ibuprofen and found similar efficacy between the 2 groups but a significant increase in oliguria and serum creatinine in patients treated with indomethacin compared with ibuprofen [30]. A Cochrane review that included 15 studies and 740 infants also found no statistical difference in the effectiveness of the 2 medications but did report that there was less evidence of renal insufficiency associated with ibuprofen compared with indomethacin [29]. Unfortunately, due to the small differences in effectiveness between indomethacin and ibuprofen, a randomized controlled trial large enough to detect a difference between the 2 drugs would not be feasible. For example, to detect a 3.75% absolute difference observed in death or PDA ligation in our adjusted analysis between the 2 groups, 5400 infants would be needed to have 80% power.
In addition, we found that, since its approval by the Food and Drug Administration in 2006, ibuprofen has been increasingly used in the medical management of PDA [16]. This is likely due to the general notion that ibuprofen had fewer renal-related side effects than indomethacin, supported by studies performed in the mid-2000s [21,31]. However, by 2010, the use of indomethacin returned to its 2006 level, likely secondary to the voluntary recall of 2 lots of ibuprofen lysine by Lundbeck Inc. in July 2010, which accounted for the majority of the national drug supply at the time [20].
There was also significant variation in the use of the 2 drugs among sites, with the majority of sites using a mixture of ibuprofen and indomethacin. Except for the unavailability of ibuprofen following the 2010 recall, we are unable to speculate on what factors may have influenced the decision to use a specific drug at each site. Interestingly, clinicians appeared to use ibuprofen more commonly in infants with an elevated baseline creatinine, likely due to concern about the effect of indomethacin on the kidney. Nonetheless, after including serum creatinine in our adjusted model, we still did not observe any significant difference in post-treatment renal insufficiency between groups.
Although we present the largest retrospective study to date on the use of ibuprofen and indomethacin in the medical management of PDA, there are important limitations to this study that should be addressed. First, the inclusion of infants by treatment rather than by diagnosis may include infants who received either ibuprofen or indomethacin for another purpose. By limiting to infants treated between postnatal days 2 and 14, we limited the population to those likely being treated for PDA and not another indication, but it is still possible that some patients without PDA remained in the study population. As only 25% of our patients <1000 g were included, and other studies have demonstrated incidence of PDA in this population as high as 57%, it is unlikely that many patients without PDA remained in our sample [32,33]. Second, we did not have access to echocardiographic data to confirm PDA closure, but instead used secondary predictors of poor outcomes including surgical ligation and mortality to demonstrate efficacy. Although this does not strictly define success of PDA closure, it does investigate clinically relevant persistent PDA, which is perhaps a more important end point. Third, we did not have access to urine output or other factors that can be used to more completely assess renal function. Lastly, there is inherent variability in outcomes by NICU. We attempted to account for this variability by including fixed effects for NICU in our adjusted models. Nevertheless, such adjustments may not remove all sources of bias and our data should be interpreted with caution especially when compared to randomized controlled trials data.
In summary, in one of the largest studies to date to investigate the use of indomethacin and ibuprofen in the medical management of PDA, we found no major discernible safety differences between the 2 drugs. The optimal pharmaceutical agent for the medical management of PDA in premature infants remains uncertain.
Highlights.
We compared outcomes for indomethacin and ibuprofen for patent ductus arteriosus.
There was extensive center-level variation in the use of each.
There were no significant differences in effectiveness or safety.
Acknowledgments
Sources of Funding: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The sponsor played no role in the study design; collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit for publication.
Abbreviations
- BPD
Bronchopulmonary dysplasia
- FiO2
Fraction of inspired oxygen
- GA
Gestational age
- PDA
Patent ductus arteriosus
- SGA
Small for gestational age
- SIP
Spontaneous intestinal perforation
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudolph AM. The changes in the circulation after birth. Their importance in congenital heart disease. Circulation. 1970;41:343–59. doi: 10.1161/01.cir.41.2.343. [DOI] [PubMed] [Google Scholar]
- 2.Smith GC. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- 3.Heymann MA, Rudolph AM. Control of the ductus arteriosus. Physiol Rev. 1975;55:62–78. doi: 10.1152/physrev.1975.55.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Campbell M. Natural history of persistent ductus arteriosus. Br Heart J. 1968;30:4–13. doi: 10.1136/hrt.30.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med. 1976;295:526–9. doi: 10.1056/NEJM197609022951003. [DOI] [PubMed] [Google Scholar]
