Abstract
Objective
To characterize the population pharmacokinetic (PK) of oral methadone in neonates requiring pharmacologic treatment of neonatal abstinence syndrome (NAS) and to develop a PK model towards an evidence-based treatment protocol.
Study design
Based on a methadone dosing protocol, serum concentrations of methadone and its metabolites were assessed via high performance liquid chromatography-tandem mass spectrometry from dried blood spots. Population PK analysis was performed to determine the volume of distribution and clearance of oral methadone. Methadone plasma concentration-time profiles were simulated from the deduced PK model to optimize the dosing regimen.
Results
There was substantial inter-individual variability in methadone concentrations. Blood concentrations of methadone were best described by a one-compartment model with first-order absorption. The population mean estimates (coefficient of variation percentage) for oral clearance and volume of distribution were 8.94 (103%) L/h/70 kg and 177 (133%) L/70 kg, respectively. Optimized dosing strategies were developed based on the simulated PK profiles. We suggest a starting dose of 0.1 mg/kg per dose every 6 hours for most patients requiring pharmacologic treatment of NAS followed by an expedited weaning phase.
Conclusions
The proposed dosing regimen may reduce the cumulative dose of opioid and shorten the length of hospitalization. Future studies should aim to validate the simulated dosing schemes with clinical data and expand our understanding of the between-patient PK variability.
Trial registration
ClinicalTrials.gov: NCT01754324
Keywords: Infant, Methadone, NAS, Neonatal Abstinence Syndrome, Neonate, NONMEM, Opioid, Opiate, Pharmacokinetic, Pregnancy, Withdrawal
Neonatal abstinence syndrome (NAS) is a condition unique to the newborn period that results from the abrupt cessation of chronic intrauterine drug exposure following birth. NAS severe enough to require pharmacologic intervention most frequently occurs following opioid exposure. Chronic in utero exposure to opioids is a public health burden due to its increasing prevalence, frequent need for pharmacotherapy to mitigate signs of withdrawal, prolonged hospitalization and excessive cost 1–3. Opioid use in the United States is highly prevalent 4,5 with a 5-fold increase during pregnancy over the last decade, affecting 5.6 per 1000 births 6,7. Much of this increase may be attributed to ubiquitous narcotic prescription for pain relief generally and in pregnancy 8–10. The incidence of NAS has tripled from 1.20 to 3.39 per 1000 hospital births and is increasing in its geographic distribution 7, 11. In a recent prospective study, as many as 57% of infants born to mothers receiving opioid maintenance therapy required pharmacologic treatment, though the presence of withdrawal symptoms has been reported to be as high as 94% 1,12.
Opioid replacement is the standard treatment for severe opioid withdrawal in neonates. Many pharmacologic treatment protocols using a variety of drugs have been proposed for the treatment of NAS 13,14. No standardized, universally accepted treatment exists, though the agents commonly used in the majority of protocols involve methadone (20%) or other opioids (63%) 15. Most infants with NAS respond well to therapy with oral methadone. Strikingly, there are minimal pharmacokinetic (PK) data available to guide pharmacologic treatment strategies with oral methadone despite several studies suggesting that it may be an equivalent, if not superior alternative, to morphine in the treatment of NAS 16–18. For this reason, the Best Pharmaceuticals for Children Act (BPCA) of the US Food and Drug Administration includes methadone on its list of priority drugs requiring additional dosing data19. The PK of oral methadone has been described for adults receiving methadone for opioid dependence and the PK of intravenous methadone used for pain control has been reported for adolescents and neonates 20–22. We aimed to assess the PK of methadone after oral administration in infants progressing through a standardized step-wise methadone tapering protocol. The use of a formal treatment protocol has been shown to decrease hospital length of stay 16. However, understanding the PK of oral methadone is required for designing evidence-based treatment protocols.
METHODS
Institutional review boards at Cincinnati Children’s Hospital Medical Center, University of Cincinnati Medical Center and Mercy Hospital Anderson (Cincinnati, Ohio) all approved the study protocol. Informed consent was obtained from the legal guardians of all the subjects enrolled. Each participant had a history of chronic intrauterine exposure to opioids and required pharmacologic treatment with oral methadone to mitigate signs of NAS. Acutely ill neonates, infants with congenital abnormalities or medical illnesses necessitating opioid treatment for conditions other than NAS, and infants who were wards of the state were excluded.
The decision to treat infants exposed to opioids in utero rested with the medical team and was predicated upon the severity of withdrawal symptoms assessed using the Finnegan Neonatal Abstinence Scoring Tool (Finnegan) in all recruitment sites. Administrators of the Finnegan were educated using the D’Apolito Reliability Training (including an interactive DVD and proctored scoring) 16,23. In general, hospital protocols dictated that Finnegan scores be assigned every 3–4 hours starting within 24 hours of age for all infants exposed to opioids. Neonates with scores ≥8 were transferred to the neonatal intensive care unit for closer observation. Particular effort was exerted to minimize external stimulation including the use of dimmed ambient lighting and swaddling per unit protocol. Pharmacologic therapy with oral methadone was initiated in neonates having three consecutive scores ≥8 or two consecutive scores ≥12 in a 24-hour period. Administrators of Finnegan scores were not masked to prior scores or treatment.
The dosing guidelines for medications used were the same at all participating institutions and for all patients enrolled in the study (Table I). In brief, all infants were started on oral methadone treatment at 0.05 mg/kg/dose every 6 hours. Infants who responded with reduced withdrawal scores over the first 24 hours were weaned to 0.04 mg/kg/dose every 6 hours (Step 2) and continued with the step-wise dosage decreases outlined (Scheme 1). The clinical team was at liberty to wean the methadone dose more quickly than scheduled per protocol if there was concern for somnolence or consistently low Finnegan scores. This occurred twice and was done after study-related blood samples were obtained. In contrast, when the Finnegan scores failed to abate over the first 24 hours of treatment, the methadone dose was increased to 0.1 mg/kg/dose every 6 hours (Step 1A) and was subsequently weaned per protocol after stabilization of withdrawal scores (Scheme 2). Infants who failed to tolerate weaning the methadone dose every 24 to 48 hours or who backslid on dosing to recapture recurrent symptoms were started on phenobarbital. Subjects receiving phenobarbital were given an oral loading dose of 10 mg/kg followed by a daily dose of 5 mg/kg. The timing of adjunctive therapy with phenobarbital initiation was at the discretion of the clinical team. For the purposes of this study, the time to capture symptoms was documented. The time to capture was defined as the time required to attain two consecutive decreases in Finnegan scores below 12 (if therapy was initiated for 2 consecutive scores ≥12) or two consecutive decreases below 8 (if therapy was initiated for 3 consecutive scores ≥8) after initiation of pharmacotherapy. Blood specimens for PK studies (clearance and volume of distribution) were obtained within 72 hours of commencement of oral methadone regardless of the dosing scheme.
Table 1.
Oral methadone dosing scheme
| Taper Step | Dose (mg/kg) | Frequency | Number of Doses |
|---|---|---|---|
| 1 | 0.05 | q6 | x4 |
| 1A* | 0.1 | q6 | x4 |
| 1B* | 0.075 | q6 | x4 |
| 1C* | 0.05 | q6 | x4 |
| 2 | 0.04 | q6 | x4 |
| 3 | 0.03 | q6 | x4 |
| 4 | 0.02 | q6 | x4 |
| 5 | 0.02 | q8 | x3 |
| 6 | 0.02 | q12 | x4 |
| 7 | 0.01 | q12 | x4 |
| 8 | 0.01 | q24 | x2 |
Scheme 2: Used only for infants that are recalcitrant to Scheme 1 (weaning directly from Step 1 to Step 2).
We used a D-optimal sparse blood sampling design with nonparametric population modeling to allow for PK variable estimation while minimizing the need for frequent blood sampling. Three or four blood specimens were collected from each patient. The first 3 specimens involved timed collections related to a single methadone dosing (just before a dose of methadone, 1–2 hours after that dose and just prior to the next dose). Most families consented to an optional fourth blood sample obtained just prior to a methadone dose after the participant failed to respond to treatment as anticipated. Concentrations of methadone and its biologically inactive metabolites in dried blood spots on Guthrie cards were determined using a high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay at the University of Colorado, Aurora, CO, as previously published 24–26. The lower limit of quantitation was 0.25 ng/mL for methadone and 0.1 ng/mL for 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP) and 2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline (EMDP).
Outcome Measures
The primary PK outcome measure was clearance of oral methadone from blood. Large between-subject variability in serum methadone concentrations has been reported in PK studies of oral methadone in adults, a finding that is presumed to be valid in infants 27,28. In order to better understand the scope of this variability in neonates and identify the sample size needed to power a full characterization study of methadone in the neonate, a goal enrollment of 20 patients was selected for this pilot study. This was sufficient to achieve precision criteria recommendations for pediatric PK studies, giving 95% confidence intervals for mean estimates of methadone clearance and volume of distribution with at least 80% power 29. Secondary outcome measures included length of hospitalization, need for adjunctive pharmacologic treatment, thirty day hospital readmission rate, sustained clinical resolution of symptoms after discharge from the hospital as assessed by 2 week follow-up phone call and associations between the serum concentrations of methadone and its metabolites (EDDP and EMDP) and clinical response to a standardized tapering protocol.