- 6.Benitz W. Learning to live with patency of the ductus arteriosus in preterm infants. J Perinatol. 2011;31:S42–8. doi: 10.1038/jp.2010.175. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe GL, Thalme B, Larsson KS. Studies on closure of the ductus arteriosus. XI. Ductal closure in utero by a prostaglandin synthetase inhibitor. Prostaglandins. 1974;8:363–8. doi: 10.1016/0090-6980(74)90110-5. [DOI] [PubMed] [Google Scholar]
- 8.Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med. 1976;295:526–9. doi: 10.1056/NEJM197609022951003. [DOI] [PubMed] [Google Scholar]
- 9.Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr. 1999;135:733–8. doi: 10.1016/s0022-3476(99)70093-4. [DOI] [PubMed] [Google Scholar]
- 10.Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol. 2006;26:93–9. doi: 10.1038/sj.jp.7211429. [DOI] [PubMed] [Google Scholar]
- 11.Gordon PV, Herman AC, Marcinkiewicz M, Gaston BM, Laubach VE, Aschner JL. A neonatal mouse model of intestinal perforation: investigating the harmful synergism between glucocorticoids and indomethacin. J Pediatr Gastroenterol Nutr. 2007;45:509–19. doi: 10.1097/MPG.0b013e3181558591. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–85. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 13.Pacifici GM. Clinical pharmacology of indomethacin in preterm infants: implications in patent ductus arteriosus closure. Paediatr Drugs. 2013;15:363–76. doi: 10.1007/s40272-013-0031-7. [DOI] [PubMed] [Google Scholar]
- 14.Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA. 1996;275:539–44. [PubMed] [Google Scholar]
- 15.van der Lugt NM, Lopriore E, Bokenkamp R, Smits-Wintjens VE, Steggerda SJ, Walther FJ. Repeated courses of ibuprofen are effective in closure of a patent ductus arteriosus. Eur J Pediatr. 2012;171:1673–7. doi: 10.1007/s00431-012-1805-6. [DOI] [PubMed] [Google Scholar]
- 16.Poon G. Ibuprofen lysine (NeoProfen) for the treatment of patent ductus arteriosus. Proc (Bayl Univ Med Cent) 2007;20:83–5. doi: 10.1080/08998280.2007.11928244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatli MM, Kumral A, Duman N, Demir K, Gurcu O, Ozkan H. Spontaneous intestinal perforation after oral ibuprofen treatment of patent ductus arteriosus in two very-low-birthweight infants. Acta Paediatr. 2004;93:999–1001. doi: 10.1111/j.1651-2227.2004.tb02702.x. [DOI] [PubMed] [Google Scholar]
- 18.Rao R, Bryowsky K, Mao J, Bunton D, McPherson C, Mathur A. Gastrointestinal complications associated with ibuprofen therapy for patent ductus arteriosus. J Perinatol. 2011;31:465–70. doi: 10.1038/jp.2010.199. [DOI] [PubMed] [Google Scholar]
- 19.Peitz GJ, Hoie EB, Hoy S, Anderson-Berry A. Repeated bowel perforations with Ibuprofen lysine: a case report. J Pediatr Pharmacol Ther. 2008;13:166–9. doi: 10.5863/1551-6776-13.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young S. Lundbeck Inc. announces the voluntary nationwide recall of two lots of NeoProfen (ibuprofen lysine) injection. U.S. Food and Drug Administration; 2010. Available at: http://www.fda.gov/Safety/Recalls/ucm220771.htm. [Google Scholar]
- 21.Thomas RL, Parker GC, Van Overmeire B, Aranda JV. A meta-analysis of ibuprofen versus indomethacin for closure of patent ductus arteriosus. Eur J Pediatr. 2005;164:135–40. doi: 10.1007/s00431-004-1596-5. [DOI] [PubMed] [Google Scholar]
- 22.Yang EM, Song ES, Choi YY. Comparison of oral Ibuprofen and intravenous indomethacin for the treatment of patent ductus arteriosus in extremely low birth weight infants. J Pediatr (Rio J) 2013;89:33–9. doi: 10.1016/j.jped.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Katakam LI, Cotten CM, Goldberg RN, Dang CN, Smith PB. Safety and effectiveness of indomethacin versus ibuprofen for treatment of patent ductus arteriosus. Am J Perinatol. 2010;27:425–9. doi: 10.1055/s-0029-1243371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Jr, Smith PB, et al. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–21. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissaud O, Guichoux J. Patent ductus arteriosus in the preterm infant: a survey of clinical practices in French neonatal intensive care units. Pediatr Cardiol. 2011;32:607–14. doi: 10.1007/s00246-011-9925-8. [DOI] [PubMed] [Google Scholar]
- 26.Guimaraes H, Rocha G, Tome T, Anatolitou F, Sarafidis K, Fanos V. Non-steroid anti-inflammatory drugs in the treatment of patent ductus arteriosus in European newborns. J Matern Fetal Neonatal Med. 2009;22 (Suppl 3):77–80. doi: 10.1080/14767050903198314. [DOI] [PubMed] [Google Scholar]
- 27.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 28.Peng C-YJ, Lee KL, Ingersoll GM. An introduction to logistic regression analysis and reporting. J Educ Res. 2002;96:3–14. [Google Scholar]
- 29.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2013;4:CD003481. doi: 10.1002/14651858.CD003481.pub5. [DOI] [PubMed] [Google Scholar]
- 30.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–81. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 31.Su PH, Chen JY, Su CM, Huang TC, Lee HS. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatr Int. 2003;45:665–70. doi: 10.1111/j.1442-200x.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 32.Tashiro J, Wang B, Sola JE, Hogan AR, Neville HL, Perez EA. Patent ductus arteriosus ligation in premature infants in the United States. J Surg Res. 2014;190:613–22. doi: 10.1016/j.jss.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–21. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]