Pharmacokinetic Analyses and Simulation
Population PK analysis was performed by non-linear mixed effects modeling with NONMEM software (version 7.2.0, ICON, Ellicot City, MD). NONMEM uses a combination of “fixed” effects (like volume of distribution and clearance) and “random” effects (like random error and differences between individuals and observations) to describe PK variables for a population. During model development, the data were assessed for fit with one- and two-compartment models. The models were then evaluated for goodness of fit and stability. Additional details and rationale of the model development including adjustment for age and maturation are described in the Appendix (available at www.jpeds.com). The final one-compartment PK model was used to simulate one thousand methadone blood concentration-time profiles in order to design a new dosing strategy. An assumed body weight of 3 kg was used as a typical value and population means for the area under the curve (AUC) at 24, 48 and 72 hours of methadone treatment were generated for dosing Schemes 1 and 2 (Table I). The AUC was used to assess the extent of drug exposure with respect to time in this cohort of patients. A target AUC for the simulated dosing regimens was determined post-hoc by analyzing the AUC population means and medians and correlating them with Finnegan scores (a marker for clinical response) to describe overall drug effect. New simulated dosing regimens were devised to achieve the target AUC in the first 24–48 hours of therapy.
Statistical Analyses of Secondary Outcomes
We examined relationships among patient demographics, length of stay, and pharmacologic variables. Spearman rank correlation was used to test the relationship between infant cord blood methadone concentrations and length of hospital stay. Wilcoxon rank sum test was used to test for differences in infant blood methadone concentrations by need for higher initial dose (Scheme 2) or inability to progress through the protocol. All statistical analyses were performed with SAS software (version 9.3, SAS institute, Cary, NC).
RESULTS
Of the 64 neonates exposed to opioids December 2012 to October 2013, 34 were excluded (26 infants did not require pharmacologic treatment, 2 were preterm, 3 were wards of the state, 3 were treated with buprenorphine) and 2 infants were missed. Eight of the remaining 28 patients declined participation in the study. As a result, twenty term neonates, comprising 71% of eligible subjects, were enrolled in the study (Table II; available at www.jpeds.com).
Table 2.
Characteristics of study participants (N=20)
| Male, N (%) | 9 (45) |
| Non-hispanic White, N (%) | 20 (100) |
| Gestational age in weeks, Median [IQR] | 38 [37.4, 39.5] |
| Birth weight in kg, Median [IQR] | 3.0 [2.6, 3.2] |
| Mode of Delivery | N (%) |
| Vaginal | 16 (80) |
| Cesarean | 4 (20) |
| Drug of exposure | N (%) |
| Methadone | 10 (50) |
| Heroin | 7 (35) |
| Buprenorphine | 6 (30) |
| Other opioids (Oxycodone, Hydrocodone) | 7 (35) |
| Benzodiazepines | 4 (20) |
| Marijuana | 4 (20) |
| Cocaine | 2 (10) |
| Tobacco | 18 (90) |
| Multiple opioids, N (%) | 12 (60) |
| Opioid plus other substances of abuse, N (%) | 8 (40) |
| Age in hours at initiation of methadone, Median [IQR] | 32.2 [27.2, 42.7] |
| Finnegan scores, Median [IQR] | 13.0 [11.0, 14.2] |
| Time to capture in days, Median [IQR] | 1.4 [0.90, 1.7] |
| Breastfeeding initiated, N (%) | 4 (20) |
| Discharge weight in kg, Median [IQR] | 3.4 [3.0, 3.7] |
Abbreviations: N (number of patients), IQR (interquartile range)
Population PK Analysis
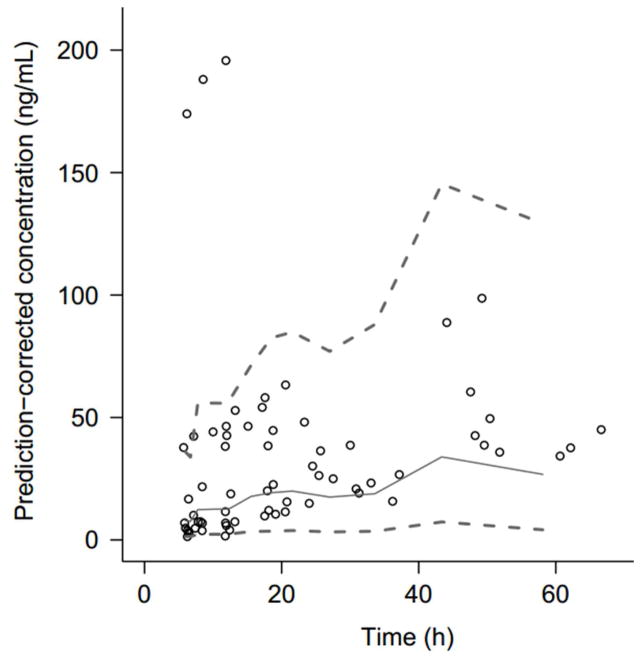
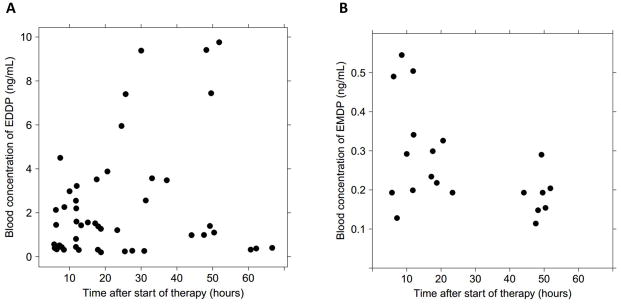
Seventy-one blood specimens were obtained from 20 patients and included 11 optional fourth blood samples. The 11 conditional specimens were collected after either therapy escalation to Scheme 2 (n=6) or after failure to tolerate weans (n=5). Specimens obtained after failure to tolerate weans were not included in the final PK model because they were confounded by increased body weight >25% in that subset of patients. Therefore, only serial PK data generated within 4 days of treatment initiation were used for the development of the final population PK model. The large fluctuation in body weight may be attributed to catch-up growth after growth restriction in utero and hyperphagia secondary to withdrawal. Patients on an ad lib feeding schedule would often consume 180–220 ml/kg/day. At the time of blood sampling, no patients were receiving phenobarbital. Concentrations of methadone were highly variable, ranging from 1.47 ng/mL to 198 ng/mL (Figure 1). Of note, the three specimens ranging between 174 and 198 ng/mL were obtained from the same subject. Methadone metabolite concentration profiles mirrored the inter-individual variability noted with the parent drug (Figure 2; available at www.jpeds.com).
Figure 1.
Observed methadone blood concentrations versus time. Solid line represents the population mean. Dashed lines represent the 5th and 95th percentiles.
Figure 2.
Observed blood concentrations of methadone metabolites versus time. (A) Blood EDDP concentration vs. time. (B) Blood EMDP concentration vs. time. Blood concentrations were below the limit of quantitation for EDDP in 6 patients and for EMDP in 14 patients.
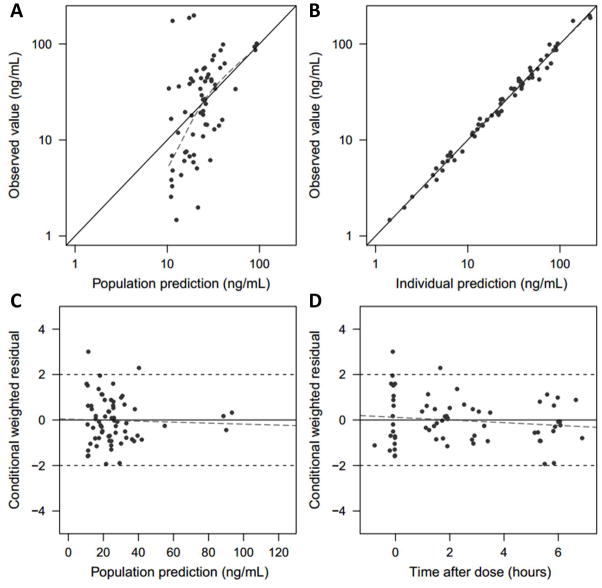
The concentration profile of methadone was best described with a one-compartment model with first order absorption and lag-time. The diagnostic plots affirm that the PK profiles are well captured in the final PK model (Figure 3; available at www.jpeds.com). Estimated PK variables are summarized in Table III as well as the median values and 95% confidence intervals from the nonparametric percentile bootstrap analysis, which indicate the stability of the model. The population mean values (coefficient of variation percentage) for clearance and volume of distribution were 8.94 (103%) L/h/70 kg and 177 (133%) L/70 kg, respectively. A PK analysis excluding blood concentrations greater than the 95th percentile was also performed; however, clearance estimates were not greatly affected (9.33 vs 8.94 L/h/70 kg) though between-subject variability decreased (87.6% vs 103%). Therefore, all data were included in the reported estimates.
Figure 3.
Goodness-of -fit plots for the final model. (A) Observed value vs. population predicted value, (B) Observed value vs. individual predicted value, (C) Conditional weighted residuals (CWRES) vs. population predicted value and (D) CWRES vs. time after the dose.
Table 3.
Variable estimates for the final population PK model
| Estimates | %RSE | Bootstrap analysis (n=1000) | ||
|---|---|---|---|---|
| Median | 95% CI | |||
| CL/F in L/h/70 kg | 8.94 | 19 | 8.37 | 4.73 – 12.8 |
| CL/F in L/h/kg | 0.37 | |||
| Vd/F in L/70 kg | 177 | 40 | 174 | 37.2 – 428 |
| Vd/F in L/kg | 2.53 | |||
| Ka in h−1 | 0.334 | 24 | 0.346 | 0.140 – 0.659 |
| Lag time in h | 0.827 | 33 | 0.837 | 0.0956 – 1.14 |
| Interindividual variability, CV% | ||||
| IIV for CL/F | 103 | 14 | 99.9 | 50.5 –132 |
| IIV for Vd/F | 133 | 19 | 130 | 79.1 – 161 |
| Residual variability, CV% | ||||
| Proportional error | 15.7 | 10 | 15.6 | 12.4 – 18.1 |
| Simulated AUC for Scheme 1 | Median | IQR | ||
| AUC0–24 h in ng·h/mL | 316 | 166–584 | ||
| AUC0–48 h in ng·h/mL | 816 | 459–1557 | ||
| AUC0–72 h in ng·h/mL | 1378 | 768–1583 | ||
| Simulated AUC for Scheme 2 | ||||
| AUC0–24 h in ng·h/mL | 333 | 183–629 | ||
| AUC0–48 h in ng·h/mL | 1257 | 697–2287 | ||
| AUC0–72 h in ng·h/mL | 2274 | 1238–4253 | ||
Abbreviations: CL (clearance), CV (coefficient of variation), F (bioavailability), IIV (inter-individual variability), ka (absorption rate constant), Vd (volume of distribution), RSE (relative standard error), CI (confidence interval), AUC (area under the concentration-time curve), IQR (Interquartile range).
PK Simulation for the Dosing Regimen Design
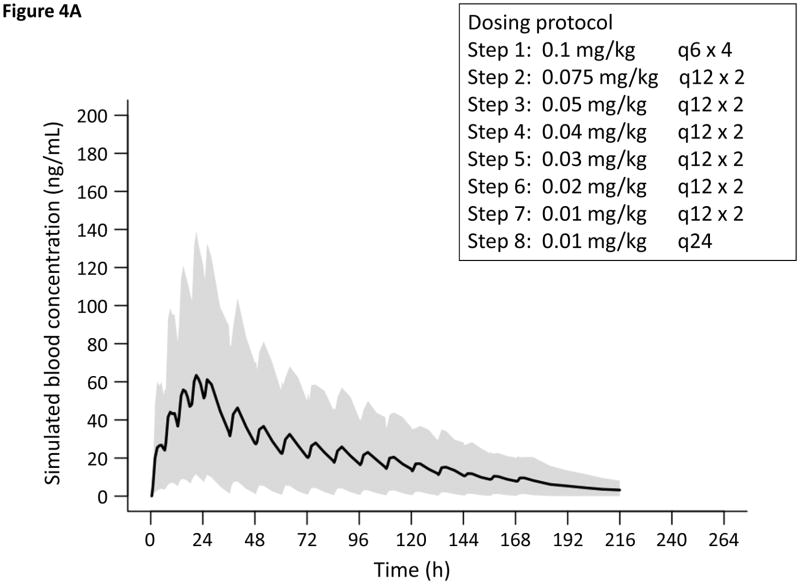
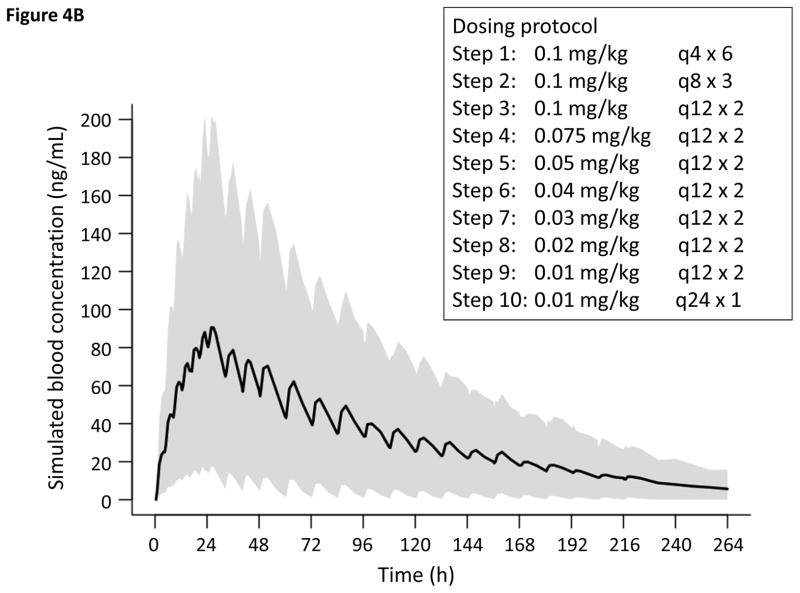
On the basis of the observed population PK and clinical response to treatment, we endeavored to model new dosing regimens (Figure 4). Infants treated with our current standard dosing protocol (Scheme 1, n = 12) had Finnegan scores that normalized within the first 48 hours, whereas it took patients treated with the higher dose schedule (Scheme 2, n = 8) approximately 72 hours for scores to stabilize. Therefore, we postulated a dose exposure between the mean and median AUC after 48 hours for Scheme 1 patients (1261 and 816 ng•h/mL, respectively) and 72 hours for Scheme 2 patients (3375 and 2274 ng•h/mL, respectively) to be a reasonable target for the mitigation of withdrawal signs. We aimed to achieve the target AUC expeditiously using two clinically convenient dosing regimens while minimizing the risk of potential adverse effects such as respiratory depression. One thousand iterations were used to fully predict the distribution of the simulated methadone concentration profiles using the final PK model and the newly-proposed dosing regimens (Figures 4) 30. From this simulation, the AUC at 24 hours for the new Scheme 1 is 985 ± 1020 ng•h/mL and the AUC at 48 hours for the new Scheme 2 is 3073 ± 2979 ng•h/mL, suggesting that the time to attain target blood concentrations could be decreased by approximately 24 hours.
Figure 4.
Simulated PK of oral methadone for a term 3-kilogram infant based on the final PK model. Solid line and gray shaded area represents the predicted mean and range (10th–90th percentile), respectively of methadone plasma concentrations from 1000 simulations. Concentration vs. time for new tapering in A, Scheme 1 and B, Scheme 2.
Secondary Outcomes
Clinical outcomes are summarized in Table IV (available at www.jpeds.com). We obtained umbilical cord blood for 9 of the 10 infants delivered to mothers on methadone at the time of delivery but the methadone concentrations (63.2 ± 55.4 ng/mL) were not significantly correlated with length of stay (Spearman ρ =0.45, p=0.22). The median length of hospitalization for all patients enrolled was 18.5 days (IQR: 16, 30). Ten participants (50%) failed to wean on at least one step of the protocol, 8 (40%) required the higher dosing protocol (Scheme 2) and 4 (20%) experienced both. Infants that required the higher dosing schedule because they could not progress through the weaning protocol had higher serum methadone concentrations at 24 hours of treatment, though these differences were not statistically significant (45.3 ± 33.9 ng/mL vs 41.2 ± 61.7 ng/mL, p=0.86). Eleven (55%) of enrolled subjects received adjunctive pharmacologic treatment (phenobarbital) but none required hospital readmission within thirty days of discharge. Eleven participants responded to follow-up phone calls to assess sustained resolution of withdrawal symptoms. The most common residual symptoms were jitteriness (8/11), fussiness (6/11) and increased muscle tone (4/11). No complaints of seizure, diarrhea or feeding difficulty were reported.
Table 4.
Clinical Outcomes
| Value | Variation or Percent | |
|---|---|---|
| Median length of hospitalization in days | 18.5 | IQR [16, 30] |
| Median length of opioid therapy in days | 14 | IQR [11, 23] |
| Mean Finnegan score 24 hr prior to initiation of methadone therapy | 10.8 | SD (1.6) |
| Mean peak Finnegan score | 15.6 | SD (3) |
| Mean time to capture in days | 1.4 | SD (0.6) |
| Patients who received standard treatment (Scheme 1) | 12 | 60% |
| Patients who received standard treatment (Scheme 1, no adjunct) | 6 | 30% |
| Patients who needed escalated dose (Scheme 2) | 8 | 40% |
| Patients who failed to progress through protocol (Backslid one or more steps) | 10 | 50% |
| Patients who needed adjunctive therapy | 11 | 55% |
| Readmission within 30 days of discharge | 0 | 0 |
Abbreviations: IQR (interquartile range), SD (standard deviation)
DISCUSSION
Our study assessed the PK of oral methadone in neonates. The population PK analysis suggests that clearance and volume of distribution normalized by allometrically scaled body weights are comparable with adult values and are congruent with recently published data on intravenous methadone 20–22,31. Ward et al reported the clearance of intravenous methadone to be 9.1 L/h/70 kg with a volume of distribution of 581 L/70 kg in neonates, and Stemland et al demonstrated a clearance of 9.33 L/h/70 kg and volume of distribution of 570 L/70 kg in adolescents 21,22. By way of comparison, Foster et al reported the clearance and volume of distribution for oral methadone in adults with opioid dependence to be 8.5 L/h/70 kg and 433 L/70 kg respectively 20. Minor differences in the observed volume of distribution as compared with studies involving intravenous methadone are likely due to differences in bioavailability and the use of a one-compartment model as opposed to the two- or three-compartment models used in other published reports.
Strengths of our study include the use of minimally invasive techniques to obtain specimens (dried blood spot sampling) and reducing disruption to patient care (sparse sampling design). This study demonstrates the feasibility of obtaining methadone concentrations from dried blood spots in the clinical setting. This modality requires much less blood from the patient (3–4 drops), does not necessitate venipuncture that is often required for traditional pharmacologic studies and appeared to be an important consideration for parents who were concerned about discomfort and phlebotomy blood loss. Additionally, blood sampling may be synchronized with clinical care in a sparse sampling design. We feel this was very important to a population of neonates who are often hypersensitive to interventions and stimulations. The high congruence with other neonatal data for intravenous methadone also strengthens confidence in the population PK findings. Subsequently, the new simulation-derived treatment protocols have been adopted by most treatment centers in the Cincinnati area, including all of the study sites.
Potential benefits of implementing the newly-proposed dosing schemes include a shortened length of time to progress through the treatment protocol by three days, decreased cumulative drug exposure by up to 10% and reduction in total drug doses by up to 48% which minimizes the chance for dosing error, lessened labor (staff time) and potentially lowered cost of care (Figure 4). An argument could be made that the high number of infants requiring management with phenobarbital (55%) might suggest the need to be more conservative with weaning protocols. However, this is likely a function of the small sample size, as it differs from its baseline use of phenobarbital in the participating institutions (27%).
In the proposed PK-based dosing protocols, our model predicts that the AUC targets will be achieved in the first 24 or 48 hours as opposed to 48 or 72 hours needed for the old treatment tapers. Down titration for both dosing schemes involve moderate decreases in dose every 24 hours based on signs or withdrawal scores until a dose of 0.01 mg/kg every 24 hours is attained, after which the medication is discontinued (Figure 4). The proposed protocol changes would increase early dose exposure and the peak methadone concentration, thereby decreasing the frequency at which treatment escalation is needed. We suggest that Scheme 2 be considered in the initial treatment of infants with scores ≥12, because the mean Finnegan score for infants requiring the dose escalation strategy during the 24 hours prior to treatment was 11 ± 1.6.
Somnolence and respiratory depression are very rare in monitored settings, but with higher initial dosing in the new treatment schemes, it would be prudent for practitioners wishing to implement the protocol to avoid extension to an outpatient treatment setting until additional safety studies are available.
With the evidence-based PK model for oral methadone proposed in this study, future investigations may focus on outcomes associated with the proposed treatment protocols, addressing the large inter-individual variability in methadone blood concentrations with more robust sampling and comparison studies with other first-line therapies such as morphine. As bedside point-of-care LC-MS/MS technology is developed and validated, future studies could also investigate the feasibility and utility of obtaining real-time drug concentrations that, in combination with model-based Bayesian interpretation, can be used to implement personalized treatment strategies 32.
Study limitations include the small sample size inherent in a pilot study like ours. Also, there is no validated, completely objective scoring system to assess clinical response to pharmacologic therapy in NAS; however, we endeavored to minimize inter-observer variability by ensuring that all staff ascribing Finnegan scores underwent training in the scoring system. Additionally, the large inter-individual variability in methadone concentrations limits the predictability of individual response to our newly-proposed methadone regimens that are based on population estimates. Though the dosing strategies offer an improved, evidence-based approach to the pharmacologic treatment of NAS with oral methadone, a larger study is needed to acquire further PK data and formulate more individualized dosing strategies. Also, a one-compartment model for oral methadone best described the PK profiles in this study, in contrast to population PK studies of intravenous methadone that reported two- and three-compartment models 22,31. The difference between sparse and frequent sampling study designs may explain this discrepancy. Only 3 or 4 blood specimens were analyzed per participant in this study, in contrast to the ≥10 plasma concentrations that were often obtained per patient in the cited studies, which enabled the authors to describe early methadone distribution to multiple compartments and the ability to utilize more complex models. However, our simpler one-compartment model adequately characterized the methadone concentration profiles and is sufficient to predict methadone concentrations and exposure (AUC) in neonates.
Based on data from this pilot study, we propose modifications to the current treatment protocol using oral methadone in order to optimize care and decrease hospital length of stay. Due to substantial inter-individual PK variability and clinical response, a larger study is warranted to address gaps in PK and pharmacodynamic knowledge, to better characterize demographic factors that are predictive of severity of withdrawal symptoms, establish pharmacogenetic data, develop biomarkers for therapeutic response to oral methadone and advance the development of bedside personalized dosing strategies.
Acknowledgments
J.W. was supported by the National Institutes of Health (5T32HD069054 at Cincinnati Children’s Hospital Medical Center). T.M. was supported by the Japan Research Foundation for Clinical Pharmacology and the Uehara Memorial Foundation.
We thank Elizabeth Tisdale, NNP, for assistance in patient recruitment and Melissa Hancock in the clinical lab and to the nursing staff at the study sites.
Abbreviations
- AUC
Area Under the Curve
- EDDP
2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine
- EMDP
2-ethyl-5-methyl-3,3-diphenyl-1-pyrroline
- NICU
Newborn Intensive Care Unit
- NAS
Neonatal Abstinence Syndrome
- PK
Pharmacokinetic
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–560. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 2.Kraft WK, van den Anker JN. Pharmacologic management of the opioid neonatal abstinence syndrome. Pediatr Clin North Am. 2012;59:1147–1165. doi: 10.1016/j.pcl.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiles JR, Isemann B, Ward LP, Vinks AA, Akinbi H. Current Management of Neonatal Abstinence Syndrome Secondary to Intrauterine Opioid Exposure. J Pediatr. 2014;165:440–446. doi: 10.1016/j.jpeds.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes MJ, Brown MS. Epidemic of prescription opiate abuse and neonatal abstinence. JAMA. 2012;307:1974–1975. doi: 10.1001/jama.2012.4526. [DOI] [PubMed] [Google Scholar]
- 5.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidem Dr S. 2008;17:997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- 6.Kellogg A, Rose CH, Harms RH, Watson WJ. Current trends in narcotic use in pregnancy and neonatal outcomes. Am J Obstet Gynecol. 2011;204:259 e251–254. doi: 10.1016/j.ajog.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 7.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307:1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 8.Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63:563–568. [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein RA, Bobo WV, Martin PR, Morrow JA, Wang W, Chandrasekhar R, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol. 2013;23:498–503. doi: 10.1016/j.annepidem.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrick SW, Dudley J, Martin PR, Harrell FE, Warren MD, Hartmann KE, et al. Prescription Opioid Epidemic and Infant Outcomes. Pediatrics. 2015;135:842–850. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015 doi: 10.1038/jp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson L, Velez M, Harrow C. The opioid exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5:47–55. [PMC free article] [PubMed] [Google Scholar]
- 14.Grim K, Harrison T, Wilder R. Management of neonatal abstinence syndrome from opioids. Clin Perinatol. 2013;40:509–524. doi: 10.1016/j.clp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol. 2006;26:15–17. doi: 10.1038/sj.jp.7211427. [DOI] [PubMed] [Google Scholar]
- 16.Hall ES, Wexelblatt SL, Crowley M, Grow JL, Jasin LR, Klebanoff MA, et al. A Multicenter Cohort Study of Treatments and Hospital Outcomes in Neonatal Abstinence Syndrome. Pediatrics. 2014;134:e527–534. doi: 10.1542/peds.2013-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patrick SW, Kaplan HC, Passarella M, Davis MM. Variation in treatment of neonatal abstinence syndrome in US Children’s Hospitals, 2004–2011. J Perinatol. 2014;34:867–872. doi: 10.1038/jp.2014.114. [DOI] [PubMed] [Google Scholar]
- 18.Brown MS, Hayes MJ, Thornton LM. Methadone versus morphine for treatment of neonatal abstinence syndrome: A prospective randomized clinical trial. J Perinatol. 2015;35:278–283. doi: 10.1038/jp.2014.194. [DOI] [PubMed] [Google Scholar]
- 19.Wiles JR, Vinks AA, Akinbi H. Federal legislation and the advancement of neonatal drug studies. J Pediatr. 2013;162:12–15. doi: 10.1016/j.jpeds.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster DJ, Somogyi AA, White JM, Bochner F. Population pharmacokinetics of (R)-, (S)-‚ and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2004;57:742–755. doi: 10.1111/j.1365-2125.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stemland CJ, Witte J, Colquhoun DA, Durieux ME, Langman LJ, Balireddy R, et al. The pharmacokinetics of methadone in adolescents undergoing posterior spinal fusion. Pediatr Anesth. 2013;23:51–57. doi: 10.1111/pan.12021. [DOI] [PubMed] [Google Scholar]
- 22.Ward RM, Drover DR, Hammer GB, Stemland CJ, Kern S, Tristani-Firouzi M, et al. The pharmacokinetics of methadone and its metabolites in neonates, infants, and children. Pediatr Anesth. 2014;24:591–601. doi: 10.1111/pan.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas K, Knobel RB. Implementing practice guidelines and education to improve care of infants with neonatal abstinence syndrome. Adv Neonatal Care. 2012;12:40–45. doi: 10.1097/ANC.0b013e318241bd73. [DOI] [PubMed] [Google Scholar]
- 24.Clavijo CF, Hoffman KL, Thomas JJ, Carvalho B, Chu LF, Drover DR, et al. A sensitive assay for the quantification of morphine and its active metabolites in human plasma and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;400:715–728. doi: 10.1007/s00216-011-4775-z. [DOI] [PubMed] [Google Scholar]
- 25.Clavijo CF, Thomas JJ, Cromie M, Schniedewind B, Hoffman KL, Christians W, et al. A low blood volume LC-MS/MS assay for the quantification of fentanyl and its major metabolites norfentanyl and despropionyl fentanyl in children. J Sep Sci. 2011;34:3568–3577. doi: 10.1002/jssc.201100422. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RR, Hoffman KL, Schniedewind Br, Clavijo C, Galinkin JL, Christians U. Comparison of the quantification of acetaminophen in plasma, cerebrospinal fluid and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. J Pharmaceut Biomed. 2013;83:1–9. doi: 10.1016/j.jpba.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fredheim O, Moksnes K, Borchgrevink P, Kaasa S, Dale O. Clinical pharmacology of methadone for pain. Acta Anaesth Scand. 2008;52:879–889. doi: 10.1111/j.1399-6576.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang F, Tong X, McCarver DG, Hines RN, Beard DA. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33:485–518. doi: 10.1007/s10928-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012;52:1601–1606. doi: 10.1177/0091270011422812. [DOI] [PubMed] [Google Scholar]
- 30.Mould DR, Upton RN. Basic concepts in population modeling, simulation and model-based drug development. CPT Pharmacometrics Syst Pharmacol. 2012;1:e6. doi: 10.1038/psp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostami-Hodjegan A, Wolff K, Hay AW, Raistrick D, Calvert R, Tucker GT. Population pharmacokinetics of methadone in opiate users: characterization of time-dependent changes. Br J Clin Pharmacol. 1999;48:43–52. doi: 10.1046/j.1365-2125.1999.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neely M, Jelliffe R. Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J Clin Pharmacol. 2010;50:842–847. doi: 10.1177/0091270009356572. [DOI] [PubMed] [Google Scholar]